Effects of Rhizobacteria Strains on Plant Growth Promotion in Tomatoes (Solanum lycopersicum)
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Rhizobacteria Strains
2.3. Plant Growth Promotion Assays
2.4. Physiological, Morphological, Chemical, and Nutritional Analysis
- Photosynthesis rate and stomatal conductance: An infrared gas analyzer (IRGA) (LCpro-SD, ADC BioScientific Ltd., Hoddesdon, UK) was used. The analysis was conducted in the culture chamber 2 h after turning on the lights. Healthy adult leaves from seven plants were randomly selected in triplicate for each treatment.
- Maximum photosystem II photochemical efficiency (Fv/Fm) and photosynthetic performance index: These were measured using a continuous excitation chlorophyll fluorimeter (Handy PEA, Hansatech Instruments Ltd., King´s Lynn, UK). In this case, the culture chamber remained in darkness for one hour before the measurement. Ten plants were randomly selected for each treatment.
- Aerial and root length: A ruler was used to measure them. The aerial and root parts were divided from the birth of the uppermost secondary root to establish both measurements.
- Aerial and root wet weight: As mentioned in the previous parameter, once the line separating the aerial and root parts was marked, they were cut and weighed separately on a scale.
- Aerial and root dry weight: The samples were dried in an oven at 65 °C for 3 days after measuring the fresh weight. After this time, the methodology described for the fresh weight was repeated.
- Total antioxidant content: The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free-radical-scavenging capacity of the leaf extracts was evaluated by adding 0.2 mL of the extract to 3.8 mL of a 0.25 mM methanolic DPPH solution. The mixture was vigorously shaken for 1 min and allowed to stand in the dark at room temperature for 30 min. The absorbance of the sample was measured using a UV–visible spectrophotometer (UV-6300PC, VWR International Europe BV, Leuven, Belgium) at a wavelength of 517 nm, with an ethanol blank serving as the reference. For the negative control, 80% methanol was employed in place of the extract. The free-radical-scavenging activity of the extracts was expressed in terms of Trolox equivalents. The results were calculated using a calibration curve and expressed in milligrams of Trolox equivalents per gram of dry weight. The linearity range of the calibration curve was 50 to 350 μg mL−1 (r = 0.998).
- Total phenolic content: Gallic acid was used as a standard. Thus, 50 μL extract was mixed with 1.5 mL of distilled water, 250 μL of Folin–Ciocalteu 2 N reagent solution, and 750 μL of 7% sodium carbonate. The obtained mixture was vortexed and incubated for 8 min at room temperature. Then, an additional 950 μL of distilled water was added and allowed to stand for 2 h at room temperature. Finally, absorbance was measured against a distilled water blank at a wavelength of 765 nm using a UV–visible spectrophotometer (UV-6300PC, VWR International Europe BV, Leuven, Belgium). Results were calculated and expressed in milligram gallic acid equivalents (mg GAE/g dry weight) using a previously established calibration curve with gallic acid concentrations from 10 to 400 μg mL−1 (r = 0.998).
- Pigment content: To prevent any acidic traces that could alter the pigment composition, 0.01 g of dry powder was combined with 2 mL of 100% acetone that had been previously saturated with calcium carbonate. After a 10 min centrifugation at 4 °C, the samples were filtered through a syringe with a 0.45 μm Millipore filter to remove impurities. Pigment quantification was conducted using a dual-beam spectrophotometer (UV-6300PC, VWR International Europe BV, Leuven, Belgium). To calculate chlorophyll a, chlorophyll b, and total carotenoids in mg g−1 dry weight, the equations given by Ruiz-Medina et al. [55] were used.
2.5. Statistical Analysis
3. Results
3.1. Soil Analysis
3.2. Physiological, Chemical, and Nutritional Analysis
3.2.1. Morphological Parameters
3.2.2. Physiological Parameters
3.2.3. Chemical Parameters
3.2.4. Nutritional Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyes-Palomino, S.; Cano-Ccoa, D. Efectos de La Agricultura Intensiva y El Cambio Climático Sobre La Biodiversidad. Rev. Investig. Altoandinas 2022, 24, 53–64. (In Spanish) [Google Scholar] [CrossRef]
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Panjiar, N.; Kumar, S.; Saxena, A.K.; Suman, A. Assessment of Genetic Diversity and Plant Growth Promoting Attributes of Psychrotolerant Bacteria Allied with Wheat (Triticum aestivum) from the Northern Hills Zone of India. Ann. Microbiol. 2015, 65, 1885–1899. [Google Scholar] [CrossRef]
- Benaissa, A. Rhizosphere: Role of Bacteria to Manage Plant Diseases and Sustainable Agriculture—A Review. J. Basic Microbiol. 2024, 64, 2300361. [Google Scholar] [CrossRef] [PubMed]
- Bhadrecha, P.; Singh, S.; Dwibedi, V. ‘A Plant’s Major Strength in Rhizosphere’: The Plant Growth Promoting Rhizobacteria. Arch. Microbiol. 2023, 205, 165. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The Role of Microbial Signals in Plant Growth and Development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus subtilis: A Plant-Growth Promoting Rhizobacterium That Also Impacts Biotic Stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in Plant Growth and Development: Roles in Abiotic Stress Tolerance and Secondary Metabolites Secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef]
- He, S.; Li, L.; Lv, M.; Wang, R.; Wang, L.; Yu, S.; Gao, Z.; Li, X. PGPR: Key to Enhancing Crop Productivity and Achieving Sustainable Agriculture. Curr. Microbiol. 2024, 81, 377. [Google Scholar] [CrossRef]
- Kour, D.; Kour, H.; Khan, S.S.; Khan, R.T.; Bhardwaj, M.; Kailoo, S.; Kumari, C.; Rasool, S.; Yadav, A.N.; Sharma, Y.P. Biodiversity and Functional Attributes of Rhizospheric Microbiomes: Potential Tools for Sustainable Agriculture. Curr. Microbiol. 2023, 80, 192. [Google Scholar] [CrossRef]
- Dame, Z.T.; Rahman, M.; Islam, T. Bacilli as Sources of Agrobiotechnology: Recent Advances and Future Directions. Green. Chem. Lett. Rev. 2021, 14, 245–270. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Yadav, A.; Yadav, A.N.; Suman, A.; Ahluwalia, A.S.; Saxena, A.K. Minerals Solubilizing and Mobilizing Microbiomes: A Sustainable Approach for Managing Minerals’ Deficiency in Agricultural Soil. J. Appl. Microbiol. 2022, 133, 1245–1272. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Rincón, L.; Aristizábal-Gutiérrez, F. Dinámica Del Ciclo Del Nitrógeno y Fósforo En Suelos. Rev. Colomb. Biotecnol. 2012, 14, 285–295. (In Spanish) [Google Scholar]
- Azeem, M.; Javed, S.; Zahoor, A.F. Bacillus Species as Potential Plant Growth Promoting Rhizobacteria for Drought Stress Resilience. Russ. J. Plant Physiol. 2023, 70, 59. [Google Scholar] [CrossRef]
- Mathur, A.; Koul, A.; Hattewar, J. Plant Growth-Promoting Rhizobacteria (PGPRs): Significant Revolutionary Tools for Achieving Long-Term Sustainability and Combating the Biotic Stress Caused by the Attack of Pathogens Affecting Crops in Agriculture. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management. Microorganisms for Sustainability; Sayyed, R., Ed.; Springer: Singapore, 2019; pp. 379–388. [Google Scholar] [CrossRef]
- Khan, A.R.; Mustafa, A.; Hyder, S.; Valipour, M.; Rizvi, Z.F.; Gondal, A.S.; Yousuf, Z.; Iqbal, R.; Daraz, U. Bacillus spp. as Bioagents: Uses and Application for Sustainable Agriculture. Biology 2022, 11, 1763. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus Species in Soil as a Natural Resource for Plant Health and Nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus spp. in Disease Suppression and Growth Growth Promotion of Field Vegetables Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Potential Use of Bacillus spp. as an Effective Biostimulant against Abiotic Stresses in Crops—A Review. Curr. Res. Biotechnol. 2023, 5, 100128. [Google Scholar] [CrossRef]
- Choudhary, M.; Meena, V.S.; Yadav, R.P.; Parihar, M.; Pattanayak, A.; Panday, S.C.; Mishra, P.K.; Bisht, J.K.; Yadav, M.R.; Nogia, M.; et al. Does PGPR and Mycorrhizae Enhance Nutrient Use Efficiency and Efficacy in Relation to Crop Productivity? In Field Crops: Sustainable Management by PGPR. Sustainable Development and Biodiversity; Maheshwari, D., Dheeman, S., Eds.; Springer: Cham, Switzerland, 2019; pp. 45–68. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere Engineering with Plant Growth-Promoting Microorganisms for Agriculture and Ecological Sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Shahwar, D.; Mushtaq, Z.; Mushtaq, H.; Alqarawi, A.A.; Park, Y.; Alshahrani, T.S.; Faizan, S. Role of Microbial Inoculants as Bio Fertilizers for Improving Crop Productivity: A Review. Heliyon 2023, 9, e16134. [Google Scholar] [CrossRef]
- Tahir, H.A.S.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant Growth Promotion by Volatile Organic Compounds Produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Morales-García, Y.E.; Baez, A.; Quintero-Hernández, V.; Molina-Romero, D.; Rivera-Urbalejo, A.P.; Pazos-Rojas, L.A.; Muñoz-Rojas, J. Bacterial Mixtures, the Future Generation of Inoculants for Sustainable Crop Production. In Field Crops: Sustainable Management by PGPR. Sustainable Development and Biodiversity; Maheshwari, D., Dheeman, S., Eds.; Springer: Cham, Switzerland, 2019; pp. 11–44. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Babalola, O.O. The Rhizosphere Microbial Complex in Plant Health: A Review of Interaction Dynamics. J. Integr. Agric. 2022, 21, 2168–2182. [Google Scholar] [CrossRef]
- Bhat, B.A.; Tariq, L.; Nissar, S.; Islam, S.T.; Islam, S.U.; Mangral, Z.; Ilyas, N.; Sayyed, R.Z.; Muthusamy, G.; Kim, W.; et al. The Role of Plant-Associated Rhizobacteria in Plant Growth, Biocontrol and Abiotic Stress Management. J. Appl. Microbiol. 2022, 133, 2717–2741. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, F.M.A.; Islam, M.A.; Rubel, M.H.; Mukharjee, S.K.; Kumar, M.; Bhattacharya, P.; Ahmed, F. Effects of Halotolerant Rhizobacteria on Rice Seedlings under Salinity Stress. Sci. Total Environ. 2023, 892, 163774. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Fiaz, S.; Hafeez, S.; Zahra, S.; Shah, A.N.; Gul, B.; Aziz, O.; Mahmood-Ur-Rahman; Fakhar, A.; Rafique, M.; et al. Plant Growth-Promoting Rhizobacteria Eliminate the Effect of Drought Stress in Plants: A Review. Front. Plant Sci. 2022, 13, 875774. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Y.L.; Wu, X.H.; Yang, X.; Wang, T.; Peng, W.X. Physiological Response of Avena sativa to Low-Temperature Stress Is Promoted by Bacillus amyloliquefaciens GL18 and Its Functional Genes. Russ. J. Plant Physiol. 2022, 69, 161. [Google Scholar] [CrossRef]
- Mukhtar, T.; Rehman, S.U.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna, A.S.; Chaudhary, H.J.; Solieman, T.H.I.; Ibrahim, A.A.; et al. Mitigation of Heat Stress in Solanum lycopersicum l. by ACC-Deaminase and Exopolysaccharide Producing Bacillus cereus: Effects on Biochemical Profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef]
- Li, G.; Peng, T.; Qu, F.; Wang, J.; Long, Y.; Hu, X. Bacillus methylotrophicus Could Improve the Tolerance and Recovery Ability of the Tomato to Low-Temperature Stress and Improve Fruit Quality. Agronomy 2023, 13, 1902. [Google Scholar] [CrossRef]
- Pereira, F. Rhizobacteria as Bioprotectants Against Stress Conditions. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management. Microorganisms for Sustainability; Sayyed, R., Arora, N., Reddy, M., Eds.; Springer: Singapore, 2019; pp. 157–177. [Google Scholar] [CrossRef]
- Arikan, Ş.; Pirlak, L. Einfluss von Wachstumsfördernden Rhizobacteria (PGPR) Auf Wachstum, Ertrag Und Fruchtqualität Bei Sauerkirschen (Prunus Cerasus L.). Erwerbs-Obstbau 2016, 58, 221–226. [Google Scholar] [CrossRef]
- Habibi, S.; Djedidi, S.; Prongjunthuek, K.; Mortuza, M.F.; Ohkama-Ohtsu, N.; Sekimoto, H.; Yokoyoma, T. Physiological and Genetic Characterization of Rice Nitrogen Fixer PGPR Isolated from Rhizosphere Soils of Different Crops. Plant Soil 2014, 379, 51–66. [Google Scholar] [CrossRef]
- Naqqash, T.; Hameed, S.; Imran, A.; Hanif, M.K.; Majeed, A.; van Elsas, J.D. Differential Response of Potato toward Inoculation with Taxonomically Diverse Plant Growth Promoting Rhizobacteria. Front. Plant Sci. 2016, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, L.A.; Sáenz-Mata, J.; Fortis-Hernández, M.; Navarro-Muñoz, C.E.; Palacio-Rodríguez, R.; Preciado-Rangel, P. Plant-Growth-Promoting Rhizobacteria Improve Germination and Bioactive Compounds in Cucumber Seedlings. Agronomy 2023, 13, 315. [Google Scholar] [CrossRef]
- Chauhan, A.; Saini, R.; Sharma, J.C. Plant Growth Promoting Rhizobacteria and Their Biological Properties for Soil Enrichment and Growth Promotion. J. Plant Nutr. 2021, 45, 273–299. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Swapnil, P. Plant Growth-Promoting Rhizobacteria as a Green Alternative for Sustainable Agriculture. Sustainability 2021, 13, 10986. [Google Scholar] [CrossRef]
- Aloo, B.N.; Tripathi, V.; Makumba, B.A.; Mbega, E.R. Plant Growth-Promoting Rhizobacterial Biofertilizers for Crop Production: The Past, Present, and Future. Front. Plant Sci. 2022, 13, 1002448. [Google Scholar] [CrossRef]
- Amaresan, N.; Jayakumar, V.; Kumar, K.; Thajuddin, N. Plant Growth-Promoting Effects of Proteus mirabilis Isolated from Tomato (Lycopersicon esculentum Mill) Plants. Natl. Acad. Sci. Lett. 2021, 44, 453–455. [Google Scholar] [CrossRef]
- Batista, B.D.; Dourado, M.N.; Figueredo, E.F.; Hortencio, R.O.; Marques, J.P.R.; Piotto, F.A.; Bonatelli, M.L.; Settles, M.L.; Azevedo, J.L.; Quecine, M.C. The Auxin-Producing Bacillus thuringiensis RZ2MS9 Promotes the Growth and Modifies the Root Architecture of Tomato (Solanum lycopersicum cv. Micro-Tom). Arch. Microbiol. 2021, 203, 3869–3882. [Google Scholar] [CrossRef]
- Dashti, N.H.; Al-Sarraf, N.Y.A.; Cherian, V.M.; Montasser, M.S. Isolation and Characterization of Novel Plant Growth-Promoting Rhizobacteria (PGPR) Isolates from Tomato (Solanum lycopersicum L.) Rhizospherical Soil: A Novel IAA Producing Bacteria. Kuwait J. Sci. 2021, 48, 2. [Google Scholar] [CrossRef]
- Délano-Frier, J.P.; Flores-Olivas, A.; Valenzuela-Soto, J.H. Bio-Inoculation of Tomato (Solanum lycopersicum L.) and Jalapeño Pepper (Capsicum annuum L.) with Enterobacter Sp. DBA51 Increases Growth and Yields under Open-Field Conditions. Agronomy 2024, 14, 702. [Google Scholar] [CrossRef]
- Dong, W.; Liu, H.; Ning, Z.; Bian, Z.; Zeng, L.; Xie, D. Inoculation with Bacillus cereus DW019 Modulates Growth, Yield and Rhizospheric Microbial Community of Cherry Tomato. Agronomy 2023, 13, 1458. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Ghorbani, M.; Moudrý, J.; Konvalina, P.; Kopecký, M. Impacts of Environmental Factors and Nutrients Management on Tomato Grown under Controlled and Open Field Conditions. Agronomy 2023, 13, 916. [Google Scholar] [CrossRef]
- FAOSTAT Database. Food and Agriculture Organization Statistics. 2021. Available online: https://www.fao.org/faostat/en/ (accessed on 11 April 2024).
- Costa, J.; Heuvelink, E.P. The Global Tomato Industry. In Tomatoes; Heuvelink, E.P., Ed.; CABI: Wallingford, UK, 2018; pp. 1–26. [Google Scholar]
- Bosa, M.S.; Déniz, P.O. Evolución Del Cultivo Del Tomate En Canarias Desde La Incorporación a La Unión Europea (1986–2001). Estud. Agrosociales Pesq. 2002, 196, 133–152. [Google Scholar]
- Adedayo, A.A.; Babalola, O.O.; Prigent-Combaret, C.; Cruz, C.; Stefan, M.; Kutu, F.; Glick, B.R. The Application of Plant Growth-Promoting Rhizobacteria in Solanum lycopersicum Production in the Agricultural System: A Review. Peer J 2022, 10, e13405. [Google Scholar] [CrossRef]
- Idaszkin, Y.L.; Polifroni, R.; Mesa-Marín, J. Isolation of Plant Growth Promoting Rhizobacteria from Spartina Densiflora and Sarcocornia perennis in San Antonio Polluted Salt Marsh, Patagonian Argentina. Estuar. Coast. Shelf Sci. 2021, 260, 107488. [Google Scholar] [CrossRef]
- MAGRAMA. Métodos Oficiales de Análisis, Tomo III. Ministerio de Agricultura, Pesca y Alimentación; Secretaria General de Alimentación, Dirección General de Política Alimentaria: Madrid, Spain, 1994.
- Hernández-Bolaños, E.; Montesdeoca-Flores, D.; Abreu-Yanes, E.; Barrios, M.L.; Abreu-Acosta, N. Evaluating Different Methodologies for Bioprospecting Actinomycetes in Canary Islands Soils. Curr. Microbiol. 2020, 77, 2510–2522. [Google Scholar] [CrossRef]
- Gayathiri, E.; Bharathi, B.; Priya, K. Study of the Enumeration of Twelve Clinical Important Bacterial Populations at 0.5 McFarland Standard. Int. J. Creat. Res. Thoughts 2018, 6, 880–893. [Google Scholar]
- Hoagland, D.R. Optimum Nutrient Solutions for Plants. Science 1920, 52, 562–564. [Google Scholar] [CrossRef]
- Ruiz-Medina, M.A.; Sansón, M.; González-Rodríguez, Á.M. Changes in Antioxidant Activity of Fresh Marine Macroalgae from the Canary Islands during Air-Drying Process. Algal Res. 2022, 66, 102798. [Google Scholar] [CrossRef]
- de O Nunes, P.S.; De Medeiros, F.H.; De Oliveira, T.S.; de Almeida Zago, J.R.; Bettiol, W. Bacillus subtilis and Bacillus licheniformis Promote Tomato Growth. Braz. J. Microbiol. 2023, 54, 397–406. [Google Scholar] [CrossRef]
- Kouam, I.D.; Mabah, J.; Germain Ntsoli, P.; Tchamani, L.; Yaouba, A.; Katte, B.; Bitom, D. Growth Promotion Potential of Bacillus spp. isolates on two tomato (Solanum lycopersicum L.) varieties in the West region of Cameroon. Open Agric. 2023, 8, 20220154. [Google Scholar] [CrossRef]
- Santoyo, G.; Equihua, A.; Flores, A.; Sepulveda, E.; Valencia-Cantero, E.; Sanchez-Yañez, J.M.; de los Santos-Villalobos, S. Plant Growth Promotion by ACC Deaminase-Producing Bacilli Under Salt Stress Conditions. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol. Bacilli in Climate Resilient Agriculture and Bioprospecting; Islam, M., Rahman, M., Pandey, P., et al., Eds.; Springer: Cham, Switzerland, 2019; pp. 81–95. [Google Scholar] [CrossRef]
- Hernández-Forte, I.; Pérez-Pérez, R.; Taulé-Gregorio, C.B.; Fabiano-González, E.; Battistoni-Urrutia, F.; Nápoles-García, M.C. New Bacteria Genera Associated with Rice (Oryza sativa L.) in Cuba Promote the Crop Growth. Agron. Mesoam. 2022, 33, 47223. [Google Scholar] [CrossRef]
- Gholamalizadeh, R.; Khodakaramian, G.; Ebadi, A.A. Assessment of Rice Associated Bacterial Ability to Enhance Rice Seed Germination and Rice Growth Promotion. Braz. Arch. Biol. Technol. 2017, 60, e17160410. [Google Scholar] [CrossRef]
- Tahir, M.; Naeem, M.A.; Shahid, M.; Khalid, U.; Farooq, A.B.U.; Ahmad, N.; Ahmad, I.; Arshad, M.; Waqar, A. Inoculation of PqqE Gene Inhabiting Pantoea and Pseudomonas Strains Improves the Growth and Grain Yield of Wheat with a Reduced Amount of Chemical Fertilizer. J. Appl. Microbiol. 2020, 129, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Q.; Li, Y.; Guan, G.; Chen, S. Paenibacillus Strains with Nitrogen Fixation and Multiple Beneficial Properties for Promoting Plant Growth. PeerJ 2019, 7, e7445. [Google Scholar] [CrossRef]
- Kaur, G.; Reddy, S.M. Influence of P-Solubilizing Bacteria on Crop Yield and Soil Fertility at Multilocational Sites. Eur. J. Soil Biol. 2014, 61, 35–40. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Vadlamudi, S.; Samineni, S.; Sameer Kumar, C.V. Plant Growth-Promotion and Biofortification of Chickpea and Pigeonpea through Inoculation of Biocontrol Potential Bacteria, Isolated from Organic Soils. Springerplus 2016, 5, 1882. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Olivares, B.O.; Calero, J.; Rey, J.C.; Lobo, D.; Landa, B.B.; Gómez, J.A. Correlation of Banana Productivity Levels and Soil Morphological Properties Using Regularized Optimal Scaling Regression. CATENA 2022, 208, 105718. [Google Scholar] [CrossRef]
- Masmoudi, F.; Alsafran, M.; Jabri, H.A.L.; Hosseini, H.; Trigui, M.; Sayadi, S.; Tounsi, S.; Saadaoui, I. Halobacteria-Based Biofertilizers: A Promising Alternative for Enhancing Soil Fertility and Crop Productivity under Biotic and Abiotic Stresses—A Review. Microorganisms 2023, 11, 1248. [Google Scholar] [CrossRef]
- Osman, H.E.M.; Nehela, Y.; Elzaawely, A.A.; El-Morsy, M.H.; El-Nagar, A. Two Bacterial Bioagents Boost Onion Response to Stromatinia Cepivora and Promote Growth and Yield via Enhancing the Antioxidant Defense System and Auxin Production. Horticulturae 2023, 9, 780. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Xu, M.; Gan, Y. Chromium-Induced Reactive Oxygen Species Accumulation by Altering the Enzymatic Antioxidant System and Associated Cytotoxic, Genotoxic, Ultrastructural, and Photosynthetic Changes in Plants. Int. J. Mol. Sci. 2020, 21, 728. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Méndez, D.; Fuentes-Ocampo, L.; Diaz-Cervantes, E. Estudio Computacional de La Capacidad Antioxidante de Tuna (Opuntia Streptacantha). Investig. Desarro. Cienc. Tecnol. Aliment. 2023, 8, 904–908. [Google Scholar] [CrossRef]
- Hernández- Herrera, K.P.; Salgado-Chávez, J.A. Contenido de Fenoles Totales y Actividad Antioxidante de Extractos Foliares de Ipomoea Pes-Caprae (Convolvulaceae). Temas Agrar. 2022, 27, 354–365. [Google Scholar] [CrossRef]
- Lone, R.; Shuab, R.; Kamili, A.N. (Eds.) Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Wang, P.; Grimm, B. Connecting Chlorophyll Metabolism with Accumulation of the Photosynthetic Apparatus. Trends Plant Sci. 2021, 26, 484–495. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital Roles of Carotenoids in Plants and Humans to Deteriorate Stress with Its Structure, Biosynthesis, Metabolic Engineering and Functional Aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant Carotenoids: Recent Advances and Future Perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef]
- Montesdeoca-Flores, D.; Alfayate-Casañas, C.; Hernández-Bolaños, E.; Hernández-González, M.; Estupiñan-Afonso, Z.; Abreu-Acosta, N. Effect of Biofertilizers and Rhizospheric Bacteria on Growth and Root Ultrastucture of Lettuce. Hortic. Environ. Biotechnol. 2024, 65, 15–28. [Google Scholar] [CrossRef]
- Vera-Sánchez, J.J. Principios de La Movilidad Acropetal de Nutrientes Por El Tejido Conductivo de Las Plantas. Bachelor’s Thesis, Universidad Técnica de Babahoyo, Babahoyo, Ecuador, 2020. [Google Scholar]
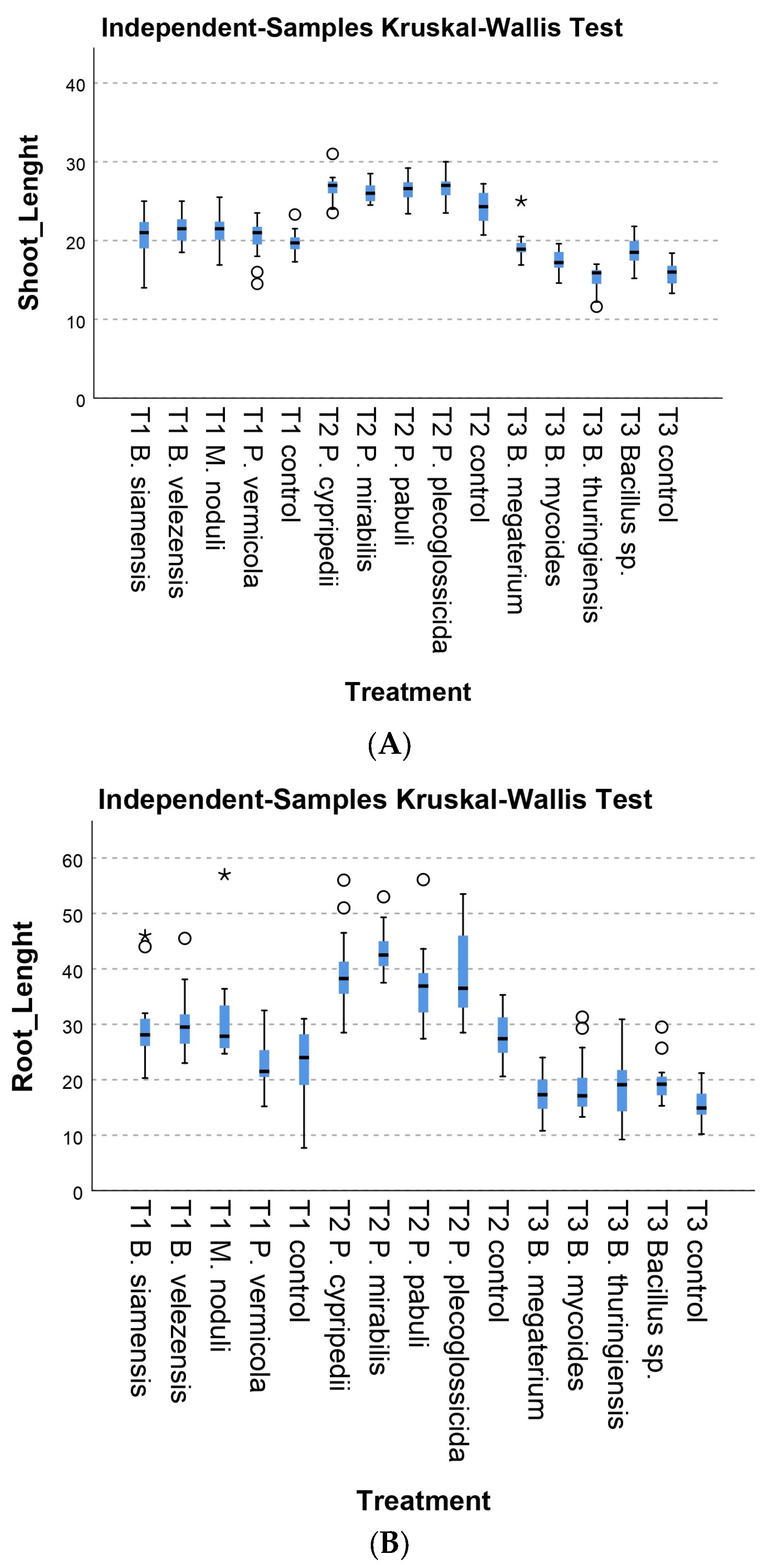
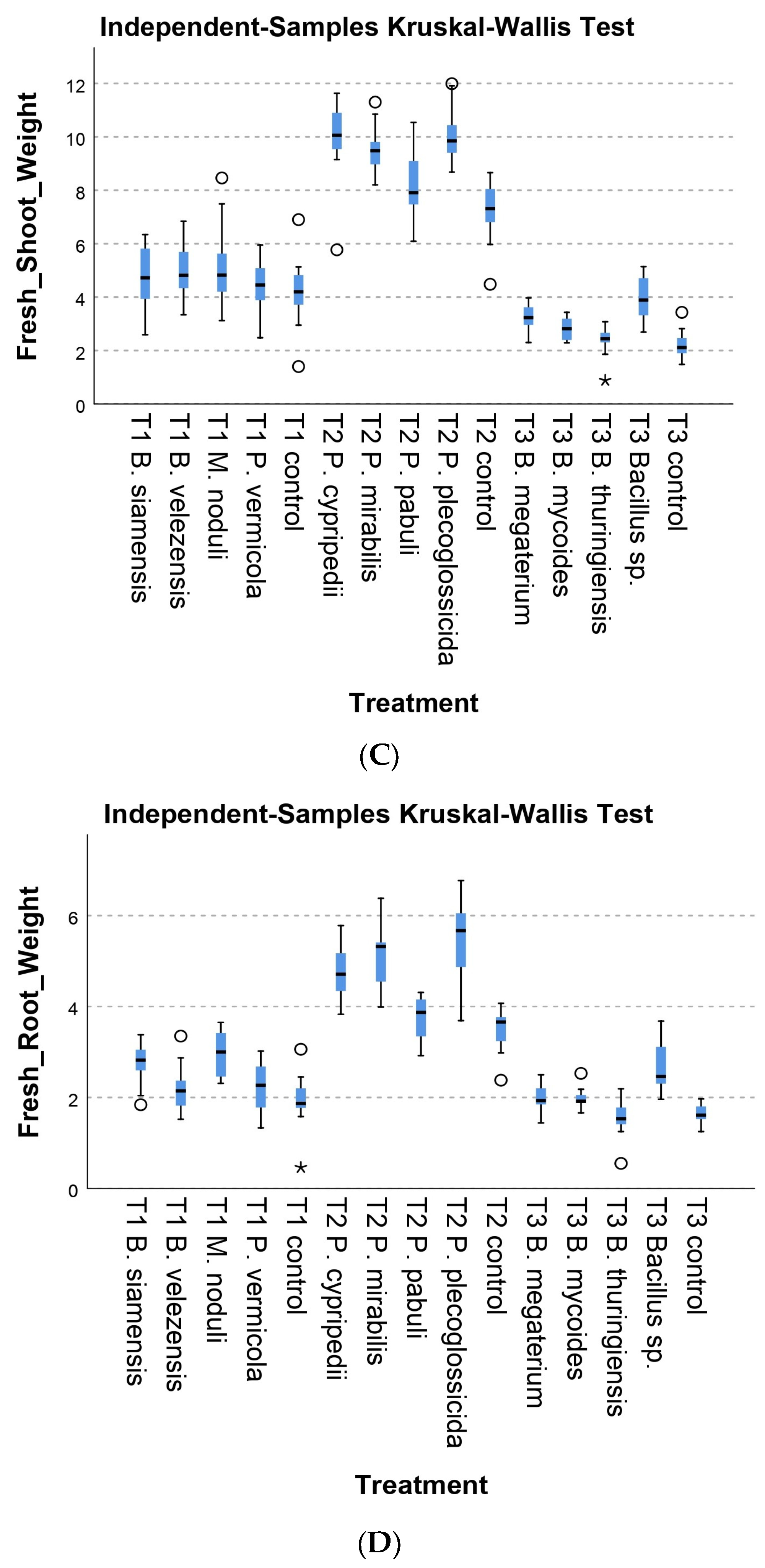
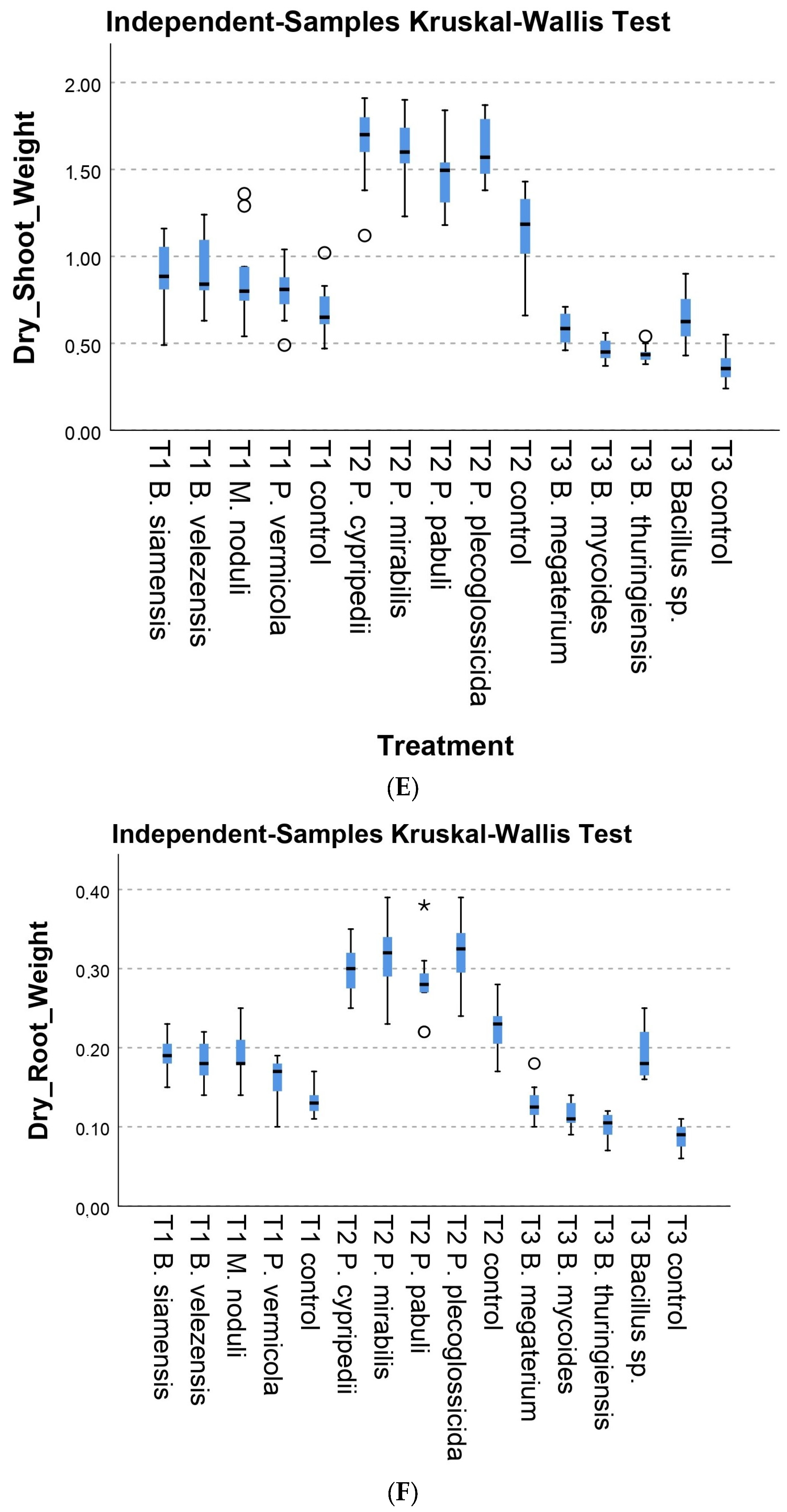


| Strain | Species | P Sol. | K Sol. | N2 Fix. | IAA μg mL−1 | PSU (7 Days) |
|---|---|---|---|---|---|---|
| 16 | Pseudomonas plecoglossicida | ++ | + | − | 2.53 ± 3.42 | 15.81 ± 9.78 |
| 17 | Providencia vermicola | + | + | + | 101.77 ± 4.44 | 28.2 ± 3.01 |
| 19 | Mitsuaria noduli | − | + | + | 36.43 ± 2.05 | 40.89 ± 7.22 |
| 27 | Proteus mirabilis | + | + | + | 5.29 ± 2.22 | 47.78 ± 10.97 |
| 32 | Pantoea cypripedii | ++ | + | + | 0 ± 0 | 58.76 ± 3.18 |
| 47 | Paenibacillus pabuli | + | − | + | 0 ± 0 | 70.48 ± 1.59 |
| 1SEF | Bacillus siamensis | + | − | + | 13.83 ± 0.55 | 52.24 ± 13.94 |
| 4PIN | Bacillus velezensis | + | + | + | 21.82 ± 0.38 | 56.99 ± 6.54 |
| 1CRN | Bacillus mycoides | + | + | + | 12.88 ± 0.86 | 39.24 ± 6.08 |
| 6AB | Bacillus sp. | + | + | − | 18.43 ± 0.48 | 51.05 ± 5.80 |
| 8AB4 | Bacillus megaterium | + | + | + | 14.89 ± 1.31 | 61.01 ± 3.72 |
| 3GRA | Bacillus thuringiensis | + | + | − | 12.55 ± 0.06 | 57.03 ± 6.51 |
| pH | % MO | P2O5 | Ca | Mg | Ca/Mg | K | Na | CE mS/cm | % SAT |
|---|---|---|---|---|---|---|---|---|---|
| 7.2 | 1.0 | 21 | 10.2 | 3.7 | 2.7 | 1.0 | 1.5 | 0.99 | 42 |
| Strain | g kg−1 | mg kg−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | Na | Fe | Mn | Cu | Zn | B | |
| Aerial portion | |||||||||||
| Control 1 | 14.00 a | 2.32 a | 12.92 b | 17.70 c | 4.00 b | 2208 c | 716 a | 75 a | 0 a | 4 a | 23 d |
| P. vermicola | 16.20 b | 2.54 b | 16.52 d | 15.33 a | 4.19 c | 2869 d | 1227 d | 101 c | 0 a | 5 b | 19 b |
| M. noduli | 16.90 c | 2.69 c | 12.06 a | 16.10 ab | 3.77 a | 1433 a | 1309 e | 95 b | 0 a | 3 a | 16 a |
| B. siamensis | 16.30 b | 2.77 c | 13.58 c | 15.67 ab | 3.83 a | 1652 b | 1116 b | 94 b | 33 c | 8 c | 20 c |
| B. velezensis | 16.80 c | 2.99 d | 12.94 b | 16.31 b | 4.11 c | 1536 a | 1201 c | 93 b | 6 b | 10 d | 25 e |
| Root portion | |||||||||||
| Control 1 | 18.80 b | 2.48 a | 24.75 b | 6.42 c | 9.30 b | 11,100 d | 3272 b | 466 b | 4 b | 27 c | 0 a |
| P. vermicola | 20.46 c | 2.59 a | 24.55 ab | 6.08 b | 9.15 b | 11,139 d | 3585 c | 579 d | 37 d | 17 a | 0 a |
| M. noduli | 16.10 a | 2.83 b | 23.93 a | 6.34 bc | 7.46 a | 8374 a | 3909 d | 526 c | 3 b | 23 b | 0 a |
| B. siamensis | 18.70 b | 2.82 b | 25.15 b | 5.77 a | 7.81 a | 9809 c | 3809 d | 646 e | 27 c | 18 a | 0 a |
| B. velezensis | 18.10 b | 2.83 b | 24.64 ab | 6.86 d | 7.69 a | 8863 b | 3040 a | 358 a | 2 a | 22 b | 0 a |
| Strain | g kg−1 | mg kg−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | Na | Fe | Mn | Cu | Zn | B | |
| Aerial portion | |||||||||||
| Control 2 | 18.90 d | 2.85 c | 15.95 b | 19.19 d | 3.95 a | 1982 e | 593 b | 86 e | 0 a | 7 a | 22 d |
| P. plecoglossicida | 14.90 b | 2.67 ab | 17.75 c | 15.68 a | 3.94 a | 1628 c | 350 a | 50 a | 0 a | 12 c | 16 ab |
| P. mirabilis | 14.10 a | 2.75 bc | 19.90 d | 16.13 ab | 3.94 a | 1710 d | 651 c | 73 c | 0 a | 18 d | 16 b |
| P. cypripedii | 15.07 c | 2.55 a | 14.53 a | 16.68 b | 3.84 a | 1156 a | 764 d | 80 d | 0 a | 7 ab | 14 a |
| P. pabuli | 14.99 bc | 2.62 ab | 16.02 b | 17.44 c | 3.83 a | 1347 b | 381 a | 65 b | 0 a | 8 b | 19 c |
| Root portion | |||||||||||
| Control 2 | 18.97 d | 3.20 d | 27.12 e | 7.33 c | 9.82 c | 10,980 e | 3989 c | 795 e | 11 d | 38 d | 0 a |
| P. plecoglossicida | 15.47 a | 1.56 a | 16.18 a | 5.84 b | 5.06 a | 4481 a | 3214 b | 261 a | 0 a | 13 a | 0 a |
| P. mirabilis | 16.26 b | 1.98 b | 20.27 c | 7.22 c | 6.56 b | 6641 c | 5050 d | 424 c | 2 b | 39 d | 0 a |
| P. cypripedii | 17.02 c | 1.91 b | 18.28 b | 5.07 a | 5.18 a | 5515 b | 2860 a | 346 b | 8 c | 19 b | 0 a |
| P. pabuli | 16.21 b | 2.18 c | 23.41 d | 7.30 c | 11.04 d | 7539 d | 5426 e | 453 d | 1 a | 22 c | 0 a |
| Strain | g kg−1 | mg kg−1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | Na | Fe | Mn | Cu | Zn | B | |
| Aerial portion | |||||||||||
| Control 3 | 18.09 c | 1.82 d | 15.58 d | 15.67 d | 2.85 d | 1784 d | 331 d | 199 e | 0 a | 5 d | 24 e |
| Bacillus sp. | 20.16 d | 1.70 c | 15.12 c | 13.15 c | 2.68 c | 1625 c | 222 c | 113 b | 0 a | 7 e | 14 c |
| B. mycoides | 15.40 b | 1.27 b | 10.14 b | 10.17 a | 1.83 a | 963 a | 120 a | 92 a | 0 a | 1 b | 11 a |
| B. thuringiensis | 15.75 b | 1.13 a | 8.22 a | 12.07 b | 2.11 b | 1269 b | 511 e | 126 d | 3 b | 0 a | 12 b |
| B. megaterium | 14.81 a | 2.12 e | 15.73 d | 17.04 e | 3.14 e | 1887 e | 150 b | 151 d | 0 a | 3 c | 23 d |
| Root portion | |||||||||||
| Control 3 | 25.20 d | 2.67 c | 38.99 d | 4.11 a | 8.35 c | 13,346 c | 1880 b | 1549 e | 4 b | 44 c | 0 a |
| Bacillus sp. | 19.53 a | 1.22 a | 16.27 a | 5.40 c | 4.80 a | 5244 a | 4387 d | 659 a | 1 a | 17 a | 0 a |
| B. mycoides | 23.80 c | 3.44 d | 36.77 c | 6.93 e | 7.68 b | 12,171 b | 2014 c | 1192 d | 75 d | 78 d | 0 a |
| B. thuringiensis | 21.10 b | 2.33 b | 34.56 b | 6.25 d | 8.35 c | 13,808 d | 2034 c | 700 b | 5 b | 37 b | 0 a |
| B. megaterium | 22.40 bc | 2.80 c | 35.00 b | 5.12 b | 8.43 c | 13,047 c | 1715 a | 945 c | 18 c | 45 c | 0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Amador, E.; Montesdeoca-Flores, D.T.; Abreu-Acosta, N.; Luis-Jorge, J.C. Effects of Rhizobacteria Strains on Plant Growth Promotion in Tomatoes (Solanum lycopersicum). Plants 2024, 13, 3280. https://doi.org/10.3390/plants13233280
Hernández-Amador E, Montesdeoca-Flores DT, Abreu-Acosta N, Luis-Jorge JC. Effects of Rhizobacteria Strains on Plant Growth Promotion in Tomatoes (Solanum lycopersicum). Plants. 2024; 13(23):3280. https://doi.org/10.3390/plants13233280
Chicago/Turabian StyleHernández-Amador, Eduardo, David Tomás Montesdeoca-Flores, Néstor Abreu-Acosta, and Juan Cristo Luis-Jorge. 2024. "Effects of Rhizobacteria Strains on Plant Growth Promotion in Tomatoes (Solanum lycopersicum)" Plants 13, no. 23: 3280. https://doi.org/10.3390/plants13233280
APA StyleHernández-Amador, E., Montesdeoca-Flores, D. T., Abreu-Acosta, N., & Luis-Jorge, J. C. (2024). Effects of Rhizobacteria Strains on Plant Growth Promotion in Tomatoes (Solanum lycopersicum). Plants, 13(23), 3280. https://doi.org/10.3390/plants13233280







