Abstract
Caffeoyl coenzyme A-O-methyltransferase (CCoAOMT) has a critical function in the lignin biosynthesis pathway. However, its functions in cotton are not clear. In this research, we observed 50 CCoAOMT genes from four cotton species, including two diploids (Gossypium arboretum, 9, and Gossypium raimondii, 8) and two tetraploids (Gossypium hirsutum, 16, and Gossypium barbadense, 17), performed bioinformatic analysis, and focused on the involvement and functions of GhCCoAOMT7 in lignin synthesis of Gossypium hirsutum. CCoAOMT proteins were divided into four subgroups based on the phylogenetic tree analysis. Motif analysis revealed that all CCoAOMT proteins possess conserved Methyltransf_3 domains, and conserved structural features were identified based on the genes’ exon-intron organization. A synteny analysis suggested that segmental duplications were the primary cause in the expansion of the CCoAOMT genes family. Transcriptomic data analysis of GhCCoAOMTs revealed that GhCCoAOMT2, GhCCoAOMT7, and GhCCoAOMT14 were highly expressed in stems. Subcellular localization experiments of GhCCoAOMT2, GhCCoAOMT7, and GhCCoAOMT14 showed that GhCCoAOMT2, GhCCoAOMT7, and GhCCoAOMT14 were localized in the nucleus and plasma membrane. However, there are no cis-regulatory elements related to lignin synthesis in the GhCCoAOMT7 gene promoter. GhCCoAOMT7 expression was inhibited by virus-induced gene silencing technology to obtain gene silencing lines, the suppression of GhCCoAOMT7 expression resulted in a 56% reduction in the lignin content in cotton stems, and the phloroglucinol staining area corresponding to the xylem was significantly decreased, indicating that GhCCoAOMT7 positively regulates lignin synthesis. Our results provided fundamental information regarding CCoAOMTs and highlighted their potential functions in cotton lignin biosynthesis and lignification.
1. Introduction
Lignin, a significant polymeric organic compound in plants, is the second most abundant organic compound after cellulose and a primary constituent of plant cell walls. Its multifaceted contributions to plant growth and development encompass the maintenance of cell wall integrity [1], augmentation of mechanical strength [2], stress resistance [3], heightened pathogen resistance [4], and facilitation of water transport [5,6]. Lignin’s economic significance in agriculture, industry, and commercial applications is substantial. In agriculture, it aids in soil improvement, pesticide substitution, and plant growth promotion, enhancing crop yield and quality. In industry, lignin’s applications are extensive, playing a crucial role in paper manufacturing, fuel and pigment production, and biofuel sectors. In commercial applications, due to its antioxidant and antibacterial properties, lignin is also utilized in the pharmaceutical and food industries. In summary, lignin is not only a vital component of ecological cycles but also provides sustainable raw materials for multiple industries. The main challenges to lignin production in cotton crops are environmental conditions (soil type, temperature, water availability, and stress conditions), the agronomic practices employed in cotton cultivation (fertilizer application and irrigation methods), and cotton varieties exhibit significant genetic variability affecting lignin biosynthesis pathways. The biosynthesis of lignin is a complex biochemical process that involves multiple genes and enzymes.
Lignin is a complex polymer of phenylpropanoid monomers synthesized by the polymerization of three hydroxyl cinnamyl alcohols: p-coumaryl, coniferyl, and sinapyl alcohols. Since lignin has high thermal stability and good adhesion, it can be used as a natural binder for soil improvement. It can be categorized into three types based on the different monomers of lignin: syringyl lignin (S lignin), guaiacyl lignin (G lignin) and hydroxyphenyl lignin (H lignin) [7,8]. O-methyltransferases (OMTs) play key roles in the synthesis of these lignin monomers and are categorized into caffeoyl-coenzyme A O-methyltransferases (CCoAOMTs) and caffeic acid O-methyltransferases (COMTs). While COMTs are involved in the syringyl pathway, CCoAOMTs participate in the syringyl and guaiacyl pathways [9]. COMTs, in coordination with ferulate-5-hydroxylase, catalyze the methylation of 5’-OH on the aromatic ring of lignin aldehydes and lignin alcohols.
CCoAOMTs were a class of S-adenosine-L-methionine methyltransferases. They catalyze the transfer of the methyl group of S-adenosine methionine to the benzene ring C3 position of the lignin monomer, converting caffeoyl coenzyme A to feruloyl coenzyme A, providing substrates for syringyl lignin synthesis [10,11,12]. CCoAOMT genes encode for CCoAOMT enzymes [13,14]. To date, RNAi, gene knockout, and biological stress have been successfully implemented to modulate lignin content by targeting CCoAOMT genes in various plants, affecting plant development and abiotic stress resistance. For example, RNAi was implemented to downregulate the SmCCoAOMT of Salvia miltiorrhiza, resulting in a significant decrease in lignin content, and affecting the accumulation of phenolic acids [15]. Li et al. demonstrated that lignin concentration in transgenic maize could be reduced by 22.4% through downregulation of the ZmCoA gene, similarly using RNAi. Histological staining of lignin with Wiesner reagent revealed a slightly higher staining intensity in the xylem and sclerenchyma of RNAi plants than in WT. These results have laid the foundation for the breeding of maize with reduced lignin content [16]. Moreover, a CCoAOMT gene (PrCCoAOMT), involved in the biosynthesis of G lignin in coniferous gymnosperms such as Pinus radiata, was successfully cloned. Suppression of this gene in lignin polymers consisting of p-hydroxyphenyl (H), catechyl (C), and guaiacyl (G) subunits [17]. It has been demonstrated that inhibition of AtCCoAOMT enhanced the vulnerability of Arabidopsis thaliana (A. thaliana) roots to fungal pathogens [18]. Liu et al. investigated the CCoAOMTs at the transcriptome level under abiotic stress in Panicum virgatum, and showed that PvCCoAOMT is involved in lignin biosynthesis and could be highly induced by drought and cold stresses [19]. Wei et al. found that the ABA/MeJA signaling pathway was activated in response to both biotic and abiotic stress via DfCCoAOMT genes. They demonstrated that DfCCoAOMT14 overexpression in the stem of Dendrocalamus farinosus resulted in a notable increase in lignin content, xylem thickness, and resistance to drought conditions [20]. CCoAOMTs were shown to be linked to the abiotic stress response in jute and are involved in lignin production. Such genes could potentially be utilized to genetically enhance the fiber quality of jute [21]. Liao et al. demonstrated that CsCCoAOMT1 was involved in the biosynthesis of citrus polymethoxylated flavones [22]. Moreover, it has been reported that CCoAOMT1 directly acts on the K259 residue that is conserved in bHLH010 and bHLH089, promoting the nuclear localization and function of bHLH transcription factors. This effectively inhibits the expression of downstream genes by reducing the accumulation of bHLHs transcription factors in the nucleus to facilitate pollen development [23]. Following the pioneering discoveries of CCoAOMT genes in cell suspension cultures of parsley and carrot by Kuhnl [24] and Pakusch [25], subsequent investigations into the CCoAOMT gene family have been conducted in model organisms, such as A. thaliana [26], rice [27], tobacco [28,29], wheat [30], sorghum [31], and poplar [32] with the increase in the availability of plant genomic data. Studies have demonstrated that the CCoAOMT gene family exhibits a high degree of sequence and functional conservation across plant species. For instance, research in tea and Populus has revealed the conserved role of this gene family in plant growth, development, and phenylpropanoid metabolism, suggesting that the CCoAOMT gene family has undergone stringent selective pressures during plant evolution, maintaining critical biological properties. However, there is limited information available regarding the CCoAOMT gene family in cotton, despite its significance as a cash crop.
In this study, the cotton CCoAOMT gene family was analyzed using bioinformatics approaches. Their phylogeny, physicochemical properties, gene structure, cis-elements in their promoters, chromosome distribution, and collinearity were comprehensively analyzed. Concurrently, the expression profiles of GhCCoAOMT gene family members were assessed in different tissues of cotton by employing qRT-PCR analyses, virus-induced gene silencing assays, lignin staining, in conjunction with the quantitative determination of lignin, cellulose, hemicellulose, and pectin. It was found that a reduction in GhCCoAOMT7 expression in cotton stems resulted in reduced lignin staining in the xylem of cotton stems and a decrease in lignin content, suggesting that GhCCoAOMT7 was involved in lignin biosynthesis. These findings strongly suggest the involvement of GhCCoAOMT7 in lignin biosynthesis, thereby enhancing our understanding of GhCCoAOMT7 functions and laying the foundation for future research on CCoAOMTs in cotton.
2. Results
2.1. Identification and Phylogenetic Analysis of the CCoAOMT Family Members in Cotton
In total, 16 CCoAOMT genes were identified in Gossypium hirsutum (G. hirsutum), 17 in Gossypium barbadense (G. barbadense), 9 in Gossypium arboretum (G. arboretum), and 8 in Gossypium raimondii (G. Raimondii). All these identified CCoAOMT genes were named by the chromosomal locations. The information for these identified CCoAOMT genes was summarized in Table A1, including chromosome location, isoelectric point (pI), amino acid length, molecular weight (MW), and projected subcellular localization. The pI, MW, and protein length of the projected CCoAOMT proteins varied from 11.91 to 36.2 kDa, 4.42 to 9.69, and 108 to 318 amino acids, respectively.
The phylogenetic tree, which revealed the evolutionary relationships between CCoAOMT proteins from multiple species, included a total of 71 full-length proteins from G. raimondii (8), G. arboretum (9), G. barbadense (17), G. hirsutum (16), Oryza sativa (6), N. tabacum (8), and A. thaliana (7) (Figure 1). The seventy-one CCoAOMT proteins from these seven species were clustered into four branches. Twenty-eight CCoAOMTs were clustered in group I (four GrCCoAOMTs, seven GbCCoAOMTs, three GaCCoAOMTs, seven GhCCoAOMTs, five NbCCoAOMTs, AtCCoAOMT1, and OsCCoAOMT1). Fourteen CCoAOMTs were clustered in group II, including GrCCoAOMT7, three AtCCoAOMTs, three GaCCoAOMTs, three GhCCoAOMTs, and four GbCCoAOMTs. Additionally, fourteen CCoAOMTs were clustered in group III, including two GaCCoAOMTs, two GrCCoAOMTs, four GbCCoAOMTs, four GhCCoAOMTs, AtCCoAOMT7, and NbCCoAOMT14. Fifteen in group IV, including five OsCCoAOMTs, two GbCCoAOMTs, two GhCCoAOMTs, two NtCCoAOMTs, two AtCCoAOMTs, GaCCoAOMT8, and GrCCoAOMT4.
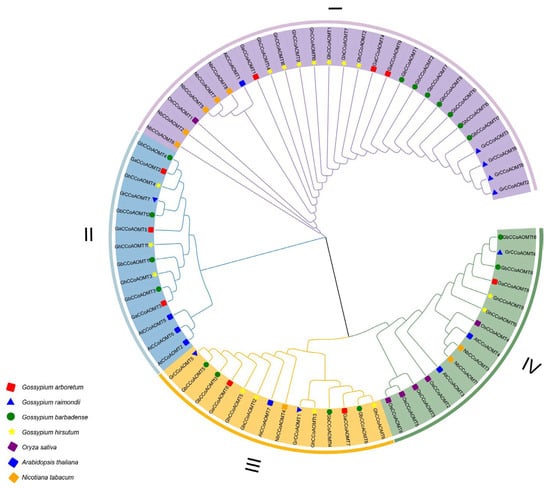
Figure 1.
Phylogenetic relationship of CCoAOMTs proteins from Gossypium hirsutum, Gossypium barbadense, Gossypium arboretum, Gossypium raimondii, Oryza sativa, Nicotiana tabacum, and Arabidopsis thaliana.
2.2. CCoAOMTs Family Motif and Gene Structure Analysis
The CCoAOMT gene family member properties were more thoroughly assessed through a combined phylogenetic tree, gene structure, and motif analysis. According to the clustering of the CCoAOMT gene family in the five examined species, the CCoAOMT gene family members in the four cotton species were divided into four groups: I, II, III, and IV (Figure 2A). As is shown in Figure 2B, all CCoAOMTs in group I contained two copies of motifs 3. The CCoAOMTs in Group II contained a single motif 6, and some CCoAOMTs included motif 10, which was absent in other genes from other groups, such as GhCCoAOMT3, GaCCoAOMT3, GbCCoAOMT3, GhCCoAOMT11, and GbCCoAOMT11. Group III included relatively similar but diverse motif structures, such as motif 5, motif 4, motif 1, motif 8, motif 3, motif 7, and motif 2, arranged in the order of most frequently present to less frequently present. Group IV members possess motif 7, which was absent in other classes. Overall, motifs 1–4 were postulated to play crucial roles in keeping the structural stability and gene functional specificity of CCoAOMT proteins. Furthermore, using the Gene Structure Display Server 2.0 to plot the gene structures (Figure 2C). The results clearly revealed that the exon numbers significantly varied between the CCoAOMT gene family members from one to nine, which may be related to splicing site mutations. We also found that all paralogs present in the same branch of the phylogenetic tree shared similar numbers of exons/introns.
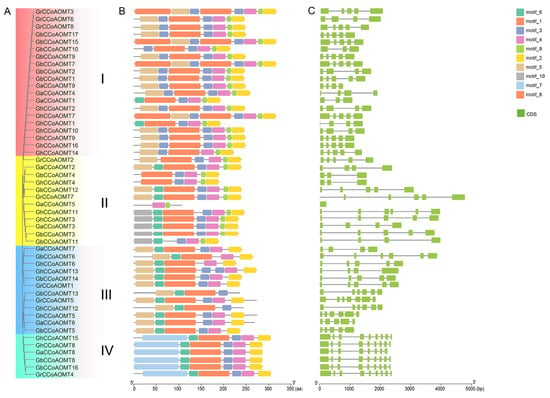
Figure 2.
Comprehensive analysis of the structural properties of the cotton CCoAOMT genes family. (A) Phylogenetic tree of the cotton CCoAOMT genes family. (B) Conserved motif of the four cotton CCoAOMT proteins. (C) Gene structure of the four cotton CCoAOMT proteins.
2.3. Chromosomal Distribution and Synteny Analysis of CCoAOMTs
The chromosomal locations of the CCoAOMTs were determined in the chromosomes of the At and Dt cotton sub-genomes (Figure A1). In G. arboretum, the nine GaCCoAOMTs were mainly distributed on seven chromosomes, namely Chr3, Chr4, Chr5, Chr6, Chr8, Chr12, and Chr13. Only Chr4 contained three GaCCoAOMTs, and the rest of the chromosomes contained only one GaCCoAOMT. In G. raimondii, six GrCCoAOMTs were distributed on six chromosomes, namely Chr4, Chr5, Chr8, Chr10, Chr12, and Chr13. In G. hirsutum, a total of 16 GhCCoAOMTs were located in the 11 chromosomes. While three GhCCoAOMTs were located in the A04 chromosome. In G. barbadense, a total of 17 GbCCoAOMTs were distributed on 11 chromosomes, with three GbCCoAOMTs located on the A04 and D04 chromosomes. On the contrary, no CCoAOMT genes are present on the rest of the chromosomes, such as Chr1, Chr2, Chr7, Chr9, and Chr11. The uneven chromosomal distribution of CCoAOMT genes indicated significant genetic diversification during the evolutionary process.
Three processes are often involved in the evolution of gene families: tandem duplication, whole genome duplication, and fragment segmental duplication [33]. Detection of gene duplication events in the genomes of upland cotton using MCScan X revealed that all 4 GaCCoAOMT gene pairs in G. arboretum, all 7 pairs of GrCCoAOMT genes in G. raimondii, all 19 pairs of GbCCoAOMT genes in G. barbadense, and all 26 pairs of GhCCoAOMT genes in upland cotton were derived from segmental duplications. This indicated that segmental duplications were the primary mode of the GhCCoAOMT gene family expansion in upland cotton (Figure 3A). In addition, a total of 39 duplicated gene pairs were identified among the 50 cotton CCoAOMT genes. Computed the Ka/Ks ratios between CCoAOMT duplicate gene pairs. The results showed that only the KA/KS ratios of GhCCoAOMT6—GaCCoAOMT7 were greater than one. Meanwhile, the Ka/Ks ratios of the other 38 CCoAOMT gene pairs investigated were below one, suggesting that purifying selection was applied to them (Table A2).
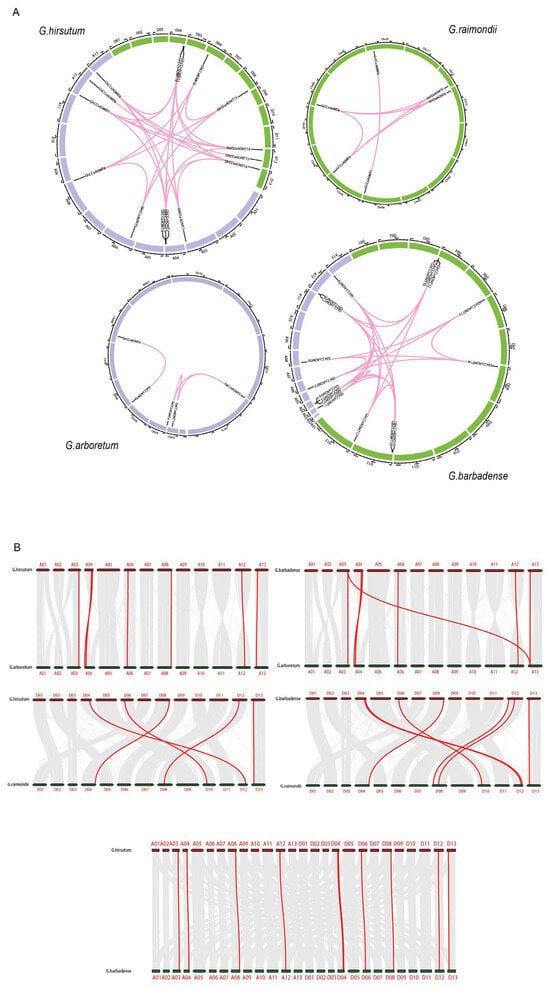
Figure 3.
Synteny analysis of the CCoAOMT genes. (A) Genomic synteny analysis of GaCCoAOMTs, GrCCoAOMTs, GhCCoAOMTs, and GbCCoAOMTs. Purple and green blocks represent the A-subgenome and D-subgenome, respectively. Numbers along each chromosome block represent the sequence lengths in megabases. Red lines indicate the duplicated CCoAOMT pairs. (B) Synteny and collinearity relationships between G. hirsutum and G. arboreum, between G. hirsutum and G. raimondii, between G. barbadense and G. arboretum, between G. barbadense and G. raimondii, and between G. hirsutum and G. barbadense. Gray lines depict the collinearity between different genomes, and red lines depict the collinearity of the CCoAOMT genes between different genomes.
Allotetraploid species, G. hirsutum and G. barbadense, were produced by the hybridization between diploid A and D genome species [34]. Thus, we performed a collinearity analysis of the A and D genomes between the G. hirsutum and G. barbadense with G. arboretum and G. raimondii (Figure 3B). A high collinearity was observed at the whole-genome level between G. barbadense and G. hirsutum with their diploid progenitors. Furthermore, seven of the nine CCoAOMT genes in the A genome of upland cotton were highly collinear with those of G. arboretum, and five of the seven genes in the D genome were highly collinear with those of G. raimondii, indicating that the other two genes (GhCCoAOMT11 and GhCCoAOMT16) were lost during the tetraploidization of G. hirsutum. Six of the nine genes in the A genome of G. barbadense were highly collinear with those of G. arboretum, and seven of the eight genes in the D genome of G. barbadense were highly collinear with those of. G. raimondii, indicating that the other three genes (GbCCoAOMT6, GbCCoAOMT11, and GbCCoAOMT15) were lost during the tetraploidization of G. barbadense. Fiftenn genes of G. hirsutum were highly collinear with those of G. barbadense, speculating that GhCCoAOMT14 was formed after the genome duplication.
2.4. Cis-Element Analysis of CCoAOMTs
Cis-elements in the promoter region upstream of a gene often control the expression of that gene. Promoters of putative CCoAOMT genes were identified and examined for the presence of cis-elements to analyze the potential involvement of CCoAOMT genes in the responses to various conditions. In this study, we examined the promoter region 1500 bp upstream of the CCoAOMT transcription start sites (ATG codon). The findings revealed that in addition to core elements such as TATA-box, O2-site, and other commonly present elements, the promoter of the CCoAOMT genes contained 17 additional cis-element types (Figure 4, Table A3). Most were light signal transduction-related elements, such as AE-Box, ACE, GATA-motif, etc. There were plant hormone-responsive elements involved in Methyl Jasmonate (TGACG-motif), auxin (AUXRR-core and TGA-element), gibberellin (TATC-box), and abscisic acid (ABRE) signal transduction and responses. Elements associated with plant growth and development, such as meristem development (CAT-box), endosperm development (GCN4-motif), and palisade cell differentiation (HD-zip). Various stress-responsive cis-elements were also discovered, such as defense and wound response (TC-rich), drought response (MBS), low-temperature response (LTR), and plant hypoxia and anaerobic response (GC-motif and ARE) elements. In addition, we found that most CCoAOMTs in group I contained the AC-I or AC-II cis-element, except for GhCCoAOMT7, GaCCoAOMT4, and GbCCoAOMT15. AC-I and AC-II are important cis-elements involved in plant lignin synthesis [35,36,37], suggesting that the lignin production may be connected to these genes.
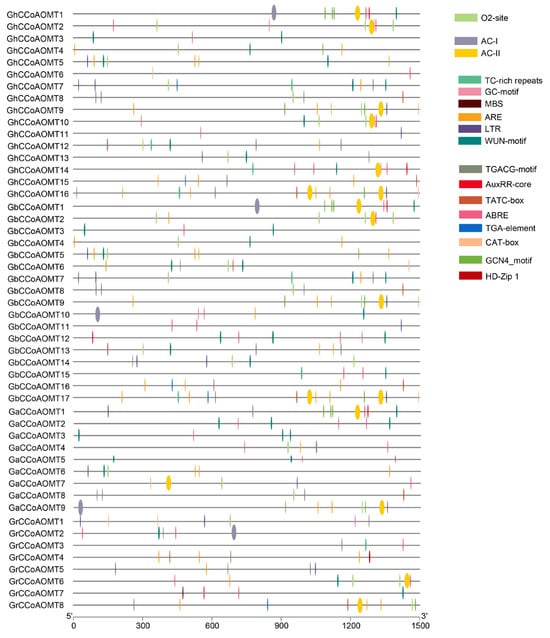
Figure 4.
Cis-elements present in the CCoAOMTs promoters.
2.5. Subcellular Localization of CCoAOMT Proteins
To further study the functions of CCoAOMT proteins, the subcellular localization of CCoAOMT family members was predicted using the CELLO v.2.5 online tool. Based on the predicted subcellular localization, twenty-nine of the CCoAOMT proteins were exclusively localized into the cytoplasm, five CCoAOMT proteins were localized in the chloroplast, one CCoAOMT protein was localized in the mitochondrion, four CCoAOMT proteins were localized in the nucleus, and eleven CCoAOMT proteins were predicted to be localized in the cytoplasm, plasma membrane, chloroplasts, and mitochondria (Table A1). Generally, a gene’s function correlates with its corresponding protein’s subcellular location [38,39]. We generated pGhCCoAOMT2::eGFP, pGhCCoAOMT7::eGFP, and pGhCCoAOMT14::eGFP vectors and expressed these fusion proteins in N. tabacum leaf cells. The pGhCCoAOMT2::eGFP, pGhCCoAOMT7::eGFP, and pGhCCoAOMT14::eGFP fusion vector’s fluorescence signal was detected in the nucleus and plasma membrane. In contrast, the empty vector eGFP protein displayed a fluorescence signal in the cytoplasm, plasma membrane, and nucleus, indicating that GhCCoAOMT2, GhCCoAOMT7, and GhCCoAOMT14 were located in the nucleus and plasma membrane (Figure 5).
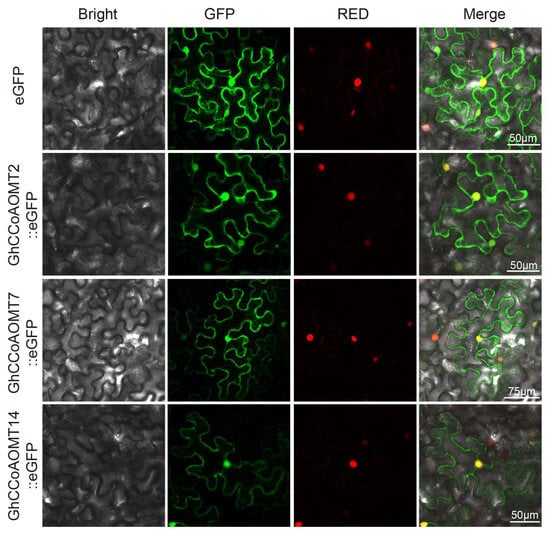
Figure 5.
Subcellular localization of CCoAOMT proteins.
2.6. Expression Analysis of the GhCCoAOMT Genes in Different Tissues in G. hirsutum
The spatiotemporal gene expression patterns greatly correlate with the gene’s biological function. The expression patterns of GhCCoAOMT genes were analyzed to explore their roles in cotton growth and development. The expression of most GhCCoAOMTs was low in various tissues. GhCCoAOMT1, GhCCoAOMT4, and GhCCoAOMT11 were not expressed in almost all tissues. Certain GhCCoAOMT genes, such as GhCCoAOMT2, GhCCoAOMT3, GhCCoAOMT7, GhCCoAOMT9, GhCCoAOMT10, GhCCoAOMT14, and GhCCoAOMT16, were expressed in all tissues. Among them, GhCCoAOMT2 was highly expressed in stems, leaves, stamen, and 15dpa ovules, especially in the stamen. GhCCoAOMT7 was most highly expressed in stems. GhCCoAOMT10 was highly expressed in leaves, stems, stamens, and 15dpa ovules (Figure 6A). In addition, we selected several genes expressed in various tissues for qRT-PCR analysis to validate the accuracy of these results. The qRT-PCR results were highly consistent with those mentioned above (Figure 6B). These findings provide a reference for our further research on the biological function of GhCCoAOMTs.
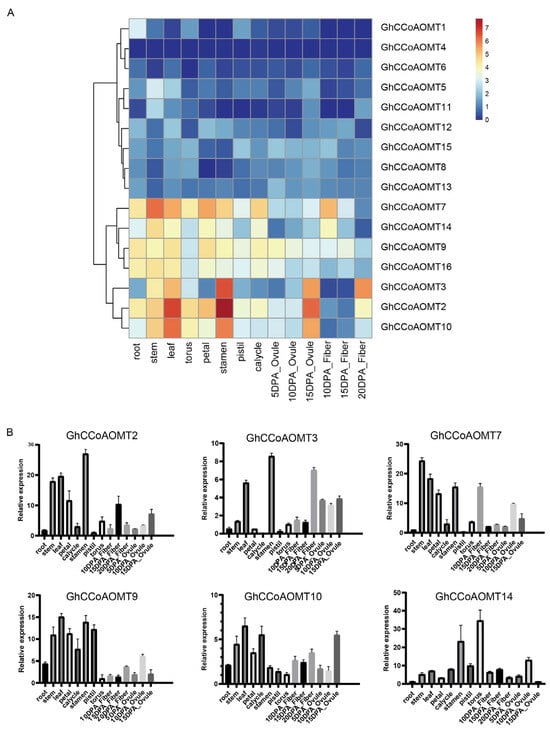
Figure 6.
Expression patterns of CCoAOMTs in (A) ten plant tissues (root, stem, leaf, torus, petal, stamen, pistil, calycle, ovule, fiber). Color scale represents FPKM normalized log2 transformed counts where blue represents low expression and red represents high expression. (B). Relative expression of GhCCoAOMT2, GhCCoAOMT3, GhCCoAOMT7, GhCCoAOMT9, GhCCoAOMT10, and GhCCoAOMT14 in different tissues assessed by qRT-PCR and calculated by the 2−ΔΔCt method. Mean values and standard deviations were obtained from three technical replicates and biological replicates. Error bars represent the standard deviation estimated by three independent experiments.
2.7. Silencing of GhCCoAOMT7 Affects Lignin Synthesis in Cotton
According to the analysis of family gene expression, GhCCoAOMT7 was highly expressed in stems. Although it belongs to group I, its promoter is the only promoter that does not contain AC-I or AC-II cis-elements related to lignin synthesis. Therefore, we selected the GhCCoAOMT7 gene for further study to determine whether it is related to lignin biosynthesis.
The TRV::GhCCoAOMT7 vector was generated to downregulate the expression of GhCCoAOMT7, and it was transformed into Agrobacterium. Three weeks post agroinfiltration, the TRV::PDS vector resulted in an albino phenotype, indicating the success of the VIGS assay (Figure 7A). To further evaluate the VIGS assay’s effectiveness, we utilized qRT-PCR to detect the expression levels of GhCCoAOMT7 in plants transformed with the TRV::GhCCoAOMT7 and TRV:00 vectors. As demonstrated in Figure 7B, the expression level of GhCCoAOMT7 was significantly decreased in TRV::GhCCoAOMT7-treated plants, implying that the gene had been successfully silenced. Then, the stems from the TRV::GhCCoAOMT7 and TRV::00 cotton plants were collected for chemical phloroglucinol staining. The lignin staining results indicated that the TRV::GhCCoAOMT7 cotton exhibited a reduced and low-intensity cross-section staining in comparison to the TRV::00 plants (Figure 7C).
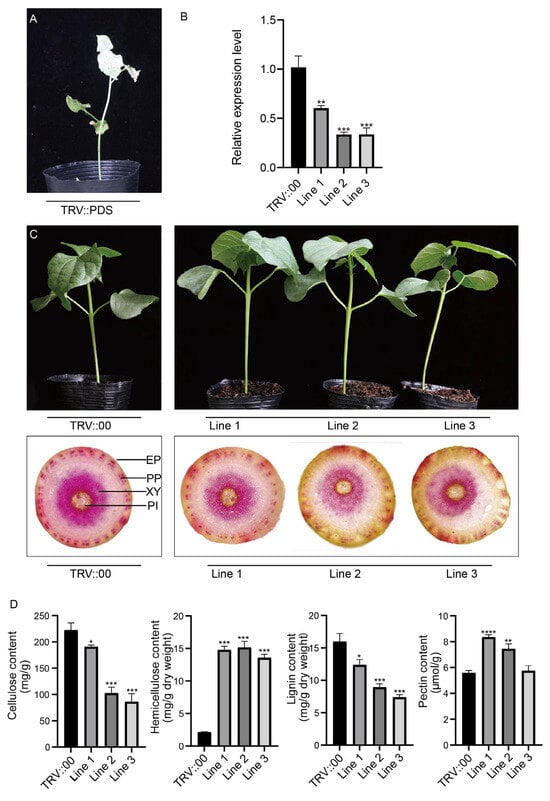
Figure 7.
Silencing of GhCCoAOMT7 reduces cotton stem lignin content. (A) corresponds to the TRV::PDS positive control. (B) Relative expression of GhCCoAOMT7 in the cotton stem was assessed by qRT–PCR and calculated by the 2−ΔΔCt method. Mean values and standard deviations were obtained from three biological and three technical replicates (n = 9). Error bars indicate the standard deviation estimated by three independent experiments. (C) TRV::00 and TRV::GhCCoAOMT7 silenced cotton plant growth statues and stem phloroglucinol staining. EP, Epidermis; PP, Phloem fibers; XY, Secondary xylem; PI, Pith cells. (D) Determination of cellulose, hemicellulose, lignin, and pectin in the TRV::00 and TRV::GhCCoAOMT7 cotton stems. Asterisks (* p < 0.05; ** p < 0.01; *** p < 0.001; and **** p < 0.0001) indicate significant differences from the blank control (pTRV:00).
The cell wall of cotton is mainly composed of cellulose, hemicellulose, lignin, and pectin. In order to determine these components, the stems of TRV::00 and TRV::GhCCoAOMT7 cotton plants were collected. As shown in Figure 7D, the content of cellulose and lignin in stems decreased significantly in the TRV::GhCCoAOMT7 plants, while the content of hemicellulose increased. However, pectin content did not change significantly, which may be due to the compensatory regulatory function between lignin and cellulose. Those results suggested that the GhCCoAOMT7 gene may be involved in lignin biosynthesis.
3. Discussion
The primary constituent of the cell wall, lignin, plays key roles in the plant’s responses to both biotic and abiotic stressors [12]. The functions of the CCoAOMT gene family in the lignin synthesis pathway have been studied in A. thaliana [26], sorghum [31], and other plants. In this research, we used bioinformatics tools to systematically identify and characterize the cotton CCoAOMT family genes, and focus on validating the role of GhCCoAOMT7 in cotton.
3.1. Characterization of CCoAOMTs in Cotton
Allotetraploid cotton genetic resources were obtained by interspecific hybridization between G. arboretum and G. raimondii [40]. While G. barbadense D-subgenomes and G. hirsutum D-subgenomes source from the genome of G. raimondii [39], the G. barbadense A-subgenome, and G. hirsutum A-subgenome descended from common descent, G. arboretum [37,40]. This study identified 9, 8, 17, and 16 CCoAOMT genes in G. arboreum, G. raimondii, G. barbadense, and G. hirsutum, respectively. These were clustered based on the phylogenetic analysis into four groups. Analysis of CCoAOMT gene duplications indicated that segmental duplications are the primary mode of expansion in cotton for the CCoAOMT gene family. Four cotton species were used to further study the Ka and Ks and the Ka/Ks ratios of CCoAOMT segmentally duplicated gene pairs. The Ka/Ks ratios of most of the duplicated gene pairs of CCoAOMT in cotton were less than one, and CCoAOMT genes in four cotton species had undergone purifying selection during the evolutionary process [41]. Among all sixteen GhCCoAOMTs of G. hirsutum, nine GhCCoAOMTs were located in the G. hirsutum A-subgenom, and seven GhCCoAOMTs in the G. hirsutum D-subgenome. Similarly, in G. barbadense, nine GbCCoAOMTs were located in the G. barbadense A-subgenome, and eight GbCCoAOMTs were located in the G. barbadense D-subgenome. The above observations indicated that CCoAOMTs of the D genome appear to be lost to varying degrees during cotton polyploidization. This finding not only deepens our understanding of the genomic structure and evolution of cotton, but also provides a basis for further research into the functions and regulatory mechanisms of the CCoAOMT genes. Further analysis of the distribution and evolutionary features of these genes revealed significant differences in the number of CCoAOMT genes in the different subgenomes, indicating dynamic changes in the different subgenomes during evolution.
3.2. Regulatory Mechanisms and Functions of CCoAOMTs
The function of related gene families can be studied more precisely by gene structure analysis, phylogenetic analysis, and their subcellular localization [42]. The cotton CCoAOMTs gene structure differed greatly, consisting of a maximum of nine exons and a minimum of just one exon. The varied exon-intron architectures can lead to gene function diversification [43]. The gene structure analysis of the CCoAOMT family in cotton was carried out using the acquired NWK data and motif files. Although the motif types in each class were almost identical, individual genes were structurally unique from other CCoAOMT genes. The comparatively conserved function of the CCoAOMT genes potentially derived from the presence of the highly conserved motifs 1–4 found in cotton. Additional motifs can be gradually incorporated or removed throughout evolution to alter the functions of genes in plants. By subcellular localization analysis of cotton CCoAOMT proteins, it was found that most proteins were located in the cytoplasm, certain proteins were predicted to be located in the cytoplasm, plasma membrane, chloroplasts, and mitochondria, and a few proteins were located in the nucleus. We performed an in vivo subcellular localization study of GhCCoAOMT2, GhCCoAOMT7, and GhCCoAOMT14 through their transient expression in N. benthamiana leaf cells. We determined that GhCCoAOMT2, GhCCoAOMT7, and GhCCoAOMT14 were localized in the nucleus and the plasma membrane. This is the first time CCoAOMTs have been observed to be localized to plants’ nucleus and plasma membrane since their discovery, possibly due to differences between plant species.
The promoter sequence upstream of the transcription start site houses cis-elements that control downstream gene expression in response to various biotic or abiotic stressors in order to adjust to various growth conditions [44]. Plant hormone responsive cis-elements and other cis-elements linked to stress response were found in the promoters of CCoAOMT gene family members. Every gene contained a different set of cis-elements that were involved in plant growth and development regulation or resistance to stress. In this study, we analyzed the expression of identified GhCCoAOMT family genes in different cotton tissues using transcriptome data and validated the results with qRT-PCR experiments. We found that GhCCoAOMT14 in the torus showed opposite expression patterns in the transcriptome and qRT-PCR results. This discrepancy is probably due to technical differences. RNA-Seq captures the expression patterns of the entire transcriptome, including low and medium abundance transcripts, while qRT-PCR is a quantitative technique that targets specific loci and genes and requires highly purified RNA samples with strict quality control during RNA extraction and reverse transcription. RNA-Seq is more sensitive for detecting low-abundance transcripts and may identify transcripts that are too low in abundance to be fully detected by qRT-PCR [45,46]. Therefore, we consider the transcriptome results as the primary reference in this study.
3.3. The Function of GhCCoAOMT7 in Cotton
Our study provides insights into the role of GhCCoAOMT7 in lignin biosynthesis in cotton stems and emphasizes the importance of this gene as a potential breeding target. The results suggest a close correlation between the high expression of GhCCoAOMT7 in stems and lignin biosynthesis, consistent with its clustering in the phylogenetic tree. Although the promoter of GhCCoAOMT7 does not contain typical cis-elements associated with lignin biosynthesis (such as AC-I or AC-II) [35,36], its crucial role in lignin synthesis was experimentally confirmed [37]. Through gene silencing experiments, we observed a significant decrease in lignin biosynthesis in the transgenic cotton plants (TRV::GhCCoAOMT7), characterized by a reduction in the staining area and intensity of the xylem. These phenomena suggest that GhCCoAOMT7 plays a key role in the lignin biosynthesis process in cotton. In addition, quantitative analysis of cell wall components indicated changes in the content of cellulose, hemicellulose, and lignin, further supporting the functional contribution of GhCCoAOMT7. This finding not only reveals the involvement of GhCCoAOMT7 in lignin synthesis but also provides important insights into the complex mechanisms coordinating the synthesis of lignin components. The investigation of GhCCoAOMT7 offers new molecular targets for genetic improvement and breeding strategies in cotton. Increasing lignin content can increase plant stress resistance and improve fiber quality in cotton production [38]. Therefore, precise regulation of GhCCoAOMT7 expression could facilitate the optimization of lignin biosynthesis and thus improve cotton growth and yield. Future research should further investigate the interactions between GhCCoAOMT7 and other genes related to lignin biosynthesis, as well as its functional variations under different environmental conditions.
In summary, this study confirms the significant role of GhCCoAOMT7 in lignin biosynthesis in cotton, laying a foundation for further exploration of the mechanisms involved in lignin synthesis and its applications in cotton breeding. This not only deepens our understanding of the biological functions of CCoAOMT genes in plant growth and development but also provides theoretical support for future strategies in cotton improvement.
4. Materials and Methods
4.1. Identification and Phylogenetic Analysis of CCoAOMT Proteins in Cotton
The genomes of four different cotton species, two diploids (G. arboretum, CRI; G. raimondii, JGI), and two allotetraploids (G. hirsutum, NBI; G. barbadense, ZJU) were download from the online database CottonFGD (https://cottonfgd.net/) [47]. The A. thaliana, Oryza sativa, and N. tabacum CCoAOMT family protein sequences were downloaded from the A. thaliana genome database (https://www.arabidopsis.org/), and NCBI (https://www.ncbi.nlm.nih.gov/). The hidden Markov model profile of the Methyltransf_3 domain (PF01596) was obtained to search for all CCoAOMT proteins in the four cotton species genomes (https://www.ncbi.nlm.nih.gov/). The Pfam (https://pfam.xfam.org/) and SMART website(http://smart.embl-heidelberg.de/) were used to confirm that the predicted proteins contained the Methyltransf_3 domain [48]. Alignment of A. thaliana, G. raimondii, G. arboretum, G. barbadense, G. hirsutum, Oryza sativa, and N. tabacum CCoAOMT protein domain sequences was conducted using the MUSCLE method of MEGA7.0 with the default parameters [49]. Using the neighbor-joining (NJ) approach, phylogenetic analysis was performed on the aligned sequences. Using MEGA 7.0 and the P-distance model, a pairwise deletion option, and 1000 bootstrap repetitions, the consensus tree was built [50]. Finally, Adobe Illustrator CS6 (Version 16) software was used to more clearly illustrate the subfamilies and visually edit the phylogenetic trees.
4.2. Bioinformatics Analysis of CCoAOMT Proteins in Cotton
The program ExPASy was utilized to calculate the isoelectric point (pI) as well as the molecular weight (MW) of each of the CCoAOMT proteins [51,52]. CELLO v.2.5 was used for subcellular localization predictions [39]. The data of the cotton genome chromosomes were procured from the online repository CottonFGD (https://cottonfgd.net/). The Multiple Collinearity Scan tool was employed to examine synteny and collinearity [53]. Using the TBtools Version 1.115, the non-synonymous (Ka) and synonymous (Ks) substitution rates were computed to estimate the selection pressure acting on the cotton CCoAOMTs during their evolution. Positive, neutral, and purifying selection are generally indicated by Ka/Ks > 1, Ka/Ks = 1, and Ka/Ks < 1 [54]. Using the OmicStudio to create the circos plot and label the chromosomal locations of the CCoAOMT proteins [55]. Gene structure data of the CCoAOMT gene family members were downloaded from the online website CottonFGD, and the GSDS2.0 web server (https://gsds.gao-lab.org/) was used to graphically examine their gene structures [56]. The web program MEME predicted the conserved motifs of the proteins of all gene family members encoded. The findings were visualized using TBtools, and the maximum number of motifs was set to 10. The remaining parameters were left at their default values. Upstream promoter region sequences (1500 bp) of all CCoAOMTs, starting from the initiation codon (ATG), were retrieved. The PlantCARE database was used to identify the cis-elements within these sequences [57].
4.3. Expression Pattern Analysis
Based on the GhCCoAOMT gene ID in G. hirsutum identified by the gene family evolutionary tree, transcriptome data were obtained from different cotton tissues (root, stem, leaf, torus, petal, stamen, pistil, calycle) and developmental stages (5, 10, 15 DPA ovule, and 10, 15, 20 DPA fiber) retrieved from CottonFGD (https://cottonfgd.net/) to gain further insights into the expression patterns of GhCCoAOMTs. TBtools Version 1.115 software was used to visualize the heatmap of the GhCCoAOMTs expression and FPKM normalized log2 transformed counts.
4.4. Subcellular Localization Experiments
Using the primers listed in Table A4, the coding sequences of GhCCoAOMT2, GhCCoAOMT7, and GhCCoAOMT14 were extracted from CCRI24 cDNA by PCR, and the resulting fragments were ligated to the pCAMBIA-2300-35S::eGFP vector to form the pCAMBIA-2300-35S::GhCCoAOMT7-eGFP vector. The recombinant vector was transfected into the Agrobacterium tumefaciens strain GV3101, which was then injected into tobacco leaves to be transiently expressed. The inoculated tobacco plants were grown in 12 h of darkness and 24 h of light. An aDmi8 inverted phase microscope (Leica) was used to observe the resulting eGFP fluorescence.
4.5. Plant Material and Virus-Induced Gene Silencing
The recombinant vector pTRV::GhCCoAOMT7 was constructed by inserting a 300 bp fragment of GhCCoAOMT7 into the pTRV2 vector. Separate transformations of the vectors containing the recombinant vector (pTRV::GhCCoAOMT7), the positive control vector (pTRV::PDS), and the empty vector (pTRV::00) were carried out in GV3101 Agrobacterium Competent Cells. Cotton seedlings whose cotyledons have just begun to flatten were selected and were watered prior to the agroinfiltration. A tiny puncture was created in the cotyledon’s epidermis using a sterile needle, and then a syringe was used to inject the bacterial solution into it until the entire cotyledon was filled with the solution. The CCRI24 seedlings were kept in the dark for 24 h following agroinfiltration. All of the seedlings were then moved to a greenhouse where they were cultured under 16 h of light and 8 h of darkness at 25 °C.
4.6. RNA Extraction and qRT-PCR Detection
The samples’ total RNA was extracted using the fast pure plant total RNA isolation kit (Vazyme Biotech, Nanjing, China). Transcript all-in-one first-strand cDNA synthesis supermix (TransGen Biotech, Beijing, China) for qPCR was used to create the cDNA from the reverse transcription of RNA. ABI 7500 real-time PCR equipment (Applied Biosystems, Foster City, CA, USA) was used to carry out qRT-PCR using the SYBR premix ex taq (TakaRa, Shiga, Japan). This experiment involved three separate biological replicates as well as three technical repeats. Table A4 contains a list of primers utilized in this investigation.
4.7. Lignin Content Determination and Histochemical Staining
A tissue staining technique was employed to analyze the potential involvement of GhCCoAOMT7 in lignin biosynthesis in cotton stem tissues. Stem sections from cotton carrying pTRV::GhCCoAOMT7 and pTRV::00 constructs were selected to prepare slides and were mounted. The sections were then subjected to staining using a phloroglucinol lignin staining solution. During the staining process, the lignin was first acidified using a lignin acidification solution prior to staining with phloroglucinol staining solution. Subsequently, the differences in lignin deposition in the stem cortex were observed and examined under a microscope [58].
A kit was used for the determination of cellulose, hemicellulose, pectin, and lignin contents [59]. For hemicellulose and lignin determination, the stems were dried to a constant weight at 80 °C, then crushed and passed through a 50 mesh sieve, and 0.05 g was weighed and placed into 1.5 mL EP tubes. A characteristic absorption peak at 280 nm is observed after acetylation of phenolic and hydroxyl groups in lignin. The absorbance value at 280 nm is positively correlated with lignin content. Hemicellulose has a specific absorption peak at 530 nm, and its content was measured after treatment. Samples for cellulose and pectin determination were each weighed at 0.1 and 0.05 g, respectively, and after processing, their absorbance was measured at 620 nm and 530 nm, respectively, to determine the content of cellulose and pectin.
5. Conclusions
In summary, this study conducted a comprehensive analysis of CCoAOMT genes in four cotton species, resulting in the identification of 50 CCoAOMT genes. Based on sequence similarities, the genes were categorized into five groups by phylogenetic analysis. Analysis of exon-intron structures and conserved motifs in cotton CCoAOMT genes revealed their high conservation during evolution. They exhibited uneven distribution across chromosomes. Collinearity analysis demonstrated varying degrees of gene loss among the CCoAOMT gene family members during cotton evolution. Promoter cis-element analysis revealed the presence of regulatory elements associated with plant growth and stress responses, and transcriptomic data analysis indicated the high expression of GhCCoAOMT7 in stems. Subcellular localization analysis of GhCCoAOMT7 revealed its nuclear and plasma membrane localization. Silencing of GhCCoAOMT7 resulted in decreased lignin content in cotton stems, as confirmed by lignin staining, determination of lignin content, and qRT-PCR. This study confirms the crucial role of GhCCoAOMT7 in lignin biosynthesis in cotton and provides new molecular targets for genetic improvement and breeding strategies. Increasing lignin content can increase the stress resistance of cotton and improve fiber quality. Regulating the expression of GhCCoAOMT7 could enable precise modulation of lignin biosynthesis and thus optimize cotton growth and yield. This finding provides an important basis for understanding the functions of CCoAOMT genes in plant growth and development and has significant implications for theoretical support of cotton improvement strategies.
Author Contributions
Conceptualization, S.F. and L.W.; writing—original draft preparation, L.M.; methodology, J.W.; software, K.Q. and Y.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the China Agriculture Research System (CARS-15-03), Natural Science Foundation of Xinjiang Uygur Autonomous Region (2022D01A158), and Agricultural Science and Technology Innovation Program of Chinese Academy of Agricultural Sciences (CAAS-ASTIP-CCRI).
Data Availability Statement
All data are incorporated into the article and its Appendix A and Appendix B.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Figure A1.
Chromosomal distribution of CCoAOMTs genes in cotton.
Appendix B

Table A1.
The basic information and characteristics of the CCoAOMT genes in G.arboreum, G. barbadense, G. hirsutum, and G. raimondii.
Table A1.
The basic information and characteristics of the CCoAOMT genes in G.arboreum, G. barbadense, G. hirsutum, and G. raimondii.
| Gene Name | Gene ID | Chromosome | AA Size | MW (Da) | Isoelectric Point | Prediction of Subcellular Localization |
|---|---|---|---|---|---|---|
| GhCCoAOMT1 | Gh_A03G1660 | A03 | 194 | 21,852.3 | 6.94 | Cytoplasmic |
| GhCCoAOMT2 | Gh_A04G1032 | A04 | 248 | 28,009 | 5.97 | Cytoplasmic |
| GhCCoAOMT3 | Gh_A04G1207 | A04 | 191 | 21,654.7 | 4.66 | Cytoplasmic |
| GhCCoAOMT4 | Gh_A04G1208 | A04 | 234 | 26,668.4 | 4.81 | Cytoplasmic, PlasmaMembrane, Chloroplast |
| GhCCoAOMT5 | Gh_A06G0620 | A06 | 275 | 31,036 | 7.5 | Cytoplasmic |
| GhCCoAOMT6 | Gh_A08G2183 | A08 | 229 | 25,631.2 | 6.15 | Chloroplast |
| GhCCoAOMT7 | Gh_A12G0204 | A12 | 318 | 36,179.5 | 9.46 | Mitochondrial, Cytoplasmic, Nuclear |
| GhCCoAOMT8 | Gh_A12G1072 | A12 | 287 | 31,906.4 | 8.82 | Chloroplast |
| GhCCoAOMT9 | Gh_A13G0363 | A13 | 250 | 28,239.2 | 5.18 | Cytoplasmic |
| GhCCoAOMT10 | Gh_D04G1586 | D04 | 248 | 28,073 | 5.39 | Cytoplasmic |
| GhCCoAOMT11 | Gh_D04G1818 | D04 | 248 | 28,511.8 | 6.69 | Cytoplasmic |
| GhCCoAOMT12 | Gh_D06G0703 | D06 | 245 | 28,037.5 | 9.55 | Mitochondrial |
| GhCCoAOMT13 | Gh_D08G2549 | D08 | 274 | 31,102.5 | 6.69 | PlasmaMembrane, Cytoplasmic |
| GhCCoAOMT14 | Gh_D12G1196 | D12 | 306 | 34,079 | 9.16 | Chloroplast, Nuclear |
| GhCCoAOMT15 | Gh_D13G0408 | D13 | 250 | 28,287.2 | 5.18 | Cytoplasmic |
| GhCCoAOMT16 | Gh_D12G0206 | D12 | 223 | 25,039.7 | 5.36 | Cytoplasmic |
| GaCCoAOMT1 | Ga03G2453 | A03 | 194 | 21,852.3 | 6.94 | Cytoplasmic |
| GaCCoAOMT2 | Ga04G0015 | A04 | 241 | 27,513.4 | 4.73 | Cytoplasmic |
| GaCCoAOMT3 | Ga04G0016 | A04 | 234 | 26,668.4 | 4.81 | Cytoplasmic |
| GaCCoAOMT4 | Ga04G0313 | A04 | 260 | 29,427.8 | 7.13 | Cytoplasmic |
| GaCCoAOMT5 | Ga05G3423 | A05 | 108 | 11,912.8 | 5.08 | PlasmaMembrane, Chloroplast |
| GaCCoAOMT6 | Ga06G0722 | A06 | 269 | 30,296 | 5.85 | Cytoplasmic |
| GaCCoAOMT7 | Ga08G2876 | A08 | 242 | 27,170 | 5.02 | Cytoplasmic, Chloroplast |
| GaCCoAOMT8 | Ga12G1724 | A12 | 287 | 31,932.5 | 8.82 | Chloroplast |
| GaCCoAOMT9 | Ga13G0397 | A13 | 250 | 28,330.3 | 5.91 | Cytoplasmic |
| GrCCoAPMT1 | Gorai.004G283700 | D04 | 237 | 26,632.4 | 5.69 | Cytoplasmic, Chloroplast |
| GrCCoAPMT2 | Gorai.005G237100 | D05 | 240 | 27,340.2 | 6.17 | Cytoplasmic |
| GrCCoAPMT3 | Gorai.008G024000 | D08 | 318 | 36,161.6 | 9.69 | Mitochondrial, Cytoplasmic, Nuclear |
| GrCCoAPMT4 | Gorai.008G133100 | D08 | 306 | 33,994.9 | 9.32 | Chloroplast |
| GrCCoAPMT5 | Gorai.010G081000 | D10 | 274 | 30,720.3 | 4.97 | Cytoplasmic, Chloroplast |
| GrCCoAPMT6 | Gorai.012G149800 | D12 | 248 | 28,073 | 5.39 | Cytoplasmic |
| GrCCoAPMT7 | Gorai.012G175500 | D12 | 241 | 27,482.3 | 4.66 | Cytoplasmic |
| GrCCoAPMT8 | Gorai.013G045300 | D13 | 250 | 28,356.3 | 5.39 | Cytoplasmic |
| GbCCoAOMT1 | GB_A03G2207 | A03 | 247 | 28,000 | 6.17 | Cytoplasmic |
| GbCCoAOMT2 | GB_A04G1485 | A04 | 248 | 28,025 | 5.97 | Cytoplasmic |
| GbCCoAOMT3 | GB_A04G1725 | A04 | 234 | 26,668.4 | 4.81 | Cytoplasmic |
| GbCCoAOMT4 | GB_A04G1726 | A04 | 191 | 21,626.6 | 4.42 | PlasmaMembrane, Chloroplast, Cytoplasmic |
| GbCCoAOMT5 | GB_A06G0796 | A06 | 238 | 26,897.9 | 4.93 | Cytoplasmic |
| GbCCoAOMT6 | GB_A08G2894 | A08 | 267 | 29,974 | 6.11 | Cytoplasmic |
| GbCCoAOMT7 | GB_A12G0214 | A12 | 318 | 36,223.6 | 9.46 | Mitochondrial, Cytoplasmic, Nuclear |
| GbCCoAOMT8 | GB_A12G1419 | A12 | 287 | 31,906.4 | 8.82 | Chloroplast |
| GbCCoAOMT9 | GB_A13G0414 | A13 | 250 | 28,308.3 | 5.39 | Cytoplasmic |
| GbCCoAOMT10 | GB_D04G1874 | D04 | 215 | 24,341 | 5.38 | Cytoplasmic |
| GbCCoAOMT11 | GB_D04G2117 | D04 | 190 | 21,780.8 | 4.95 | Cytoplasmic |
| GbCCoAOMT12 | GB_D04G2118 | D04 | 241 | 27,553.4 | 4.74 | Cytoplasmic |
| GbCCoAOMT13 | GB_D06G0782 | D06 | 236 | 26,900.1 | 8.95 | Mitochondrial, Cytoplasmic, Chloroplast |
| GbCCoAOMT14 | GB_D08G2886 | D08 | 242 | 27,287.2 | 5.39 | Cytoplasmic, Chloroplast |
| GbCCoAOMT15 | GB_D12G0230 | D12 | 318 | 36,094.4 | 9.31 | Cytoplasmic, Mitochondrial |
| GbCCoAOMT16 | GB_D12G1416 | D10 | 287 | 32,005.5 | 8.64 | Cytoplasmic, Chloroplast |
| GbCCoAOMT17 | GB_D13G0404 | D11 | 250 | 28,356.3 | 5.39 | Cytoplasmic |

Table A2.
The non-synonymous (Ka) to synonymous substitution ratio (Ks) in duplicated CCoAOMT gene pairs in cotton.
Table A2.
The non-synonymous (Ka) to synonymous substitution ratio (Ks) in duplicated CCoAOMT gene pairs in cotton.
| Gene Pairs | Ka_Ks | Gene Pairs | Ka_Ks | ||
|---|---|---|---|---|---|
| GbCCoAOMT1 | GaCCoAOMT9 | 0.065068696 | GhCCoAOMT4 | GaCCoAOMT2 | 0.541581445 |
| GbCCoAOMT4 | GaCCoAOMT2 | 0.540163087 | GhCCoAOMT5 | GaCCoAOMT6 | 0.100265716 |
| GbCCoAOMT5 | GaCCoAOMT6 | 0.911349732 | GhCCoAOMT6 | GaCCoAOMT7 | 1.548836158 |
| GbCCoAOMT10 | GaCCoAOMT4 | 0.040931697 | GhCCoAOMT10 | GaCCoAOMT4 | 0.070833499 |
| GbCCoAOMT12 | GaCCoAOMT2 | 0.229109501 | GhCCoAOMT12 | GaCCoAOMT6 | 0.095121818 |
| GbCCoAOMT13 | GaCCoAOMT6 | 0.114461472 | GhCCoAOMT13 | GaCCoAOMT7 | 0.322398054 |
| GbCCoAOMT14 | GaCCoAOMT7 | 0.314951527 | GhCCoAOMT14 | GaCCoAOMT8 | 0.192725361 |
| GbCCoAOMT16 | GaCCoAOMT8 | 0.267528849 | GhCCoAOMT15 | GaCCoAOMT9 | 0.382526196 |
| GbCCoAOMT17 | GaCCoAOMT9 | 0.28693383 | GhCCoAOMT2 | GrCCoAPMT6 | 0.163281629 |
| GbCCoAOMT1 | GrCCoAPMT8 | 0.060498137 | GhCCoAOMT4 | GrCCoAPMT7 | 0.472390717 |
| GbCCoAOMT2 | GrCCoAPMT6 | 0.1226389 | GhCCoAOMT5 | GrCCoAPMT5 | 0.454166854 |
| GbCCoAOMT4 | GrCCoAPMT7 | 0.471143834 | GhCCoAOMT6 | GrCCoAPMT1 | 0.671213344 |
| GbCCoAOMT5 | GrCCoAPMT5 | 0.600294416 | GhCCoAOMT7 | GrCCoAPMT3 | 0.166795279 |
| GbCCoAOMT7 | GrCCoAPMT3 | 0.145483775 | GhCCoAOMT8 | GrCCoAPMT4 | 0.184354187 |
| GbCCoAOMT8 | GrCCoAPMT4 | 0.184354187 | GhCCoAOMT9 | GrCCoAPMT8 | 0.2865639 |
| GbCCoAOMT9 | GrCCoAPMT8 | 0.191069052 | GhCCoAOMT12 | GrCCoAPMT5 | 0.443452894 |
| GbCCoAOMT12 | GrCCoAPMT7 | 0.131872778 | GhCCoAOMT13 | GrCCoAPMT1 | 0.302139173 |
| GbCCoAOMT13 | GrCCoAPMT5 | 0.29703111 | GhCCoAOMT14 | GrCCoAPMT4 | 0.786156023 |
| GbCCoAOMT14 | GrCCoAPMT1 | 0.204346831 | GhCCoAOMT15 | GrCCoAPMT8 | 0.143345629 |
| GbCCoAOMT16 | GrCCoAPMT4 | 0.786002408 | |||

Table A3.
Cis-elements in the cotton CCoAOMT promoters.
Table A3.
Cis-elements in the cotton CCoAOMT promoters.
| Name | Sequence | Explain |
|---|---|---|
| GCN4_motif | TGAGTCA | cis-regulatory element involved in endosperm expression |
| AC-I | (T/C)C(T/C)(C/T)ACC(T/C)ACC | lignin correlation |
| AC-II | TCACCAACCCCC | |
| ABRE | ACGTG | cis-acting element involved in the abscisic acid responsiveness |
| AuxRR-core | GGTCCAT | cis-acting regulatory element involved in auxin responsiveness |
| TGA-element | AACGAC | |
| WUN-motif | AAATTACT | wound-responsive element |
| TATA-box | TATAA | core promoter element |
| O2-site | GATGATGTGG | |
| ARE | AAACCA | cis-acting regulatory element essential for the anaerobic induction |
| LTR | CCGAAA | cis-acting element involved in low-temperature responsiveness |
| CAT-box | GCCACT | cis-acting regulatory element related to meristem expression |
| TGACG-motif | TGACG | cis-acting regulatory element involved in the MeJA-responsiveness |
| TATC-box | TATCCCA | cis-acting element involved in gibberellin-responsiveness |
| TC-rich repeats | ATTCTCTAAC | cis-acting element involved in defense and stress responsiveness |
| MBS | CAACTG | MYB binding site involved in drought-inducibility |
| GC-motif | CCCCCG | enhancer-like element involved in anoxic specific inducibility |
| HD-zip | CTTTACCAACC | Palisade differentiation |

Table A4.
Primer sequences.
Table A4.
Primer sequences.
| Primer Name | Primer Sequence | Primer Function |
|---|---|---|
| GhCCoAOMT2-F | CAACACCACCCAAGAGCAAC | gene clone |
| GhCCoAOMT2-R | TTGACACGGCGGCAAAGGG | |
| GhCCoAOMT7-F | GAGATGGGTCCCAGTCCAGC | |
| GhCCoAOMT7-R | TCATTTAACGCGACGGCAAA | |
| GhCCoAOMT14-F | ATGACAAAAAGCGCAGCAA | |
| GhCCoAOMT14-R | TCGGCATATTGTCATTCCAT | |
| eGFP-GhCCoAOMT2-F | GGGGCCCGGGGTCGACATGGCAACCAACACCACCC | carrier conjugation |
| eGFP-GhCCoAOMT2-R | TACCGGATCCACTAGTTTTGACACGGCGGCAAAG | |
| eGFP-GhCCoAOMT7-F | GGGGCCCGGGGTCGACATGGGTCCCAGTCCAGCT | |
| eGFP-GhCCoAOMT7-R | TACCGGATCCACTAGTTTTAACGCGACGGCAAAGG | |
| eGFP-GhCCoAOMT14-F | GGGGCCCGGGGTCGACATGGCAACCAATACGCAAGAGC | |
| eGFP-GhCCoAOMT14-R | TACCGGATCCACTAGTTTTGACGCGACGGCAAAG | |
| TRV-GhCCoAOMT7-F | TAAGGTTACCGAATTCCTCGAGACCAGTGTGTATCCGAG | |
| TRV-GhCCoAOMT7-R | GCTCGGTACCGGATCCAGGGCCCTCTTTGAAATCAATTTTGT | |
| GhCCoAOMT2-YG-F | AACACACTGTGGAATGGGTCGG | qRT-PCR |
| GhCCoAOMT2-YG-R | TCACCAACAGGGAGCATGCAAA | |
| GhCCoAOMT3-YG-F | CATTGCCTGAGGATGGCAAGGT | |
| GhCCoAOMT3-YG-R | AGGCATCTGAGGGGAAGAACTCA | |
| GhCCoAOMT7-YG-F | TGTATCCGAGGGAGCCTGAA | |
| GhCCoAOMT7-YG-R | GCAGACCCAGCTCGTAGTTT | |
| GhCCoAOMT9-YG-F | GCAACCAACAAAACAGAAGAGC | |
| GhCCoAOMT9-YG-R | TTGAGGCGACGGCAAAGG | |
| GhCCoAOMT10-YG-F | AGAGCTCAGAGAGTTGACCGCT | |
| GhCCoAOMT10-YG-R | TTGTGTGCAACGCCAGCTTTTT | |
| GhCCoAOMT14-YG-F | GCAACCAATACGCAAGAGCA | |
| GhCCoAOMT14-YG-R | GACGCGACGGCAAAGGGTGA |
References
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.C.; Bonine, C.A.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Balint-Kurti, P.J. Maize Homologs of CCoAOMT and HCT, Two Key Enzymes in Lignin Biosynthesis, Form Complexes with the NLR Rp1 Protein to Modulate the Defense Response. Plant Physiol. 2016, 171, 2166–2177. [Google Scholar] [CrossRef]
- Gallego-Giraldo, L.; Posé, S.; Pattathil, S.; Peralta, A.G.; Hahn, M.G.; Ayre, B.G.; Sunuwar, J.; Hernandez, J.; Patel, M.; Shah, J.; et al. Elicitors and defense gene induction in plants with altered lignin compositions. New Phytol. 2018, 219, 1235–1251. [Google Scholar] [CrossRef]
- Barros, J.; Serk, H.; Granlund, I.; Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef]
- Fan, L.; Linker, R.; Gepstein, S.; Tanimoto, E.; Yamamoto, R.; Neumann, P.M. Progressive inhibition by water deficit of cell wall extensibility and growth along the elongation zone of maize roots is related to increased lignin metabolism and progressive stelar accumulation of wall phenolics. Plant Physiol. 2006, 140, 603–612. [Google Scholar] [CrossRef]
- Sarkanen, K.V.; Hergert, H.L. Lignins: Occurrence, Formation, Structure and Reactions; Wiley Interscience: New York, NY, USA, 1971. [Google Scholar]
- Rumpf, J.; Do, X.T.; Burger, R.; Monakhova, Y.; Schulze, M. Chapter 4—Types of lignin, properties, and structural characterization techniques. In Lignin-Based Materials for Biomedical Applications; Santos, H., Figueiredo, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 105–158. [Google Scholar]
- Fellenberg, C.; van Ohlen, M.; Handrick, V.; Vogt, T. The role of CCoAOMT1 and COMT1 in Arabidopsis anthers. Planta 2012, 236, 51–61. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Su, N.; Ling, F.; Xing, A.; Zhao, H.; Zhu, Y.; Wang, Y.; Deng, X.; Wang, C.; Xu, X.; Hu, Z.; et al. Lignin synthesis mediated by CCoAOMT enzymes is required for the tolerance against excess Cu in Oryza sativa. Environ. Exp. Bot. 2020, 175, 104059. [Google Scholar] [CrossRef]
- Kai, K.; Mizutani, M.; Kawamura, N.; Yamamoto, R.; Tamai, M.; Yamaguchi, H.; Sakata, K.; Shimizu, B. Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2008, 55, 989–999. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Qi, S.; Zhao, J.; Kong, J.; Xue, Z.; Sun, W.; Zeng, W. Genome-wide identification, expression profiling, and protein interaction analysis of the CCoAOMT gene family in the tea plant (Camellia sinensis). BMC Genom. 2024, 25, 238. [Google Scholar] [CrossRef] [PubMed]
- Meyermans, H.; Morreel, K.; Lapierre, C.; Pollet, B.; De Bruyn, A.; Busson, R.; Herdewijn, P.; Devreese, B.; Van Beeumen, J.; Marita, J.M.; et al. Modifications in lignin and accumulation of phenolic glucosides in poplar xylem upon down-regulation of caffeoyl-coenzyme A O-methyltransferase, an enzyme involved in lignin biosynthesis. J. Biol. Chem. 2000, 275, 36899–36909. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, Q.; Chen, C.; Jin, X.; Cao, X.; Wang, Z. Function Analysis of Caffeoyl-CoA O-Methyltransferase for Biosynthesis of Lignin and Phenolic Acid in Salvia miltiorrhiza. Appl. Biochem. Biotechnol. 2017, 181, 562–572. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Zhao, Y.; Xiang, Y.; Jiang, H.; Zhu, S.; Cheng, B. Downregulation of caffeoyl-CoA O-methyltransferase (CCoAOMT) by RNA interference leads to reduced lignin production in maize straw. Genet. Mol. Biol. 2013, 36, 540–546. [Google Scholar] [CrossRef]
- Wagner, A.; Tobimatsu, Y.; Phillips, L.; Flint, H.; Torr, K.; Donaldson, L.; Pears, L.; Ralph, J. CCoAOMT suppression modifies lignin composition in Pinus radiata. Plant J. 2011, 67, 119–129. [Google Scholar] [CrossRef]
- Ji, P.; Lin, M.; Chen, M.; Kashif, M.H.; Fan, Y.; Ali, T.; Dai, R.; Peng, C.; Wang, Z.; Liu, Z. Caffeoyl-coenzyme A O-methyltransferase mediates regulation of carbon flux fluctuations during phenylpropenes and lignin biosynthesis in the vegetative organ roots of Asarum sieboldii Miq. Plant Physiol. Biochem. 2023, 201, 107855. [Google Scholar] [CrossRef]
- Liu, S.J.; Huang, Y.H.; He, C.J.; Cheng, F.A.N.G.; Zhang, Y.W. Cloning, bioinformatics and transcriptional analysis of caffeoyl-coenzyme A 3-O-methyltransferase in switchgrass under abiotic stress. J. Integr. Agric. 2016, 15, 636–649. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, X.; Gu, X.; Peng, J.; Song, W.; Deng, B.; Cao, Y.; Hu, S. Genome-Wide Identification and Expression Analysis of Dendrocalamus farinosus CCoAOMT Gene Family and the Role of DfCCoAOMT14 Involved in Lignin Synthesis. Int. J. Mol. Sci. 2023, 24, 8965. [Google Scholar] [CrossRef]
- Kahie, M.A.; Wang, Y.; Fang, P.; Qi, J.; Lei, R.; Xu, J.; Lin, L.; Zhang, L.; Zhang, J.; Tao, A. Evolution and expression analysis of the caffeoyl-CoA 3-O-methyltransferase (CCoAOMT) gene family in jute (Corchorus L.). BMC Genom. 2023, 24, 204. [Google Scholar] [CrossRef]
- Liao, Z.; Liu, X.; Zheng, J.; Zhao, C.; Wang, D.; Xu, Y.; Sun, C. A multifunctional true caffeoyl coenzyme A O-methyltransferase enzyme participates in the biosynthesis of polymethoxylated flavones in citrus. Plant Physiol. 2023, 192, 2049–2066. [Google Scholar] [CrossRef]
- Lai, Z.; Wang, J.; Fu, Y.; Wang, M.; Ma, H.; Peng, S.; Chang, F. Revealing the role of CCoAOMT1: Fine-tuning bHLH transcription factors for optimal anther development. Sci. China Life Sci. 2024, 67, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Kühnl, T.; Koch, U.; Heller, W.; Wellmann, E. Elicitor induced S-adenosyl-l-methionine: Caffeoyl-CoA 3-O-methyltransferase from carrot cell suspension cultures-Science Direct. Plant Sci. 1989, 60, 21–25. [Google Scholar] [CrossRef]
- Pakusch, A.E.; Kneusel, R.E.; Matern, U. S-adenosyl-L-methionine:trans-caffeoyl-coenzyme A 3-O-methyltransferase from elicitor-treated parsley cell suspension cultures. Arch. Biochem. Biophys. 1989, 271, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Do, C.T.; Pollet, B.; Thévenin, J.; Sibout, R.; Denoue, D.; Barrière, Y.; Lapierre, C.; Jouanin, L. Both caffeoyl Coenzyme A 3-O-methyltransferase 1 and caffeic acid O-methyltransferase 1 are involved in redundant functions for lignin, flavonoids and sinapoyl malate biosynthesis in Arabidopsis. Planta 2007, 226, 1117–1129. [Google Scholar] [CrossRef]
- Zhao, H. Characterization of three rice CCoAOMT genes. Chin. Sci. Bull. Engl. Ed. 2004, 49, 5. [Google Scholar] [CrossRef]
- Pinçon, G.; Maury, S.; Hoffmann, L.; Geoffroy, P.; Lapierre, C.; Pollet, B.; Legrand, M. Repression of O-methyltransferase genes in transgenic tobacco affects lignin synthesis and plant growth. Phytochemistry 2001, 57, 1167–1176. [Google Scholar] [CrossRef]
- Song, J.L.; Wang, Z.Y.; Wang, Y.H.; Du, J.; Wang, C.Y.; Zhang, X.Q.; Chen, S.; Huang, X.L.; Xie, X.M.; Zhong, T.X. Overexpression of Pennisetum purpureum CCoAOMT Contributes to Lignin Deposition and Drought Tolerance by Promoting the Accumulation of Flavonoids in Transgenic Tobacco. Front. Plant Sci. 2022, 13, 884456. [Google Scholar] [CrossRef]
- Yang, G.; Pan, W.; Zhang, R.; Pan, Y.; Guo, Q.; Song, W.; Zheng, W.; Nie, X. Genome-wide identification and characterization of caffeoyl-coenzyme A O-methyltransferase genes related to the Fusarium head blight response in wheat. BMC Genom. 2021, 22, 504. [Google Scholar] [CrossRef]
- Rakoczy, M.; Femiak, I.; Alejska, M.; Figlerowicz, M.; Podkowinski, J. Sorghum CCoAOMT and CCoAOMT-like gene evolution, structure, expression and the role of conserved amino acids in protein activity. Mol. Genet. Genom. 2018, 293, 1077–1089. [Google Scholar] [CrossRef]
- Zhao, H.; Qu, C.; Zuo, Z.; Cao, L.; Zhang, S.; Xu, X.; Xu, Z.; Liu, G. Genome Identification and Expression Profiles in Response to Nitrogen Treatment Analysis of the Class I CCoAOMT Gene Family in Populus. Biochem. Genet. 2022, 60, 656–675. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Y.; Chen, D.; Liu, D.; Hu, M.; Dong, J.; Zhang, X.; Song, L.; Shen, F. The Catalase Gene Family in Cotton: Genome-Wide Characterization and Bioinformatics Analysis. Cells 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Wendel, J.F. Polyploidy and the evolutionary history of cotton. Adv. Agron. 2003, 78, 139–186. [Google Scholar]
- Hatton, D.; Sablowski, R.; Yung, M.H.; Smith, C.; Schuch, W.; Bevan, M. Two classes of cis sequences contribute to tissue-specific expression of a PAL2 promoter in transgenic tobacco. Plant J. 1995, 7, 859–876. [Google Scholar] [CrossRef]
- Kim, W.C.; Ko, J.H.; Han, K.H. Identification of a cis-acting regulatory motif recognized by MYB46, a master transcriptional regulator of secondary wall biosynthesis. Plant Mol. Biol. 2012, 78, 489–501. [Google Scholar] [CrossRef]
- Shen, H.; He, X.; Poovaiah, C.R.; Wuddineh, W.A.; Ma, J.; Mann, D.G.J.; Wang, H.; Jackson, L.; Tang, Y.; Neal Stewart, C., Jr.; et al. Functional characterization of the switchgrass (Panicum virgatum) R2R3-MYB transcription factor PvMYB4 for improvement of lignocellulosic feedstocks. New Phytol. 2012, 193, 121–136. [Google Scholar] [CrossRef]
- Wang, G.-L.; Wu, J.-Q.; Chen, Y.-Y.; Xu, Y.-J.; Zhou, C.-L.; Hu, Z.-Z.; Ren, X.-Q.; Xiong, A.-S. More or Less: Recent Advances in Lignin Accumulation and Regulation in Horticultural Crops. Agronomy 2023, 13, 2819. [Google Scholar] [CrossRef]
- Gillani, M.; Pollastri, G. Protein subcellular localization prediction tools. Comput. Struct. Biotechnol. J. 2024, 23, 1796–1807. [Google Scholar] [CrossRef]
- Huang, G.; Wu, Z.; Percy, R.G.; Bai, M.; Li, Y.; Frelichowski, J.E.; Hu, J.; Wang, K.; Yu, J.Z.; Zhu, Y. Genome sequence of Gossypium herbaceum and genome updates of Gossypium arboreum and Gossypium hirsutum provide insights into cotton A-genome evolution. Nat. Genet. 2020, 52, 516–524. [Google Scholar] [CrossRef]
- Cvijović, I.; Good, B.H.; Desai, M.M. The Effect of Strong Purifying Selection on Genetic Diversity. Genetics 2018, 209, 1235–1278. [Google Scholar] [CrossRef]
- Ruan, Q.; Wang, Y.; Xu, H.; Wang, B.; Zhu, X.; Wei, B.; Wei, X. Genome-wide identification, phylogenetic, and expression analysis under abiotic stress conditions of Whirly (WHY) gene family in Medicago sativa L. Sci. Rep. 2022, 12, 18676. [Google Scholar] [CrossRef]
- Bonthala, V.S.; Mayes, K.; Moreton, J.; Blythe, M.; Wright, V.; May, S.T.; Massawe, F.; Mayes, S.; Twycross, J. Identification of Gene Modules Associated with Low Temperatures Response in Bambara Groundnut by Network-Based Analysis. PLoS ONE 2016, 11, e0148771. [Google Scholar] [CrossRef] [PubMed]
- Wittkopp, P.J.; Kalay, G. Cis-regulatory elements: Molecular mechanisms and evolutionary processes underlying divergence. Nat. Rev. Genet. 2011, 13, 59–69. [Google Scholar] [CrossRef]
- Corchete, L.A.; Rojas, E.A.; Alonso-López, D.; De Las Rivas, J.; Gutiérrez, N.C.; Burguillo, F.J. Systematic comparison and assessment of RNA-seq procedures for gene expression quantitative analysis. Sci. Rep. 2020, 10, 19737. [Google Scholar] [CrossRef]
- Froussios, K.; Mourão, K.; Simpson, G.; Barton, G.; Schurch, N. Relative Abundance of Transcripts (RATs): Identifying differential isoform abundance from RNA-seq. F1000Research 2019, 8, 213. [Google Scholar] [CrossRef]
- Zhu, T.; Liang, C.; Meng, Z.; Sun, G.; Meng, Z.; Guo, S.; Zhang, R. CottonFGD: An integrated functional genomics database for cotton. BMC Plant Biol. 2017, 17, 101. [Google Scholar] [CrossRef]
- Letunic, I.; Doerks, T.; Bork, P. SMART: Recent updates, new developments and status in 2015. Nucleic Acids Res. 2015, 43, D257–D260. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: A community resource for phylogenetic analyses. In Proceedings of the 2011 TeraGrid Conference: Extreme Digital Discovery, Salt Lake City, UT, USA, 18–21 July 2011. [Google Scholar]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; de Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]
- Yupeng, W.; Haibao, T.; Debarry, J.D.; Xu, T.; Jingping, L.; Xiyin, W.; Tae-Ho, L.; Huizhe, J.; Barry, M.; Hui, G. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar]
- Zhang, Z. KaKs_Calculator 3.0: Calculating Selective Pressure on Coding and Non-coding Sequences. Genom. Proteom. Bioinform. 2022, 20, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Paterson, A.H. MCScanX-transposed: Detecting transposed gene duplications based on multiple colinearity scans. Bioinformatics 2013, 29, 1458–1460. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Rombauts, S.; Déhais, P.; Van Montagu, M.; Rouzé, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef]
- Xie, M.; Muchero, W.; Bryan, A.C.; Yee, K.; Guo, H.B.; Zhang, J.; Tschaplinski, T.J.; Singan, V.R.; Lindquist, E.; Payyavula, R.S.; et al. A 5-Enolpyruvylshikimate 3-Phosphate Synthase Functions as a Transcriptional Repressor in Populus. Plant Cell 2018, 30, 1645–1660. [Google Scholar] [CrossRef]
- Kang, B.H.; Anderson, C.T.; Arimura, S.I.; Bayer, E.; Bezanilla, M.; Botella, M.A.; Brandizzi, F.; Burch-Smith, T.M.; Chapman, K.D.; Dünser, K.; et al. A glossary of plant cell structures: Current insights and future questions. Plant Cell 2022, 34, 10–52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).