Unleashing the Potential of CRISPR/Cas9 Genome Editing for Yield-Related Traits in Rice
Abstract
1. Introduction
2. Yield and Its Component Traits
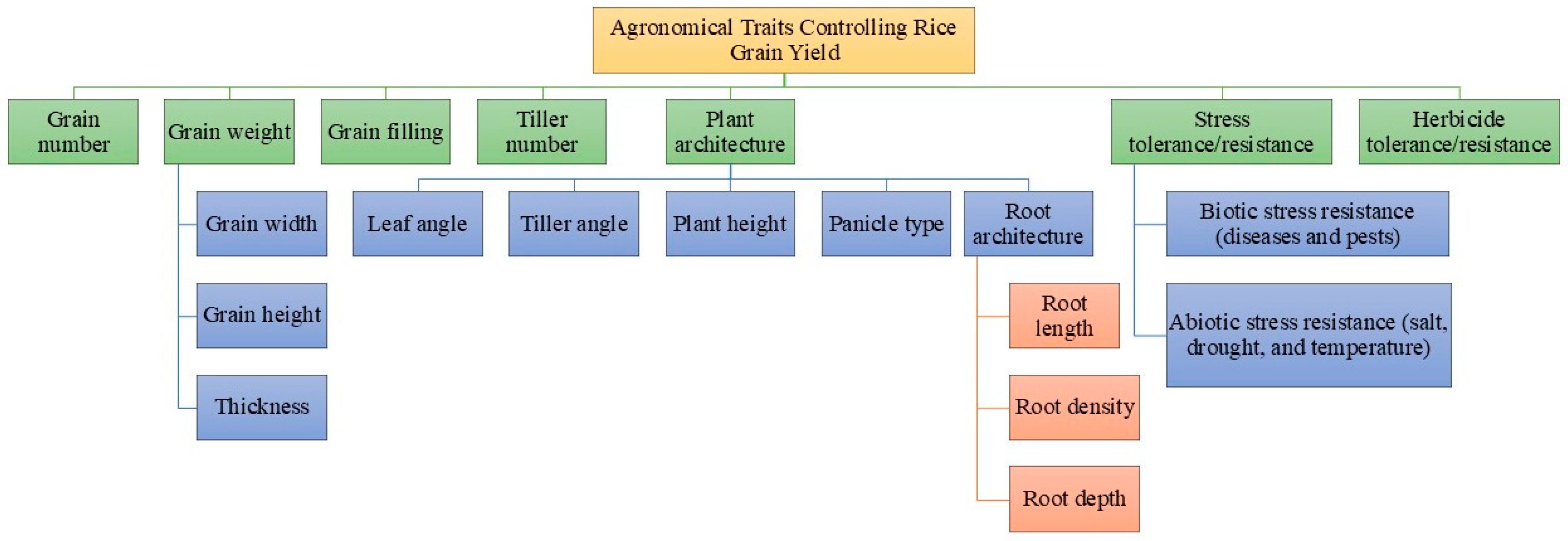
3. Agronomical Traits
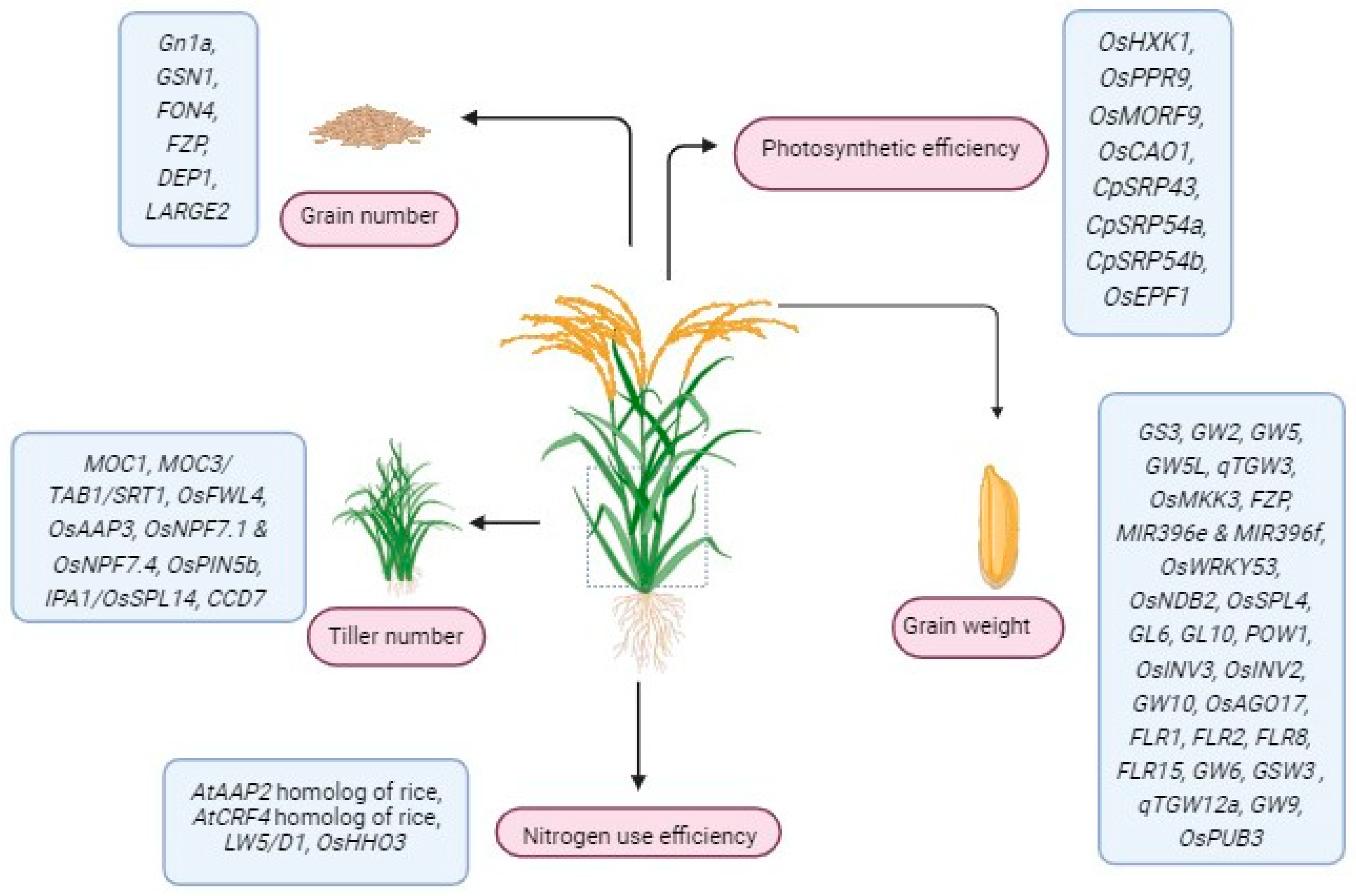
3.1. Genes Controlling Rice Grain Number
3.2. Genes Controlling Rice Grain Weight
3.3. Genes Controlling Rice Tiller Number
3.4. Genes Controlling Biotic and Abiotic Stress Resistance in Rice
3.5. Genes Controlling Herbicide Resistance in Rice
4. Physiological Traits
4.1. Genes Controlling NUE in Rice
4.2. Genes Controlling Photosynthetic Efficiency in Rice
5. Targeting Regulatory DNA Regions for Improving Rice Yield
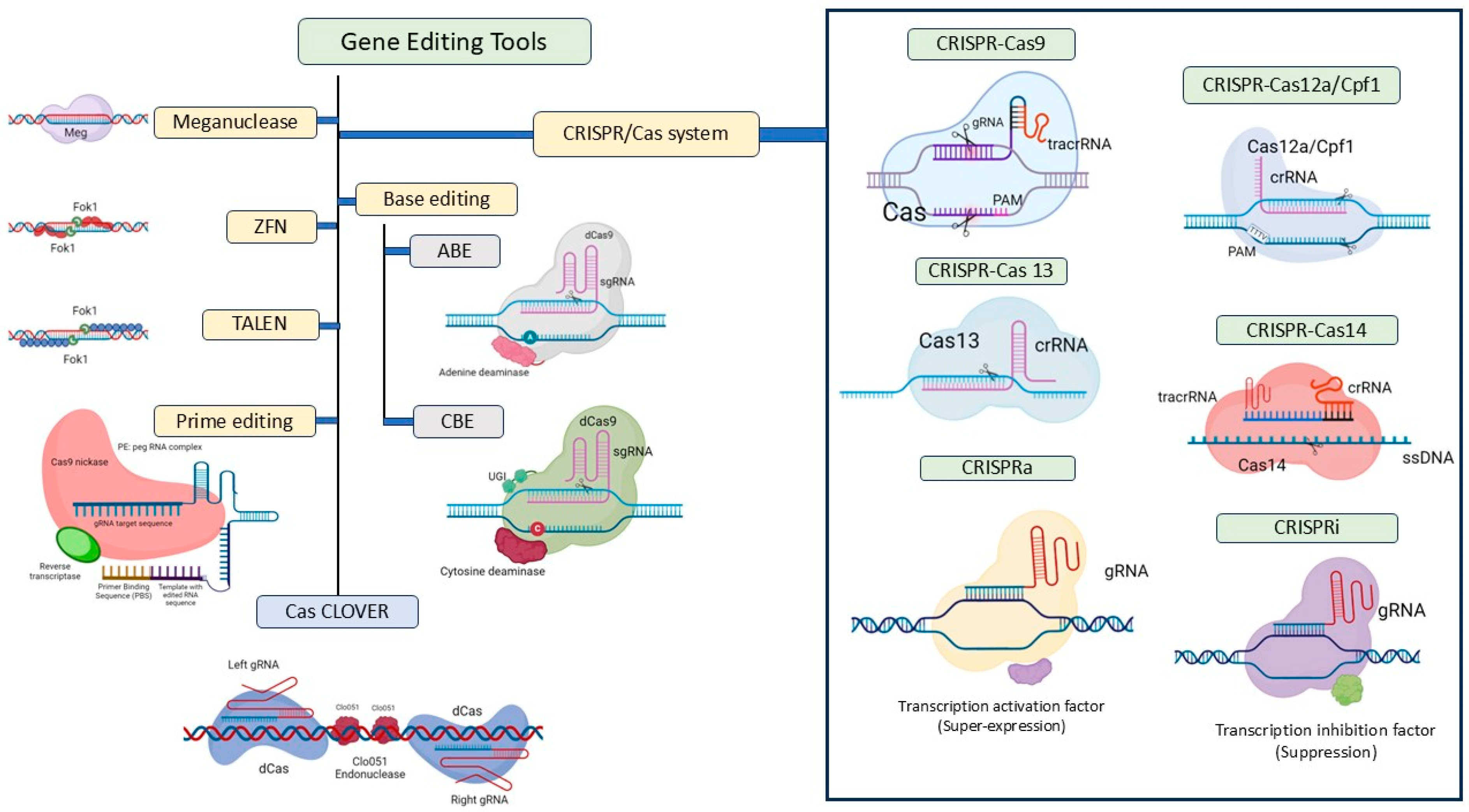
6. CRISPR-Cas: Advantages over Other Gene Editing Tools
| Gene | Gene Editing Tool | Trait | Reference |
|---|---|---|---|
| OsSPL14 | Base editing | Tiller number | [140] |
| OsEPFL9 | CRISPR-LbCpf1 (Lachnospiracae bacterium Cpf1), CRISPR-FnCpf1 (Francisella novicida Cpf1) | Stomatal development | [148,149] |
| OsDEP1 | CRISPR-LbCpf1, CRISPR-FnCpf1 | Plant architecture and grain number | [149,150,151] |
| OsGS3 | CRISPR-LbCpf1 | Grain size | [152] |
| OsDEP1 | Prime editing | Plant architecture and grain number | [153] |
| OsSPL14 | Prime editing | Tiller number | [153] |
| OsEPFL9 | CRISPR/Cas12a-RNP | Stomatal development | [145] |
| NRT1.1B | Base editing | Enhanced nitrogen use efficiency | [154] |
| C287 | Base editing | Herbicide resistance | [155] |
| GL2/OsGRF4, OsGRF3 | Base editing | Grain size and yield | [156] |
| OsACC-T1 | CRISPR–Cpf1-based base editing | Herbicide resistance | [157] |
| OsALS | Prime editing | Herbicide resistance | [158,159] |
| TFIIAg5, OsSWEET11a, OsEPSPS1 and OsALS1 | Multiplexing—quadruple prime editing | Broad spectrum resistance to bacterial blight and herbicide | [160] |
| OsSPL13, OsSPL14 and OsGS2 | Multiplex prime editing | Major yield traits—grain size and weight, plant architecture, tiller number and NUE | [160] |
| OsSWEET14 | Base editng (CBE) | Resistance to bacterial blight | [161] |
| OsDEP1, OsNRT1.1b, OsWaxyT1, OsWaxyT2 and OsWaxyT3 | Adenine base transition editor (ABE8e) | Panicle architecture, NUE, starch biosynthesis | [162] |
| OsYSA, OsNAL, OsMIR396e, and OsPYL6 | CRIPSR/Cas12i3-based multiplex direct repeat (DR)-spacer Array Genome Editing system (iMAGE) | Chloroplast development, grain number, yield | [163] |
| OsACCase | Deactivated Cas12i3 base editor | Resistant to sethoxydim herbicide | [163] |
| OsGRF4 | Prime editing | Grain yield | [164] |
7. Off-Target Effects of CRISPR/Cas9 on Plant Physiology
8. Challenges and Future Prospects in Genome Editing
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zafar, K.; Sedeek, K.E.; Rao, G.S.; Khan, M.Z.; Amin, I.; Kamel, R.; Mukhtar, Z.; Zafar, M.; Mansoor, S.; Mahfouz, M.M. Genome editing technologies for rice improvement: Progress, prospects, and safety concerns. Front. Genome Ed. 2020, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhang, Q. Genetic and molecular bases of rice yield. Annu. Rev. Plant Biol. 2010, 61, 421–442. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.-H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, J.; Ahmad, S.; Hussain, B.; Mawia, A.M.; Zeb, A.; Ju, L. Applications and potential of genome-editing systems in rice improvement: Current and future perspectives. Agronomy 2021, 11, 1359. [Google Scholar] [CrossRef]
- Endo, M.; Toki, S. Genome Editing in Rice; Springer: Cham, Switzerland, 2020; Volume 13, pp. 1–2. [Google Scholar]
- Osakabe, Y.; Osakabe, K. Genome editing with engineered nucleases in plants. Plant Cell Physiol. 2015, 56, 389–400. [Google Scholar] [CrossRef]
- Symington, L.S.; Gautier, J. Double-strand break end resection and repair pathway choice. Annu. Rev. Genet. 2011, 45, 247–271. [Google Scholar] [CrossRef]
- Hussain, B.; Lucas, S.J.; Budak, H. CRISPR/Cas9 in plants: At play in the genome and at work for crop improvement. Brief. Funct. Genom. 2018, 17, 319–328. [Google Scholar] [CrossRef]
- Vats, S.; Kumawat, S.; Kumar, V.; Patil, G.B.; Joshi, T.; Sonah, H.; Sharma, T.R.; Deshmukh, R. Genome editing in plants: Exploration of technological advancements and challenges. Cells 2019, 8, 1386. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Matsuoka, M. Identifying and exploiting grain yield genes in rice. Curr. Opin. Plant Biol. 2008, 11, 209–214. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain-filling problem in ‘super’rice. J. Exp. Bot. 2010, 61, 1–5. [Google Scholar] [CrossRef]
- Li, G.; Tang, J.; Zheng, J.; Chu, C. Exploration of rice yield potential: Decoding agronomic and physiological traits. Crop J. 2021, 9, 577–589. [Google Scholar] [CrossRef]
- Zhong, Q.; Jia, Q.; Yin, W.; Wang, Y.; Rao, Y.; Mao, Y. Advances in cloning functional genes for rice yield traits and molecular design breeding in China. Front. Plant Sci. 2023, 14, 1206165. [Google Scholar] [CrossRef] [PubMed]
- Deveshwar, P.; Prusty, A.; Sharma, S.; Tyagi, A.K. Phytohormone-mediated molecular mechanisms involving multiple genes and QTL govern grain number in rice. Front. Genet. 2020, 11, 586462. [Google Scholar] [CrossRef] [PubMed]
- Terao, T.; Nagata, K.; Morino, K.; Hirose, T. A gene controlling the number of primary rachis branches also controls the vascular bundle formation and hence is responsible to increase the harvest index and grain yield in rice. Theor. Appl. Genet. 2010, 120, 875–893. [Google Scholar] [CrossRef]
- Lu, Y.; Chuan, M.; Wang, H.; Chen, R.; Tao, T.; Zhou, Y.; Xu, Y.; Li, P.; Yao, Y.; Xu, C. Genetic and molecular factors in determining grain number per panicle of rice. Front. Plant Sci. 2022, 13, 964246. [Google Scholar] [CrossRef] [PubMed]
- Ashikari, M.; Sakakibara, H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhou, Z.; Wu, P.; Fang, M.; Pan, X.; Lin, Q.; Luo, W.; Wu, G.; Li, H. Reassessment of the four yield-related genes Gn1a, DEP1, GS3, and IPA1 in rice using a CRISPR/Cas9 system. Front. Plant Sci. 2016, 7, 377. [Google Scholar] [CrossRef]
- Paul-Victor, C.; Turnbull, L.A. The effect of growth conditions on the seed size/number trade-off. PLoS ONE 2009, 4, e6917. [Google Scholar] [CrossRef]
- Gasparis, S.; Miłoszewski, M.M. Genetic Basis of Grain Size and Weight in Rice, Wheat, and Barley. Int. J. Mol. Sci. 2023, 24, 16921. [Google Scholar] [CrossRef]
- Guo, T.; Chen, K.; Dong, N.-Q.; Shi, C.-L.; Ye, W.-W.; Gao, J.-P.; Shan, J.-X.; Lin, H.-X. GRAIN SIZE AND NUMBER1 negatively regulates the OsMKKK10-OsMKK4-OsMPK6 cascade to coordinate the trade-off between grain number per panicle and grain size in rice. Plant Cell 2018, 30, 871–888. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, S.-L.; Zhu, Y.-J.; Fan, Y.-Y.; Huang, D.-R.; Zhu, A.-K.; Zhuang, J.-Y.; Liang, Y.; Zhang, Z.-H. Control of Grain Shape and Size in Rice by Two Functional Alleles of OsPUB3 in Varied Genetic Background. Plants 2022, 11, 2530. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Xu, Q.; Qiu, Z.; Cui, Y.; Zhou, T.; Zeng, D.; Guo, L.; Qian, Q. FON4 prevents the multi-floret spikelet in rice. Plant Biotechnol. J. 2019, 17, 1007. [Google Scholar] [CrossRef]
- Lakshmi Jayaraj, K.; Thulasidharan, N.; Antony, A.; John, M.; Augustine, R.; Chakravartty, N.; Sukumaran, S.; Uma Maheswari, M.; Abraham, S.; Thomas, G. Targeted editing of tomato carotenoid isomerase reveals the role of 5′ UTR region in gene expression regulation. Plant Cell Rep. 2021, 40, 621–635. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Elbaiomy, R.G.; Chen, L.; Wang, Z.; Jiao, J.; Zhu, J.; Zhou, W.; Chen, B.; Soaud, S.A. Evolution analysis of FRIZZY PANICLE (FZP) orthologs explored the mutations in DNA coding sequences in the grass family (Poaceae). PeerJ 2022, 10, e12880. [Google Scholar] [CrossRef]
- Chen, H.; Cai, Y.; Zhang, S.; Tang, W.; Fang, X.; Zhang, Y. Identification of a novel mutant allele, fzp-15, involved in panicle branch pattern of rice (Oryza sativa). Plant Breed. 2021, 140, 595–602. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, M.; Zhang, Q.; Xu, Z.; Xu, Q. The DENSE AND ERECT PANICLE 1 (DEP1) gene offering the potential in the breeding of high-yielding rice. Breed. Sci. 2016, 66, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wang, F.; Kong, D.; Hou, D.; Huang, L.; Bi, J.; Zhang, F.; Luo, X.; Wang, J.; Liu, G. Mutation of DEP1 facilitates the improvement of plant architecture in Xian rice (Oryza sativa). Plant Breed. 2023, 142, 338–344. [Google Scholar] [CrossRef]
- Chu, H.; Qian, Q.; Liang, W.; Yin, C.; Tan, H.; Yao, X.; Yuan, Z.; Yang, J.; Huang, H.; Luo, D. The floral organ number4 gene encoding a putative ortholog of Arabidopsis CLAVATA3 regulates apical meristem size in rice. Plant Physiol. 2006, 142, 1039–1052. [Google Scholar] [CrossRef]
- Huang, L.; Hua, K.; Xu, R.; Zeng, D.; Wang, R.; Dong, G.; Zhang, G.; Lu, X.; Fang, N.; Wang, D. The LARGE2-APO1/APO2 regulatory module controls panicle size and grain number in rice. Plant Cell 2021, 33, 1212–1228. [Google Scholar] [CrossRef]
- Chen, K.; Łyskowski, A.; Jaremko, Ł.; Jaremko, M. Genetic and molecular factors determining grain weight in rice. Front. Plant Sci. 2021, 12, 605799. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y. Molecular, cellular and Yin-Yang regulation of grain size and number in rice. Mol. Breed. 2019, 39, 163. [Google Scholar] [CrossRef]
- Li, N.; Xu, R.; Li, Y. Molecular networks of seed size control in plants. Annu. Rev. Plant Biol. 2019, 70, 435–463. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Sun, S.; Yao, J.; Wang, C.; Yu, S.; Xu, C.; Li, X.; Zhang, Q. Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 19579–19584. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, L.; Mao, H.; Shao, L.; Li, X.; Xiao, J.; Ouyang, Y.; Zhang, Q. A G-protein pathway determines grain size in rice. Nat. Commun. 2018, 9, 851. [Google Scholar] [CrossRef]
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.; Zhang, Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [CrossRef]
- Zeng, Y.; Wen, J.; Zhao, W.; Wang, Q.; Huang, W. Rational improvement of rice yield and cold tolerance by editing the three genes OsPIN5b, GS3, and OsMYB30 with the CRISPR–Cas9 system. Front. Plant Sci. 2020, 10, 1663. [Google Scholar] [CrossRef]
- Yuyu, C.; Aike, Z.; Pao, X.; Xiaoxia, W.; Yongrun, C.; Beifang, W.; Yue, Z.; Liaqat, S.; Shihua, C.; Liyong, C. Effects of GS3 and GL3. 1 for grain size editing by CRISPR/Cas9 in rice. Rice Sci. 2020, 27, 405–413. [Google Scholar] [CrossRef]
- Weng, J.; Gu, S.; Wan, X.; Gao, H.; Guo, T.; Su, N.; Lei, C.; Zhang, X.; Cheng, Z.; Guo, X. Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res. 2008, 18, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, J.; Zheng, X.; Wu, F.; Lin, Q.; Heng, Y.; Tian, P.; Cheng, Z.; Yu, X.; Zhou, K. GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat. Plants 2017, 3, 17043. [Google Scholar] [CrossRef]
- Tian, P.; Liu, J.; Mou, C.; Shi, C.; Zhang, H.; Zhao, Z.; Lin, Q.; Wang, J.; Wang, J.; Zhang, X. GW5-Like, a homolog of GW5, negatively regulates grain width, weight and salt resistance in rice. J. Integr. Plant Biol. 2019, 61, 1171–1185. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, S.-J.; Wang, M.-J.; He, H.; Sun, L.; Wang, H.; Liu, X.-H.; Jiang, L.; Sun, J.-L.; Xin, X. A novel QTL qTGW3 encodes the GSK3/SHAGGY-like kinase OsGSK5/OsSK41 that interacts with OsARF4 to negatively regulate grain size and weight in rice. Mol. Plant 2018, 11, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Usman, B.; Zhao, N.; Nawaz, G.; Qin, B.; Liu, F.; Liu, Y.; Li, R. CRISPR/Cas9 guided mutagenesis of grain size 3 confers increased rice (Oryza sativa L.) grain length by regulating cysteine proteinase inhibitor and ubiquitin-related proteins. Int. J. Mol. Sci. 2021, 22, 3225. [Google Scholar] [CrossRef] [PubMed]
- Achary, V.M.M.; Reddy, M.K. CRISPR-Cas9 mediated mutation in GRAIN WIDTH and WEIGHT2 (GW2) locus improves aleurone layer and grain nutritional quality in rice. Sci. Rep. 2021, 11, 21941. [Google Scholar] [CrossRef] [PubMed]
- Qing, D.; Chen, W.; Huang, S.; Li, J.; Pan, Y.; Zhou, W.; Liang, Q.; Yuan, J.; Gan, D.; Chen, L. Editing of rice (Oryza sativa L.) OsMKK3 gene using CRISPR/Cas9 decreases grain length by modulating the expression of photosystem components. Proteomics 2023, 23, 2200538. [Google Scholar] [CrossRef]
- Ren, D.; Hu, J.; Xu, Q.; Cui, Y.; Zhang, Y.; Zhou, T.; Rao, Y.; Xue, D.; Zeng, D.; Zhang, G. FZP determines grain size and sterile lemma fate in rice. J. Exp. Bot. 2018, 69, 4853–4866. [Google Scholar] [CrossRef]
- Miao, C.; Wang, D.; He, R.; Liu, S.; Zhu, J.K. Mutations in MIR 396e and MIR 396f increase grain size and modulate shoot architecture in rice. Plant Biotechnol. J. 2020, 18, 491–501. [Google Scholar] [CrossRef]
- Tang, J.; Mei, E.; He, M.; Bu, Q.; Tian, X. Functions of OsWRKY24, OsWRKY70 and OsWRKY53 in regulating grain size in rice. Planta 2022, 255, 92. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.; Hou, L.; Zhao, S.; Zhang, N.; Lu, L.; Zhao, X. The mitochondria-localized protein OsNDB2 negatively regulates grain size and weight in rice. Crop J. 2022, 10, 1819–1824. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, H.-Z.; Wu, Y.; Zhang, H.; Lin, J.; Jiang, X.; He, Q.; Zhu, J.; Li, Y.; Yu, H. OsSPL3, an SBP-domain protein, regulates crown root development in rice. Plant Cell 2019, 31, 1257–1275. [Google Scholar] [CrossRef]
- Hu, J.; Huang, L.; Chen, G.; Liu, H.; Zhang, Y.; Zhang, R.; Zhang, S.; Liu, J.; Hu, Q.; Hu, F. The elite alleles of OsSPL4 regulate grain size and increase grain yield in rice. Rice 2021, 14, 90. [Google Scholar] [CrossRef]
- Wang, A.; Hou, Q.; Si, L.; Huang, X.; Luo, J.; Lu, D.; Zhu, J.; Shangguan, Y.; Miao, J.; Xie, Y. The PLATZ transcription factor GL6 affects grain length and number in rice. Plant Physiol. 2019, 180, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Ma, S.; Xiao, Z.; Li, F.; Wei, X.; Lin, S.; Wang, X.; Ji, Z.; Fu, Y.; Pan, J. Natural variations in grain length 10 (GL10) regulate rice grain size. J. Genet. Genom. 2022, 49, 405–413. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, R.; Xing, Y.; Xu, Y.; Xiong, D.; Wang, Y.; Yao, S. Separable regulation of POW1 in grain size and leaf angle development in rice. Plant Biotechnol. J. 2021, 19, 2517–2531. [Google Scholar] [CrossRef]
- Deng, X.; Han, X.; Yu, S.; Liu, Z.; Guo, D.; He, Y.; Li, W.; Tao, Y.; Sun, C.; Xu, P. OsINV3 and its homolog, OsINV2, control grain size in rice. Int. J. Mol. Sci. 2020, 21, 2199. [Google Scholar] [CrossRef]
- Zhan, P.; Wei, X.; Xiao, Z.; Wang, X.; Ma, S.; Lin, S.; Li, F.; Bu, S.; Liu, Z.; Zhu, H. GW10, a member of P450 subfamily regulates grain size and grain number in rice. Theor. Appl. Genet. 2021, 134, 3941–3950. [Google Scholar] [CrossRef]
- Zhong, J.; He, W.; Peng, Z.; Zhang, H.; Li, F.; Yao, J. A putative AGO protein, OsAGO17, positively regulates grain size and grain weight through OsmiR397b in rice. Plant Biotechnol. J. 2020, 18, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, D.; Yang, Z.; Jiang, S.; Qu, J.; He, W.; Liu, Z.; Xing, J.; Ma, Y.; Lin, Q. Roles of FERONIA-like receptor genes in regulating grain size and quality in rice. Sci. China Life Sci. 2021, 64, 294–310. [Google Scholar] [CrossRef]
- Shi, C.L.; Dong, N.Q.; Guo, T.; Ye, W.W.; Shan, J.X.; Lin, H.X. A quantitative trait locus GW6 controls rice grain size and yield through the gibberellin pathway. Plant J. 2020, 103, 1174–1188. [Google Scholar] [CrossRef]
- Bai, F.; Ma, H.; Cai, Y.; Shahid, M.Q.; Zheng, Y.; Lang, C.; Chen, Z.; Wu, J.; Liu, X.; Wang, L. Natural Allelic Variation in GRAIN SIZE AND WEIGHT 3 of Wild Rice Regulates the Grain Size and Weight. Plant Physiol. 2023, 193, 502–518. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Huang, Z.; Li, J.; Bao, J.; Tu, H.; Zeng, C.; Wu, Z.; Fu, H.; Xu, J.; Zhou, D. qTGW12 a, a naturally varying QTL, regulates grain weight in rice. Theor. Appl. Genet. 2021, 134, 2767–2776. [Google Scholar] [CrossRef]
- Wen, Y.; Hu, P.; Fang, Y.; Tan, Y.; Wang, Y.; Wu, H.; Wang, J.; Wu, K.; Chai, B.; Zhu, L. GW9 determines grain size and floral organ identity in rice. Plant Biotechnol. J. 2023, 22, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Zhang, Z.-H.; Fan, Y.-Y.; Huang, D.-R.; Yang, Y.-L.; Zhuang, J.-Y.; Zhu, Y.-J. Control of Grain Weight and Size in Rice (Oryza sativa L.) by OsPUB3 Encoding a U-Box E3 Ubiquitin Ligase. Rice 2022, 15, 58. [Google Scholar] [CrossRef]
- Li, X.; Qian, Q.; Fu, Z.; Wang, Y.; Xiong, G.; Zeng, D.; Wang, X.; Liu, X.; Teng, S.; Hiroshi, F. Control of tillering in rice. Nature 2003, 422, 618–621. [Google Scholar] [CrossRef]
- Yan, Y.; Ding, C.; Zhang, G.; Hu, J.; Zhu, L.; Zeng, D.; Qian, Q.; Ren, D. Genetic and environmental control of rice tillering. Crop J. 2023, 11, 1287–1302. [Google Scholar] [CrossRef]
- Shao, G.; Lu, Z.; Xiong, J.; Wang, B.; Jing, Y.; Meng, X.; Liu, G.; Ma, H.; Liang, Y.; Chen, F. Tiller bud formation regulators MOC1 and MOC3 cooperatively promote tiller bud outgrowth by activating FON1 expression in rice. Mol. Plant 2019, 12, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.-h.; Shang, F.; Lin, Q.-t.; Lou, C.; Zhang, J. Tillering and panicle branching genes in rice. Gene 2014, 537, 1–5. [Google Scholar] [CrossRef]
- Gao, Q.; Li, G.; Sun, H.; Xu, M.; Wang, H.; Ji, J.; Wang, D.; Yuan, C.; Zhao, X. Targeted mutagenesis of the rice FW 2.2-like gene family using the CRISPR/Cas9 system reveals OsFWL4 as a regulator of tiller number and plant yield in rice. Int. J. Mol. Sci. 2020, 21, 809. [Google Scholar] [CrossRef]
- Taylor, M.R.; Reinders, A.; Ward, J.M. Transport Function of Rice Amino Acid Permeases (AAPs). Plant Cell Physiol. 2015, 56, 1355–1363. [Google Scholar] [CrossRef]
- Lu, K.; Wu, B.; Wang, J.; Zhu, W.; Nie, H.; Qian, J.; Huang, W.; Fang, Z. Blocking amino acid transporter Os AAP 3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol. J. 2018, 16, 1710–1722. [Google Scholar] [CrossRef]
- Huang, W.; Nie, H.; Feng, F.; Wang, J.; Lu, K.; Fang, Z. Altered expression of OsNPF7. 1 and OsNPF7. 4 differentially regulates tillering and grain yield in rice. Plant Sci. 2019, 283, 23–31. [Google Scholar] [CrossRef]
- Song, X.; Lu, Z.; Yu, H.; Shao, G.; Xiong, J.; Meng, X.; Jing, Y.; Liu, G.; Xiong, G.; Duan, J. IPA1 functions as a downstream transcription factor repressed by D53 in strigolactone signaling in rice. Cell Res. 2017, 27, 1128–1141. [Google Scholar] [CrossRef]
- Butt, H.; Jamil, M.; Wang, J.Y.; Al-Babili, S.; Mahfouz, M. Engineering plant architecture via CRISPR/Cas9-mediated alteration of strigolactone biosynthesis. BMC Plant Biol. 2018, 18, 174. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; He, J.; Yu, W. Knocking out of carotenoid catabolic genes in rice fails to boost carotenoid accumulation, but reveals a mutation in strigolactone biosynthesis. Plant Cell Rep. 2017, 36, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Mo, T.; Wang, T.; Sun, Y.; Kumar, A.; Mkumbwa, H.; Fang, J.; Zhao, J.; Yuan, S.; Li, Z.; Li, X. The chloroplast pentatricopeptide repeat protein RCN22 regulates tiller number in rice by affecting sugar levels via the TB1-RCN22-RbcL module. Plant Commun. 2024, 101073. [Google Scholar] [CrossRef]
- Song, J.; Tang, L.; Fan, H.; Xu, X.; Peng, X.; Cui, Y.; Wang, J. Enhancing Yield and Improving Grain Quality in Japonica Rice: Targeted EHD1 Editing via CRISPR-Cas9 in Low-Latitude Adaptation. Curr. Issues Mol. Biol. 2024, 46, 3741–3751. [Google Scholar] [CrossRef]
- Ahmad, S.; Wei, X.; Sheng, Z.; Hu, P.; Tang, S. CRISPR/Cas9 for development of disease resistance in plants: Recent progress, limitations and future prospects. Brief. Funct. Genom. 2020, 19, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zou, B.; Hong, X.; Gao, M.; Yang, W.; Zhong, X.; He, Y.; Kuai, P.; Lou, Y.; Huang, J. Rice copine genes Os BON 1 and Os BON 3 function as suppressors of broad-spectrum disease resistance. Plant Biotechnol. J. 2018, 16, 1476–1487. [Google Scholar] [CrossRef] [PubMed]
- Dhadge, K.; Walia, P. Applications of CRISPR/Cas9 for biotic and abiotic stress resistance of rice (Oryza sativa). J. Adv. Biol. Biotechnol. 2024, 27, 172–179. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, S.; Jiang, N.; Zhao, X.; Bai, Z.; Liu, J.; Yao, W.; Tang, Q.; Xiao, G.; Lv, C. Engineering of rice varieties with enhanced resistances to both blast and bacterial blight diseases via CRISPR/Cas9. Plant Biotechnol. J. 2022, 20, 876–885. [Google Scholar] [CrossRef]
- Jha, U.C.; Bohra, A.; Nayyar, H. Advances in “omics” approaches to tackle drought stress in grain legumes. Plant Breed. 2020, 139, 1–27. [Google Scholar] [CrossRef]
- Kim, M.-S.; Ko, S.-R.; Jung, Y.J.; Kang, K.-K.; Lee, Y.-J.; Cho, Y.-G. Knockout mutants of OsPUB7 generated using CRISPR/Cas9 revealed abiotic stress tolerance in rice. Int. J. Mol. Sci. 2023, 24, 5338. [Google Scholar] [CrossRef] [PubMed]
- Alfatih, A.; Wu, J.; Jan, S.U.; Zhang, Z.S.; Xia, J.Q.; Xiang, C.B. Loss of rice PARAQUAT TOLERANCE 3 confers enhanced resistance to abiotic stresses and increases grain yield in field. Plant Cell Environ. 2020, 43, 2743–2754. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Peng, Z.; Long, J.; Sosso, D.; Liu, B.; Eom, J.S.; Huang, S.; Liu, S.; Vera Cruz, C.; Frommer, W.B. Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 2015, 82, 632–643. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.-G.; Zhao, K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.-S.; Li, C.; Nguyen, H.; Liu, B. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef]
- Foster, A.J.; Martin-Urdiroz, M.; Yan, X.; Wright, H.S.; Soanes, D.M.; Talbot, N.J. CRISPR-Cas9 ribonucleoprotein-mediated co-editing and counterselection in the rice blast fungus. Sci. Rep. 2018, 8, 14355. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Zhou, Z.; Chen, H.; Xie, C.; Lin, Y. A new rice breeding method: CRISPR/Cas9 system editing of the Xa13 promoter to cultivate transgene-free bacterial blight-resistant rice. Plant Biotechnol. J. 2020, 18, 313. [Google Scholar] [CrossRef]
- Macovei, A.; Sevilla, N.R.; Cantos, C.; Jonson, G.B.; Slamet-Loedin, I.; Čermák, T.; Voytas, D.F.; Choi, I.R.; Chadha-Mohanty, P. Novel alleles of rice eIF4G generated by CRISPR/Cas9-targeted mutagenesis confer resistance to Rice tungro spherical virus. Plant Biotechnol. J. 2018, 16, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Arra, Y.; Auguy, F.; Stiebner, M.; Chéron, S.; Wudick, M.M.; Miras, M.; Schepler-Luu, V.; Köhler, S.; Cunnac, S.; Frommer, W.B. Rice Yellow Mottle Virus resistance by genome editing of the Oryza sativa L. ssp. japonica nucleoporin gene OsCPR5.1 but not OsCPR5.2. Plant Biotechnol. J. 2024, 22, 1299–1311. [Google Scholar]
- Ogata, T.; Ishizaki, T.; Fujita, M.; Fujita, Y. CRISPR/Cas9-targeted mutagenesis of OsERA1 confers enhanced responses to abscisic acid and drought stress and increased primary root growth under nonstressed conditions in rice. PLoS ONE 2020, 15, e0243376. [Google Scholar] [CrossRef]
- Santosh Kumar, V.; Verma, R.K.; Yadav, S.K.; Yadav, P.; Watts, A.; Rao, M.; Chinnusamy, V. CRISPR-Cas9 mediated genome editing of drought and salt tolerance (OsDST) gene in indica mega rice cultivar MTU1010. Physiol. Mol. Biol. Plants 2020, 26, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhong, Z.; Wang, X.; Han, X.; Yu, D.; Wang, C.; Song, W.; Zheng, X.; Chen, C.; Zhang, Y. Knockout of the OsNAC006 transcription factor causes drought and heat sensitivity in rice. Int. J. Mol. Sci. 2020, 21, 2288. [Google Scholar] [CrossRef]
- Nawaz, G.; Han, Y.; Usman, B.; Liu, F.; Qin, B.; Li, R. Knockout of OsPRP1, a gene encoding proline-rich protein, confers enhanced cold sensitivity in rice (Oryza sativa L.) at the seedling stage. 3 Biotech 2019, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, Y.; Li, W.; Chen, Z.; Wang, J.; Fan, F.; Tao, Y.; Jiang, Y.; Zhu, Q.-H.; Yang, J. Creating a novel herbicide-tolerance OsALS allele using CRISPR/Cas9-mediated gene editing. Crop J. 2021, 9, 305–312. [Google Scholar] [CrossRef]
- Li, J.; Meng, X.; Zong, Y.; Chen, K.; Zhang, H.; Liu, J.; Li, J.; Gao, C. Gene replacements and insertions in rice by intron targeting using CRISPR–Cas9. Nat. Plants 2016, 2, 16139. [Google Scholar] [CrossRef]
- Hussain, A.; Ding, X.; Alariqi, M.; Manghwar, H.; Hui, F.; Li, Y.; Cheng, J.; Wu, C.; Cao, J.; Jin, S. Herbicide resistance: Another hot agronomic trait for plant genome editing. Plants 2021, 10, 621. [Google Scholar] [CrossRef]
- Romero, F.M.; Gatica-Arias, A. CRISPR/Cas9: Development and application in rice breeding. Rice Sci. 2019, 26, 265–281. [Google Scholar] [CrossRef]
- Lyu, Y.-S.; Cao, L.-M.; Huang, W.-Q.; Liu, J.-X.; Lu, H.-P. Disruption of three polyamine uptake transporter genes in rice by CRISPR/Cas9 gene editing confers tolerance to herbicide paraquat. Abiotech 2022, 3, 140–145. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Chen, B.; Mo, S.; Lian, L.; Luo, Y.; Ding, D.; Ding, Y.; Cao, Q.; Li, Y. A donor-DNA-free CRISPR/Cas-based approach to gene knock-up in rice. Nat. Plants 2021, 7, 1445–1452. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, S.; Chu, C. Improvement of nutrient use efficiency in rice: Current toolbox and future perspectives. Theor. Appl. Genet. 2020, 133, 1365–1384. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Ohtsuki, N.; Kadota, K.; Tezuka, A.; Nagano, A.J.; Kadowaki, T.; Kim, Y.; Miyao, M.; Yanagisawa, S. Gene regulatory network and its constituent transcription factors that control nitrogen-deficiency responses in rice. New Phytol. 2020, 227, 1434–1452. [Google Scholar] [CrossRef]
- Konishi, N.; Ma, J.F. Three polarly localized ammonium transporter 1 members are cooperatively responsible for ammonium uptake in rice under low ammonium condition. New Phytol. 2021, 232, 1778–1792. [Google Scholar] [CrossRef]
- Liu, K.; Sakuraba, Y.; Ohtsuki, N.; Yang, M.; Ueda, Y.; Yanagisawa, S. CRISPR/Cas9-mediated elimination of OsHHO3, a transcriptional repressor of three AMMONIUM TRANSPORTER1 genes, improves nitrogen use efficiency in rice. Plant Biotechnol. J. 2023, 21, 2169. [Google Scholar] [CrossRef]
- Wakatabi, K.; Selvaraj, M.G.; Guzmán-Prada, D.A.; Cuásquer, J.B.; López-López, K.; Endo, M.; Ishitani, M. Selection and evaluation of gene-edited knockout mutants of AtAAP2 and AtCRF4 homologs of rice for agronomic nitrogen use efficiency (ANUE). Rev. Colomb. De Cienc. Hortícolas 2023, 17, e16120. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Xu, J.; Wang, J.; Wang, L.; Zou, W.; Zeng, D.; Zhu, L.; Chen, G.; Hu, J. Leaf width gene LW5/D1 affects plant architecture and yield in rice by regulating nitrogen utilization efficiency. Plant Physiol. Biochem. 2020, 157, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, Q.; Yin, P. RNA editing machinery in plant organelles. Sci. China Life Sci. 2018, 61, 162–169. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Zhang, M.; Ai, P. A chloroplast-localized pentatricopeptide repeat protein involved in RNA editing and splicing and its effects on chloroplast development in rice. BMC Plant Biol. 2022, 22, 437. [Google Scholar] [CrossRef]
- Small, I.D.; Schallenberg-Rüdinger, M.; Takenaka, M.; Mireau, H.; Ostersetzer-Biran, O. Plant organellar RNA editing: What 30 years of research has revealed. Plant J. 2020, 101, 1040–1056. [Google Scholar] [CrossRef]
- Chen, C.-Z.; Wang, Y.-L.; He, M.-X.; Li, Z.-W.; Lan, S.; Qing, L.; Ren, D.-Y.; Jiang, H.; Li, Z.; Zhang, G.-H. OsPPR9 encodes a DYW-type PPR protein that affects editing efficiency of multiple RNA editing sites and is essential for chloroplast development. J. Integr. Agric. 2023, 22, 972–980. [Google Scholar] [CrossRef]
- Zheng, S.; Ye, C.; Lu, J.; Liufu, J.; Lin, L.; Dong, Z.; Li, J.; Zhuang, C. Improving the rice photosynthetic efficiency and yield by editing OsHXK1 via CRISPR/Cas9 system. Int. J. Mol. Sci. 2021, 22, 9554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Xie, W.; Chen, C.; Ren, D.; Hu, J.; Zhu, L.; Zhang, G.; Gao, Z.; Guo, L. OsMORF9 is necessary for chloroplast development and seedling survival in rice. Plant Sci. 2021, 307, 110907. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Lee, H.J.; Yu, J.; Bae, S.; Cho, Y.-G.; Kang, K.K. Transcriptomic and physiological analysis of OsCAO1 knockout lines using the CRISPR/Cas9 system in rice. Plant Cell Rep. 2021, 40, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Caddell, D.; Langenfeld, N.J.; Eckels, M.J.; Zhen, S.; Klaras, R.; Mishra, L.; Bugbee, B.; Coleman-Derr, D. Photosynthesis in rice is increased by CRISPR/Cas9-mediated transformation of two truncated light-harvesting antenna. Front. Plant Sci. 2023, 14, 1050483. [Google Scholar] [CrossRef]
- Rathnasamy, S.A.; Kambale, R.; Elangovan, A.; Mohanavel, W.; Shanmugavel, P.; Ramasamy, G.; Alagarsamy, S.; Marimuthu, R.; Rajagopalan, V.R.; Manickam, S. Altering Stomatal Density for Manipulating Transpiration and Photosynthetic Traits in Rice through CRISPR/Cas9 Mutagenesis. Curr. Issues Mol. Biol. 2023, 45, 3801–3814. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lu, J.; Yang, X.; Huang, L.-C.; Zhang, C.-Q.; Liu, Q.-Q.; Li, Q.-F. Gene editing of non-coding regulatory DNA and its application in crop improvement. J. Exp. Bot. 2023, 74, 6158–6175. [Google Scholar] [CrossRef]
- Basak, J.; Nithin, C. Targeting non-coding RNAs in plants with the CRISPR-Cas technology is a challenge yet worth accepting. Front. Plant Sci. 2015, 6, 1001. [Google Scholar] [CrossRef]
- Wu, B.; Luo, H.; Chen, Z.; Amin, B.; Yang, M.; Li, Z.; Wu, S.; Salmen, S.H.; Alharbi, S.A.; Fang, Z. Rice Promoter Editing: An Efficient Genetic Improvement Strategy. Rice 2024, 17, 55. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Wu, C.; Bi, J.; Chen, Y.; Jiang, C.; Cui, M.; Chen, Y.; Hou, X.; Yuan, M. Fine-tuning OsCPK18/OsCPK4 activity via genome editing of phosphorylation motif improves rice yield and immunity. Plant Biotechnol. J. 2022, 20, 2258–2271. [Google Scholar] [CrossRef]
- Dong, S.; Dong, X.; Han, X.; Zhang, F.; Zhu, Y.; Xin, X.; Wang, Y.; Hu, Y.; Yuan, D.; Wang, J. OsPDCD5 negatively regulates plant architecture and grain yield in rice. Proc. Natl. Acad. Sci. USA 2021, 118, e2018799118. [Google Scholar] [CrossRef]
- Usman, B.; Nawaz, G.; Zhao, N.; Liao, S.; Qin, B.; Liu, F.; Liu, Y.; Li, R. Programmed editing of rice (Oryza sativa L.) OsSPL16 gene using CRISPR/Cas9 improves grain yield by modulating the expression of pyruvate enzymes and cell cycle proteins. Int. J. Mol. Sci. 2020, 22, 249. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Luo, Y.; Wei, G.; Zong, W.; Zeng, W.; Xiao, D.; Zhang, H.; Song, Y.; Hao, Y.; Sun, K. Improving yield-related traits by editing the promoter of the heading date gene Ehd1 in rice. Theor. Appl. Genet. 2023, 136, 239. [Google Scholar] [CrossRef] [PubMed]
- Usman, B.; Nawaz, G.; Zhao, N.; Liao, S.; Liu, Y.; Li, R. Precise editing of the OsPYL9 gene by RNA-guided Cas9 nuclease confers enhanced drought tolerance and grain yield in rice (Oryza sativa L.) by regulating circadian rhythm and abiotic stress responsive proteins. Int. J. Mol. Sci. 2020, 21, 7854. [Google Scholar] [CrossRef]
- Song, X.; Meng, X.; Guo, H.; Cheng, Q.; Jing, Y.; Chen, M.; Liu, G.; Wang, B.; Wang, Y.; Li, J. Targeting a gene regulatory element enhances rice grain yield by decoupling panicle number and size. Nat. Biotechnol. 2022, 40, 1403–1411. [Google Scholar] [CrossRef]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Wu, X.; Scott, D.A.; Kriz, A.J.; Chiu, A.C.; Hsu, P.D.; Dadon, D.B.; Cheng, A.W.; Trevino, A.E.; Konermann, S.; Chen, S. Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat. Biotechnol. 2014, 32, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Yip, B.H. Recent advances in CRISPR/Cas9 delivery strategies. Biomolecules 2020, 10, 839. [Google Scholar] [CrossRef]
- González Castro, N.; Bjelic, J.; Malhotra, G.; Huang, C.; Alsaffar, S.H. Comparison of the feasibility, efficiency, and safety of genome editing technologies. Int. J. Mol. Sci. 2021, 22, 10355. [Google Scholar] [CrossRef]
- Hu, J.H.; Miller, S.M.; Geurts, M.H.; Tang, W.; Chen, L.; Sun, N.; Zeina, C.M.; Gao, X.; Rees, H.A.; Lin, Z. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 2018, 556, 57–63. [Google Scholar] [CrossRef]
- Kim, N.; Kim, H.K.; Lee, S.; Seo, J.H.; Choi, J.W.; Park, J.; Min, S.; Yoon, S.; Cho, S.-R.; Kim, H.H. Prediction of the sequence-specific cleavage activity of Cas9 variants. Nat. Biotechnol. 2020, 38, 1328–1336. [Google Scholar] [CrossRef]
- Walton, R.T.; Christie, K.A.; Whittaker, M.N.; Kleinstiver, B.P. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science 2020, 368, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Kancharla, N.; Javalkote, V.S.; Dasgupta, S.; Brutnell, T.P. CRISPR-Cas12a (Cpf1): A versatile tool in the plant genome editing tool box for agricultural advancement. Front. Plant Sci. 2020, 11, 584151. [Google Scholar] [CrossRef]
- Liu, S.; Sretenovic, S.; Fan, T.; Cheng, Y.; Li, G.; Qi, A.; Tang, X.; Xu, Y.; Guo, W.; Zhong, Z. Hypercompact CRISPR–Cas12j2 (CasΦ) enables genome editing, gene activation, and epigenome editing in plants. Plant Commun. 2022, 3, 100453. [Google Scholar] [CrossRef] [PubMed]
- Kavuri, N.R.; Ramasamy, M.; Qi, Y.; Mandadi, K. Applications of CRISPR/Cas13-based RNA editing in plants. Cells 2022, 11, 2665. [Google Scholar] [CrossRef]
- Harrington, L.B.; Burstein, D.; Chen, J.S.; Paez-Espino, D.; Ma, E.; Witte, I.P.; Cofsky, J.C.; Kyrpides, N.C.; Banfield, J.F.; Doudna, J.A. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science 2018, 362, 839–842. [Google Scholar] [CrossRef]
- Hess, G.T.; Tycko, J.; Yao, D.; Bassik, M.C. Methods and applications of CRISPR-mediated base editing in eukaryotic genomes. Mol. Cell 2017, 68, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Zhang, Y.; Abdullah, M.; Ma, Q.; Wang, H.; Zhang, P. Current status, challenges, and future prospects of plant genome editing in China. Plant Biotechnol. Rep. 2019, 13, 459–472. [Google Scholar] [CrossRef]
- Hua, K.; Tao, X.; Yuan, F.; Wang, D.; Zhu, J.-K. Precise A· T to G· C base editing in the rice genome. Mol. Plant 2018, 11, 627–630. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Lang, Z.; Botella, J.R.; Zhu, J.-K. Genome editing—Principles and applications for functional genomics research and crop improvement. Crit. Rev. Plant Sci. 2017, 36, 291–309. [Google Scholar] [CrossRef]
- Zhou, J.; Xin, X.; He, Y.; Chen, H.; Li, Q.; Tang, X.; Zhong, Z.; Deng, K.; Zheng, X.; Akher, S.A. Multiplex QTL editing of grain-related genes improves yield in elite rice varieties. Plant Cell Rep. 2019, 38, 475–485. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Fang, H.; Roberts, N.; Zhang, L.; Vakulskas, C.A.; Niedz, R.P.; Culver, J.N.; Qi, Y. Highly efficient genome editing in plant protoplasts by ribonucleoprotein delivery of CRISPR-Cas12a nucleases. Front. Genome Ed. 2022, 4, 780238. [Google Scholar] [CrossRef] [PubMed]
- Banakar, R.; Schubert, M.; Kurgan, G.; Rai, K.M.; Beaudoin, S.F.; Collingwood, M.A.; Vakulskas, C.A.; Wang, K.; Zhang, F. Efficiency, specificity and temperature sensitivity of Cas9 and Cas12a RNPs for DNA-free genome editing in plants. Front. Genome Ed. 2022, 3, 760820. [Google Scholar] [CrossRef] [PubMed]
- Banakar, R.; Schubert, M.; Collingwood, M.; Vakulskas, C.; Eggenberger, A.L.; Wang, K. Comparison of CRISPR-Cas9/Cas12a ribonucleoprotein complexes for genome editing efficiency in the rice phytoene desaturase (OsPDS) gene. Rice 2020, 13, 4. [Google Scholar] [CrossRef]
- Yin, X.; Biswal, A.K.; Dionora, J.; Perdigon, K.M.; Balahadia, C.P.; Mazumdar, S.; Chater, C.; Lin, H.-C.; Coe, R.A.; Kretzschmar, T. CRISPR-Cas9 and CRISPR-Cpf1 mediated targeting of a stomatal developmental gene EPFL9 in rice. Plant Cell Rep. 2017, 36, 745–757. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, Y.; You, Q.; Tang, X.; Ren, Q.; Liu, S.; Yang, L.; Wang, Y.; Liu, X.; Liu, B. Plant genome editing using FnCpf1 and LbCpf1 nucleases at redefined and altered PAM sites. Mol. Plant 2018, 11, 999–1002. [Google Scholar] [CrossRef]
- Tang, X.; Lowder, L.G.; Zhang, T.; Malzahn, A.A.; Zheng, X.; Voytas, D.F.; Zhong, Z.; Chen, Y.; Ren, Q.; Li, Q. A CRISPR–Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants 2017, 3, 17018. [Google Scholar] [CrossRef]
- Malzahn, A.A.; Tang, X.; Lee, K.; Ren, Q.; Sretenovic, S.; Zhang, Y.; Chen, H.; Kang, M.; Bao, Y.; Zheng, X. Application of CRISPR-Cas12a temperature sensitivity for improved genome editing in rice, maize, and Arabidopsis. BMC Biol. 2019, 17, 9. [Google Scholar] [CrossRef]
- Xu, R.; Qin, R.; Li, H.; Li, J.; Yang, J.; Wei, P. Enhanced genome editing in rice using single transcript unit CRISPR-LbCpf1 systems. Plant Biotechnol. J. 2019, 17, 553. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Sretenovic, S.; Ren, Q.; Jia, X.; Li, M.; Fan, T.; Yin, D.; Xiang, S.; Guo, Y.; Liu, L. Plant prime editors enable precise gene editing in rice cells. Mol. Plant 2020, 13, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, J.-K. Precise editing of a target base in the rice genome using a modified CRISPR/Cas9 system. Mol. Plant 2017, 10, 523–525. [Google Scholar] [CrossRef]
- Shimatani, Z.; Kashojiya, S.; Takayama, M.; Terada, R.; Arazoe, T.; Ishii, H.; Teramura, H.; Yamamoto, T.; Komatsu, H.; Miura, K. Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 441–443. [Google Scholar] [CrossRef]
- Hao, L.; Ruiying, Q.; Xiaoshuang, L.; Shengxiang, L.; Rongfang, X.; Jianbo, Y.; Pengcheng, W. CRISPR/Cas9-mediated adenine base editing in rice genome. Rice Sci. 2019, 26, 125–128. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Liu, Y.; Yang, B.; Wang, X.; Wei, J.; Lu, Z.; Zhang, Y.; Wu, J.; Huang, X. Base editing with a Cpf1–cytidine deaminase fusion. Nat. Biotechnol. 2018, 36, 324–327. [Google Scholar] [CrossRef]
- Butt, H.; Rao, G.S.; Sedeek, K.; Aman, R.; Kamel, R.; Mahfouz, M. Engineering herbicide resistance via prime editing in rice. Plant Biotechnol. J. 2020, 18, 2370. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zong, Y.; Xue, C.; Wang, S.; Jin, S.; Zhu, Z.; Wang, Y.; Anzalone, A.V.; Raguram, A.; Doman, J.L. Prime genome editing in rice and wheat. Nat. Biotechnol. 2020, 38, 582–585. [Google Scholar] [CrossRef]
- Gupta, A.; Liu, B.; Raza, S.; Chen, Q.-J.; Yang, B. Modularly assembled multiplex prime editors for simultaneous editing of agronomically important genes in rice. Plant Commun. 2024, 5, 100741. [Google Scholar] [CrossRef]
- Li, C.; Liu, B.; Dong, H.; Yang, B. Enhancing resistance to bacterial blight in rice using CRISPR-based base editing technology. Crop J. 2024, in press. [CrossRef]
- Li, Y.; Li, S.; Li, C.; Zhang, C.; Yan, L.; Li, J.; He, Y.; Guo, Y.; Xia, L. Fusion of a rice endogenous N-methylpurine DNA glycosylase to a plant adenine base transition editor ABE8e enables A-to-K base editing in rice plants. aBIOTECH 2024, 5, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Su, F.; Chen, F.; Yan, C.; Xia, D.; Sun, H.; Li, S.; Duan, Z.; Ma, C.; Zhang, H. Genome editing in rice using CRISPR/Cas12i3. Plant Biotechnol. J. 2024, 22, 379–385. [Google Scholar] [CrossRef]
- Zhong, Z.; Fan, T.; He, Y.; Liu, S.; Zheng, X.; Xu, Y.; Ren, J.; Yuan, H.; Xu, Z.; Zhang, Y. An improved plant prime editor for efficient generation of multiple-nucleotide variations and structural variations in rice. Plant Commun. 2024, 5, 100976. [Google Scholar] [CrossRef]
- Zhao, H.; Wolt, J.D. Risk associated with off-target plant genome editing and methods for its limitation. Emerg. Top. Life Sci. 2017, 1, 231–240. [Google Scholar] [PubMed]
- Brazelton, V.A., Jr.; Zarecor, S.; Wright, D.A.; Wang, Y.; Liu, J.; Chen, K.; Yang, B.; Lawrence-Dill, C.J. A quick guide to CRISPR sgRNA design tools. GM Crops Food 2015, 6, 266–276. [Google Scholar] [CrossRef]
- Lei, Y.; Lu, L.; Liu, H.-Y.; Li, S.; Xing, F.; Chen, L.-L. CRISPR-P: A web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant 2014, 7, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Kadam, U.S.; Shelake, R.M.; Chavhan, R.L.; Suprasanna, P. Concerns regarding ‘off-target’activity of genome editing endonucleases. Plant Physiol. Biochem. 2018, 131, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Qin, R.; Li, H.; Li, D.; Li, L.; Wei, P.; Yang, J. Generation of targeted mutant rice using a CRISPR-Cpf1 system. Plant Biotechnol. J. 2017, 15, 713–717. [Google Scholar] [CrossRef]
- Osakabe, Y.; Watanabe, T.; Sugano, S.S.; Ueta, R.; Ishihara, R.; Shinozaki, K.; Osakabe, K. Optimization of CRISPR/Cas9 genome editing to modify abiotic stress responses in plants. Sci. Rep. 2016, 6, 26685. [Google Scholar] [CrossRef]
- Anders, C.; Niewoehner, O.; Duerst, A.; Jinek, M. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature 2014, 513, 569–573. [Google Scholar] [CrossRef]
- Kaya, H.; Mikami, M.; Endo, A.; Endo, M.; Toki, S. Highly specific targeted mutagenesis in plants using Staphylococcus aureus Cas9. Sci. Rep. 2016, 6, 26871. [Google Scholar] [CrossRef] [PubMed]
- Kleinstiver, B.P.; Prew, M.S.; Tsai, S.Q.; Topkar, V.V.; Nguyen, N.T.; Zheng, Z.; Gonzales, A.P.; Li, Z.; Peterson, R.T.; Yeh, J.-R.J. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature 2015, 523, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, C.; Fu, Y.; Liu, Q.; Jiao, X.; Wang, K. Expanding the range of CRISPR/Cas9 genome editing in rice. Mol. Plant 2016, 9, 943–945. [Google Scholar] [CrossRef]
- Hu, X.; Meng, X.; Liu, Q.; Li, J.; Wang, K. Increasing the efficiency of CRISPR-Cas9-VQR precise genome editing in rice. Plant Biotechnol. J. 2018, 16, 292–297. [Google Scholar] [CrossRef]
- Meng, X.; Hu, X.; Liu, Q.; Song, X.; Gao, C.; Li, J.; Wang, K. Robust genome editing of CRISPR-Cas9 at NAG PAMs in rice. Sci. China. Life Sci. 2018, 61, 122–125. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Wang, F.; Zhao, S.; Song, J.; Feng, F.; Zhao, J.; Yang, J. Expanding base editing scope to near-PAMless with engineered CRISPR/Cas9 variants in plants. Mol. Plant 2021, 14, 191–194. [Google Scholar] [CrossRef] [PubMed]
- El-Mounadi, K.; Morales-Floriano, M.L.; Garcia-Ruiz, H. Principles, applications, and biosafety of plant genome editing using CRISPR-Cas9. Front. Plant Sci. 2020, 11, 56. [Google Scholar] [CrossRef]
- Pan, C.; Sretenovic, S.; Qi, Y. CRISPR/dCas-mediated transcriptional and epigenetic regulation in plants. Curr. Opin. Plant Biol. 2021, 60, 101980. [Google Scholar] [CrossRef]
- Xu, L.; Sun, B.; Liu, S.; Gao, X.; Zhou, H.; Li, F.; Li, Y. The evaluation of active transcriptional repressor domain for CRISPRi in plants. Gene 2023, 851, 146967. [Google Scholar] [CrossRef]
- Tripathi, L.; Ntui, V.O.; Tripathi, J.N.; Norman, D.; Crawford, J. A new and novel high-fidelity genome editing tool for banana using Cas-CLOVER. Plant Biotechnol. J. 2023, 21, 1731. [Google Scholar] [CrossRef]
- Tripathi, L.; Ntui, V.O.; Tripathi, J.N. Application of CRISPR/Cas-based gene-editing for developing better banana. Front. Bioeng. Biotechnol. 2024, 12, 1395772. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Lebbink, J.H.; Kanaar, R.; Geijsen, N.; Van Der Oost, J. Genome editing by natural and engineered CRISPR-associated nucleases. Nat. Chem. Biol. 2018, 14, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Demorest, Z.L.; Coffman, A.; Baltes, N.J.; Stoddard, T.J.; Clasen, B.M.; Luo, S.; Retterath, A.; Yabandith, A.; Gamo, M.E.; Bissen, J. Direct stacking of sequence-specific nuclease-induced mutations to produce high oleic and low linolenic soybean oil. BMC Plant Biol. 2016, 16, 225. [Google Scholar] [CrossRef] [PubMed]
| Gene | Coding Product/Protein | Modification | Pathway | Phenotype | Reference |
|---|---|---|---|---|---|
| Gn1a | Cytokinin oxidase/dehydrogenase | Gene disruption | Cytokinin biosynthesis | Increased panicle size and flower number per panicle | [17,18] |
| GSN1 |
OsMPK1 (Mitogen-activated protein kinase (MAPK) phosphatase enzyme) | Gene disruption | MAPK signaling pathway | Denser panicles and smaller grains | [21] |
| FON4 | Receptor-like kinase | Gene Knockout | CLV pathway | Increased spikelet number per panicle | [23,29] |
| FZP | AP2/ERF transcription factor | Gene disruption | Different phytohormone-mediated signaling pathways | Increased grain numbers | [26] |
| DEP1 | G-protein γ subunit | Gene disruption | G-protein signaling pathway | Dense erect panicle and increased grain number and density | [18,28] |
| LARGE2 | HECT-domain E3 ubiquitin ligase OsUPL2 | Gene disruption | Functions with APO1 and APO2 (positively regulates grain number and panicle number) | Large panicles with increased grain number | [30] |
| Gene | Coding Product/Protein | Modification | Pathway | Phenotype | Reference |
|---|---|---|---|---|---|
| GS3 | G protein γ subunit | Gene disruption | G-protein signaling | Increased grain size and quality | [18,37,38,43] |
| GW2 (GRAIN WIDTH and WEIGHT2) | RING-type E3 ubiquitin ligase | Gene knockout | Ubiquitin-proteasome pathway | Improved grain filling and larger grain architecture | [44] |
| GW5 | Calmodulin binding protein | Gene knockout | BR signaling | Increased grain width and weight | [40] |
| GW5L | Calmodulin binding protein | Gene knockout | BR signaling | Increased grain width and weight | [41] |
| qTGW3 | OsSK41/OsGSK5 | Gene disruption | Auxin signaling | Larger grain size | [42] |
| OsMKK3 | Mitogen-Activated Protein Kinase Kinase 3 | Gene knockout * | MAPK signaling | Decreases grain length | [45] |
| FZP | ERF domain | Gene knockout * | Ethylene biosynthesis | Smaller grains and degenerated sterile lemmas | [46] |
| MIR396e and MIR396f | Transcription factor | Gene disruption | GA biosynthesis | Increased grain size and altered plant architecture | [47] |
| OsWRKY53 | Transcription factor | Gene disruption * | BA signaling and MAPK cascades | Smaller grains | [48] |
| OsNDB2 | Type II NADPH dehydrogenase | Gene knockout | Alternative respiratory pathway in mitochondria | Increased grain size and 1000-grain weight | [49] |
| OsSPL4 | SQUAMOSA PROMOTER BINDING PROTEIN-LIKEs | Gene disruption | Transcription factor | Increased grain number and size | [3,50,51] |
| GL6 | Plant AT-rich sequence- and zinc-binding (PLATZ) transcription factor | Gene disruption | RNA polymerase III transcription machinery | Short grains with increase in number | [52] |
| GL10 | MADS56 | Gene knockout * | Gibberellic acid (GA) signaling pathway | Shorter grain length, lower grain weight and delayed flowering | [53] |
| POW1 (PUT ON WEIGHT 1) | Homeodomain-like protein | Gene disruption | BR pathway | Increased grain size and leaf angle | [54] |
| OsINV3 and OsINV2 | Invertase | Gene knockout * | Sucrose metabolism | Smaller grain size | [55] |
| GW10 | Cytochrome P450 subfamily 89A2 homology protein | Gene knockout | BR pathway | Increased grain number with smaller grains | [56] |
| OsAGO17 | Argonaute (AGO) protein—component of the RNA-induced silencing complex (RISC) | Gene knockout * | sRNA pathway | Decreased grain size and weight | [57] |
| FLR1, FLR2, FLR8 FLR15 | FERONIA-like receptor protein kinases (FER) | Gene disruption Gene disruption * | FER pathway | Larger grain size Smaller grain size | [58] |
| GW6 (GRAIN WIDTH 6) | GA-regulated GAST family protein | Gene knockout * | Gibberellin pathway | Reduced grain size and weight | [59] |
| GSW3 (GRAIN SIZE AND WEIGHT 3) | GTPase-regulated protein | Gene knockout | Gibberellin pathway | Increased grain length, width and 1,000-grain weight | [60] |
| qTGW12a | Multidrug and toxic compound extrusion (MATE) transporter | Gene knockout * | Various regulatory pathways | Reduction in grain weight | [61] |
| GW9 | Nucleus-localized protein containing both C2H2 zinc finger (C2H2-ZnF) and VRN2-EMF2-FIS2-SUZ12 (VEFS) domains | Gene knockout/disruption | GW2 ubiquitination pathway | Large grains with increased plant height | [62] |
| OsPUB3 | U-box E3 ubiquitin ligase | Gene knockout * | Ubiquitin-proteasome pathway | Decreased grain weight and size | [63] |
| Gene | Coding Product/Protein | Modification | Pathway | Phenotype | References |
|---|---|---|---|---|---|
| MOC1 | GRAS family transcription factor | Gene knockout * | Transcription factor | Reduced tillering | [66] |
| MOC3/ TAB1/SRT1 | Homeobox domain -containing protein | Gene knockout * | Transcription factor | Reduced tillering | [66] |
| OsFWL4 | Cysteine-rich protein | Gene disruption | Transcription factor | Increased tiller number and grain length | [68] |
| OsAAP3 | Amino acid permeases | Gene knockout | Amino acid transport (regulate the concentrations of amino acids) | Increased tiller number | [69,70] |
| OsNPF7.1 and OsNPF7.4 | Nitrate and di/tripeptide transporter | Gene knockout (differential expression in the presence of nitrogen) | Nitrogen transport | Plant architecture, NUE and tiller number | [71] |
| OsPIN5b | Endoplasmic reticulum localized protein | Gene knockout | Auxin balance and transport | Longer balance and transport tiller numbers | [37] |
| IDEAL PLANT ARCHITECTURE1 (IPA1)/ OsSPL14 | Squamosa promoter binding protein | Gene disruption | Strigolactone signaling pathway | Tiller number varied according to the changes induced in the OsmiR156 target region | [18,72] |
| CCD7 (CAROTENOID CLEAVAGE DIOXYGENASE 7) | Carotenoid cleavage dioxygenase | Gene disruption, gene knockout | Strigolactone biosynthesis | Increased tillering and reduced height | [73,74] |
| TB1 (TEOSINTE BRANCHED1) | TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTORS (TCP) family transcription factor | Gene knockout | Strigolactone signaling pathway | Dwarf phenotype with increased tiller numbers | [75] |
| EHD1 | B-type response regulator | Gene disruption | Flowering pathway | Enhanced yield and improved grain quality | [76] |
| Trait | Gene | Gene Function/Coding Product | Improved Phenotype | Reference |
|---|---|---|---|---|
| Biotic stress tolerance/resistance | OsSWEET13 | Sucrose transporter gene | Bacterial blight disease resistance | [84] |
| OsERF922 | Ethylene responsive factors | Increased resistance to blast disease | [85] | |
| OsSWEET11, OsSWEET13, OsSWEET14 | Sucrose transporter genes | Broad-spectrum resistance to bacterial blight | [86] | |
| ALB1, RSY1 | Melanin biosynthetic polyketide synthase | Rice blast resistance | [87] | |
| Xa13 | Recessive resistant allele of Os8N3, a member of the NODULIN3 (N3) gene family | Bacterial blight disease resistance | [88] | |
| eIF4G | Translation initiation factor 4 gamma gene | Resistance to Rice Tungro Spherical Virus (RTSV) | [89] | |
| OsCPR5.1 | Nucleoporin | Resistance to Rice Yellow Mottle Virus (RYMV) | [90] | |
| Abiotic stress tolerance/resistance | OsERA1 | β-subunit of farnesyltransferase | Drought tolerance | [91] |
| OsDST (Drought and salt tolerance) | DST protein | Drought and salt tolerance | [92] | |
| OsNAC006 | NAC transcription factor | Heat tolerance | [93] | |
| OsPRP1 | Proline-rich protein | Cold tolerance | [94] | |
| Herbicide resistance | OsALS | Acetolactate synthase | Significant tolerance to herbicides | [95] |
| OsEPSPS | 5-enolpyruvylshikimate-3-phosphate synthase | Resistance to glyphosate | [96] |
| Gene | Coding Product/Protein | Modification | Function | Phenotype | Reference |
|---|---|---|---|---|---|
| AtAAP2 homolog of rice AtCRF4 homolog of rice | Amino acid permease Transcription factor | Gene knockout | N transportation N uptake in roots | Shorter plants, increased panicle numbers, and dry biomass weight | [106] |
| LW5/D1 | α subunit of G-protein | Gene disruption | N uptake and transport | Affect plant architecture and grain size by regulating N-transfer | [107] |
| OsHHO3 | NIGT1 family protein | Gene knockout | Nitrate signaling | Enhanced growth and increased shoot and root dry mass | [105] |
| Gene | Coding Product/ Protein | Modification | Pathway/Function | Phenotype | References |
|---|---|---|---|---|---|
| OsHXK1 | Hexokinase | Gene knockout | Different phytohormone-mediated signaling pathways | High photosynthetic efficiency and yield | [112] |
| OsPPR9 | DYW-PPR (Pentatricopeptide repeat) | Gene disruption * | RNA editing | Affects chloroplast growth and development | [111] |
| OsMORF9 | MORFs (multiple organellar RNA editing factors) | Gene knockout * | RNA editing | Biogenesis of chloroplast ribosomes and chloroplast development | [113] |
| OsCAO1 | Chlorophyllide a oxygenase | Gene knockout * | Chlorophyll degradation and ROS scavenging | Degradation and ROS senescence | [114] |
| CpSRP43, CpSRP54a, CpSRP54b | CpSRP (signal recognition particle) | Gene knockout | CpSRP pathway | Increased photosynthesis per photon absorbed | [115] |
| OsEPF1 | Epidermal patterning factor | Gene knockout | Stomatal development and patterning | Enhanced the stomatal conductance and photosynthetic efficiency | [116] |
| Gene | Modification | Improved Phenotype | Reference |
|---|---|---|---|
| OsCPK18/OsCPK4 | Modification of the phosphorylation motif of OsCPK18/OsCPK4 | Improved disease resistance and yield | [120] |
| OsPDCD5 | Improved plant architecture (plant height, panicle type, grain shape) | Enhanced yield | [121] |
| OsSPL16 | Upregulation of pyruvate enzymes and cell cycle enzymes | Larger grain size and increased yield | [122] |
| (Ehd1) Early heading date 1 | Modification of promoter at multiple sites | Delayed heading date and improved yield-related traits | [123] |
| OsPYL9 (Pyrabactin resistance 1-like 9) | Upregulation of circadian rhythm and abiotic stress-responsive proteins | Increased yield and drought tolerance | [124] |
| FZP | Deletion of −157 to −45 bp in UTR (untranslated region) upstream of FZP 5′ | Grain number | [26] |
| IPA1/OsSPL14 | Deletion of An-1 (transcription factor) binding site of IPA1 (54-base pair cis-regulatory region) | Balances the trade-off between grains per panicle and tiller numbers, leading to increased grain yield | [125] |
| Cas Protein | Host | Target | PAM Site | Cut Type | Unique Features | Reference |
|---|---|---|---|---|---|---|
| Cas9 | Streptococcus pyogenes | Double-strand DNA (dsDNA) | NGG | DSB | Versatile, suitable for multiplexing | [133] |
| Cas12a (Cpf1) | Prevotella and Francisella 1 | dsDNA | TTTN (AT-rich region) | Staggered cut | Staggers DSBs and promotes HDR mechanism | [134] |
| Cas12j2 (CasΦ) | Huge phages | dsDNA | NTTV | Staggered cut | Small and compact | [135] |
| Cas13a | Leptotrichia shahii | Single-strand RNA | None | RNA cleavage | RNA targeting | [136] |
| Cas14 | Uncultivated Archaea | Single-strand DNA (ssDNA) | None | ssDNA cleavage | Small and specific for ssDNA | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiruppathi, A.; Salunkhe, S.R.; Ramasamy, S.P.; Palaniswamy, R.; Rajagopalan, V.R.; Rathnasamy, S.A.; Alagarswamy, S.; Swaminathan, M.; Manickam, S.; Muthurajan, R. Unleashing the Potential of CRISPR/Cas9 Genome Editing for Yield-Related Traits in Rice. Plants 2024, 13, 2972. https://doi.org/10.3390/plants13212972
Thiruppathi A, Salunkhe SR, Ramasamy SP, Palaniswamy R, Rajagopalan VR, Rathnasamy SA, Alagarswamy S, Swaminathan M, Manickam S, Muthurajan R. Unleashing the Potential of CRISPR/Cas9 Genome Editing for Yield-Related Traits in Rice. Plants. 2024; 13(21):2972. https://doi.org/10.3390/plants13212972
Chicago/Turabian StyleThiruppathi, Archana, Shubham Rajaram Salunkhe, Shobica Priya Ramasamy, Rakshana Palaniswamy, Veera Ranjani Rajagopalan, Sakthi Ambothi Rathnasamy, Senthil Alagarswamy, Manonmani Swaminathan, Sudha Manickam, and Raveendran Muthurajan. 2024. "Unleashing the Potential of CRISPR/Cas9 Genome Editing for Yield-Related Traits in Rice" Plants 13, no. 21: 2972. https://doi.org/10.3390/plants13212972
APA StyleThiruppathi, A., Salunkhe, S. R., Ramasamy, S. P., Palaniswamy, R., Rajagopalan, V. R., Rathnasamy, S. A., Alagarswamy, S., Swaminathan, M., Manickam, S., & Muthurajan, R. (2024). Unleashing the Potential of CRISPR/Cas9 Genome Editing for Yield-Related Traits in Rice. Plants, 13(21), 2972. https://doi.org/10.3390/plants13212972





