Effects of Straw Addition on Soil Priming Effects Under Different Tillage and Straw Return Modes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Description
2.2. Experimental Material
2.3. Incubation Experiment
2.4. Soil Sampling and Analysis
2.5. Calculation Methods
2.6. Data Analysis
3. Results
3.1. Effect of Different Tillage Modes on Soil Physicochemical Property
3.2. Effect of Straw Addition on Soil Organic Carbon Mineralization and the Apparent Priming Effect
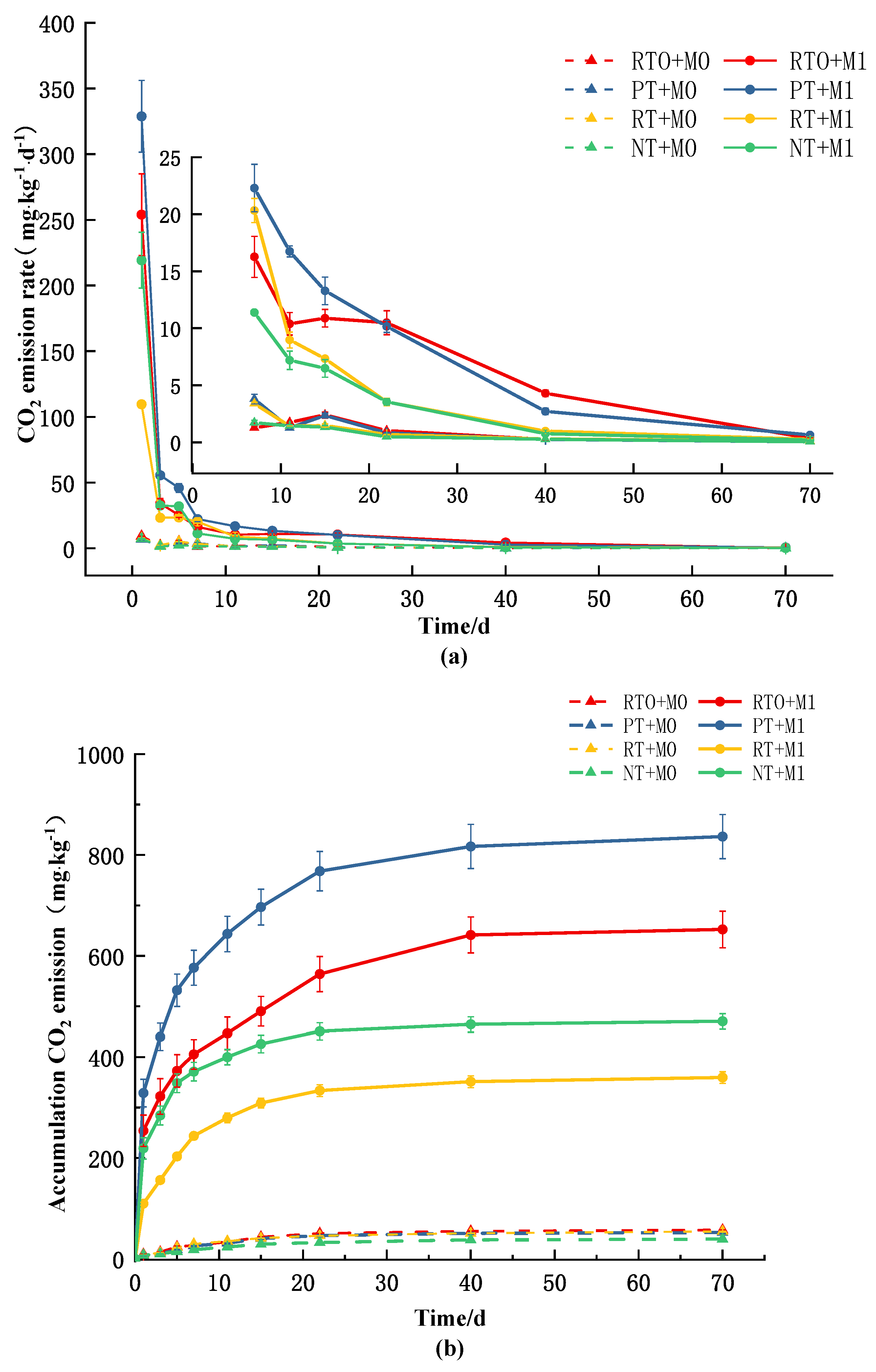
3.2.1. Effect on the Rate of Soil CO2 Emission and Its Cumulative Emission
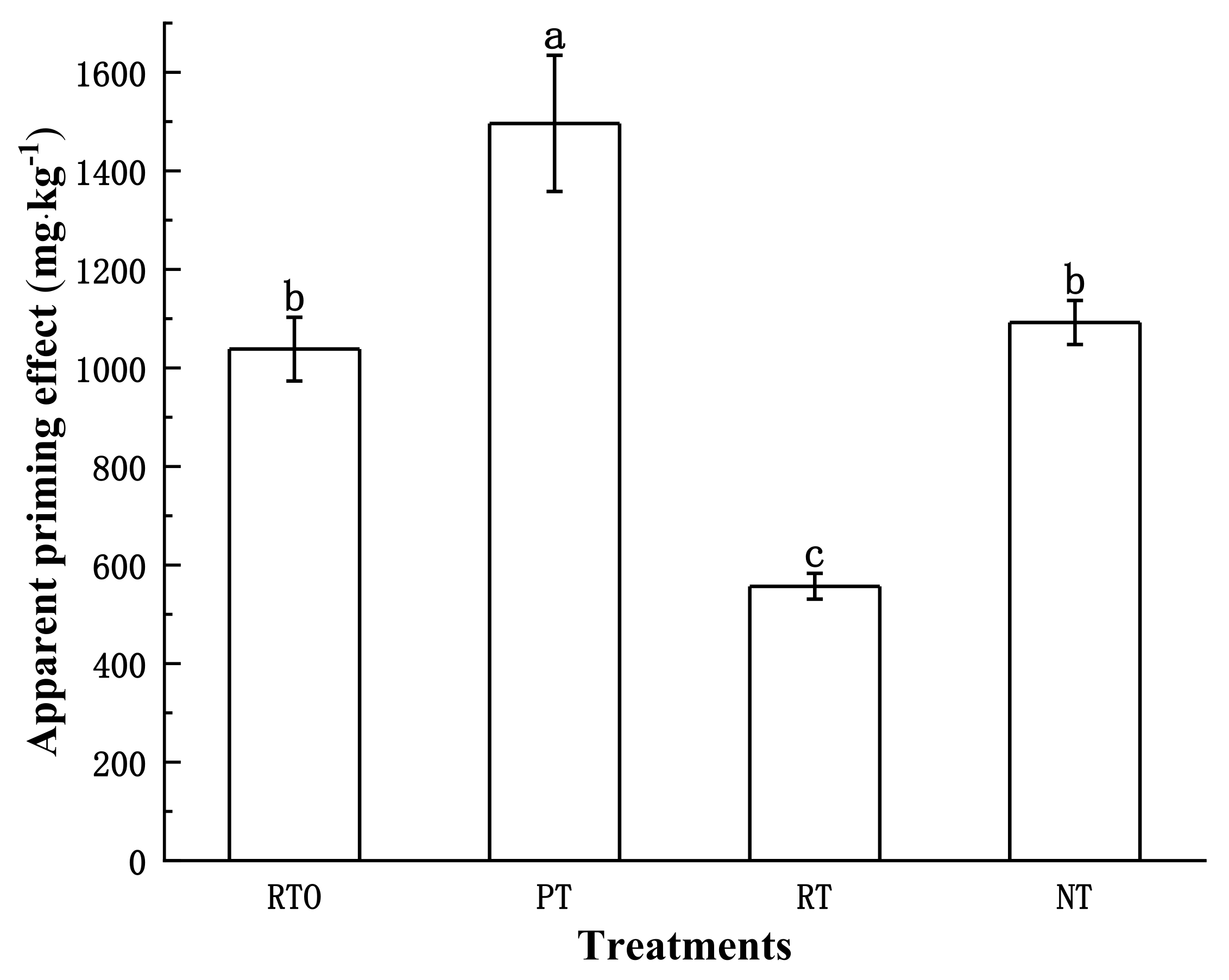
3.2.2. Apparent Priming Effect
3.3. Effect of Straw Addition on Soil Organic Carbon and Its Active Components Under Different Tillage Modes
3.3.1. Soil Organic Carbon
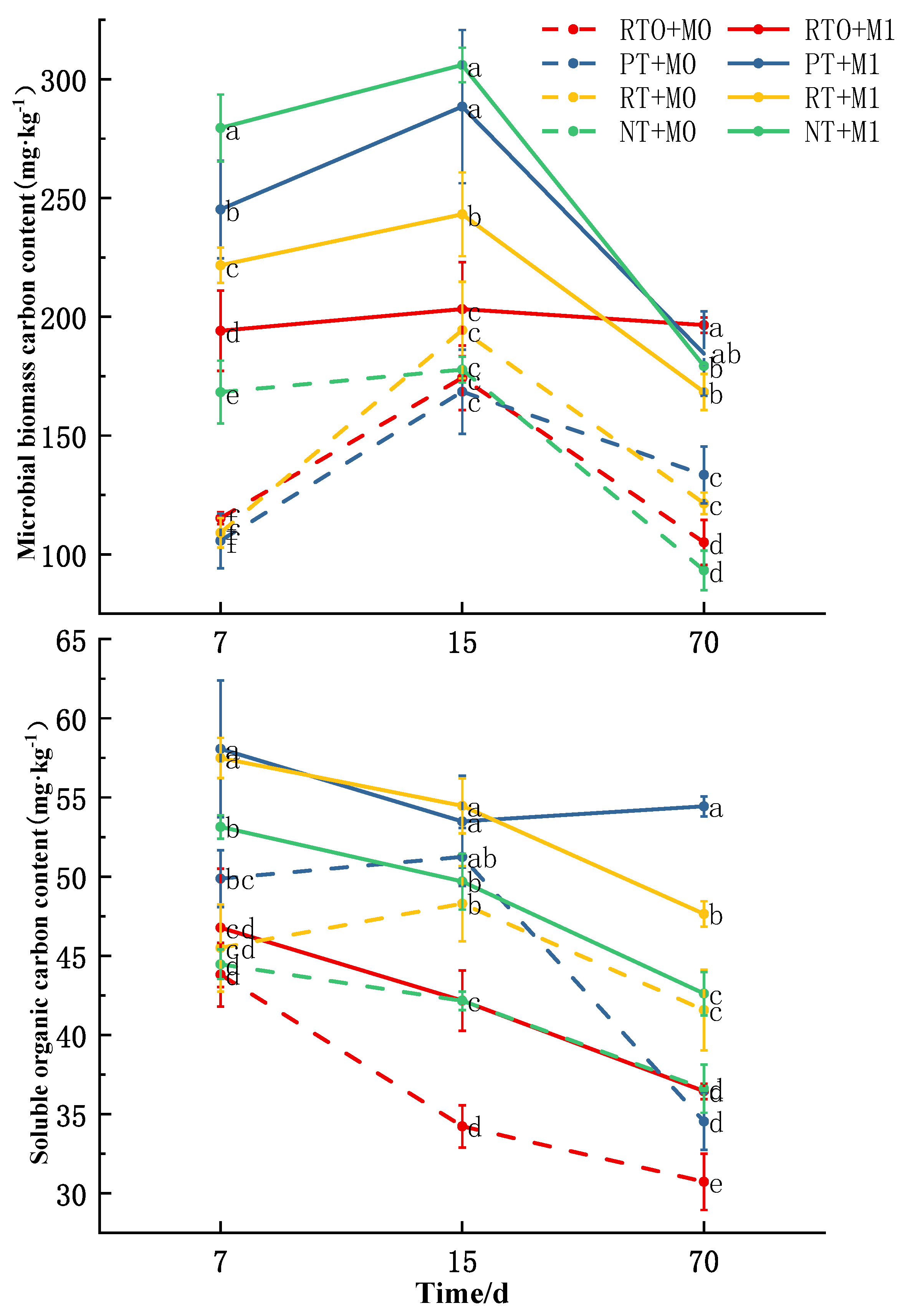
3.3.2. Soil Microbial Biomass Carbon (MBC) Content
3.3.3. Soil Dissolved Organic Carbon (DOC) Content
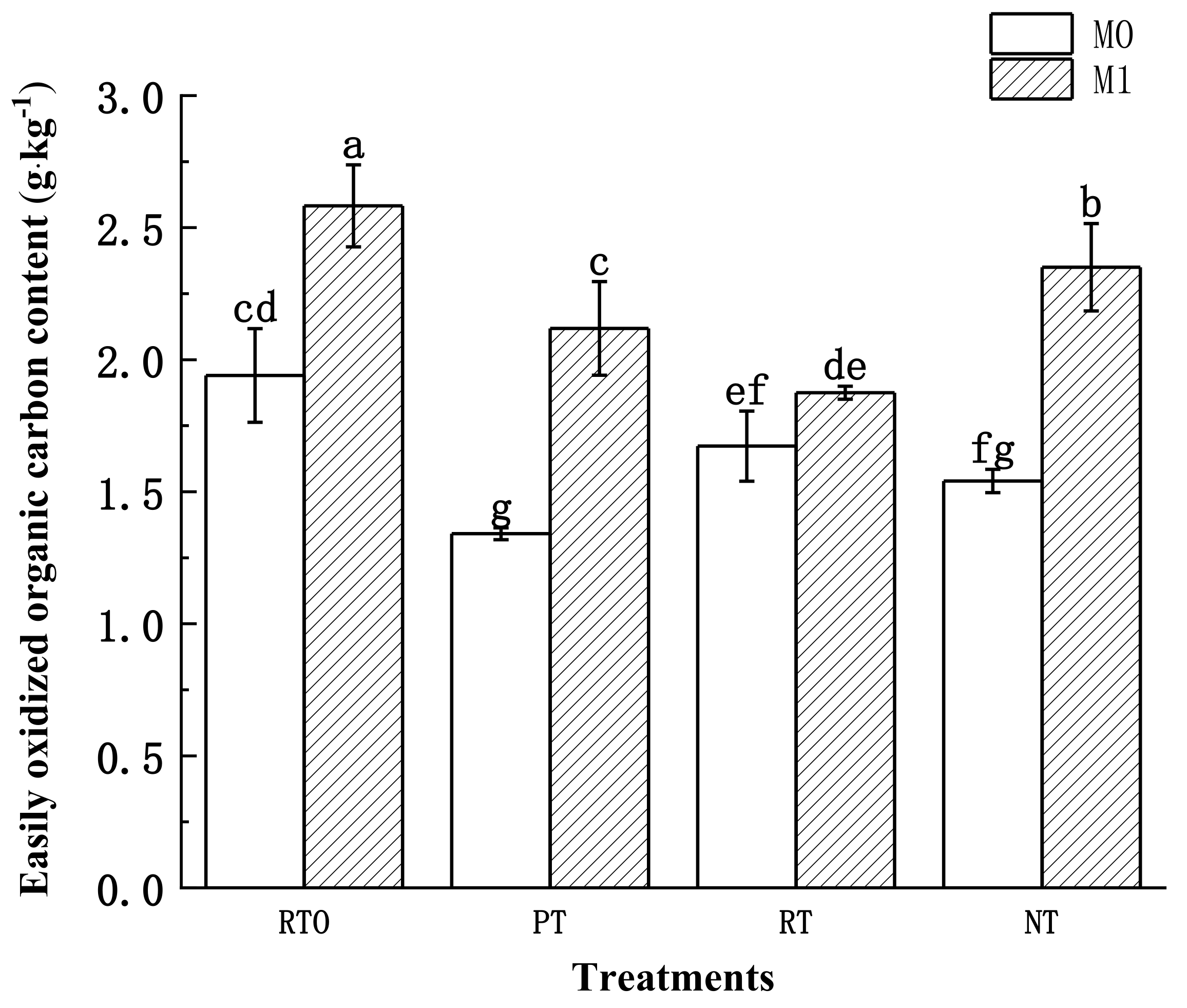
3.3.4. Soil Easily Oxidized Organic Carbon (EOC) Content
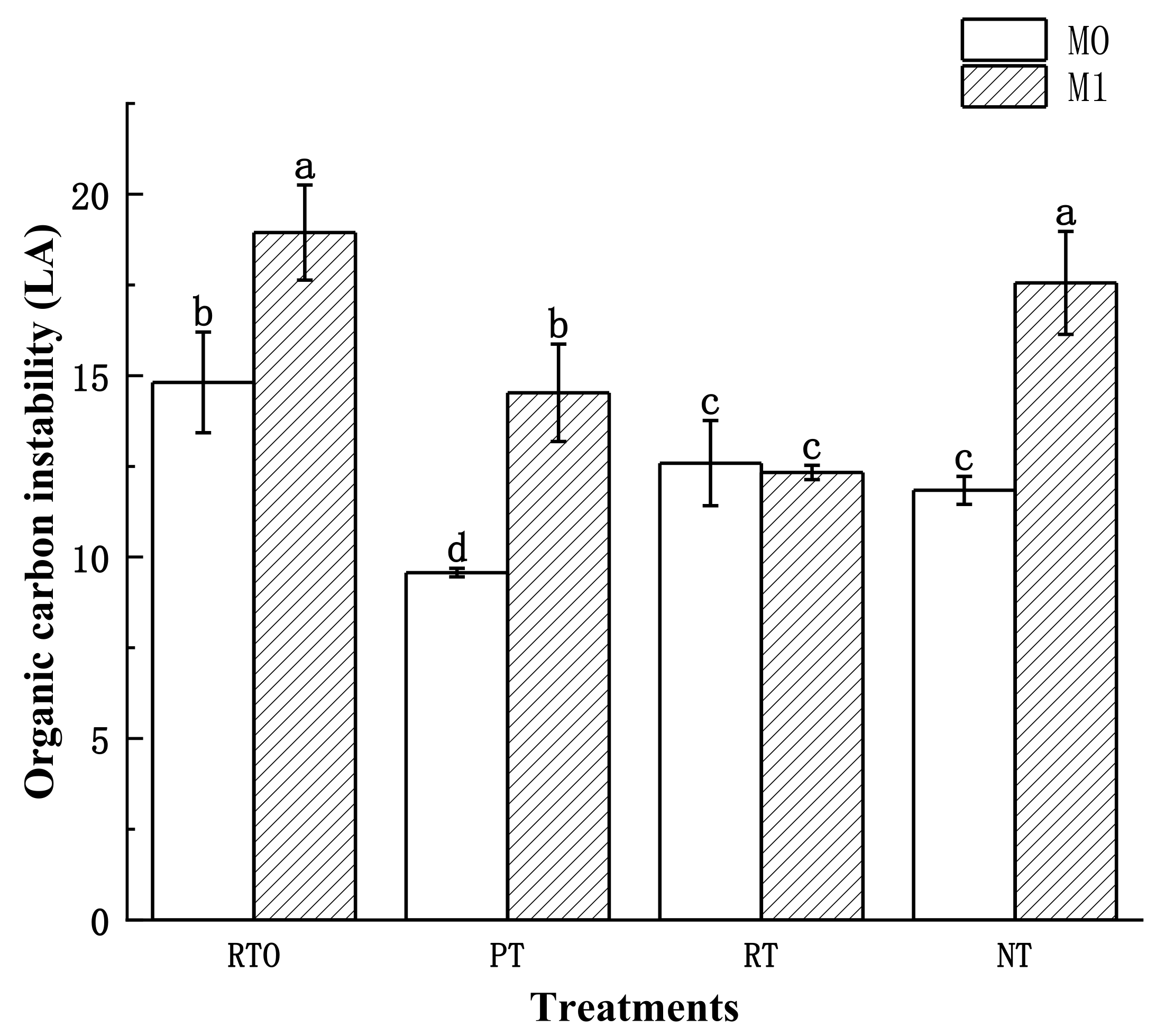
3.3.5. Soil Organic Carbon Instability (LA)
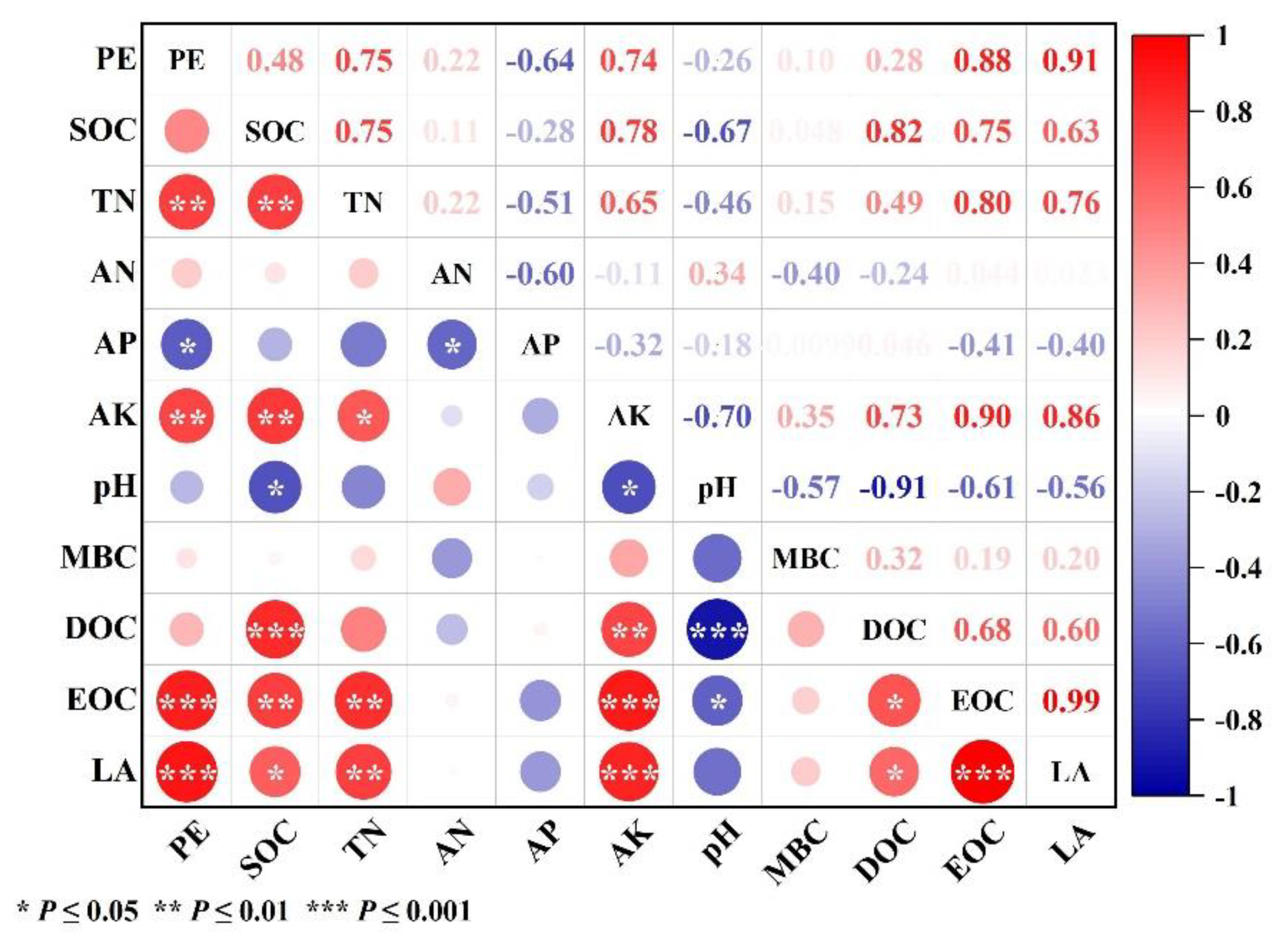
3.4. Impact Factor of Priming Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenka, S.; Trivedi, P.; Singh, B.; Singh, B.P.; Pendall, E.; Bass, A.; Lenka, N.K. Effect of crop residue addition on soil organic carbon priming as influenced by temperature and soil properties. Geoderma 2019, 347, 70–79. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil. Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Li, L.-J.; Zhu-Barker, X.; Ye, R.; Doane, T.A.; Horwath, W.R. Soil microbial biomass size and soil carbon influence the priming effect from carbon inputs depending on nitrogen availability. Soil Biol. Biochem. 2018, 119, 41–49. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Jin, Z.Q.; Shah, T.R.; Zhang, L.; Liu, H.Y.; Peng, S.B.; Nie, L.X. Effect of straw returning on soil organic carbon in rice-wheat rotation system: A review. Food Energy Secur. 2020, 9, e200. [Google Scholar] [CrossRef]
- Lehtinen, T.; Schlatter, N.; Baumgarten, A.; Bechini, L.; Krüger, J.; Grignani, C.; Zavattaro, L.; Costamagna, C.; Spiegel, H. Effect of crop residue incorporation on soil organic carbon and greenhouse gas emissions in European agricultural soils. Soil Use Manag. 2014, 30, 524–538. [Google Scholar] [CrossRef]
- Poeplau, C.; Kätterer, T.; Bolinder, M.A.; Börjesson, G.; Berti, A.; Lugato, E. Low stabilization of aboveground crop residue carbon in sandy soils of Swedish long-term experiments. Geoderma 2015, 237, 246–255. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Geng, P.; Wang, X.; Li, X.; Cai, P.X.; Zhan, X.M.; Han, X.R. Improvement of Active Organic Carbon Distribution and Soil Quality with the Combination of Deep Tillage and No-Tillage Straw Returning Mode. Agronomy 2023, 13, 2398. [Google Scholar] [CrossRef]
- Kirkby, C.A.; Richardson, A.E.; Wade, L.J.; Passioura, J.B.; Batten, G.D.; Blanchard, C.; Kirkegaard, J.A. Nutrient availability limits carbon sequestration in arable soils. Soil Biol. Biochem. 2014, 68, 402–409. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, Y.L.; Bei, S.K.; Li, X.L.; Reinsch, S.; Zhang, H.Y.; Zhang, J.L. Contrasting impacts of manure and inorganic fertilizer applications for nine years on soil organic carbon and its labile fractions in bulk soil and soil aggregates. Catena 2020, 194, 104739. [Google Scholar] [CrossRef]
- Liu, B.; Xia, H.; Jiang, C.C.; Riaz, M.; Yang, L.; Chen, Y.F.; Fan, X.P.; Xia, X.E. 14 year applications of chemical fertilizers and crop straw effects on soil labile organic carbon fractions, enzyme activities and microbial community in rice-wheat rotation of middle China. Sci. Total Environ. 2022, 841, 156608. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, D.S.; Ladd, J.N. Microbial biomass in soil: Measurement and turnover. Soil Biochem. 1981, 5, 415–471. [Google Scholar]
- Post, W.M.; Kwon, K.C. Soil Carbon sequestration and land use change: Processes and potential. Glob. Change Biol. 2010, 6, 317–327. [Google Scholar] [CrossRef]
- Wang, X.H.; Yang, H.S.; Liu, J.; Wu, J.S.; Chen, W.P.; Wu, J.; Zhu, L.Q.; Bian, X.M. Effects of ditch-buried straw return on soil organic carbon and rice yields in a rice-wheat rotation system. Catena 2015, 127, 56–63. [Google Scholar] [CrossRef]
- Zhang, X.F.; Xin, X.L.; Zhu, A.N.; Zhang, J.B.; Yang, W.H. Effects of tillage and residue managements on organic C accumulation and soil aggregation in a sandy loam soil of the North China Plain. Catena 2017, 156, 176–183. [Google Scholar] [CrossRef]
- Troyer, I.D.; Amery, F.; Moorleghem, C.V.; Smolders, E.; Merckx, R. Tracing the source and fate of dissolved organic matter in soil after incorporation of a 13C-labelled residue: A batch incubation study. Soil Biol. Biochem. 2011, 43, 513–519. [Google Scholar] [CrossRef]
- Schulz, E. Influence of site conditions and management on different soil organic matter (som) pools. Arch. Agron. Soil Sci. 2004, 50, 33–47. [Google Scholar] [CrossRef]
- Yan, D.; Wang, D.; Yang, L. Long-term effect of chemical fertilizer, straw, and manure on labile organic matter fractions in a paddy soil. Biol. Fertil. Soils 2007, 44, 93–101. [Google Scholar] [CrossRef]
- Kan, Z.R.; Virk, A.L.; Wu, G.; Qi, J.Y.; Ma, S.T.; Wang, X.; Zhao, X.; Lal, R.; Zhang, H.L. Priming effect intensity of soil organic carbon mineralization under no-till and residue retention. Appl. Soil Ecol. 2020, 147, 103445. [Google Scholar] [CrossRef]
- Wang, H.; Hu, G.Q.; Xu, W.H.; Boutton, T.W.; Zhuge, Y.P.; Bai, E. Effects of nitrogen addition on soil organic carbon mineralization after maize stalk addition. Eur. J. Soil Biol. 2018, 89, 33–38. [Google Scholar] [CrossRef]
- Sarker, J.R.; Singh, B.P.; Fang, Y.Y.; Cowie, A.L.; Dougherty, W.J.; Collins, D.; Dalal, R.C.; Singh, B.K. Tillage history and crop residue input enhanced native carbon mineralisation and nutrient supply in contrasting soils under long-term farming systems. Soil Tillage Res. 2019, 193, 71–84. [Google Scholar] [CrossRef]
- Sarker, J.R.; Singh, B.P.; Cowie, A.L.; Fang, Y.; Collins, D.; Dougherty, W.J.; Singh, B.K. Carbon and nutrient mineralisation dynamics in aggregate-size classes from different tillage systems after input of canola and wheat residues. Soil Biol. Biochem. 2018, 116, 22–38. [Google Scholar] [CrossRef]
- Jenkinson, D.S. Studies on the decomposition of c14 labelled organic matter in soil. Soil Sci. 1971, 111, 64–70. [Google Scholar] [CrossRef]
- Chen, R.R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.G.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Glob. Change Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Dimassi, B.; Mary, B.; Fontaine, S.; Perveen, N.; Revaillot, S.; Cohan, J.P. Effect of nutrients availability and long-term tillage on priming effect and soil C mineralization. Soil Biol. Biochem. 2014, 78, 332–339. [Google Scholar] [CrossRef]
- Yan, S.S.; Song, J.M.; Fan, J.S.; Yan, C.; Dong, S.K.; Ma, C.M.; Gong, Z.P. Changes in soil organic carbon fractions and microbial community under rice straw return in Northeast China. Glob. Ecol. Conserv. 2020, 22, e00962. [Google Scholar] [CrossRef]
- Liu, S.D.; Yi, S.J.; Ma, Y.C. Effects of Straw Mulching on Soil Temperature and Maize Growth in Northeast China. Teh. Vjesn. Tech. Gaz. 2022, 29, 1805–1810. [Google Scholar]
- Hu, N.J.; Wang, B.J.; Gu, Z.H.; Tao, B.R.; Zhang, Z.W.; Hu, S.J.; Zhu, L.Q.; Meng, Y.L. Effects of different straw returning modes on greenhouse gas emissions and crop yields in a rice-wheat rotation system. Agric. Ecosyst. Environ. 2016, 223, 115–122. [Google Scholar] [CrossRef]
- Tian, P.; Sui, P.X.; Lian, H.L.; Wang, Z.Y.; Meng, G.X.; Sun, Y.; Wang, Y.Y.; Su, Y.H.; Ma, Z.Q.; Qi, H.; et al. Maize Straw Returning Approaches Affected Straw Decomposition and Soil Carbon and Nitrogen Storage in Northeast China. Agronomy 2019, 9, 818. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Naresh, R.K.; Gupta, R.K.; Panwar, A.S.; Mahajan, N.C.; Singh, R.; Mandal, A. Effect of tillage and straw return on carbon footprints, soil organic carbon fractions and soil microbial community in different textured soils under rice-wheat rotation: A review. Rev. Environ. Sci. Bio-Technol. 2020, 19, 103–115. [Google Scholar] [CrossRef]
- Yang, F.K.; He, B.L.; Zhang, L.G.; Zhang, G.P.; Gao, Y.P. An Approach to Improve Soil Quality: A Case Study of Straw Incorporation with a Decomposer Under Full Film-Mulched Ridge-Furrow Tillage on the Semiarid Loess Plateau, China. J. Soil Sci. Plant Nutr. 2020, 20, 125–138. [Google Scholar] [CrossRef]
- Wang, D.D.; Zhu, Z.K.; Shahbaz, M.; Chen, L.; Liu, S.L.; Inubushi, K.; Wu, J.S.; Ge, T.D. Split N and P addition decreases straw mineralization and the priming effect of a paddy soil: A 100-day incubation experiment. Biol. Fertil. Soils 2019, 55, 701–712. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.-Q.; Zhang, Y.; Zhu, Z.-K.; Wu, J.-S.; Ge, T.-d.; Li, Y.-H. Effect of Long-term Straw Returning on the Mineralization and Priming Effect of Rice Root-carbon. Huan Jing Ke Xue 2022, 43, 4372–4378. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Blair, G.; Lefroy, R.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Hamer, U.; Marschner, B. Priming effects in different soil types induced by fructose, alanine, oxalic acid and catechol additions. Soil Biol. Biochem. 2005, 37, 445–454. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Wang, S.; He, T.; Liu, L. Fresh carbon and nitrogen inputs alter organic carbon mineralization and microbial community in forest deep soil layers. Soil Biol. Biochem. 2014, 72, 145–151. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Thiessen, S.; Gleixner, G.; Wutzler, T.; Reichstein, M. Both priming and temperature sensitivity of soil organic matter decomposition depend on microbial biomass—An incubation study. Soil Biol. Biochem. 2013, 57, 739–748. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Nazaries, L.; Singh, B.K.; Singh, B.P. Microbial mechanisms of carbon priming effects revealed during the interaction of crop residue and nutrient inputs in contrasting soils. Glob. Change Biol. 2018, 24, 2775–2790. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Riaz, M.; Al-Wabel, M.I.; Arif, M.S.; Yasmeen, T.; Hussain, Q.; Roohi, M.; Fahad, S.; Ali, K.; Arif, M. Higher biochar rate strongly reduced decomposition of soil organic matter to enhance C and N sequestration in nutrient-poor alkaline calcareous soil. J. Soils Sediments 2021, 21, 148–162. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.C.; Li, J.; Xu, Y.; Wang, X.L. Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 2019, 14, e0216006. [Google Scholar]
- Wang, X.; Wang, X.X.; Geng, P.; Yang, Q.; Chen, K.; Liu, N.; Fan, Y.L.; Zhan, X.M.; Han, X.R. Effects of different returning method combined with decomposer on decomposition of organic components of straw and soil fertility. Sci. Rep. 2021, 11, 15495. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Rudrappa, L.; Singh, D.; Swarup, A.; Bhadraray, S. Long-term impact of fertilizers on soil organic carbon pools and sequestration rates in maize–wheat–cowpea cropping system. Geoderma 2008, 144, 370–378. [Google Scholar] [CrossRef]
- Yang, X.; Meng, J.; Lan, Y.; Chen, W.F.; Yang, T.X.; Yuan, J.; Liu, S.N.; Han, J. Effects of maize stover and its biochar on soil CO2 emissions and labile organic carbon fractions in Northeast China. Agric. Ecosyst. Environ. 2017, 240, 24–31. [Google Scholar] [CrossRef]
- Dou, F.; Wright, A.L.; Hons, F.M. Dissolved and Soil Organic Carbon after Long-Term Conventional and No-Tillage Sorghum Cropping. Commun. Soil Sci. Plant Anal. 2008, 39, 667–679. [Google Scholar] [CrossRef]
- Zhu, K.; Ran, H.Y.; Wang, F.F.; Ye, X.; Niu, L.A.; Schulin, R.; Wang, G. Conservation tillage facilitated soil carbon sequestration through diversified carbon conversions. Agric. Ecosyst. Environ. 2022, 337, 108080. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Hu, N.J.; Zhang, Z.W.; Xu, J.L.; Tao, B.R.; Meng, Y.L. Short-term responses of soil organic carbon and carbon pool management index to different annual straw return rates in a rice-wheat cropping system. Catena 2015, 135, 283–289. [Google Scholar] [CrossRef]
- Chen, Z.M.; Wang, H.Y.; Liu, X.W.; Zhao, X.L.; Lu, D.J.; Zhou, J.M.; Li, C.Z. Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice-wheat cropping system. Soil Tillage Res. 2017, 165, 121–127. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Griffiths, H.; Chamberlain, P.M.; Stott, A.W.; Tanner, E.V.J. Soil priming by sugar and leaf-litter substrates: A link to microbial groups. Appl. Soil Ecol. 2009, 42, 183–190. [Google Scholar] [CrossRef]
- Xia, L.L.; Wang, S.W.; Yan, X.Y. Effects of long-term straw incorporation on the net global warming potential and the net economic benefit in a rice-wheat cropping system in China. Agric. Ecosyst. Environ. 2014, 197, 118–127. [Google Scholar] [CrossRef]
- Wang, N.; Yu, J.G.; Zhao, Y.H.; Chang, Z.Z.; Shi, X.X.; Ma, L.N.Q.; Li, H.B. Straw enhanced CO2 and CH4 but decreased N2O emissions from flooded paddy soils: Changes in microbial community compositions. Atmos. Environ. 2018, 174, 171–179. [Google Scholar] [CrossRef]
- Chen, W.H.; Yuan, W.; Wang, J.; Wang, Z.Y.; Zhou, Z.P.; Liu, S.P. No-Tillage Combined with Appropriate Amount of Straw Returning Increased Soil Biochemical Properties. Sustainability 2022, 14, 4875. [Google Scholar] [CrossRef]
- Liu, X.Q.; Liu, H.R.; Ren, D.Y.; Liu, C.R.; Zhang, Y.S.; Wang, S.Q.; Li, Z.H.; Zhang, M.C. Interlinkages between soil properties and keystone taxa under different tillage practices on the North China Plain. Appl. Soil Ecol. 2022, 178, 104551. [Google Scholar] [CrossRef]
- Zhu, F.N.; Lin, X.X.; Guan, S.; Dou, S. Deep incorporation of corn straw benefits soil organic carbon and microbial community composition in a black soil of Northeast China. Soil Use Manag. 2022, 38, 1266–1279. [Google Scholar] [CrossRef]
- Mu, X.Y.; Zhao, Y.L.; Liu, K.; Ji, B.Y.; Guo, H.B.; Xue, Z.W.; Li, C.H. Responses of soil properties, root growth and crop yield to tillage and crop residue management in a wheat-maize cropping system on the North China Plain. Eur. J. Agron. 2016, 78, 32–43. [Google Scholar] [CrossRef]
- Zhang, L.G.; Chen, X.; Xu, Y.J.; Jin, M.C.; Ye, X.X.; Gao, H.J.; Chu, W.Y.; Mao, J.D.; Thompson, M.L. Soil labile organic carbon fractions and soil enzyme activities after 10 years of continuous fertilization and wheat residue incorporation. Sci. Rep. 2020, 10, 11318. [Google Scholar] [CrossRef]
- He, L.Y.; Lu, S.X.; Wang, C.G.; Mu, J.; Zhang, Y.L.; Wang, X.D. Changes in soil organic carbon fractions and enzyme activities in response to tillage practices in the Loess Plateau of China. Soil Tillage Res. 2021, 209, 104940. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Gao, S.F.; Lu, C.Y.; Li, X.Y.; Li, F.; Wang, T.Y. Effects of different tillage and fertilization management practices on soil organic carbon and aggregates under the rice-wheat rotation system. Soil Tillage Res. 2021, 212, 105071. [Google Scholar] [CrossRef]
- Sun, H.Y.; Wang, C.X.; Wang, X.D.; Rees, R.M. Changes in soil organic carbon and its chemical fractions under different tillage practices on loess soils of the Guanzhong Plain in north-west China. Soil Use Manag. 2013, 29, 344–353. [Google Scholar] [CrossRef]
- Huo, L.L.; Zou, Y.C.; Lyu, X.G.; Zhang, Z.S.; Wang, X.H.; An, Y. Effect of Wetland Reclamation on Soil Organic Carbon Stability in Peat Mire Soil Around Xingkai Lake in Northeast China. Chin. Geogr. Sci. 2018, 28, 325–336. [Google Scholar] [CrossRef]
- Wang, J.; Fu, X.; Zhao, F.Z.; Sainju, U.M. Response of Soil Carbon Fractions and Dryland Maize Yield to Mulching. Soil Sci. Soc. Am. J. 2018, 82, 371–381. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Prakash, V.; Kundu, S.; Srivastva, A.K.; Gupta, H.S.; Mitra, S. Long term effects of fertilization on carbon and nitrogen sequestration and aggregate associated carbon and nitrogen in the Indian sub-Himalayas. Nutr. Cycl. Agroecosyst. 2010, 86, 1–16. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Hu, N.J.; Yang, M.F.; Zhan, X.H.; Zhang, Z.W. Effects of Different Tillage and Straw Return on Soil Organic Carbon in a Rice-Wheat Rotation System. PLoS ONE 2014, 9, e88900. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kumar, A.; Kuzyakov, Y.; Börjesson, G.; Blagodatskaya, E. Priming effects induced by glucose and decaying plant residues on SOM decomposition: A three-source 13C/14C partitioning study. Soil Biol. Biochem. 2018, 121, 138–146. [Google Scholar] [CrossRef]
- Xiao, M.L.; Shahbaz, M.; Liang, Y.; Yang, J.; Wang, S.; Chadwicka, D.R.; Jones, D.; Chen, J.P.; Ge, T.D. Effect of microplastics on organic matter decomposition in paddy soil amended with crop residues and labile C: A three-source-partitioning study. J. Hazard. Mater. 2021, 416, 126221. [Google Scholar] [CrossRef]
- Song, X.; Liu, X.; Liang, G.; Li, S.; Li, J.; Zhang, M.; Zheng, F.; Ding, W.; Wu, X.; Wu, H. Positive priming effect explained by microbial nitrogen mining and stoichiometric decomposition at different stages. Soil Biol. Biochem. 2022, 175, 108852. [Google Scholar] [CrossRef]
- Xiao, D.; He, X.Y.; Wang, G.H.; Xu, X.C.; Hu, Y.J.; Chen, X.B.; Zhang, W.; Su, Y.R.A.; Wang, K.L.; Soromotin, A.V.; et al. Network analysis reveals bacterial and fungal keystone taxa involved in straw and soil organic matter mineralization. Appl. Soil Ecol. 2022, 173, 104395. [Google Scholar] [CrossRef]
- Kan, Z.R.; Chen, Z.; Wei, Y.X.; Virk, A.L.; Bohoussou, Y.N.; Lal, R.; Zhao, X.; Zhang, H.L. Contribution of wheat and maize to soil organic carbon in a wheat-maize cropping system: A field and laboratory study. J. Appl. Ecol. 2022, 59, 2716–2729. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: Mechanisms and thresholds. Biol. Fertil. Soils 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Dikgwatlhe, S.B.; Kong, F.L.; Chen, Z.D.; Lal, R.; Zhang, H.L.; Chen, F. Tillage and residue management effects on temporal changes in soil organic carbon and fractions of a silty loam soil in the North China Plain. Soil Use Manag. 2014, 30, 496–506. [Google Scholar] [CrossRef]
| RTO | RT | PT | NT | |
|---|---|---|---|---|
| SOC (g·kg−1) | 14.18 ± 0.17 c | 14.49 ± 0.11 b | 15.52 ± 0.04 a | 13.75 ± 0.06 d |
| TN (g·kg−1) | 1.65 ± 0.04 b | 1.62 ± 0.03 b | 1.74 ± 0.03 a | 1.65 ± 0.03 b |
| AHN (mg·kg−1) | 120.60 ± 0.76 a | 112.08 ± 4.26 b | 115.29 ± 6.22 ab | 111.07 ± 2.65 b |
| AP (mg·kg−1) | 27.34 ± 0.33 c | 35.88 ± 0.68 a | 28.08 ± 1.07 c | 32.30 ± 0.50 b |
| AK (mg·kg−1) | 178.28 ± 5.53 b | 180.24 ± 2.21 b | 212.88 ± 9.00 a | 184.66 ± 6.63 b |
| MBC (mg·kg−1) | 91.16 ± 6.11 c | 98.48 ± 0.72 b | 101.99 ± 10.08 ab | 105.55 ± 9.54 a |
| DOC (mg·kg−1) | 39.41 ± 1.75 d | 47.91 ± 1.52 b | 52.41 ± 0.65 a | 42.47 ± 1.91 c |
| EOC (g·kg−1) | 1.98 ± 0.13 c | 1.87 ± 0.11 c | 3.09 ± 0.16 a | 2.21 ± 0.01 b |
| LA | 16.29 ± 1.47 c | 14.84 ± 1.11 c | 24.91 ± 1.64 a | 19.12 ± 0.08 b |
| pH | 5.70 ± 0.07 a | 5.35 ± 0.04 b | 5.20 ± 0.01 c | 5.46 ± 0.13 b |
| Time | Treatment | SOC |
|---|---|---|
| (g·kg−1) | ||
| 0 d | RTO | 14.18 ± 0.17 c |
| PT | 15.52 ± 0.04 a | |
| RT | 14.49 ± 0.11 b | |
| NT | 13.75 ± 0.06 d | |
| 70 d | RTO + M0 | 15.04 ± 0.16 f |
| RTO + M1 | 16.22 ± 0.12 c | |
| PT + M0 | 15.35 ± 0.09 e | |
| PT + M1 | 16.71 ± 0.16 b | |
| RT + M0 | 14.97 ± 0.08 f | |
| RT + M1 | 17.08 ± 0.09 a | |
| NT + M0 | 14.56 ± 0.01 g | |
| NT + M1 | 15.74 ± 0.06 d | |
| Mode | *** | |
| Input | *** | |
| Mode × Input | *** | |
| Target | MBC | DOC |
|---|---|---|
| Mode | *** | *** |
| Input | *** | *** |
| Time | *** | *** |
| Mode × Input | *** | * |
| Mode × Time | *** | ** |
| Input × Time | *** | * |
| Mode × Input × Time | *** | *** |
| Target | Accumulation CO2 Emission | SOC | EOC | LA |
|---|---|---|---|---|
| Mode | *** | *** | *** | *** |
| Input | *** | *** | *** | *** |
| Mode × Input | *** | *** | ** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, P.; Wang, H.; Zhao, Z.; Li, X.; Wang, Y.; Zhan, X.; Han, X. Effects of Straw Addition on Soil Priming Effects Under Different Tillage and Straw Return Modes. Plants 2024, 13, 3188. https://doi.org/10.3390/plants13223188
Cai P, Wang H, Zhao Z, Li X, Wang Y, Zhan X, Han X. Effects of Straw Addition on Soil Priming Effects Under Different Tillage and Straw Return Modes. Plants. 2024; 13(22):3188. https://doi.org/10.3390/plants13223188
Chicago/Turabian StyleCai, Peixuan, Haixia Wang, Zhihui Zhao, Xue Li, Ying Wang, Xiumei Zhan, and Xiaori Han. 2024. "Effects of Straw Addition on Soil Priming Effects Under Different Tillage and Straw Return Modes" Plants 13, no. 22: 3188. https://doi.org/10.3390/plants13223188
APA StyleCai, P., Wang, H., Zhao, Z., Li, X., Wang, Y., Zhan, X., & Han, X. (2024). Effects of Straw Addition on Soil Priming Effects Under Different Tillage and Straw Return Modes. Plants, 13(22), 3188. https://doi.org/10.3390/plants13223188






