Abstract
Recruitment poses significant challenges for narrow endemic plant species inhabiting extreme environments like vertical cliffs. Investigating seed traits in these plants is crucial for understanding the adaptive properties of chasmophytes. Focusing on the Iberian endemic genus Petrocoptis A. Braun ex Endl., a strophiole-bearing Caryophyllaceae, this study explored the relationships between seed traits and climatic variables, aiming to shed light on the strophiole’s biological role and assess its classificatory power. We analysed 2773 seeds (557 individuals) from 84 populations spanning the genus’ entire distribution range. Employing cluster and machine learning algorithms, we delineated well-defined morphogroups based on seed traits and evaluated their recognizability. Linear mixed-effects models were utilized to investigate the relationship between climate predictors and strophiole area, seed area and the ratio between both. The combination of seed morphometric traits allows the division of the genus into three well-defined morphogroups. The subsequent validation of the algorithm allowed 87% of the seeds to be correctly classified. Part of the intra- and interpopulation variability found in strophiole raw and relative size could be explained by average annual rainfall and average annual maximum temperature. Strophiole size in Petrocoptis could have been potentially driven by adaptation to local climates through the investment of more resources in the production of bigger strophioles to increase the hydration ability of the seed in dry and warm climates. This reinforces the idea of the strophiole being involved in seed water uptake and germination regulation in Petrocoptis. Similar relationships have not been previously reported for strophioles or other analogous structures in Angiosperms.
1. Introduction
The singularity, remarkable diversity and ecology of rock-dwelling plants have piqued the interest of the scientific community at least since the middle of the last century [1,2]. Given the abundance of endemic and/or threatened rupicolous taxa and the particularities of rocky habitats [3,4,5], an increasing number of authors have contributed to enlarging our knowledge about plant life in rocky habitats worldwide (e.g., [6,7,8]) and in Mediterranean area (e.g., [9,10,11]). Rocky outcrops are fragmented environments where a great diversity of specialist plant taxa—often with narrow distribution areas—occur. These outcrops are proposed to be a result of the climatic and topographic changes throughout the Cenozoic [2,12,13], when the Alpine orogeny led to the formation of most Indo-European mountain chains which, together with the existing ones, have been shaped by erosive forces since then. These processes were intensified during the Pleistocene climatic oscillations and resulted in a diversity of rocky microenvironments, which played a key role as refugia for the flora both in the glacial and interglacial periods [14,15]. Consequently, rocky habitats would have acted as reservoirs of relict biodiversity [16] and promoted diversification of rock specialist plant lineages [17,18]. In this context, some widely distributed plant lineages probably underwent transitions towards small ecologically and taxonomically isolated populations and/or taxa [19,20,21]. The inherent disjunction of rocky habitats leads to diversification processes, and therefore, a geographic pattern may be found across the range of taxonomically coherent accepted species, in which different groups—either genetic or morphological—might be identified in different regions [22,23]. Thus, those species may be composed of a mosaic of biological units with a variable degree of isolation that are potentially responding to different adaptive pressures. Given this uncertainty, the study of rupicolous genera is needed to shed light on their evolutionary history, understand their adaptation strategies and promote their conservation.
The Iberian Peninsula, as part of the Mediterranean biodiversity hotspot, hosts a high richness of rupicolous taxa and, together with its great geological and topographical complexity, provides an ideal study location for the abovementioned purpose. Due to its complex palaeoclimatic and palaeogeographic history and to the huge variety of rocky environments available (e.g., [24,25,26,27]), the Iberian Peninsula lodges a high diversity of rock-dwelling taxa, many of which are endemic [5]. Among the 30 largest genera of the Iberian endemic flora [28], the following ones stand out as eminently rupicolous: Armeria Willd. (Plumbaginaceae, ca. 55% of the species present in the Iberian Peninsula are rupicolous, based on estimations according to our own unpublished data), Teucrium L. (Lamiaceae, ca. 51%), Saxifraga L. (Saxifragaceae, ca. 90%), Erodium L’Hér. (Geraniaceae, ca. 61%), Campanula L. (Campanulaceae, ca. 52%), Scrophularia L. (Scrophulariaceae, ca. 52%), Hieracium L. (Asteraceae, ca. 62%) and Chaenorhinum (DC.) Rchb. (Plantaginaceae, ca. 53%). Accordingly, rocky outcrops of the Iberian Peninsula would have acted as important centres of diversity for many genera or infrageneric groups (e.g.,: [10,11,29,30,31,32,33,34]), as well as a centre of origin for others such as the endemic Iberian rock-dwelling genera Rivasmartinezia Fern; Prieto & Cires (Apiaceae), Dethawia Endl. (Apiaceae), Phalacrocarpum (DC.) Willk. (Asteraceae) and Petrocoptis A. Braun ex Endl. (Caryophyllaceae), our study model.
Petrocoptis is an Iberian-endemic genus that comprises between 4 and 12 chasmophytic species, depending on the authors (see Section 4). It is phylogenetically closely related to Silene L. and Agrostemma L. [35,36,37], from which it differs, among other traits, by the presence of a strophiole, a characteristic small tuft of hairs by the hilum of its seed [38]. Strophiole morphology has also been used as a taxonomic character to distinguish species within the genus [39]. Nearly all Petrocoptis species are included in conservation lists at European and Spanish levels—either national or regional—due to their restricted ecology and the small distribution area of the taxa [40,41]. However, the different taxonomic treatments available for the genus sometimes differ strongly among them, which interferes with the appropriate evaluation of the conservation status of the taxa concerned. Consequently, it is essential to resolve the taxonomic uncertainty that affects Petrocoptis. In this respect, a phylogenomic analysis of the whole genus is being performed at the moment by our team, and thus, a taxonomic objective is excluded from this work. Moreover, comprehensive knowledge of the genus evolution and its current fragmented distribution pattern linked to habitat specificity should be integrated into a meaningful taxonomic treatment.
Despite its restricted distribution range, Petrocoptis inhabits cliffs comprising a wide altitudinal range (from 20 to more than 2000 m a.s.l.) and climatic spectrum (temperate to Mediterranean macrobioclimates, following [42]). Under these broad conditions, individuals may be phenotypically plastic, expressing the optimal phenotype in different environments without genetic differentiation [43,44,45], or populations may differentiate genetically so as to become locally adapted [46,47,48]. Although plants are thought to be generally plastic [49], populations can differ greatly in their plasticity levels [50,51,52,53], especially when habitat heterogeneity and/or isolation limit gene flow among them [54]. In addition, it has been observed that plasticity can be facilitated or limited by climatic variables [55,56]. Additionally, it is probable that phenotypic plasticity and precipitation or temperature are correlated, with benign conditions favouring greater phenotypic plasticity [57,58] due to a wider range of morphological and physiological variations [59]. On the contrary, stressful conditions may place restrictions on phenotypic plasticity [60]. In the case of Petrocoptis, prior research showed high plasticity in germination throughout most of the species of the genus [61], but further research is needed to evaluate plasticity in seed morphology traits. All mentioned features make the genus Petrocoptis an ideal study case to understand plant specialization in rocky habitats.
The ecology of chasmophytic plants is relevant to understanding their past, present and future distribution. Although spatially open, a vertical cliff is a biologically closed community [2] because the space that separates one cliff from another is often inadequate for plant establishment. Therefore, range expansion is usually extremely difficult for plants living on vertical cliffs. In these habitats, the suitable holes on rock surfaces available for seedling establishment are very scarce and, when they originate, tend to be occupied by the seeds of species already present nearby. Consequently, medium- and long-distance dispersal is unfavoured, and population isolation is enhanced. Moreover, chasmophytes would disappear from a cliff without the presence of specialized traits that favour the dispersal and establishment of the progeny. Therefore, the seeds of chasmophyte plants are of primary research interest. Wind is believed to be the main dispersal agent for plants, but it is dependent on the lightness of the diaspore, either seeds or fruits [62,63]. However, there are cliff specialist plants with no wind-compatible syndrome. Some of them have a diaspore with a fatty-rich appendage, the elaiosome, which is widely thought to function as a nutritional reward for mutualistic ants that indirectly disperse the diaspores (i.e., myrmecochory [64]). This structure has been described in certain Iberian rupicolous plants [65], which has led to the associated presence of an elaiosome or other structures resembling it with the myrmecochory syndrome [66,67]. However, each case has to be investigated independently in search of empirical evidence.
An analogous structure, which has been traditionally used to distinguish Petrocoptis from other Caryophyllaceae genera, is the strophiole [68]. It is an outgrowth of the hilum region that restricts water movement into and out of the seed [69,70]. As stated, Petrocoptis strophiole is composed of a very conspicuous tuft of either cylindrical or claviform hairs of variable size [39,71], which have hygroscopic properties and become mucilaginous on wetting [72]. In addition to its function as a regulatory structure of the seed water balance, it has also been considered an adaptive trait related to seed dispersal. For example, refs. [67,73] have considered the strophiole as a structure related to myrmecochory. Alternatively, refs. [72,74] discussed that the hygroscopy of the strophiole facilitates adherence within the damp crevices where Petrocoptis lives, promoting a specialization to these habitats. An exhaustive analysis of seed traits, including the strophiole, is needed to fully understand the role that its particularly heavy seed plays in the dispersal and rock specialization of the genus Petrocoptis.
The aim of this study was to investigate the morphology of the seeds of Petrocoptis in order to (1) assess whether the measured morphometric seed traits can contribute to the description of seed morphogroups that hold taxonomic significance); (2) explore whether a relationship can be established between these traits and climate; and (3) try to obtain further clues on the biological role that the strophiole may play in Petrocoptis. Taking into account intrapopulation variability associated with phenotypic plasticity and a potential high interpopulation variability due to the high degree of population isolation, seed morphology may have taxonomic value. Additionally, a correlation between strophiole-related traits and climate can be expected, considering that this structure is suspected to play a key role in water uptake and regulation.
2. Results
2.1. Clustering and Recognisable Morphological Groups
Based on all nine analysed morphological traits, the grouping of population means into three clusters received strong support. A total of 16 out of the 27 indices calculated by the NbClust algorithm converged k = 3 as the optimal number of clusters. The remaining 11 indices showed much weaker support, with less than 4 of them agreeing on a different number of clusters (Table S2). Despite the high inter-population variability, the variation between all clusters (between cluster sum of squares, BCSS) was higher than the variation within them (within-cluster sum of squares, WCSS): BCSS/WCSS = 1.364.
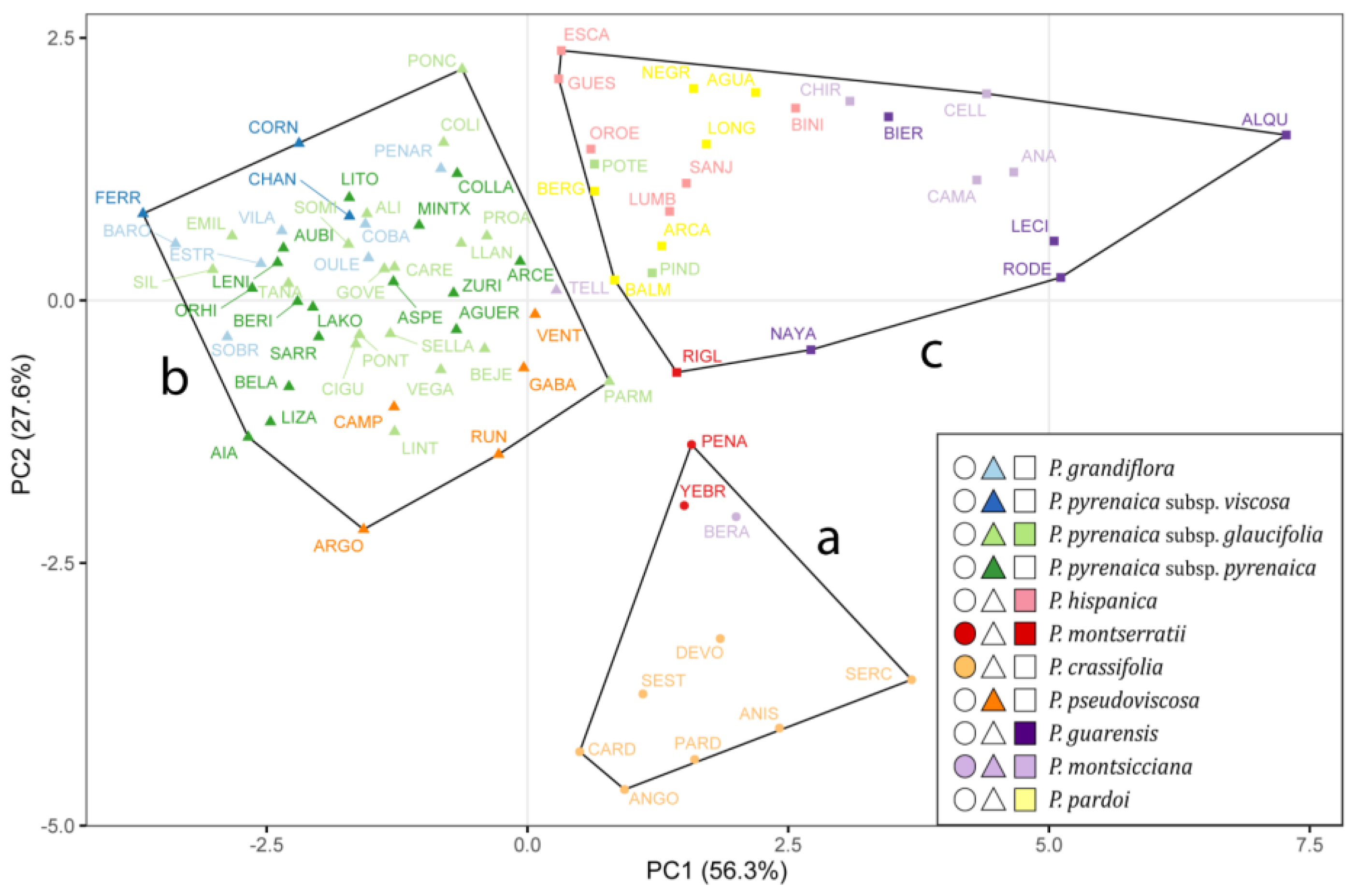
The first two principal components (PC1 and PC2) were the ones that best illustrated the separation among clusters, explaining 56.27% and 27.59% (83.86% in total) of the population variance, respectively (Figure 1). PC1 showed a high positive correlation with the variables related to the raw size of both the seed and the strophiole, while PC2 was positively correlated with the strophiole proportional size and seed roundness (Figure S1). As expected, the width and length variables of both seed and strophiole showed a high correlation with their respective areas. Therefore, they were discarded. The correlation coefficients between the remaining five traits did not exceed the value of 0.74 for any pair. Thus, seed area, seed roundness, strophiole area, strophiole roundness and strophiole relative size were retained for clustering analyses.
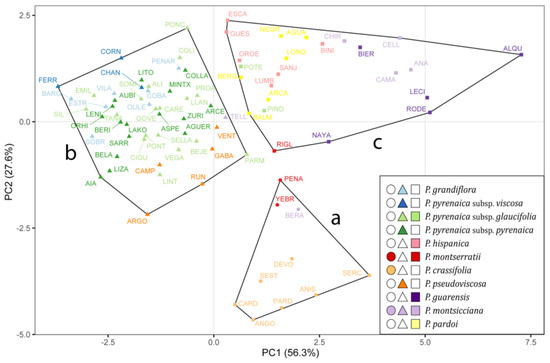
Figure 1.
Cluster grouping of 84 populations of Petrocoptis A. Braun ex Endl. following k-means algorithm based on 9 morphological seed traits. Colours depict prior identification following the taxonomic proposal by Montserrat & Fernández-Casas [39], and symbol shapes indicate seed morphogroups: circles, cluster a; triangles, cluster b; squares, cluster c.
Cluster “a” (circles in Figure 1), characterized by high seed area, low strophiole area and low strophiole relative size, included populations from Petrocoptis crassifolia Rouy, Petrocoptis montserratii Fern.Casas (except for Riglos, RIGL) and Beranuy (BERA), identified as Petrocoptis montsicciana O.Bolòs & Rivas Mart Cluster “b” (triangles), consisting of individuals showing low seed area, low strophiole area and medium strophiole relative size, included all populations of Petrocoptis grandiflora Rothm., Petrocoptis pseudoviscosa Fern.Casas, Petrocoptis pyrenaica subsp. pyrenaica (Bergeret) A.Braun ex Walp., Petrocoptis pyrenaica subsp. viscosa (Rothm.) P. Monts. & Fern. Casas and Petrocoptis pyrenaica subsp. glaucifolia (Lag.) P. Monts. & Fern. Casas, except for the populations from El Pindal (PIND) and Potes (POTE) of the latter. Likewise, this cluster included the population from Teller (TELL), identified as P. montsicciana. Cluster “c” (squares), characterized by a medium seed area, a high strophiole area and a high strophiole relative size, included all the populations from Petrocoptis guarensis Fern.Casas, Petrocoptis hispanica (Willk.) Pau, Petrocoptis pardoi Pau, P. montsicciana (except those mentioned above) and the populations from Riglos (RIGL, P. montserratii), El Pindal (PIND, P. pyrenaica subsp. glaucifolia) and Potes (POTE, P. pyrenaica subsp. glaucifolia).
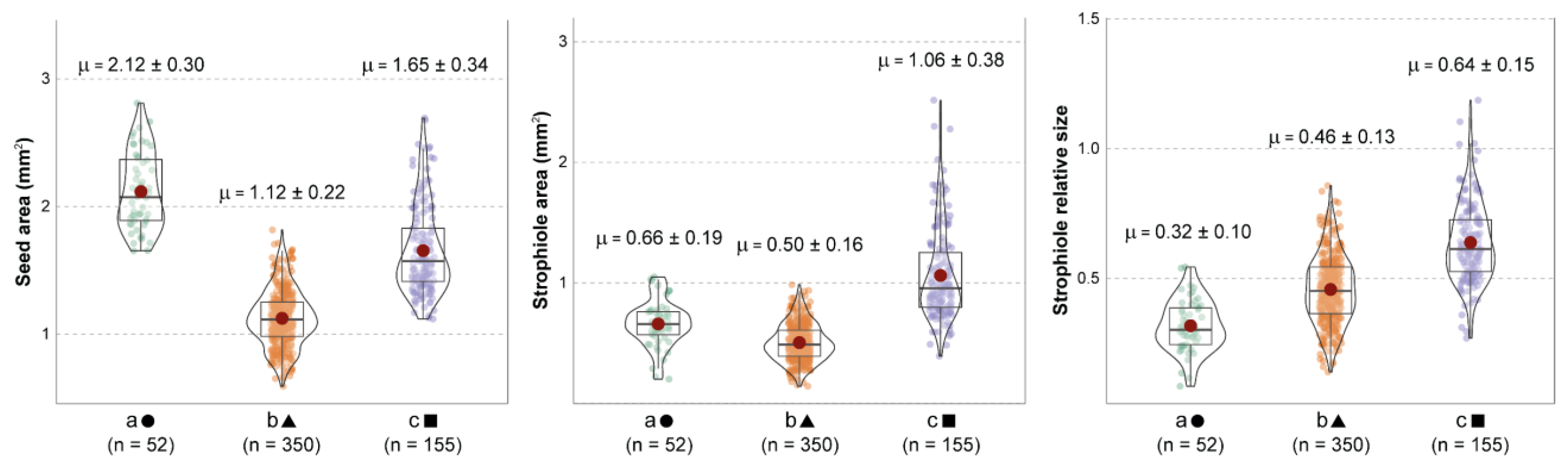
The subsequent one-way Welch’s ANOVA showed significant evidence of morphological differences among the three groups when combining seed area, strophiole area and strophiole relative size (Figure 2). Both seed and strophiole roundness showed few or no differences across groups.
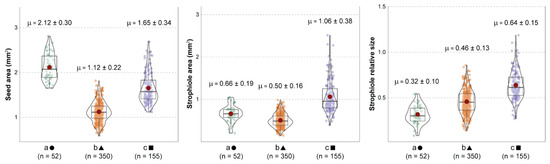
Figure 2.
Comparison among the three k-means morphogroups (a, b and c), indicating the distribution, mean values and standard deviation of seed area (left), strophiole area (center) and strophiole relative size (right). Red dots indicate mean values and grey bars indicate median values. Every Games–Howell pairwise test showed significant differences.
Furthermore, the random forest model displayed an overall accuracy of 0.8723, which means that ca. 87% of the seeds were correctly classified (Table 1). Despite group size inequality, the significant difference between the no-information rate and the overall accuracy outlined a good performance across groups. The balanced accuracy of classification for each group was also high and consistent (ca. 91% for group a, 89% for group b and 85% for group c). The traditional taxonomic classification selected as reference for the genus in 9 species or 11 taxa (including subspecies, [39]) showed worse predictive results: ca. 63% and 48% of correct classifications, respectively (Tables S2–S7). The more synthetic taxonomic proposals by Walters [38], which classified the genus in five species or seven taxa, and Mayol & Rosselló [71,75], with four species or seven taxa, obtained slightly better predictive results: ca. 68%, 54%, 77% and 69% correct classifications, respectively. Nonetheless, their classification showed worse predictive results than our cluster arrangement.

Table 1.
Confusion matrix (left) and statistics (right) of the model-trained random forest classification.
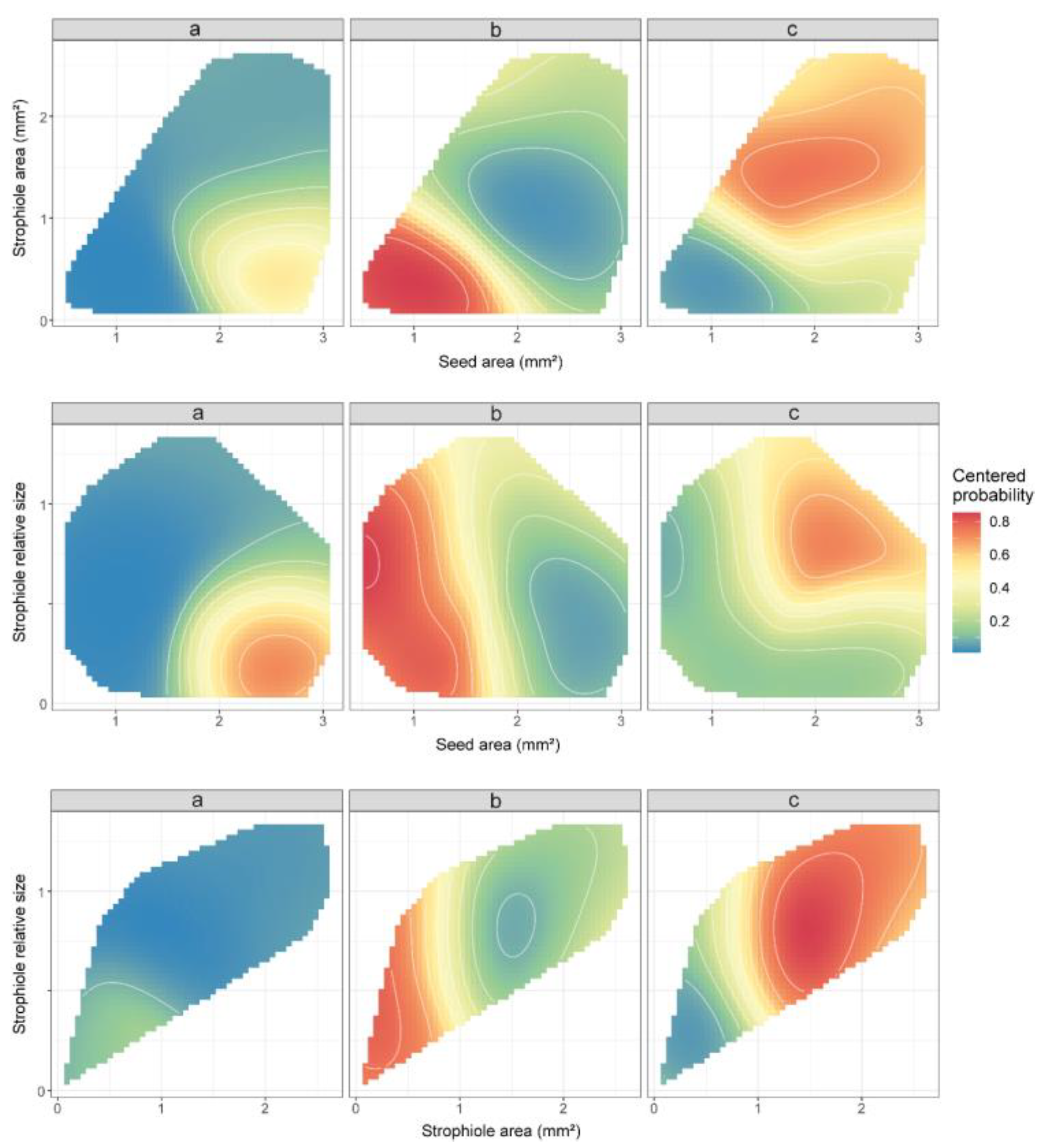
In line with previous ANOVA results, the variables with the highest classifying importance under the random forest model assumptions turned out to be the seed area, the strophiole area and the strophiole relative size. When combining these morphological traits, the support vector machine algorithm created a space of classification probabilities of a random seed in a given group (Figure 3). Thus, the highest probability of classifying a seed as part of cluster “a” (>0.6) was obtained when the seed area exceeded 2 mm2 and the strophiole relative size was below the threshold of 0.5. In the case of cluster “b”, any seed with a seed area of <1.75 mm2 and a strophiole area <1 mm2 was classified as such with a high probability (>0.8). Last, the highest values of classificatory probability within cluster “c” (>0.7) were obtained when the strophiole relative size exceeded the threshold of 1.
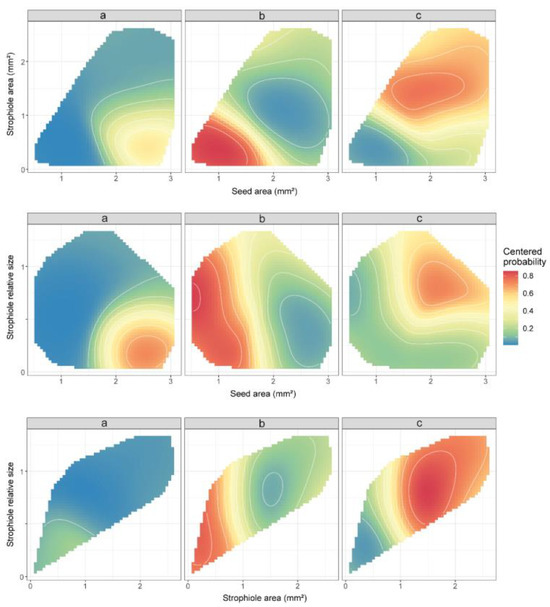
Figure 3.
Partial dependence plots of the three morphogroups (a, b and c), based on seed area, strophiole area and strophiole relative size for the seed morphology data, derived from support vector machine (svm) algorithm. Colours depict the predicted classification probabilities of a Petrocoptis seed within a given group based on its morphological traits of interest. C and gamma hyperparameters were set as default: C = 1; γ = 1/(data dimension). Values are restricted to lie within the convex hull of their training values in order to avoid extrapolation.
2.2. Climate—Seed Morphology Relationship
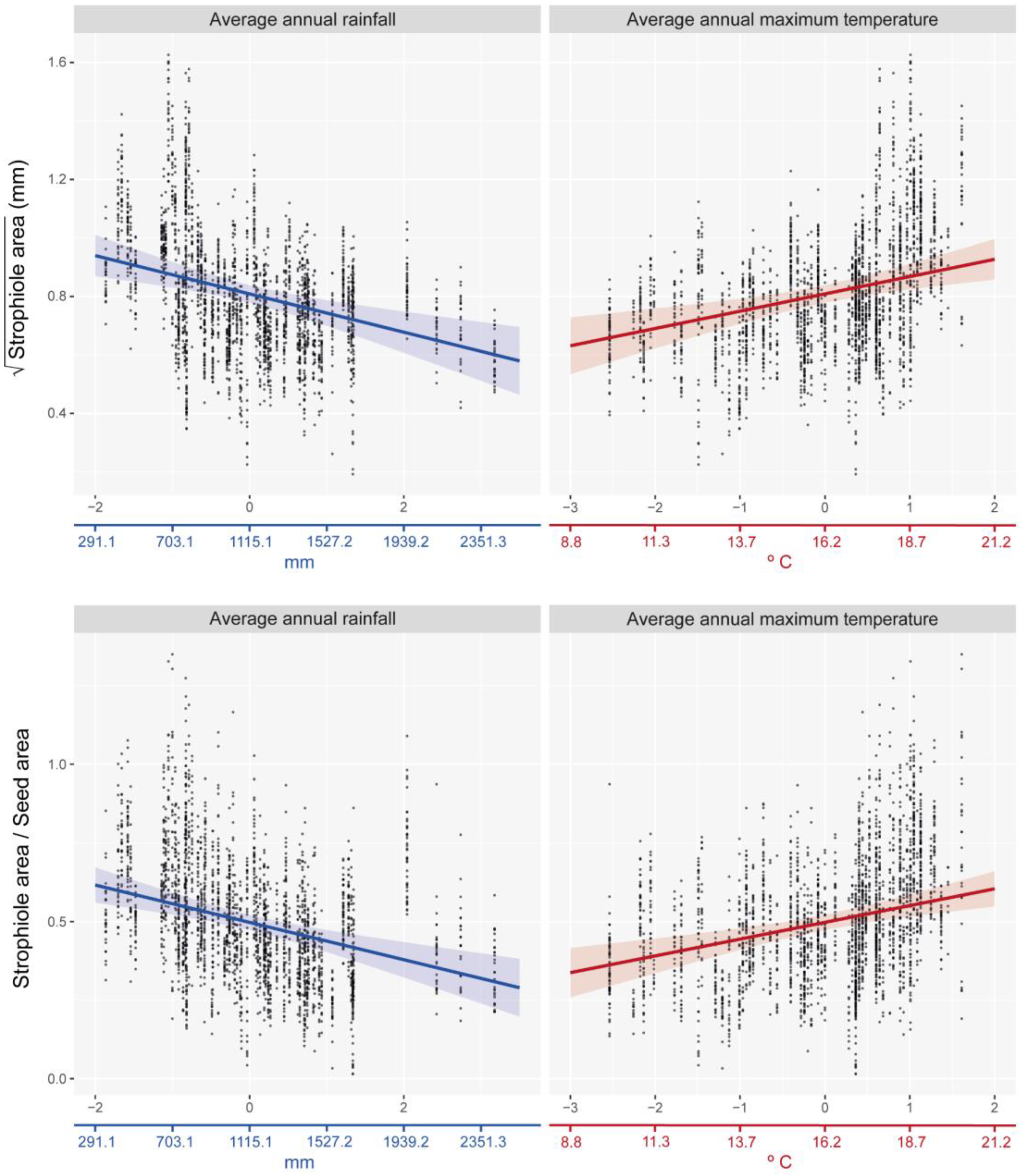
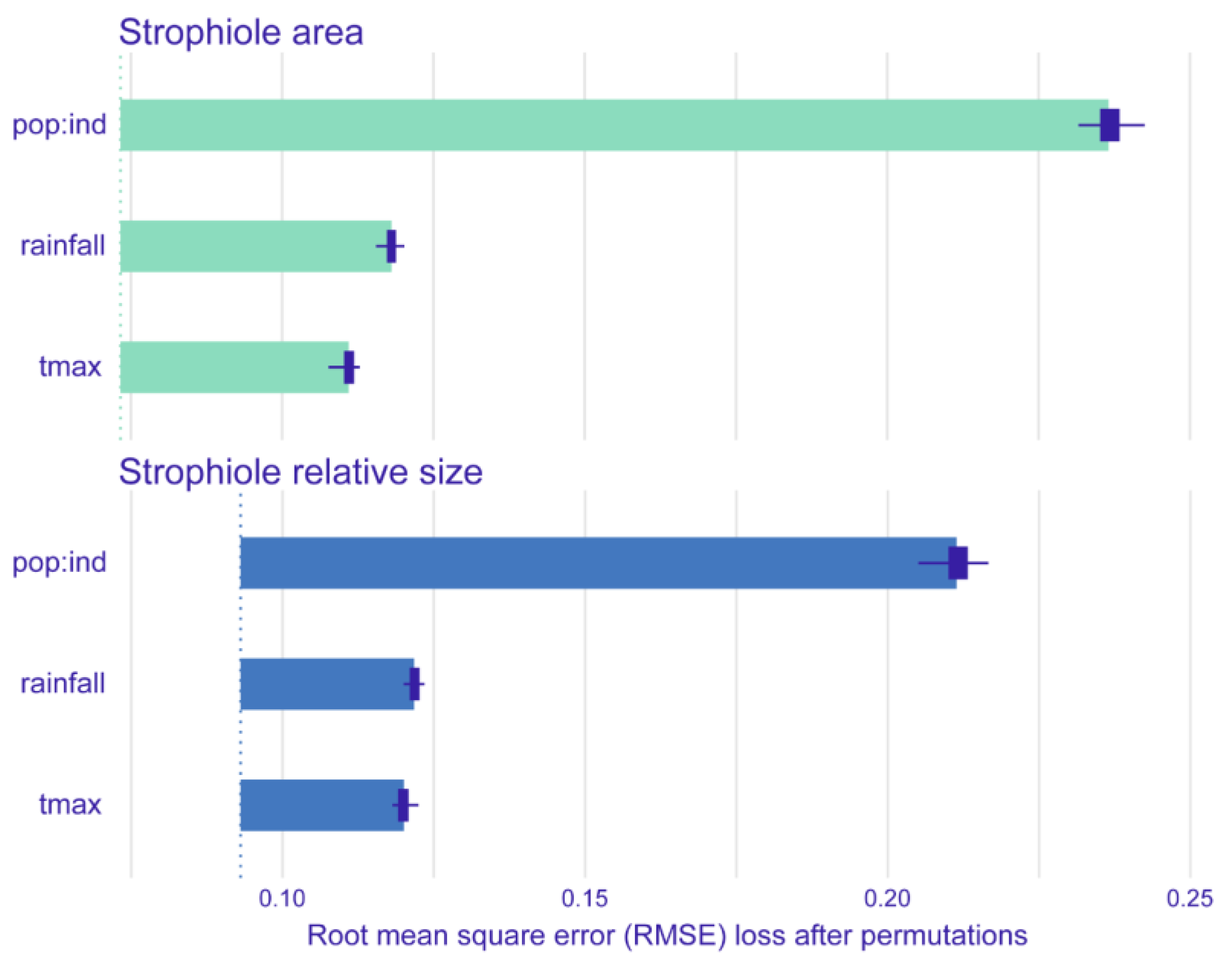
The best-fitted linear mixed effects model showed that the strophiole area of Petrocoptis seeds is sensitive to climate. Average annual rainfall was the climatic variable that explained most of the variability of the strophiole area (ΔAIC = 6.427; Table 2) and showed a negative correlation with it (Figure 4, Table 3). The average annual maximum temperature was the other most explanatory variable within the best model (ΔAIC = 4.517) and showed a positive correlation with the strophiole area. Furthermore, the relative size of the strophiole (i.e., strophiole area/seed area), a variable calculated to control the seed area effect on the strophiole, was consistent with the raw size model. Again, average annual rainfall explained most of the variability (ΔAIC = 7.334) and was negatively correlated. Temperature remained a driver of strophiole relative size (average annual maximum temperature ΔAIC = 7.019) and was positively correlated. On the other hand, the best-fitted and most parsimonious seed area model did not include any of the considered climatic variables and suggested that there is no evidence of a climate-driven seed size variation in our dataset. Exploring the variable importance through RMSE loss function, nested population and individual levels was the most important variable of both strophiole area and strophiole relative size models (ΔRMSE = 0.162 and 0.119, respectively; Figure 5), followed by average annual rainfall (ΔRMSE = 0.117 and 0.090) and average annual maximum temperature (ΔRMSE = 0.124 and 0.092).

Table 2.
Backward ∆AIC model selection of both strophiole area (a) and strophiole relative size (b). Average annual rainfall (rainfall) and average annual maximum temperature (tmax) were included as fixed effects in all models. For the best model (Mbest), null model (Mnull) and partial models (M1, M2), the following parameters are provided: Akaike Information Criterion (AICi) values, AIC differences between Mbest and M1, M2 and Mnull, degrees of freedom (df) and the marginal coefficient of determination (R2 marg.).
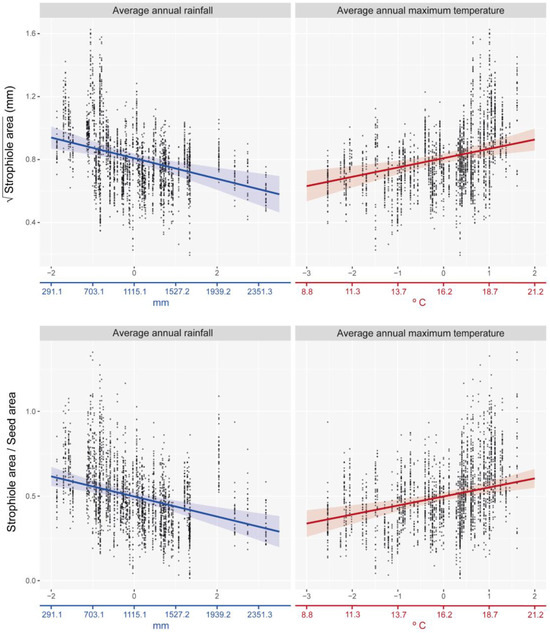
Figure 4.
Distribution of observed data values and best-fitted linear mixed-effects models (lme) between climate variables and strophiole area (up) and strophiole relative size (down). Coloured X-axis is adjusted to show its original scale (prior standardization) for illustrative purposes.

Table 3.
Summary of the best-supported models of strophiole area (a) and strophiole relative size (b). Each model includes average annual rainfall (rainfall) and average annual maximum temperature (tmax) as fixed effects. Variability at the population (pop) and individual (ind) levels is included as random effects. For each climate predictor and intercept, the following parameters are provided: coefficient estimates (Coefficient) with indication of the 95% confidence interval (95% CI), standard error (SE), t-statistic (t), degrees of freedom (df) and statistical significance (p-value).
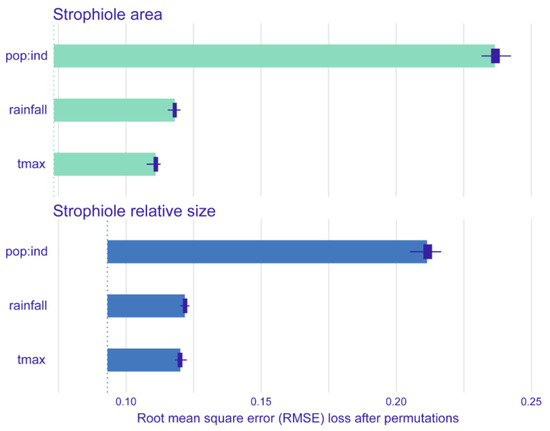
Figure 5.
Mean variable importance of best-fitted linear mixed-effects models of strophiole area (up) and strophiole relative size (down). Dotted lines indicate RMSE value of the full models, and bars depict RMSE loss when removing one variable at a time. RMSE loss was calculated after 50 permutations. Variables included: nested population and individual levels (pop:ind), average annual rainfall (rainfall) and average annual maximum temperature (tmax).
When implementing the grouping factor within the best climate models, it turned out to be the most fixed effect that collected the greatest amount of variance of both strophiole area (ΔAIC = 34.953, ΔRMSE = 0.110) and strophiole relative size (ΔAIC = 13.977, ΔRMSE = 0.042).
3. Discussion
3.1. On the Taxonomic Value of Morphometric Seed Traits in Petrocoptis Seeds
The success of sexual reproduction and plant establishment, subsequent diversification and local adaptation ultimately rely on seeds [76]. Therefore, it is expected that seed-related traits may have undergone strong selective pressure and may keep a high phylogenetic signal. In fact, seed morphology has been used for classification purposes across several genera of Caryophyllaceae, where the taxonomic significance of vegetative and floral structures is scarce [38,77,78,79,80,81,82]. The presence of a strophiole separates Petrocoptis from its sister genera, and intraspecific classifications within the genus have always relied on seed morphology [38,39,71]. In this section, we focus on the taxonomic value of the studied seed traits, but other morphological traits may also have complementary taxonomic value.
Despite the high intra- and inter-population variability found in the measured Petrocoptis seed traits, it was possible to arrange an overall coherent set of three morphological groups implementing the K-means clustering algorithm. It is remarkable that most of the 11 taxa included in Montserrat & Fernández-Casas [39], a widely used taxonomic treatment of the genus, are coherently captured within any of the three groups: P. crassifolia in cluster “a” (●), P. grandiflora, P. pseudoviscosa, P. pyrenaica subsp. pyrenaica and P. pyrenaica subsp. viscosa in cluster “b” (▲) and P. guarensis, P. hispanica and P. pardoi in cluster “c”(■). Among the 20 P. pyrenaica subsp. glaucifolia populations analysed, only two—El Pindal (PIND) and Potes (POTE), which are geographically close to each other (Figure 6)—were classified in a different cluster. For P. montsicciana, the two northernmost populations—Beranuy (BERA) and Teller (TELL)—are recovered in our analysis apart from the rest and arranged in distinct clusters. The position of P. montserratii within the cluster arrangement remains unclear, as the three populations studied were split into two distinct clusters. Nevertheless, Figure 1 shows that these three populations occupy a nearby space in the ordination, indicating that they may share common morphological features, somehow intermediate among the three recovered clusters, although they have been separated by the K-means clustering algorithm. Summarizing, the resulting clustering of populations means, according to measured seed morphometric traits, resembles the taxonomic backbone selected as reference [39] in a synthetic way, so a given seed will be classified in the same cluster as all seeds belonging to the same species or subspecies. However, no differences were observed in the quantitative study of the seed traits that allow an analytical separation of all nine species (or eleven taxa, including the three subspecies, Table 4).
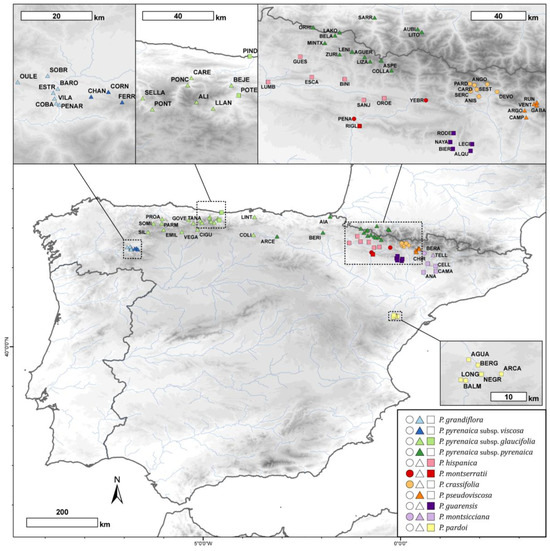
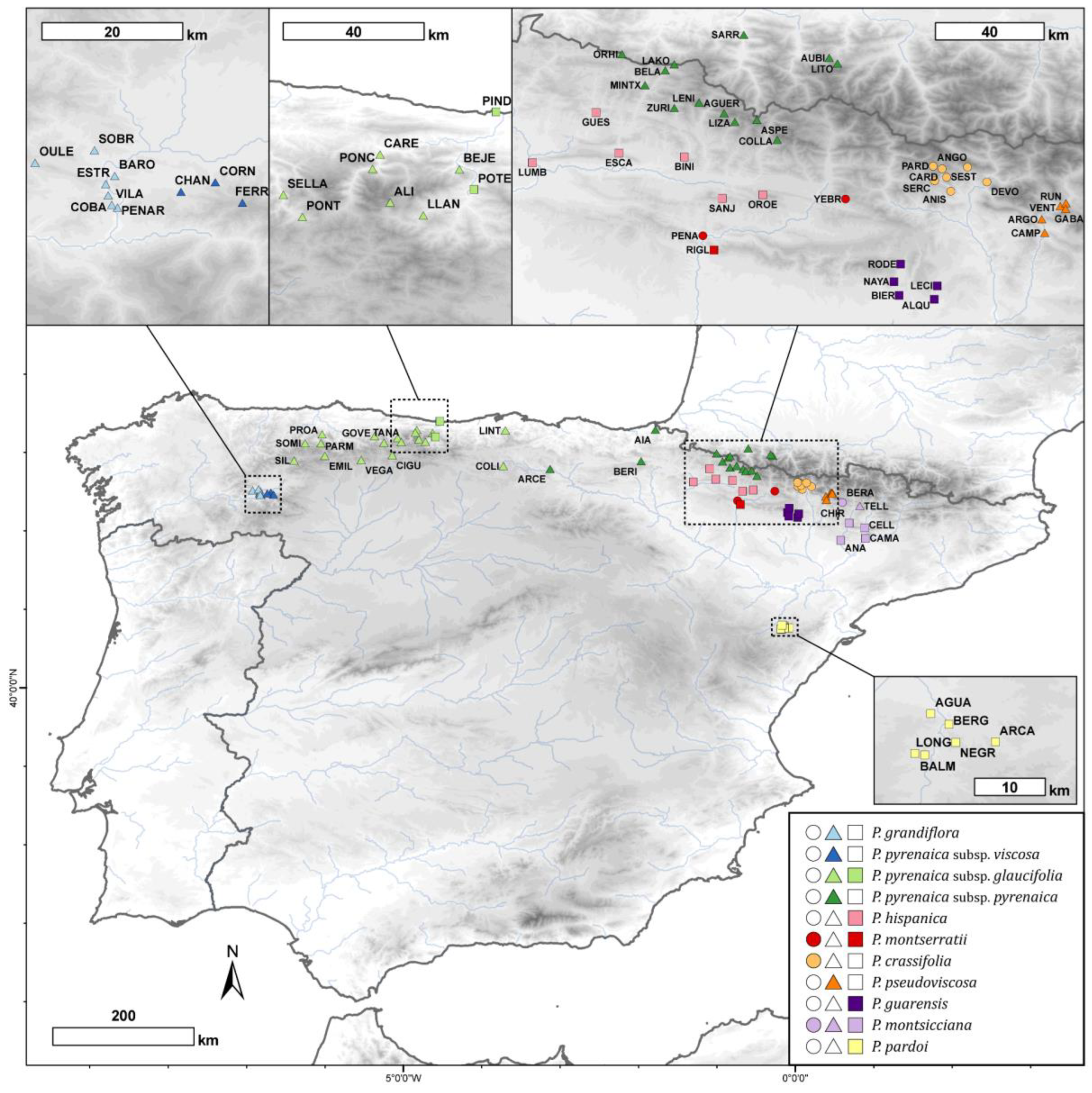
Figure 6.
Geographic distribution of the Petrocoptis sampled populations. Symbol shapes indicate seed morphogroups (see Section 2 and Figure 1: circles, cluster a; triangles, cluster b; squares, cluster c) and colours depict prior identification following the taxonomic proposal by Montserrat & Fernández Casas [39]. Population codes follow Table S1.

Table 4.
Comparison of the main taxonomic treatments proposed for the genera Petrocoptis and Silene (subg. Petrocoptis) and the assignment to each of the three morphogroups (a ●, b▲ and c ■) proposed in the present study. The classification accuracy of our seed data set, obtained by each of the random forest models, is indicated.
Walters [38] and Mayol & Rosselló [71,75] proposed more synthetic taxonomic classifications, as did our seed morphology analysis (Figure 1). Nonetheless, some of our results are not congruent with the specific and infraspecific arrangements proposed by these authors. The case of P. hispanica is notable because both Walters [38] and Mayol & Roselló [71,75] grouped this species with taxa included herein in cluster “b” (▲), like P. pseudoviscosa and P. pyrenaica (low seed area, low strophiole area, medium strophiole relative size), whereas our classification (based only on seed morphology) placed it closer to the species included in cluster “c” (■) (medium seed area, high strophiole area, high strophiole relative size). Similarly, our analysis does not support any difference in seed traits between P. grandiflora and P. pyrenaica, clearly differentiated by both Walters [38] and Mayol & Rosselló [71,75].
Further molecular work is required to trace these seed traits on an extensive phylogeny and see if they are congruent with it or, instead, if they are a case of adaptive convergence. Studies of other morphological and molecular characters are being conducted to try to propose a taxonomic treatment for the genus based on global evidence.
3.2. Relationship Between Climate and Morphological Traits of Petrocoptis Seeds
This is the first comprehensive study on patterns of seed traits and climate in Petrocoptis. Although there are some studies that explore possible relationships between climate-related variability and the morphology of plant diaspora in the literature [83,84,85,86,87], to our knowledge, no previous study has used data from representative natural populations collected throughout the entire distribution range of a chasmophytic genus.
Petrocoptis species live in limestone cliffs with some degree of temporal drought. Therefore, water availability in these populations varies depending primarily on local climate conditions, such as precipitation (directly or indirectly through infiltration) and evaporation linked to high temperatures. It has been described that seed size may increase drought tolerance [88], but this pattern does not fit the case of Petrocoptis at a climatic mesoscale level. The seed area of Petrocoptis showed no association with any of the climatic variables considered; thus, no evidence was found directly supporting the idea that the high intra- and interpopulation variability displayed by this trait is influenced by current or recent local climate. Instead, this variability could likely be explained by other factors, such as the existence of kinship networks that regulate seed size through maternal effects [89,90,91] or rock surface topographic heterogeneity, which affect microclimate components and, thus, resource availability at a microscale [3]. This can directly influence the vigour of individual plants growing in exceptionally favourable spots or that of an entire population (in case it grows in a particularly favourable cliff) and, thus, the size of the resulting mature seeds. Likewise, populations may be genetically distinct for this trait due to genetic drift or local adaptations [46,47,48].
Although it would appear reasonable to assume that the strophiole variability within Petrocoptis has taxonomic significance, ecological aspects should not be disregarded. The analysis of the strophiole identified a general and ubiquitous trend of differentiation of this structure throughout the entire distribution range and climatic spectrum of the genus. Even though Minuto et al. [82] hypothesized a correlation between the habitat in which the species grows and strophiole morphology in Moehringia L. (another strophiole-bearing Caryophyllaceae), similar relationships have not been previously explored when studying the strophiole or other analogous seed structures in Angiosperms. This strong trait–environment relationship opens the possibility that strophiole size variation has likely been driven by adaptation to local climate variables. Both raw strophiole area and its relative size exhibit clinal variation from cool/wet to warm/dry climates. Strophioles grown under low precipitation and/or warm temperature conditions are significantly bigger than those found in plants from high precipitation and/or cool temperature areas. Furthermore, the same trend is found when relative size is calculated (i.e., controlled the allometric effect that the seed size could have on it). This means that considering two individuals of the same seed size, the one growing under lower precipitation/warmer temperature conditions invests more resources in developing its strophiole than the one growing under higher precipitation/colder temperature. The ability of the strophiole to capture water helps the seed to hydrate in the overhanging cliffs with low water availability throughout the year. Our results point to an adaptive response through the investment of more resources in the production of bigger strophioles under dry and warm climates, increasing the hydration ability of the seed. Most populations and species integrating cluster “c” (■) —P. pardoi, P. montsicciana and P. guarensis—grow under Mediterranean macrobioclimate conditions [42], whereas those integrating cluster “b” (▲)—particularly P. pyrenaica and P. grandiflora—are more influenced by the temperate oceanic bioclimate (see below). However, Petrocoptis species present very marked geographic distribution patterns that strongly overlap with climatic gradients, which may lead to misinterpretation of the significant relationship found here between strophiole size and climate. Consequently, further research is needed (i.e., common garden experiments simulating climatic gradients) to test this hypothesis and clarify whether these relationships are or are not spurious.
Moreover, in the case that the effect of climate on the strophiole is confirmed, we are aware that it can be either a plastic- or allelic-dependent response. For perennial plants growing in regions showing important inter-annual climatic differences (as is the case of Petrocoptis in north Iberian mountains), it does not seem that allele selection through generation is the only adaptive pressure operating regarding climate, as it may take many years to modify allele frequencies and not every year the pressure may have the same intensity and direction. Common garden experiments simulating climatic gradients may help to clarify this issue, as they allow to test to what extent seeds of different origins present a fixed strophiole morphology independently of the climate (low plasticity and high genetically determined trait) or a variable behaviour according to climate (high plasticity and low genetically determined trait).
3.3. On the Biological Role of the Strophiole in Petrocoptis
Our results support the hypothesis that the strophiole is involved in the regulation of seed water balance. This outgrowth of the hilum region is capable of catching and retaining water so that it could be subsequently supplied to the seed [69]. Strophioles can regulate water movements in response to changes in external moisture but in an opposite way as compared to the hilum. While the hilum can open and close repeatedly in correspondence, respectively, with low and high environmental humidity, the strophiole remains closed at very low humidity [92,93,94]. Furthermore, variations in seed hydration may result from simultaneous fluctuations in temperature and humidity, which is likely what causes strophiole rupture in the field [94]. This is relevant on the face of the often overhanging limestone cliffs where Petrocoptis lives, a potential water-limited habitat [3] where fast and efficient seed hydration would improve germination and plant establishment. Furthermore, it appears to be critical for populations living under Mediterranean macrobioclimate conditions characterized by seasonal precipitation and summer water scarcity. In this context, it is likely that in the course of the evolution of the genus, the hygroscopic ability of the strophiole and its adaptation to water unavailability have secondarily favoured adhesion to the vertical surface of the rock and establishment in crevices where the soil is scarce or non-existent, as some researchers have already observed [72,74]. Following this hypothesis, this character could be understood as an exaptation [95]. The establishment of Petrocoptis in the rocky outcrops has probably benefited from these strophiole properties, along with the ability of the flower stems to reorient themselves towards the wall once the fruit has ripened, favouring the introduction of the seeds into the nearby cracks (i.e., geoautochory, active geocarpy according to [96,97,98]). Probably, apart from geoautochory, gravity (barochory), ombrohydrochory (rain-operated seed dispersal [99]) and even the intervention of certain ants, specialized or not, also act in Petrocoptis species. Under these premises, it is likely that Petrocoptis strophiole provides other adaptive advantages in addition to those discussed in this article.
The potential function of the strophiole in Petrocoptis as an ant attraction and reward structure needs to be discussed. Following the enunciation of the myrmecochory syndrome [64], it has recently been generalized for European and Iberian species that the presence of excrescences in the plant diaspora (i.e., elaiosomes) has a meaning related to its dispersal via ants [66,67]. The elaiosome is a lipid-rich appendage that can vary greatly in form, colour, hardness and size that can be found on seeds, fruits and other angiosperm organs [64,100]. Therefore, it brings together a broad spectrum of analogous structures (e.g., strophiole, aril) observed throughout different organs that share being mainly composed of lipids [67]. However, this characteristic has not been observed nor tested in the strophiolar tuft of Petrocoptis; neither has the case of the dispersal potential of ants in the case of Petrocoptis seeds. While it is not a focus of this study, exploring the relationship between Petrocoptis strophiole and ant-mediated dispersal will be part of our future research objectives.
This study is the first to interpret the seed strophiole of Petrocoptis as a potentially climate-related water uptake structure, which is especially important under adverse (dry) climatic conditions. Further physiological studies may help to deeply understand how this physical process occurs and how water uptake is regulated.
4. Materials and Methods
4.1. Plant Material: The Genus Petrocoptis
Petrocoptis is composed of chasmophytic chamaephytes endemic to the northern Iberian Peninsula. They live in the crevices of overhanging limestone cliffs, which are harsh environments for plant life. Both the delimitation of the genus and the number of accepted infrageneric taxa have changed over the last century (between four and twelve species, depending on the author), as studies of morphological traits [39,71,75,101,102,103] and both biochemical (isozymes; [104]) and genetic markers analyses (ITS, rps16 intron; [105]) were carried out. Some of the traits that have traditionally been shown to be taxonomically important in the genus Petrocoptis, as well as other taxa within the family Caryophyllaceae, are found in the seed. It has a subreniform appearance and a large hygroscopic strophiolar tuft made of hairs that conceal an opening in the testa.
Based exclusively on practical reasons and in order to facilitate communication, in the first part of this study, we followed an easily available taxonomic treatment (included within the reference work Flora iberica http://www.floraiberica.es/, accessed on 10 November 2024), proposed by Montserrat & Fernández-Casas [39], in which the infrageneric classification of Petrocoptis consists of 9 species: P. crassifolia Rouy, P. grandiflora Rothm., P. guarensis Fern. Casas, P. hispanica (Willk.) Pau, P. montserratii Fern. Casas, P. montsicciana O. Bolòs & Rivas Mart., P. pardoi Pau, P. pseudoviscosa Fern. Casas and P. pyrenaica. Within the latter, 3 subspecies are recognized: P. pyrenaica subsp. glaucifolia (Lag.) P. Monts. & Fern. Casas, P. pyrenaica subsp. pyrenaica (Bergeret) A. Braun ex Walp. and P. pyrenaica subsp. viscosa (Rothm.) P. Monts. & Fern. Casas.
4.2. Sampling
Based on intensive field and herbarium investigations, 557 individuals from 84 populations of Petrocoptis were selected, and germplasm material was collected and preserved in silica gel. These populations are evenly distributed throughout the entire distribution range of the genus, and they cover both its whole altitudinal gradient and climatic spectrum (Figure 6). A total of 81 of the 84 populations were located in Spain, and 3 populations occurred on the French Pyrenees. Population information and specimen voucher numbers are listed in Table S1. The voucher specimens were deposited in the herbarium of the Pyrenean Institute of Ecology-CSIC (JACA, herbarium codes following standard abbreviations from [106,107]). In each of the 84 populations, a minimum of three individuals were randomly selected for morphometric measurements. When more than three individuals had ripened dehiscent capsular fruit, the sampling was extended to a maximum of ten individuals per population. Conversely, the underrepresented populations were completed with previously collected herbarium material from BC, BIO, JACA, LEB, MA, SALA, SANT, VAL and VIT collections.
4.3. Measurement and Analysis of Morphological Traits
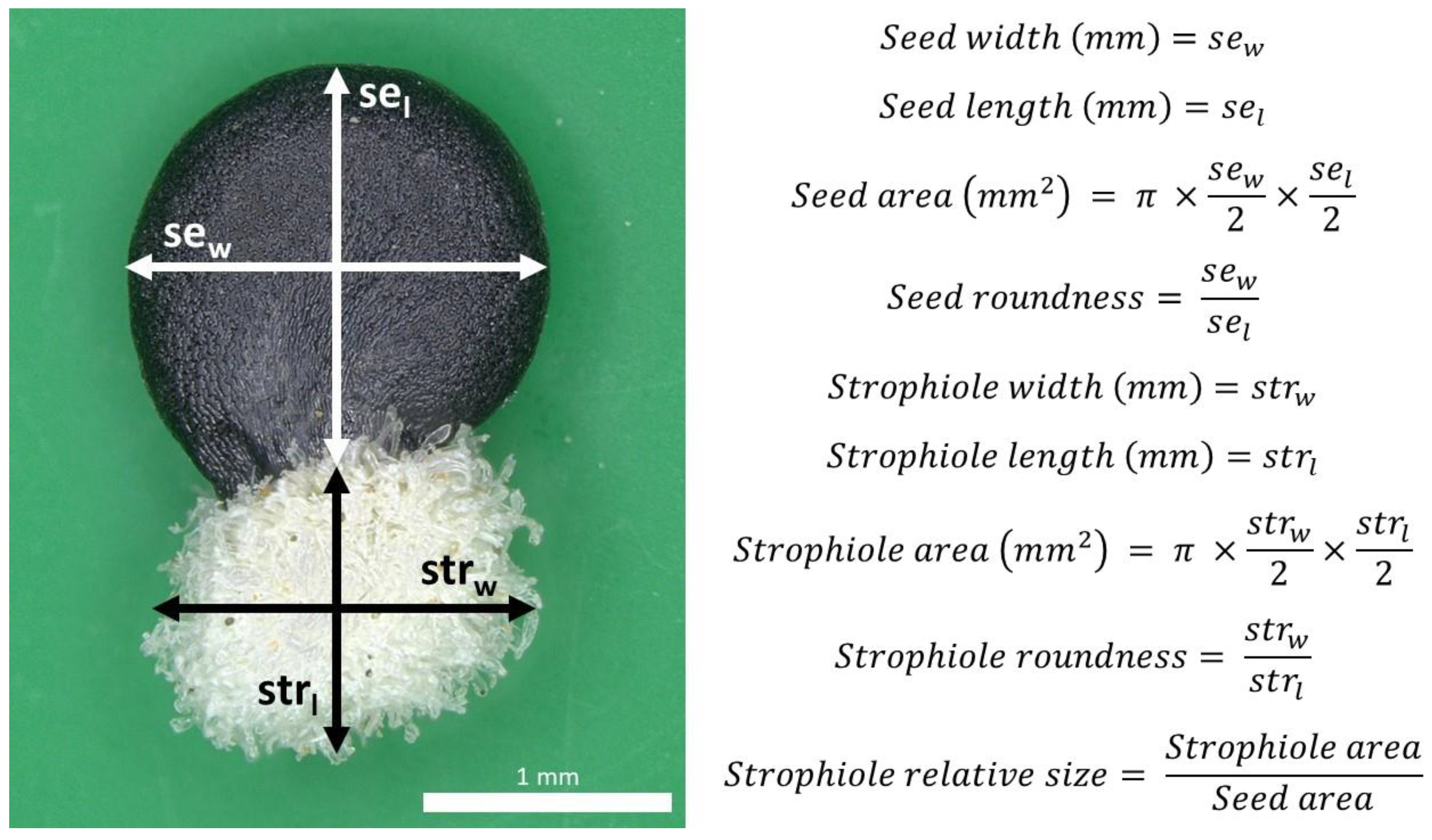
In total, 2773 seeds from 557 individuals were processed. Nine quantitative seed traits were selected following previous taxonomic accounts available for Petrocoptis [39] and other related taxa of the tribe Sileneae [108,109]. Seed digital images were captured with a Leica MC190 camera connected to an HD NIKON SMZ800 stereo microscope at 10× magnification with a resolution of 171 pixels mm−1. Then, images were semi-automatically processed with the software Leica Application Suite v4.12.0: seed and strophiole diameters were measured using software manual tools, and their respective area, roundness and strophiole relative size were automatically calculated (Figure 7).
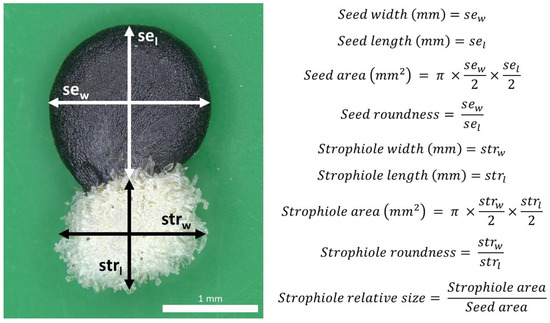
Figure 7.
Petrocoptis seed traits of interest.
For further clustering analysis, each population was considered as an operational taxonomic unit (OTU), and a data matrix was built using population mean values of all 9 morphological traits. We selected k-means as the cluster analysis technique to arrange populations based on seed morphology, as it is the most commonly used unsupervised machine learning algorithm for partitioning a given data set [110,111]. We applied a square root transformation to variables seed area and strophiole area and then we scaled/standardized the data to avoid the clustering algorithm dependence on an arbitrary variable unit. Euclidean distance was selected as a measurement of dissimilarity between observations. The NbClust R package (version 3.0.1) [112] provided 30 indices that helped determine the number of clusters (k) that will be generated in the final solution. Finally, the best k-means algorithm (k = 3; see Section 2) was carried out using the k-means function implemented in the stats R package and displayed with package factoextra (version 1.0.7) [113].
Then, we assessed the morphological recognisability of the three groups (those identified by the best k-means algorithm) in order to allow their identification and description. Thus, we estimated the normal fitted density distributions of 9 quantitative morphological traits for the 3 groups to assess the discontinuity and overlap of the variation of these morphological traits. One-way Welch’s ANOVA was performed to test the statistical significance of morphological differences among the groups proposed by k-means algorithm, and Games–Howell test was selected for pairwise comparisons. Both analyses were computed and displayed using ggstatsplot R package (version 0.12.2) [114]. Then, the coherence and predictability of each group were examined following Breiman’s random forest algorithm for classification [115,116], using randomForest R package (version 4.7.1.1) [117]. Then, we compared our results with the traditional taxonomic groups proposed by Montserrat & Fernández-Casas [39], Walters [38] and Mayol & Rosselló [71,75]. Although there are other taxonomic treatments available [101,102,118], they do not differ substantially from these ones. In order to test the predictive and classifying capacity of the model, it was trained with a subset of the raw seed data (70%) and later validated with the rest of them (30%). Given the high correlation between the length and width with the area of both the seed and strophiole, only the areas were included. In this way, the most explanatory variables were selected, discarding the redundant ones. The models were evaluated taking into account their confusion matrices, calculated using caret R package (version 6.0.94) [119] and the following general statistics:
(1) accuracy (Equation (1));
Accuracy = (True Positive + False Positive)/(True Positive + False Positive + True Negative + False Negative)
(2) No information rate (i.e., how often the model would be wrong if it always predicted the majority class);
(3) Cohen’s Kappa: a measure of how well the classifier performed as compared to how well it would have randomly performed [120,121]. The following specific statistics for each class (i.e., the three different clusters) were also explored:
(4) Sensitivity (Equation (2)):
Sensitivity = (True Positive)/(True Positive + False Negative)
(5) Specificity (Equation (3)):
Specificity = (True Negative)/(False Positive + True Negative)
(6) Balanced accuracy (Equation (4)):
Balanced Accuracy = (Sensitivity + Specificity)/2
Finally, the classificatory importance of each of the predictor variables (i.e., the diagnostic ability of each of them) was evaluated using the vip R package (version 0.4.1) [122]. The probability prediction of classification in a given cluster was explored following a support vector machine approach (SVM), implemented in e1071 R package (version 1.7.14) [123], selecting a radial basis kernel and setting C and gamma (γ) hyperparameters as default (C = 1, γ = 1/data dimension). The results were displayed as partial dependence plots and implemented in the pdp R package (version 0.8.1) [124] on a probability scale.
4.4. Identification of Relationships Among Seed Traits and Climatic Variables
We explored the relationship between climate and the seed traits and the following three results of interest: strophiole area, seed area and the ratio established between them. Linear mixed effects models (lme) were fitted between the traits and a set of climatic variables in order to determine the amount of variability explained by the environment. For this analysis, all 2773 Petrocoptis seeds were used as samples, and the genus’ perspective was followed as the reference taxonomic unit. Climate data were extracted for each population location from the Climatic Digital Atlas of the Iberian Peninsula [125], with a spatial resolution of 200 m. The following variables were considered as fixed effects: average annual rainfall, mean annual temperature, average annual maximum and minimum temperatures and average annual solar radiation. Furthermore, as observations were not independent nor exhaustive, individual and population levels were nested and included as random effects. We standardized all predictor variables to be comparable (i.e., equally weighted within the model), with a mean value of 0 and a standard deviation of 1, and seed trait values were transformed using square roots in order to achieve a normal fitted distribution of residuals. Then, linear mixed-effects models were performed to evaluate which predictor feature proposed a priori explained most of the variability. Each model, from the full one (with all the variables) to the null one (without any of them), was compared following the Akaike Information Criterion (AIC; [126,127]. Finally, after choosing the best one, we performed a backward model selection, starting with the best model and generating new simpler models by removing one variable at a time to determine the amount of variability of the seed traits that each could explain. The importance of each explanatory variable was evaluated by the calculation of a loss function that quantified the root-mean-squared-error (RMSE) goodness-of-fit measure drop of a given model after removing one variable at a time. It allowed us to compare the dependence of the models on climatic variables to make accurate predictions and to quantify the importance of random effects corresponding to the individual and population levels. This approach was performed by applying the feature_importance function on a previously created model explainer (explain function), setting 50 as the desired number of permutations. Algorithms were implemented in DALEX (version 2.4.3) and ingredients (version 2.3.0) R packages [128,129].
Finally, the K means grouping factor was added to the best model as a fixed effect in order to compare the amount of variability explained by cluster arrangement and climatic variables and to explore the relationship between seed morphology and climate across morphogroups.
5. Conclusions
The combination of seed morphometric traits of Petrocoptis alone allows the division of the genus into three well-defined morphogroups with known seed characteristics: one made up of large seeds (>2 mm2) with low strophiole relative size (<0.5), a second one composed by small seeds < 1.75 mm2) with small strophioles (<1 mm2) and a third one that includes seeds of high strophiole relative size (>1). Most of the taxa included in the taxonomic treatment that was used as reference [39] are coherently captured within any of the three groups, although no differences were observed in the quantitative study that allowed an analytical separation of all nine species (or eleven taxa, including the three subspecies proposed under P. pyrenaica). However, our results suggest that part of the intra- and interpopulation variability found in strophiole raw and relative size can be explained by climatic variables. We identified a general and ubiquitous trend of differentiation of this structure throughout the entire distribution range and climatic spectrum of the genus. This strong trait–environment relationship opens the possibility that strophiole size variation has likely been driven by adaptation to local climatic variables. Both raw strophiole area and its relative size exhibit clinal variation from cool/wet to warm/dry climates. This fact points to an adaptive response through the investment of more resources in the production of bigger strophioles in dry and warm climates, therefore increasing the hydration ability of the seed under these conditions. Similar relationships have not been previously explored when studying the strophiole or other analogous seed structures in Angiosperms. Our results lead us to reinterpret strophiole, in this case, as a climate-related structure involved in the regulation of seed water balance. It is likely that the adaptation of the strophiole to water unavailability, together with its hygroscopicity, secondarily favoured plant establishment and survival of Petrocoptis species in the crevices of vertical limestone cliffs throughout the course of the evolution of the genus.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13223208/s1, Figure S1: Variable intercorrelations and correlations between variables and the first 4 principal components; Table S1: Overview of the studied Petrocoptis populations and their characteristics; Table S2: Values of indices for each partition of the dataset obtained with a number of clusters; Tables S3–S8: Confusion matrices, overall statistics and statistics by class of the model-trained random forest algorithm following the taxonomic classification of the genus considered as reference.
Author Contributions
J.C.-Y.: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing—original draft, writing—review and editing. Á.L.R.-R.: formal analysis, investigation. B.H.: formal analysis, investigation, methodology. A.A.: funding acquisition, methodology, project administration, resources, writing—review and editing. M.M.M.-O.: conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—original draft, writing—review and editing. P.T.: conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the project PRIOCONEX, funded by FUNDACIÓN BIODIVERSIDAD (MINISTERIO PARA LA TRANSICIÓN ECOLÓGICA Y EL RETO DEMOGRÁFICO, SPANISH GOVERNMENT, Resolución 10/11/2020) and by the MINISTERIO DE CIENCIA, INNOVACIÓN Y UNIVERSIDADES through a PhD scholarship [FPU21/00305 to J.C-Y].
Data Availability Statement
The data presented in this study are contained within the article and Supplementary Materials and openly available in Zenodo at https://doi.org/10.5281/zenodo.13972509.
Acknowledgments
We would like to thank everyone who has contributed to the project, especially those who have made the intense period of seed collection possible. We also thank Ordesa y Monte Perdido and Picos de Europa National Parks for their support, as well as biodiversity and environment authorities of Galicia, Asturias, Castilla y León, Cantabria, Euskadi, Navarra, Aragón, Catalunya and Comunitat Valenciana for their collaboration and for granting collecting permits. Lastly, we would like to thank all herbaria that have provided material for this study: JACA, SALA, MA, VIT, VAL, LEB, BIO, SANT and BC (in order of contributions).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rioux, J.; Quézel, P. Contribution a l’etude Des Groupements Rupicoles Endemiques Des Alpes-Maritimes. Vegetatio 1949, 2, 1–13. [Google Scholar] [CrossRef]
- Davis, P.H. Cliff Vegetation in the Eastern Mediterranean. J. Ecol. 1951, 39, 63–93. [Google Scholar] [CrossRef]
- Larson, D.W.; Matthes, U.; Kelly, P.E. Cliff Ecology: Pattern and Process in Cliff Ecosystems; Cambridge University Press: Cambridge, UK, 2000; ISBN 9780521554893. [Google Scholar]
- Lavergne, S.; Garnier, E.; Debussche, M. Do Rock Endemic and Widespread Plant Species Differ under the Leaf–Height–Seed Plant Ecology Strategy Scheme? Ecol. Lett. 2003, 6, 398–404. [Google Scholar] [CrossRef]
- Buira, A.; Cabezas, F.; Aedo, C. Disentangling Ecological Traits Related to Plant Endemism, Rarity and Conservation Status in the Iberian Peninsula. Biodivers. Conserv. 2020, 29, 1937–1958. [Google Scholar] [CrossRef]
- Pérez-Gutiérrez, M.A.; Romero-García, A.T.; Salinas, M.J.; Blanca, G.; Fernández, M.C.; Suárez-Santiago, V.N. Phylogeny of the Tribe Fumarieae (Papaveraceae s.l.) Based on Chloroplast and Nuclear DNA Sequences: Evolutionary and Biogeographic Implications. Am. J. Bot. 2012, 99, 517–528. [Google Scholar] [CrossRef]
- Ebersbach, J.; Muellner-Riehl, A.N.; Michalak, I.; Tkach, N.; Hoffmann, M.H.; Röser, M.; Sun, H.; Favre, A. In and out of the Qinghai-Tibet Plateau: Divergence Time Estimation and Historical Biogeography of the Large Arctic-Alpine Genus Saxifraga, L. J. Biogeogr. 2017, 44, 900–910. [Google Scholar] [CrossRef]
- Machado, T.M.; Loiseau, O.; Paris, M.; Weigand, A.; Versieux, L.M.; Stehmann, J.R.; Lexer, C.; Salamin, N. Systematics of Vriesea (Bromeliaceae): Phylogenetic Relationships Based on Nuclear Gene and Partial Plastome Sequences. Bot. J. Linn. Soc. 2020, 192, 656–674. [Google Scholar] [CrossRef]
- Crespo, M.B.; Martínez-Azorín, M.; Alonso, M.Á.; Sáez, L. Two New Calcicolous Species of Pinguicula Sect. Pinguicula (Lentibulariaceae) Growing on Rocky Habitats of the Iberian Peninsula. Phytotaxa 2020, 456, 269–284. [Google Scholar] [CrossRef]
- Bobo-Pinilla, J.; Peñas, J.; Padilla-García, N.; Martínez-Ortega, M.M. Phylogeny and Phylogeography of Arenaria Section Pseudomoehringia. J. Syst. Evol. 2021, 59, 298–315. [Google Scholar] [CrossRef]
- Otero, A.; Fernández-Mazuecos, M.; Vargas, P. Evolution in the Model Genus Antirrhinum Based on Phylogenomics of Topotypic Material. Front. Plant. Sci. 2021, 12, 631178. [Google Scholar] [CrossRef]
- de Queiroz, L.P.; Cardoso, D.; Fernandes, M.F.; Moro, M.F. Diversity and Evolution of Flowering Plants of the Caatinga Domain. In Caatinga: The Largest Tropical Dry Forest Region in South America; Springer: Berlin/Heidelberg, Germany, 2018; pp. 23–63. [Google Scholar] [CrossRef]
- Médail, F. Plant Biogeography and Vegetation Patterns of the Mediterranean Islands. Bot. Rev. 2022, 88, 63–129. [Google Scholar] [CrossRef]
- Holderegger, R.; Thiel-Egenter, C. A Discussion of Different Types of Glacial Refugia Used in Mountain Biogeography and Phylogeography. J. Biogeogr. 2009, 36, 476–480. [Google Scholar] [CrossRef]
- Gentili, R.; Bacchetta, G.; Fenu, G.; Cogoni, D.; Abeli, T.; Rossi, G.; Salvatore, M.C.; Baroni, C.; Citterio, S. From Cold to Warm-Stage Refugia for Boreo-Alpine Plants in Southern European and Mediterranean Mountains: The Last Chance to Survive or an Opportunity for Speciation? Biodiversity 2015, 16, 247–261. [Google Scholar] [CrossRef]
- Cooper, A. Plant Species Coexistence in Cliff Habitats. J. Biogeogr. 1997, 24, 483–494. [Google Scholar] [CrossRef]
- Pérez-Gutiérrez, M.A.; Romero-García, A.T.; Fernández, M.C.; Blanca, G.; Salinas-Bonillo, M.J.; Suárez-Santiago, V.N. Evolutionary History of Fumitories (Subfamily Fumarioideae, Papaveraceae): An Old Story Shaped by the Main Geological and Climatic Events in the Northern Hemisphere. Mol. Phylogenet. Evol. 2015, 88, 75–92. [Google Scholar] [CrossRef]
- Nobis, M.; Klichowska, E.; Vintsek, L.; Wróbel, A.; Nobis, A.; Zalewska-Gałosz, J.; Nowak, A. Evolutionary Response of Cold-Adapted Chasmophytic Plants to Quaternary Climatic Oscillations in the Mountains of Central Asia (a World Hotspot of Biodiversity). Divers. Distrib. 2023, 29, 1458–1477. [Google Scholar] [CrossRef]
- Lavergne, S.; Thompson, J.D.; Garnier, E.; Lavergne, M.D.; Thompson, S.; Lavergne, S.; Thompson, J.D.; Garnier, E.; Debussche, M. The Biology and Ecology of Narrow Endemic and Widespread Plants: A Comparative Study of Trait Variation in 20 Congeneric Pairs. Oikos 2004, 107, 505–518. [Google Scholar] [CrossRef]
- Thompson, J.D. Plant Evolution in the Mediterranean, 2nd ed.; Oxford University Press: Oxford, UK, 2020; ISBN 0198835140. [Google Scholar]
- Minuto, L.; Grassi, F.; Casazza, G. Ecogeographic and Genetic Evaluation of Endemic Species in the Maritime Alps: The Case of Moehringia lebrunii and M. sedoides (Caryophyllaceae). Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2006, 140, 146–155. [Google Scholar] [CrossRef]
- Dixon, C.J.; Gutermann, W.; Schönswetter, P.; Schneeweiss, G.M. Taxonomy and Nomenclature of the Polymorphic European High Mountain Species Androsace (L.) Lapeyr. (Primulaceae). PhytoKeys 2016, 75, 93. [Google Scholar] [CrossRef]
- Jiménez-López, F.J.; Ortiz, M.A.; Berjano, R.; Talavera, S.; Terrab, A. High Population Genetic Substructure in Hypochaeris leontodontoides (Asteraceae), an Endemic Rupicolous Species of the Atlas Mountains in NW Africa. Alp. Bot. 2016, 126, 73–85. [Google Scholar] [CrossRef]
- Postigo Mijarra, J.M.; Barrón, E.; Gómez Manzaneque, F.; Morla, C. Floristic Changes in the Iberian Peninsula and Balearic Islands (South-West Europe) during the Cenozoic. J. Biogeogr. 2009, 36, 2025–2043. [Google Scholar] [CrossRef]
- Barrón, E.; Rivas-Carballo, R.; Postigo-Mijarra, J.M.; Alcalde-Olivares, C.; Vieira, M.; Castro, L.; Pais, J.; Valle-Hernández, M. The Cenozoic Vegetation of the Iberian Peninsula: A Synthesis. Rev. Palaeobot. Palynol. 2010, 162, 382–402. [Google Scholar] [CrossRef]
- Jiménez-Moreno, G.; Fauquette, S.; Suc, J.P. Miocene to Pliocene Vegetation Reconstruction and Climate Estimates in the Iberian Peninsula from Pollen Data. Rev. Palaeobot. Palynol. 2010, 162, 403–415. [Google Scholar] [CrossRef]
- Quesada, C.Q.; Oliveira, J.T. The Geology of Iberia. A Geodynamic Approach—Volume 3: The Alpine Cycle; Springer Nature: Cham, Switzerland, 2019; ISBN 978-3-030-11294-3. [Google Scholar]
- Buira, A.; Aedo, C.; Medina, L. Spatial Patterns of the Iberian and Balearic Endemic Vascular Flora. Biodivers. Conserv. 2017, 26, 479–508. [Google Scholar] [CrossRef]
- Vargas, P.; Morton, C.M.; Jury, S.L. Biogeographic Patterns in Mediterranean and Macaronesian Species of Saxifraga (Saxifragaceae) Inferred from Phylogenetic Analyses of ITS Sequences. Am. J. Bot. 1999, 86, 724–734. [Google Scholar] [CrossRef]
- Fiz, O.; Vargas, P.; Alarcón, M.L.; Aldasoro, J.J. Phylogenetic Relationships and Evolution in Erodium (Geraniaceae) Based on TrnL-TrnF Sequences. Syst. Bot. 2006, 31, 739–763. [Google Scholar] [CrossRef]
- Vargas, P.; Carrió, E.; Guzmán, B.; Amat, E.; Güemes, J. A Geographical Pattern of Antirrhinum (Scrophulariaceae) Speciation since the Pliocene Based on Plastid and Nuclear DNA Polymorphisms. J. Biogeogr. 2009, 36, 1297–1312. [Google Scholar] [CrossRef]
- Alarcón, M.; Vargas, P.; Sáez, L.; Molero, J.; Aldasoro, J.J. Genetic Diversity of Mountain Plants: Two Migration Episodes of Mediterranean Erodium (Geraniaceae). Mol. Phylogenet. Evol. 2012, 63, 866–876. [Google Scholar] [CrossRef]
- Scheunert, A.; Heubl, G. Diversification of Scrophularia (Scrophulariaceae) in the Western Mediterranean and Macaronesia—Phylogenetic Relationships, Reticulate Evolution and Biogeographic Patterns. Mol. Phylogenet. Evol. 2014, 70, 296–313. [Google Scholar] [CrossRef]
- Navarro, T. Systematics and Biogeography of the Genus Teucrium (Lamiaceae). In Teucrium Species: Biology and Applications; Springer Nature: Cham, Switzerland, 2020; pp. 1–38. [Google Scholar] [CrossRef]
- Oxelman, B.; Lidén, M.; Rabeler, R.K.; Popp, M. A Revised Generic Classification of the Tribe Sileneae (Caryophyllaceae). Nord. J. Bot. 2000, 20, 743–748. [Google Scholar] [CrossRef]
- Greenberg, A.K.; Donoghue, M.J. Molecular Systematics and Character Evolution in Caryophyllaceae. Taxon 2011, 60, 1637–1652. [Google Scholar] [CrossRef]
- Jafari, F.; Zarre, S.; Gholipour, A.; Eggens, F.; Rabeler, R.K.; Oxelman, B. A New Taxonomic Backbone for the Infrageneric Classification of the Species-Rich Genus Silene (Caryophyllaceae). Taxon 2020, 69, 337–368. [Google Scholar] [CrossRef]
- Walters, S.M. Flora Europaea, Vol.1: Psilotaceae to Platanaceae; Tutin, T.G., Heywood, V.H., Burges, N.A., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1993; pp. 139–246. [Google Scholar]
- Montserrat, P.; Fernández-Casas, J.; Petrocoptis, A. Braun ex Endl. Flora Iberica Vol.2 Platanaceae-Plumbaginaceae (partim); Castroviejo, S., Laínz, M., López-González, G., Montserrat, P., Muñoz-Garmendia, F., Paiva, J., Villar, L., Eds.; Real Jardín Botánico CSIC: Madrid, Spain, 1990; pp. 304–312. [Google Scholar]
- Bañares, Á.; Blanca, G.; Güemes, J.; Moreno-Saiz, J.C.; Ortiz, S. (Eds.) Atlas y Libro Rojo de La Flora Vascular Amenazada de España. Adenda 2010; Dirección General de Medio Natural y Política Forestal (Ministerio de Medio Ambiente, y Medio Rural y Marino)—Sociedad Española de Biología de la Conservación de Plantas: Madrid, Spain, 2010. [Google Scholar]
- Moreno-Saiz, J.; Iriondo-Alegría, J.; Martínez-García, F.; Martínez-Rodríguez, J.; Salazar-Mendías, C. (Eds.) Atlas y Libro Rojo de La Flora Vascular Amenazada de España. Adenda 2017; Ministerio para la Transición Ecológica—Sociedad Española de Biología de la Conservación de Plantas: Madrid, Spain, 2019. [Google Scholar]
- Rivas-Martínez, S.; Penas, Á.; Del Río, S.; Díaz González, T.E.; Rivas-Sáenz, S. Bioclimatology of the Iberian Peninsula and the Balearic Islands. In The Vegetation of the Iberian Peninsula; Springer: Cham, Switzerland, 2017; Volume 12, pp. 29–80. ISBN 978-3-319-54784-8. [Google Scholar]
- Bradshaw, A.D. Evolutionary Significance of Phenotypic Plasticity in Plants. Adv. Genet. 1965, 13, 115–155. [Google Scholar] [CrossRef]
- Schlichting, C.D. The Evolution of Phenotypic Plasticity in Plants. Annu. Rev. Ecol. Syst. 1986, 17, 667–693. [Google Scholar] [CrossRef]
- Schlichting, C.D.; Smith, H. Phenotypic Plasticity: Linking Molecular Mechanisms with Evolutionary Outcomes. Evol. Ecol. 2002, 16, 189–211. [Google Scholar] [CrossRef]
- Futuyma, D.J.; Moreno, G. The Evolution of Ecological Specialization. Annu. Rev. Ecol. Evol. Syst. 1988, 19, 207–233. [Google Scholar] [CrossRef]
- Kawecki, T.J.; Ebert, D. Conceptual Issues in Local Adaptation. Ecol. Lett. 2004, 7, 1225–1241. [Google Scholar] [CrossRef]
- Gould, B.; Moeller, D.A.; Eckhart, V.M.; Tiffin, P.; Fabio, E.; Geber, M.A. Local Adaptation and Range Boundary Formation in Response to Complex Environmental Gradients across the Geographical Range of Clarkia xantiana ssp. Xantiana. J. Ecol. 2014, 102, 95–107. [Google Scholar] [CrossRef]
- Sultan, S.E. Phenotypic Plasticity for Plant Development, Function and Life History. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef]
- Valladares, F.; Wright, S.J.; Lasso, E.; Kitajima, K.; Pearcy, R.W. Plastic Phenotypic Response to Light of 16 Congeneric Shrubs from a Panamanian Rainforest. Ecology 2000, 81, 1925–1936. [Google Scholar] [CrossRef]
- Sultan, S.E. Phenotypic Plasticity for Fitness Components in Polygonum Species of Contrasting Ecological Breadth. Ecology 2001, 82, 328–343. [Google Scholar] [CrossRef]
- Gianoli, E.; Valladares, F. Studying Phenotypic Plasticity: The Advantages of a Broad Approach. Biol. J. Linn. Soc. 2012, 105, 1–7. [Google Scholar] [CrossRef]
- Dostál, P.; Fischer, M.; Prati, D. Phenotypic Plasticity Is a Negative, Though Weak, Predictor of the Commonness of 105 Grassland Species. Glob. Ecol. Biogeogr. 2016, 25, 464–474. [Google Scholar] [CrossRef]
- Palacio-López, K.; Beckage, B.; Scheiner, S.; Molofsky, J. The Ubiquity of Phenotypic Plasticity in Plants: A Synthesis. Ecol. Evol. 2015, 5, 3389–3400. [Google Scholar] [CrossRef]
- Valladares, F.; Gianoli, E.; Gómez, J.M. Ecological Limits to Plant Phenotypic Plasticity. New Phytol. 2007, 176, 749–763. [Google Scholar] [CrossRef]
- Murren, C.J.; Auld, J.R.; Callahan, H.; Ghalambor, C.K.; Handelsman, C.A.; Heskel, M.A.; Kingsolver, J.G.; Maclean, H.J.; Masel, J.; Maughan, H.; et al. Constraints on the Evolution of Phenotypic Plasticity: Limits and Costs of Phenotype and Plasticity. Heredity 2015, 115, 293–301. [Google Scholar] [CrossRef]
- Atkin, O.K.; Scheurwater, I.; Pons, T. High Thermal Acclimation Potential of Both Photosynthesis and Respiration in Two Lowland Plantago Species in Contrast to an Alpine Congeneric. Glob. Chang. Biol. 2006, 12, 500–515. [Google Scholar] [CrossRef]
- Emery, R.J.N.; Chinnappa, C.C.; Chmielewski, J.G. Specialization, Plant Strategies, and Phenotypic Plasticity in Populations of Stellaria longipes along an Elevational Gradient. Int. J. Plant Sci. 1994, 155, 203–219. [Google Scholar] [CrossRef]
- Hulshof, C.M.; Violle, C.; Spasojevic, M.J.; Mcgill, B.; Damschen, E.; Harrison, S.; Enquist, B.J. Intra-Specific and Inter-Specific Variation in Specific Leaf Area Reveal the Importance of Abiotic and Biotic Drivers of Species Diversity across Elevation and Latitude. J. Veg. Sci. 2013, 24, 921–931. [Google Scholar] [CrossRef]
- Stotz, G.C.; Salgado-Luarte, C.; Escobedo, V.M.; Valladares, F.; Gianoli, E. Global Trends in Phenotypic Plasticity of Plants. Ecol. Lett. 2021, 24, 2267–2281. [Google Scholar] [CrossRef]
- Tejero, P.; Otamendi, M.; Arrieta, M.; Etxeberria, M.; Agut, A.; Hermosilla, B.; Navarro, L.; Martínez-Ortega, M.M.; Calvo-Yuste, J.; Malvar-Ferreras, T.; et al. Informe Científico-Técnico Del Proyecto PRIOCONEX; Aranzadi: Cizur Menor, Spain, 2022. [Google Scholar]
- Ridley, H.N. The Dispersal of Plants Throughout the World; L. Reeve & Co: Ashford, UK, 1930. [Google Scholar]
- Rishbeth, J. The Flora of Cambridge Walls. J. Ecol. 1948, 36, 136. [Google Scholar] [CrossRef]
- Sernander, R. Entwurf Einer Monographie der Europäischen Myrmekochoren; Almqvist & Wiksells: Uppsala, Sweden, 1906. [Google Scholar]
- García, M.B.; Espadaler, X.; Olesen, J.M. Extreme Reproduction and Survival of a True Cliffhanger: The Endangered Plant Borderea chouardii (Dioscoreaceae). PLoS ONE 2012, 7, e44657. [Google Scholar] [CrossRef]
- Lengyel, S.; Gove, A.D.; Latimer, A.M.; Majer, J.D.; Dunn, R.R. Convergent Evolution of Seed Dispersal by Ants, and Phylogeny and Biogeography in Flowering Plants: A Global Survey. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 43–55. [Google Scholar] [CrossRef]
- Ortega-Olivencia, A.; Rodríguez-Riaño, T.; López, J.; Valtueña, F.J. Elaiosome-Bearing Plants from the Iberian Peninsula and the Balearic Islands. Biodivers. Conserv. 2021, 30, 1137–1163. [Google Scholar] [CrossRef]
- Castroviejo, S.; Laínz, M.; López González, G.; Montserrat, P.; Muñoz Garmendia, F.; Paiva, J.; Villar, L. (Eds.) . Flora Iberica Vol. 2; Real Jardín Botánico CSIC: Madrid, Spain, 1990. [Google Scholar]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9781461446927. [Google Scholar]
- Leubner, G. The Seed Biology Place. Available online: http://www.seedbiology.eu (accessed on 10 November 2024).
- Mayol, M.; Rosselló, J.A. A Synopsis of Silene Subgenus Petrocoptis (Caryophyllaceae). Taxon 1999, 48, 471–482. [Google Scholar] [CrossRef]
- Navarro, L.; Guitián, J. Seed Germination and Seedling Survival of Two Threatened Endemic Species of the Northwest Iberian Peninsula. Biol. Conserv. 2003, 109, 313–320. [Google Scholar] [CrossRef]
- Prieto-Mossi, J.; García-Mut, L.; Estrelles Perpiñá, E.; Ibars Almonacil, A.M. Protocolo de Germinación y Cultivo de Petrocoptis pardoi Pau (CARYOPHYLLACEAE). Botanic asPPECTS 2017, 3, 7–12. [Google Scholar]
- García, M.B.; Antor, R.J.; Villar, L. Reproductive Biology of Petrocoptis crassifolia Rouy (Caryophyllaceae), a Chasmophilous Endemic Plant of the Central Pyrenes. Bot. Helv. 1993, 103, 133–140. [Google Scholar]
- Mayol, M.; Rosselló, J.A. Two New Combinations in Silene (Caryophyllaceae). An. Jardín Botánico Madr. 2000, 57, 404. [Google Scholar]
- Donohue, K.; Rubio De Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, Postgermination Adaptation, and Species Ecological Ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Wofford, B.E. External Seed Morphology of Arenaria (Caryophyllaceae) of the Southeastern United States. Syst. Bot. 1981, 6, 126. [Google Scholar] [CrossRef]
- Wyatt, R. Intraspecific Variation in Seed Morphology of Arenaria uniflora (Caryophyllaceae). Syst. Bot. 1984, 9, 423. [Google Scholar] [CrossRef]
- Montserrat-Martí, J.M. Morfología de Las Semillas de Moehringia Gr. intricata (Caryophyllaceae). Lagascalia 1988, 15, 195–203. [Google Scholar]
- Ocaña, M.E.; Fernández González, I.; Pastor Díaz, J.E. Fruit and Seed Morphology in Paronychia Miller from South-West Spain. Lagascalia 1997, 19, 521–528. [Google Scholar]
- Yildiz, K. Seed Morphology of Caryophyllaceae Species from Turkey (North Anatolia). Pak. J. Bot. 2002, 34, 161–171. [Google Scholar]
- Minuto, L.; Fior, S.; Roccotiello, E.; Casazza, G. Seed Morphology in Moehringia, L. and Its Taxonomic Significance in Comparative Studies within the Caryophyllaceae. Plant Syst. Evol. 2006, 262, 189–208. [Google Scholar] [CrossRef]
- Prentice, H.C. Climate and Clinal Variation in Seed Morphology of the White Campion, Silene latifolia (Caryophyllaceae). Biol. J. Linn. Soc. 1986, 27, 179–189. [Google Scholar] [CrossRef]
- Zhou, X.; He, Z.-B.; Kang, H.-Z.; Sun, X.; Liu, C.-J. Variations of Seed Morphology Related to Climate for Quercus variabilis across Temperate-Subtropical China. Chin. J. Plant Ecol. 2013, 37, 481–491. [Google Scholar] [CrossRef]
- Padonou, E.A.; Ahossou, O.; Okou, F.; Assogbadjo, A.; Glèlè, K.; Lykke, A.M.; Sinsin, B. Impact of Climate on Seed Morphology and Plant Growth of Caesalpinia bonduc L. in West Africa. Int. J. Agron. Agric. Res. 2015, 6, 86–96. [Google Scholar]
- Leslie, A.B.; Beaulieu, J.M.; Mathews, S. Variation in Seed Size Is Structured by Dispersal Syndrome and Cone Morphology in Conifers and Other Nonflowering Seed Plants. New Phytol. 2017, 216, 429–437. [Google Scholar] [CrossRef]
- Mira, S.; Arnal, A.; Pérez-Garciá, F. Habitat-Correlated Seed Germination and Morphology in Populations of Phillyrea angustifolia, L. (Oleaceae). Seed Sci. Res. 2017, 27, 50–60. [Google Scholar] [CrossRef]
- Martínez-López, M.; Tinoco-Ojanguren, C.; Martorell, C. Drought Tolerance Increases with Seed Size in a Semiarid Grassland from Southern Mexico. Plant Ecol. 2020, 221, 989–1003. [Google Scholar] [CrossRef]
- Correns, C. Vererbungsversuche Mit Blass(Gelb)Grünen. Z. Indukt. Abstammungs- Vererbungsl. 1908; 1, 291–329. [Google Scholar] [CrossRef]
- Roach, D.A.; Wulff, R.D. Maternal Effects in Plants. Annu. Rev. Ecol. Syst. 1987, 18, 209–235. [Google Scholar] [CrossRef]
- Li, N.; Li, Y. Maternal Control of Seed Size in Plants. J. Exp. Bot. 2015, 66, 1087–1097. [Google Scholar] [CrossRef]
- Hagon, M.W.; Ballard, L.A.T. Reversibility of Strophiolar Permeability to Water in Seeds of Subterranean Clover (Trifolium subterraneum L.). Aust. J. Biol. Sci. 1970, 23, 519–528. [Google Scholar] [CrossRef]
- Ballard, L. Strophiolar Water Conduction in Seeds of the Trifolieae Induced by Action on the Testa at Non-Strophiolar Sites. Funct. Plant Biol. 1976, 3, 465–469. [Google Scholar] [CrossRef]
- Kigel, J.; Galili, G. Seed Development and Germination; Kigel, J., Galili, G., Eds.; Routledge: New York, NY, USA, 1995; ISBN 9780203740071. [Google Scholar]
- Gould, S.J.; Vrba, E.S. Exaptation—A Missing Term in the Science of Form. Paleobiology 1982, 8, 4–15. [Google Scholar] [CrossRef]
- Barker, N.P. A Review and Survey of Basicarpy, Geocarpy and Amphicarpy in the African and Madagascan Flora. Ann. Mo. Bot. Gard. 2005, 92, 445–462. [Google Scholar]
- Llorens, L. Un Nuevo Endemismo de La Isla de Menorca: Apium bermejoi. Folia Botanica Miscellanea 1982, 3, 27–33. [Google Scholar]
- Ellison, A.M.; Gotelli, N.J. Evolutionary Ecology of Carnivorous Plants. Trends Ecol. Evol. 2001, 16, 623–629. [Google Scholar] [CrossRef]
- Müller-Schneider, P.; Lhotská, M. Zur Terminologie der Verbreitungsbiologie der Blütenpflanzen. Folia Geobot. Phytotaxon. 1971, 6, 407–417. [Google Scholar] [CrossRef]
- Gorb, E.; Gorb, S. Seed Dispersal by Ants in a Deciduous Forest Ecosystem; Springer Netherlands: Dordrecht, The Netherlands, 2003; ISBN 978-90-481-6317-5. [Google Scholar]
- Rothmaler, W. Monographie Der Gattung Petrocoptis. Bot. Jahrb. Syst. Pflanzengesch. Pflanzengeogr. 1941, 72, 117–130. [Google Scholar]
- Merxmüller, H.; Grau, J. Ergänzende Studien an Petrocoptis (Caryophyllaceae). Collect. Bot. 1968, 7, 787–797. [Google Scholar]
- de Bolòs, O.; Rivas-Martínez, S. Comentarios Sobre El Género Petrocoptis. Petrocoptis Montsicciana Sp. Nova. An. Inst. Botánico Cavanilles 1970, 26, 53–60. [Google Scholar]
- Mayol, M.; Rosselló, J.A. Seed Isozyme Variation in Petrocoptis A. Braun (Caryophyllaceae). Biochem. Syst. Ecol. 2001, 29, 379–392. [Google Scholar] [CrossRef]
- Cires, E.; Prieto, J.A.F. Phylogenetic Relationships of Petrocoptis A. Braun Ex Endl. (Caryophyllaceae), a Discussed Genus from the Iberian Peninsula. J. Plant Res. 2015, 128, 223–238. [Google Scholar] [CrossRef]
- Holmgren, P.K.; Holmgren, N.H.; Barnett, L.C. Index Herbariorum. Part I: The Herbaria of the World; Regnum vegetabile. New York Botanical Gardens; Bohn Stafleu van Loghum: Houten, Netherlands, 1990; p. 120. [Google Scholar]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. Am. J. Plant Sci. 2017, 8. [Google Scholar]
- Talavera, S.; Silene, L. Flora iberica Vol. 2 Platanaceae-Plumbaginaceae (partim); Castroviejo, S., Laínz, M., López-González, G., Montserrat, P., Muñoz-Garmendia, F., Paiva, J., Villar, L., Eds.; Real Jardín Botánico, CSIC: Madrid, Spain, 1990; pp. 313–406. [Google Scholar]
- Eggens, F. Systematics in Sileneae (Caryophyllaceae)—Taxonomy and Phylogenetic Patterns; Acta Universitatis Upsaliensis: Uppsala, Sweden, 2006. [Google Scholar]
- Hartigan, J.A.; Wong, M.A. A K-Means Clustering Algorithm. Appl. Stat. 1979, 28, 100–108. [Google Scholar] [CrossRef]
- Ahmed, M.; Seraj, R.; Islam, S.M.S. The K-Means Algorithm: A Comprehensive Survey and Performance Evaluation. Electronics 2020, 9, 1295. [Google Scholar] [CrossRef]
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. NbClust: An R Package for Determining the Relevant Number of Clusters in a Data Set. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Extract and Visualize the Results of Multivariate Data Analyses [R Package Factoextra Version 1.0.7]; Gesis: Mannheim, Germany, 2020. [Google Scholar]
- Patil, I. Visualizations with Statistical Details: The “ggstatsplot” Approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Breiman, L. Manual on Setting Up, Using, and Understanding Random Forests v3; Statistics Department University of California Berkeley, CA, USA, 2002.
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 2, 18–22. [Google Scholar]
- Rothmaler, W. Flora Europaea Vol.1: Lycopodiaceae to Platanaceae; Tutin, T.G., Heywood, V.H., Burges, N.A., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1964. [Google Scholar]
- Kuhn, M. Building Predictive Models in R Using the Caret Package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Cohen, J. A Coefficient of Agreement for Nominal Scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Sim, J.; Wright, C.C. The Kappa Statistic in Reliability Studies: Use, Interpretation, and Sample Size Requirements. Phys. Ther. 2005, 85, 257–268. [Google Scholar] [CrossRef]
- Greenwell, B.M.; Boehmke, B.C. Variable Importance Plots—An Introduction to the Vip Package. R J. 2020, 12, 343. [Google Scholar] [CrossRef]
- Meyer, D.; Dimitriadou, E.; Hornik, K.; Weingessel, A.; Leisch, F.; Chang, C.-C.; Lin, C.-C. E1071: Misc Functions of the Department of Statistics, Probability Theory Group (Formerly: E1071), TU Wien. CRAN.R-Project; 2023.
- Greenwell, B.M. Pdp: An R Package for Constructing Partial Dependence Plots. R J. 2017, 9, 421. [Google Scholar] [CrossRef]
- Ninyerola, M.; Pons, X.; Roure, J.M. Atlas Climático Digital de La Peninsula Ibérica. Metodología y Aplicaciones En Bioclimatología y Geobotánica; Universitat Autónoma de Barcelona: Barcelona, Spain, 2005; ISBN 932860-8-7. [Google Scholar]
- Sakamoto, Y.; Ishiguro, M.; Kitagawa, G. Akaike Information Criterion Statistics; D. Reidel: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Cavanaugh, J.E. Unifying the Derivations for the Akaike and Corrected Akaike Information Criteria. Stat. Probab. Lett. 1997, 33, 201–208. [Google Scholar] [CrossRef]
- Biecek, P. DALEX: Explainers for Complex Predictive Models. J. Mach. Learn. Res. 2018, 19, 1–48. [Google Scholar]
- Biecek, P.; Baniecki, H.; Ingredients: Effects and Importances of Model Ingredients. CRAN 2023. Available online: https://cran.r-project.org/web/packages/ingredients/ingredients.pdf (accessed on 10 November 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).