Chitosan, Methyl Jasmonate, and Silicon Induce Resistance to Angular Leaf Spot in Common Bean, Caused by Pseudocercospora griseola, with Expression of Defense-Related Genes and Enzyme Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth
2.2. Pathogen Inoculation
2.3. Treatment with Chemical Elicitors
2.4. Total RNA Extraction and cDNA Synthesis
2.5. Quantitative Real-Time PCR and Data Analysis
3. Results
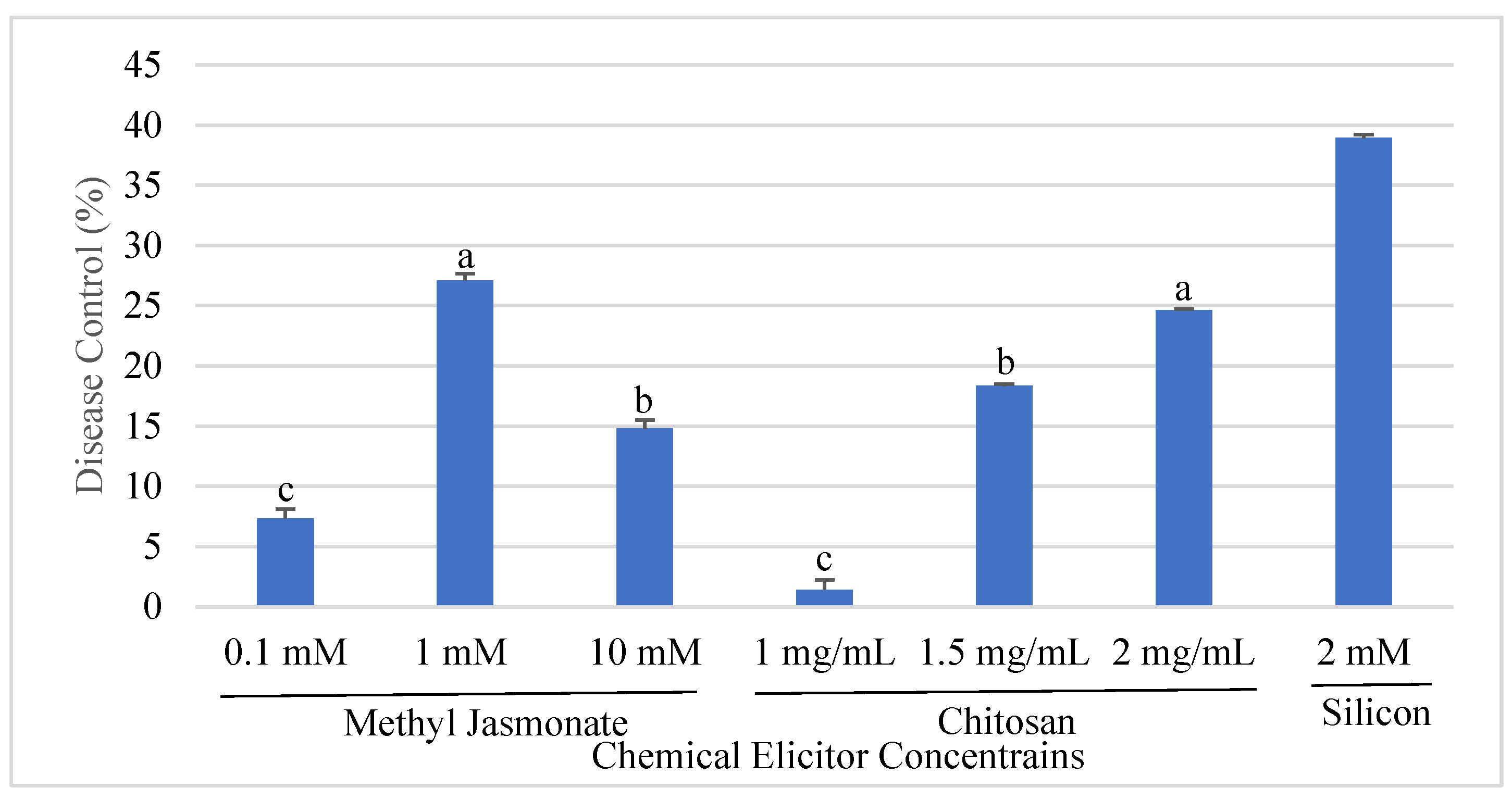
3.1. Effect of Chemical Elicitors on Disease Development of Angular Leaf Spot
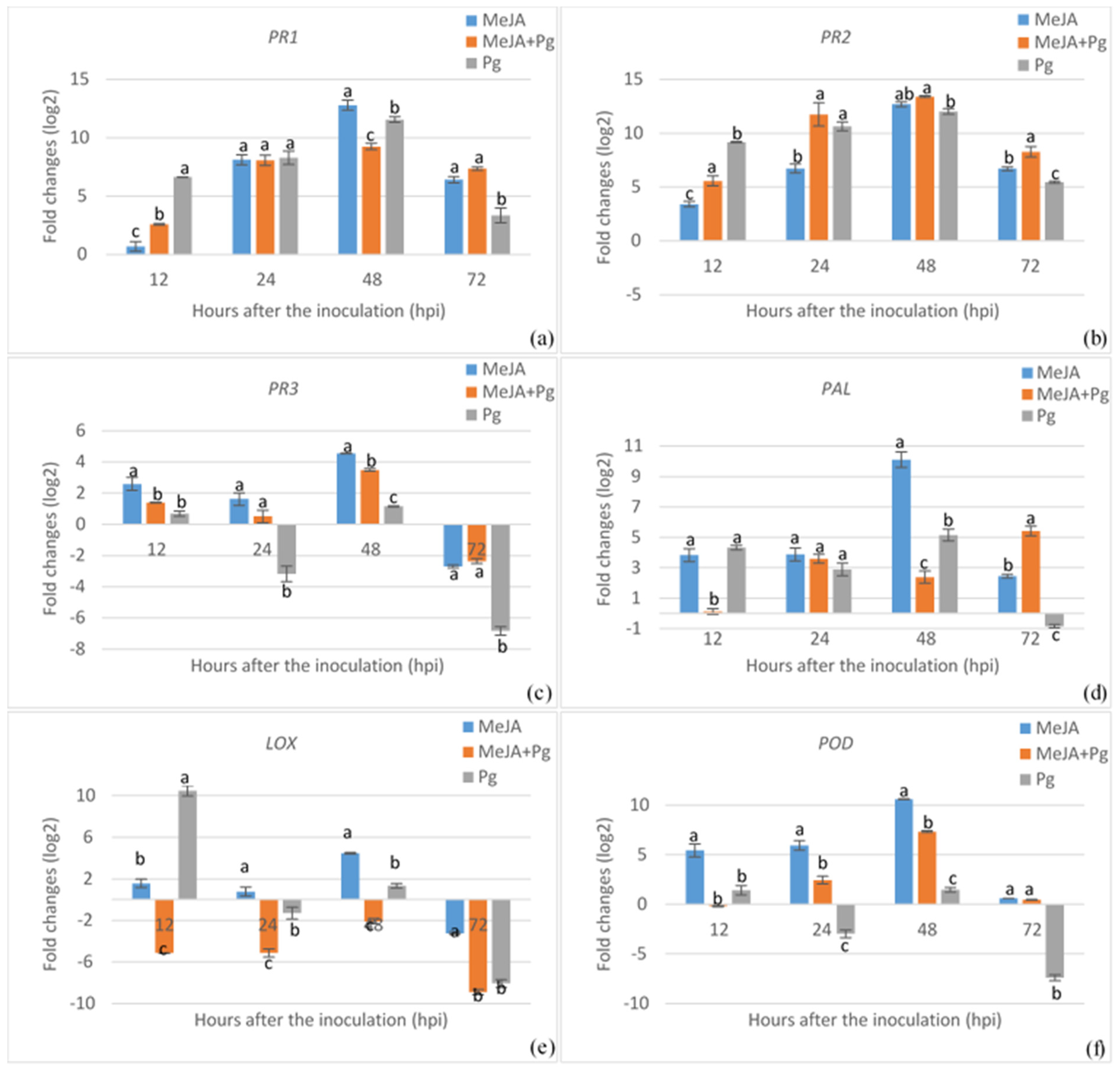
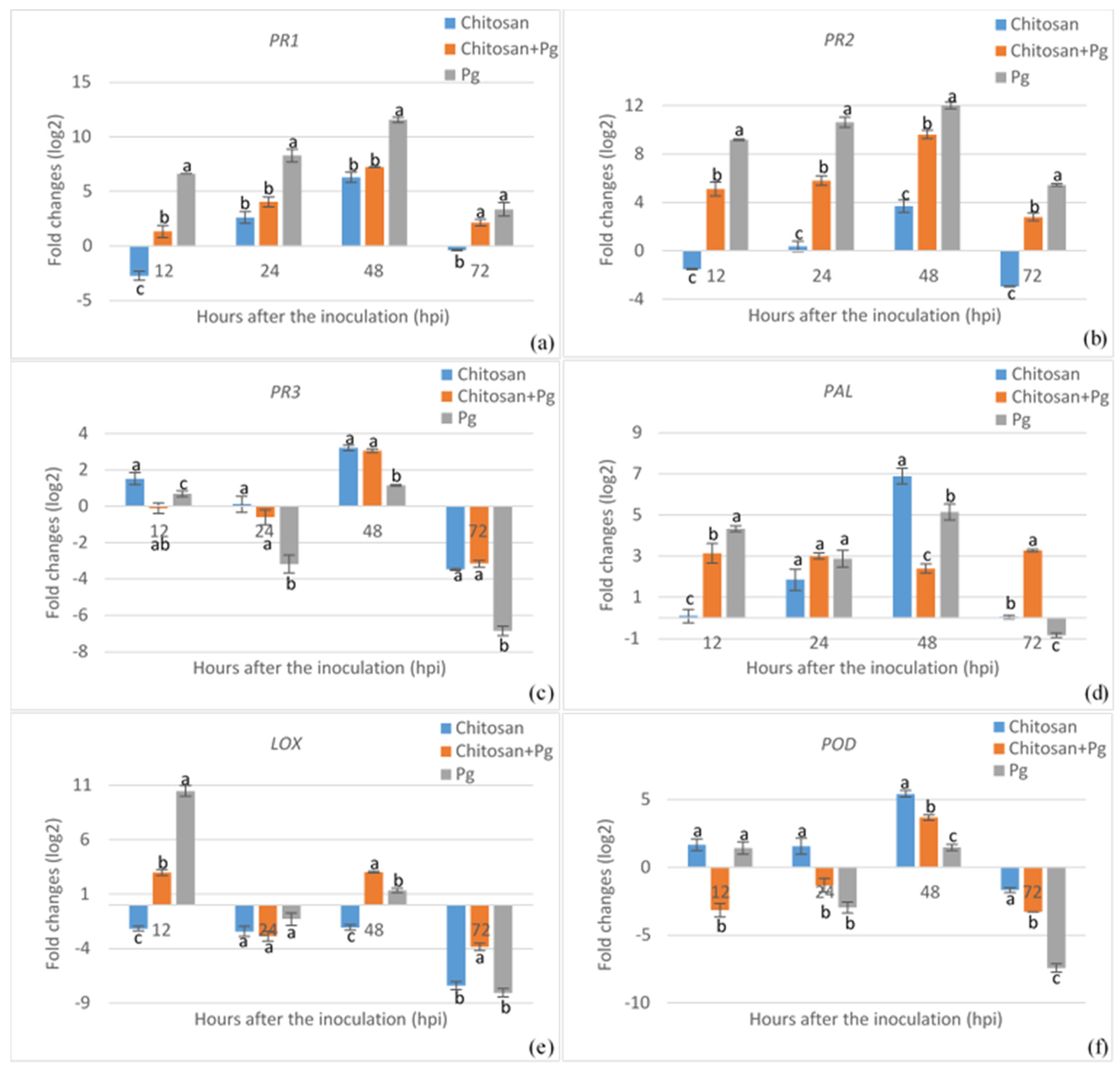
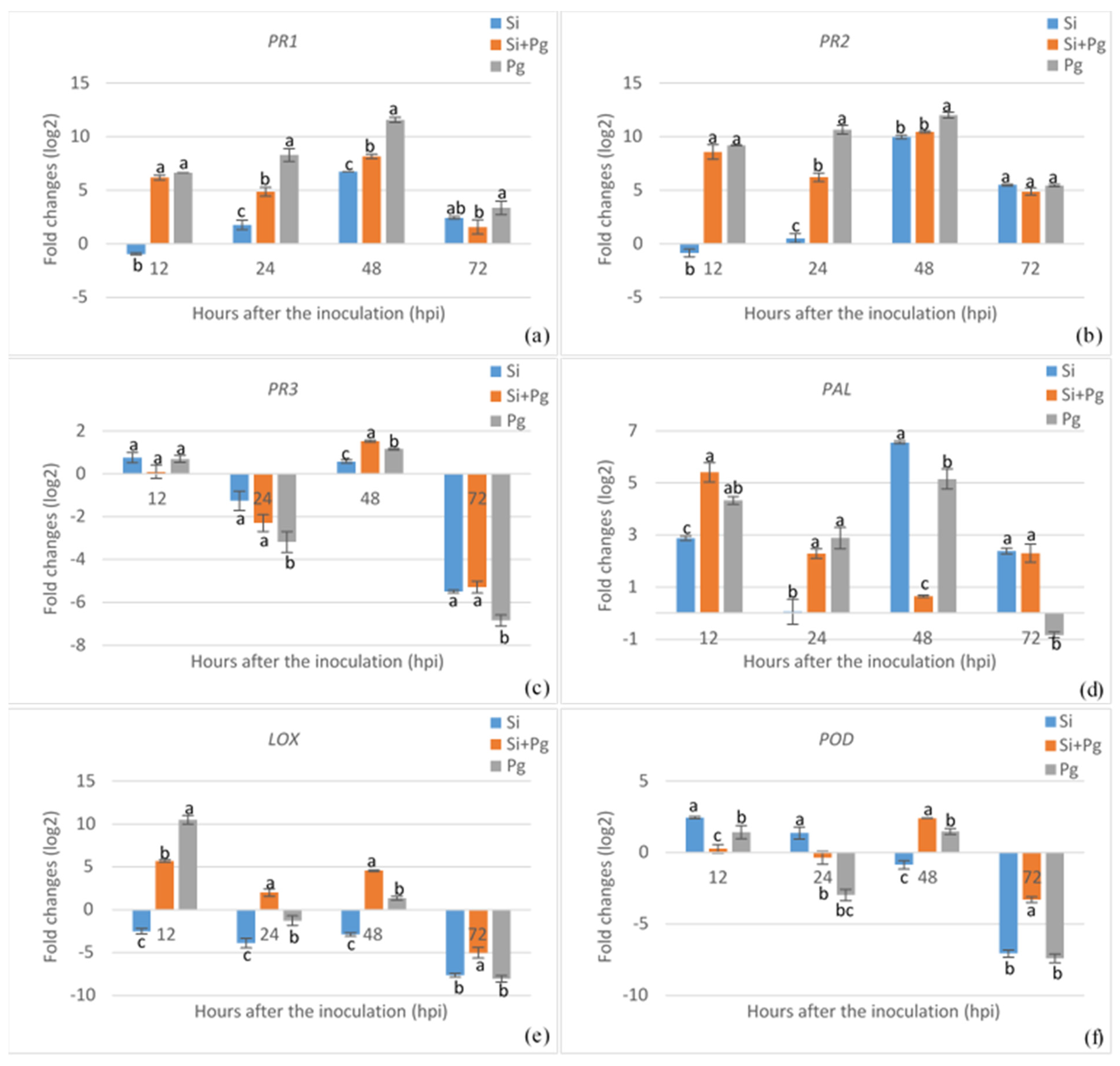
3.2. Expression Analysis of Defense-Related Genes Associated with Elicitor Treatment and Pathogen Inoculation in Common Bean
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, H.F.; Steadman, J.R.; Hall, R.; Foster, R.L. Compendium of Bean Diseases, 2nd ed.; APS Press: St. Paul, MN, USA, 2005; p. 109. [Google Scholar]
- Opio, F.; Ugen, M.A.; Kyamanywa, S.; David, S.; Mugisa-Mutetikka, M.; Mukiibi, J.K. Beans; Agriculture in Uganda: Kampala, Uganda, 2001; Volume 2, pp. 162–187. [Google Scholar]
- Singh, S.P.; Schwartz, H.F. Breeding common bean for resistance to diseases: A review. Crop Sci. 2010, 50, 2200–2223. [Google Scholar] [CrossRef]
- Garcia, P.A.V.; Carneiro, M.S.; Sartorado, A. Phaeoisariopsis griseola virulence pattern and RAPD diversity. Ann. Rep. Bean Improv. Coop. 2006, 49, 209–210. [Google Scholar]
- Ddamulira, G. Pathogen variability and new sources of resistance to angular leaf spot among bean landraces in Uganda. Afr. J. Food Agric. Nutr. Dev. 2019, 19, 13905–13927. [Google Scholar] [CrossRef]
- Mahuku, G.S.; Jara, C.; Cuasquer, J.B.; Castellanos, G. Genetic variability within Phaeoisariopsis griseola from Central America and its implications for resistance breeding of common bean. Plant Pathol. 2002, 51, 594–604. [Google Scholar] [CrossRef]
- Ddamulira, G.; Mukankusi, C.M.; Ochwo-Ssemakula, M.; Edema, R.; Sseruwagi, P.; Gepts, P.L. Identification of new sources of resistance to angular leaf spot among Uganda common bean landraces. Can. J. Plant Breed. 2014, 2, 55–65. [Google Scholar]
- Mahuku, G.S.; Jara, C.; Cajiao, C.; Beebe, S. Sources of resistance to angular leaf spot (Phaeoisariopsis griseola) in common bean core collection, wild Phaseolus vulgaris and secondary gene pool. Euphytica 2003, 130, 303–313. [Google Scholar] [CrossRef]
- Sartorato, A.; Alzate-Marin, A.L. Analysis of the pathogenic variability of Phaeoisariopsis griseola in Brazil. Annu. Rep. Bean Improv. Coop. 2004, 47, 235–237. [Google Scholar]
- Abadio, A.K.R.; Lima, S.S.; Santana, M.F.; Salomão, T.M.F.; Sartorato, A.; Mizubuti, E.S.G.; de Queiroz, M.V. Genetic diversity analysis of isolates of the fungal bean pathogen Pseudocercospora griseola from central and southern Brazil. Genet. Mol. Res. 2012, 11, 1272–1279. [Google Scholar] [CrossRef]
- Palacıoğlu, G.; Bayraktar, H.; Özer, G. Genetic variability of Colletotrichum lindemuthianum isolates from Turkey and resistance of Turkish bean cultivars. Span. J. Agric. Res. 2020, 18, e1005. [Google Scholar] [CrossRef]
- Palacıoğlu, G.; Özer, G.; Yeken, M.Z.; Çiftçi, V.; Bayraktar, H. Resistance sources and reactions of common bean (Phaseolus vulgaris L.) cultivars in Turkey to anthracnose disease. Genet. Resour. Crop Evol. 2021, 68, 3373–3381. [Google Scholar] [CrossRef]
- Walters, D.R.; Newton, A.C.; Lyon, G.D. Induced Resistance for Plant Defense: A Sustainable Approach to Crop Protection; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular mechanisms of chitosan interactions with fungi and plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [PubMed]
- Debnath, D.; Samal, I.; Mohapatra, C.; Routray, S.; Kesawat, M.S.; Labanya, R. Chitosan: An autocidal molecule of plant pathogenic fungus. Life 2022, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Steurbaut, W. Chemically modified chitosans as antimicrobial agents against some plant pathogenic bacteria and fungi. Plant Prot. Sci. 2010, 46, 149–158. [Google Scholar] [CrossRef]
- Silva, S., Jr.; Stamford, N.P.; Lima, M.A.B.; Arnaud, T.M.S.; Pintado, M.M.; Sarmento, B.F. Characterization and inhibitory activity of chitosan on hyphae growth and morphology of Botrytis cinerea plant pathogen. Int. J. Appl. Res. Nat. Prod. 2014, 7, 31–38. [Google Scholar]
- Sunpapao, A.; Pornsuriya, C. Effects of chitosan treatments on para rubber leaf fall disease caused by Phytophthora palmivora Butler-a laboratory study. Songklanakarin J. Sci. Technol. 2014, 36, 507–512. [Google Scholar]
- Hassan, O.; Chang, T. Chitosan for eco-friendly control of plant disease. Asian J. Plant Pathol. 2017, 11, 53–70. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef]
- Moret, A.; Muñoz, Z.; Garcés, S. Control of powdery mildew on cucumber cotyledons by chitosan. J. Plant Pathol. 2009, 91, 375–380. [Google Scholar]
- Gutiérrez-Martínez, P.; Bautista-Baños, S.; Berúmen-Varela, G.; Ramos-Guerrero, A.; Hernández-Ibañez, A.M. In vitro response of Colletotrichum to chitosan. Effect on incidence and quality on tropical fruit. Enzymatic expression in mango. Acta Agron. 2017, 66, 282–289. [Google Scholar] [CrossRef]
- Chun, S.C.; Chandrasekaran, M. Chitosan and chitosan nanoparticles induced expression of pathogenesis-related proteins genes enhances biotic stress tolerance in tomato. Int. J. Biol. Macromol. 2019, 125, 948–954. [Google Scholar] [CrossRef]
- Wong, M.Y.; Surendran, A.; Saad, N.M.; Burhanudin, F. Chitosan as a Biopesticide against Rice (Oryza sativa) Fungal Pathogens, Pyricularia oryzae and Rhizoctonia solani. Pertanika J. Trop. Agric. Sci. 2020, 43, 275–287. [Google Scholar]
- De Vega, D.; Holden, N.; Hedley, P.E.; Morris, J.; Luna, E.; Newton, A. Chitosan primes plant defence mechanisms against Botrytis cinerea, including expression of Avr9/Cf-9 rapidly elicited genes. Plant Cell Environ. 2021, 44, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Avanci, N.C.; Luche, D.D.; Goldman, G.H.; Goldman, M.H.D.S. Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. Genet. Mol. Res. 2010, 9, 484–505. [Google Scholar] [CrossRef] [PubMed]
- Santino, A.; Taurino, M.; De Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a) biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Pozo, M.J.; Van Loon, L.C.; Pieterse, C.M. Jasmonates-signals in plant-microbe interactions. J. Plant Growth Regul. 2005, 23, 211–222. [Google Scholar] [CrossRef]
- Laredo Alcalá, E.I.; Martínez Hernández, J.L.; Iliná, A.; Guillen Cisneros, L.; Hernández Castillo, F.D. Application of jasmonic acid as an inducer of plant resistance to pathogens. Rev. Mex. Cienc. Agrícolas 2017, 8, 673–683. [Google Scholar] [CrossRef]
- Andrade, A.; Vigliocco, A.; Alemano, S.; Miersch, O.; Botella, M.A.; Abdala, G. Endogenous jasmonates and octadecanoids in hypersensitive tomato mutants during germination and seedling development in response to abiotic stress. Seed Sci. Res. 2005, 15, 309–318. [Google Scholar] [CrossRef]
- Motallebi, P.; Tonti, S.; Niknam, V.; Ebrahimzadeh, H.; Pisi, A.; Nipoti, P.; Hashemi, M.; Prodi, A. Induction of basal resistance by methyl jasmonate against Fusarium culmorum in bread wheat. Cereal Res. Commun. 2017, 45, 248–259. [Google Scholar] [CrossRef]
- Rohwer, C.L.; Erwin, J.E. Horticultural applications of jasmonates. J. Hortic. Sci. Biotechnol. 2008, 83, 283–304. [Google Scholar] [CrossRef]
- Duan, Z.; Lv, G.; Shen, C.; Li, Q.; Qin, Z.; Niu, J. The role of jasmonic acid signalling in wheat (Triticum aestivum L.) powdery mildew resistance reaction. Eur. J. Plant Pathol. 2014, 140, 169–183. [Google Scholar] [CrossRef]
- Akbari-Vafaii, A.; Ketabchi, S.; Moradshahi, A. Effect of methyl jasmonate (MeJA) on biochemical responses of wheat seedlings infected by Fusarium culmorum. Arch. Phytopathol. Pflanzenschutz 2014, 47, 1893–1904. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, X.; Zhang, Z.; Gu, S.; Li, G.; Shi, H. Defense to Sclerotinia sclerotiorum in oilseed rape is associated with the sequential activations of salicylic acid signaling and jasmonic acid signaling. Plant Sci. 2012, 184, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Tian, S. Resistant responses of tomato fruit treated with exogenous methyl jasmonate to Botrytis cinerea infection. Sci. Hortic. 2012, 142, 38–43. [Google Scholar] [CrossRef]
- Gaige, A.R.; Ayella, A.; Shuai, B. Methyl jasmonate and ethylene induce partial resistance in Medicago truncatula against the charcoal rot pathogen Macrophomina phaseolina. Physiol. Mol. Plant Pathol. 2010, 74, 412–418. [Google Scholar] [CrossRef]
- Fauteux, F.; Remus-Borel, W.; Menzies, J.G.; Belanger, R.R. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 2005, 249, 1–6. [Google Scholar] [CrossRef]
- Yongchao, L.; Miroslav, N.; Richard, B.; Haijun, G.; Alin, S. Silicon in agriculture. In From Theory to Practice; Springer: Berlin, Germany, 2015. [Google Scholar]
- Rodrigues, F.A.; Datnoff, L.E. Silicon and Plant Diseases; Springer International Publishing: Cham, Switzerland, 2015; p. 11751. [Google Scholar]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on plant–pathogen interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef]
- Bekker, T.F.; Kaiser, C.; Merwe, R.; Labuschagne, N. In-vitro inhibition of mycelial growth of several phytopathogenic fungi by soluble potassium silicate. S. Afr. J. Plant Soil. 2009, 23, 169–172. [Google Scholar] [CrossRef]
- Shen, G.H.; Xue, Q.H.; Tang, M.; Chen, Q.; Wang, L.N.; Duan, C.M.; Xue, L.; Zhao, J. Inhibitory effects of potassium silicate on five soil-borne phytopathogenic fungi in vitro. J. Plant Dis. Prot. 2010, 117, 180–184. [Google Scholar] [CrossRef]
- Domiciano, G.P.; Cacique, I.S.; Chagas Freitas, C.; Filippi, M.C.; Damatta, F.M.; Do Vale, F.X. Alterations in gas exchange and oxidative metabolism in rice leaves infected by Pyricularia oryzae are attenuated by silicon. Phytopathology 2015, 105, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, K.J.T.; Araujo, L.; Resende, R.S.; Schurt, D.A.; Silva, W.L.D.; Rodrigues, F.D.Á. Silicon, acibenzolar-S-methyl and potassium phosphite in the control of brown spot in rice. Bragantia 2016, 75, 212–221. [Google Scholar] [CrossRef][Green Version]
- Lepolu Torlon, J.; Heckman, J.R.; Simon, J.E.; Wyenandt, C.A. Silicon soil amendments for suppressing powdery mildew on pumpkin. Sustainability 2016, 8, 293. [Google Scholar] [CrossRef]
- Whan, J.A.; Dann, E.K.; Aitken, E.A. Effects of silicon treatment and inoculation with Fusarium oxysporum f. sp. vasinfectum on cellular defences in root tissues of two cotton cultivars. Ann. Bot. 2016, 118, 219–226. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, Y.; Fang, P.; Zuo, X.; Wang, J.; Li, J.; Qian, W.; Mei, J. Silicon alleviates the disease severity of sclerotinia stem rot in rapeseed. Front. Plant Sci. 2021, 12, 721436. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Duarte, H.S.S.; Rezende, D.C.; Filho, J.W.; Korndörfer, G.H.; Zambolim, L. Foliar spray of potassium silicate on the control of angular leaf spot on beans. J. Plant Nutr. 2010, 33, 2082–2093. [Google Scholar] [CrossRef]
- Lemes, E.M.; Mackowiak, C.L.; Blount, A.; Marois, J.J.; Wright, D.L. Effect of silicon applications on soybean rust development under greenhouse and field conditions. Plant Dis. 2011, 95, 317–324. [Google Scholar] [CrossRef]
- Ashtiani, F.A.; Kadir, J.; Nasehi, A.; Rahaghi, S.R.H.; Sajili, H. Effect of Silicon on Rice Blast Disease. Pertanika J. Trop. Agric. Sci. 2012, 35, 1–12. [Google Scholar]
- Dallagnol, L.J.; Rodrigues, F.A.; Tanaka, F.A.O.; Amorim, L.; Camargo, L.E.A. Effect of potassium silicate on epidemic components of powdery mildew on melon. Plant Pathol. 2012, 61, 323–330. [Google Scholar] [CrossRef]
- Palacıoğlu, G.; Özer, G.; Yeken, M.Z.; Ören, E.; Çiftçi, V.; Bayraktar, H. Investigation of Common Bean (Phaseolus vulgaris L.) Varieties in Türkiye for Resistance Sources to Angular Leaf Spot Disease (Pseudocercospora griseola). Turk. J. Agric. Res. 2022, 9, 295–303. [Google Scholar] [CrossRef]
- Liao, H.; Yan, X.; Rubio, G.; Beebe, S.E.; Blair, M.W.; Lynch, J.P. Genetic mapping of basal root acquisition efficiency in common bean. Funct. Plant Biol. 2004, 31, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Van Schoonhoven, A.; Pastor-Corrales, M.A. Standard System for the Evaluation of Bean Germplasm; CIAT: Cali, Colômbia, 1987; p. 56. [Google Scholar]
- Oliveira, M.B.; de Andrade, R.V.; Grossi-de-Sá, M.F.; Petrofeza, S. Analysis of genes that are differentially expressed during the Sclerotinia sclerotiorum–Phaseolus vulgaris interaction. Front. Microbiol. 2015, 6, 1162. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Castro-Guerrero, N.A.; Isidra-Arellano, M.C.; Mendoza-Cozatl, D.G.; Valdés-López, O. Common bean: A legume model on the rise for unraveling responses and adaptations to iron, zinc, and phosphate deficiencies. Front. Plant Sci. 2016, 7, 600. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plants against pathogen infection: A review. Int. Sch. Res. Not. 2013, 1, 762412. [Google Scholar] [CrossRef]
- Orzali, L.; Corsi, B.; Forni, C.; Riccioni, L. Chitosan in agriculture: A new challenge for managing plant disease. In Biological Activities and Application of Marine Polysaccharides; Emad, S., Ed.; IntechOpen: London, UK, 2017; Volume 10, pp. 17–36. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent advances of chitosan applications in plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef]
- Dodgson, J.L.; Dodgson, W. Comparison of effects of chitin and chitosan for control of Colletotrichum sp. on cucumbers. J. Pure Appl. Microbiol. 2017, 11, 87–94. [Google Scholar] [CrossRef]
- Fitza, K.N.; Payn, K.G.; Steenkamp, E.T.; Myburg, A.A.; Naidoo, S. Chitosan application improves resistance to Fusarium circinatum in Pinus patula. S. Afr. J. Bot. 2013, 85, 70–78. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Lobos, T.; Cardemil, L.; Nunes-Nesi, A.; Retamales, J.; Jaakola, L.; Alberdi, M.; Ribera-Fonseca, A. Methyl jasmonate: An alternative for improving the quality and health properties of fresh fruits. Molecules 2016, 21, 567. [Google Scholar] [CrossRef]
- Kępczyńska, E.; Król, P. The phytohormone methyl jasmonate as an activator of induced resistance against the necrotroph Alternaria porri f. sp. solani in tomato plants. J. Plant Interact. 2012, 7, 307–315. [Google Scholar] [CrossRef]
- Thomma, B.P.; Eggermont, K.; Broekaert, W.F.; Cammue, B.P. Disease development of several fungi on Arabidopsis can be reduced by treatment with methyl jasmonate. Plant Physiol. Biochem. 2000, 38, 421–427. [Google Scholar] [CrossRef]
- Król, P.; Igielski, R.; Pollmann, S.; Kępczyńska, E. Priming of seeds with methyl jasmonate induced resistance to hemi-biotroph Fusarium oxysporum f. sp. lycopersici in tomato via 12-oxo-phytodienoic acid, salicylic acid, and flavonol accumulation. J. Plant Physiol. 2015, 179, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Amer, G.M.; El-Shamy, S.S.; Ghozlan, M.H. Effect of methyl jasmonate ın controllıng Alternarıa alternata and enhancıng the defense mechanısm actıvıty of tomato. J. Agric. Environ. Sci. 2024, 23, 1–24. [Google Scholar] [CrossRef]
- Saavedra, G.M.; Sanfuentes, E.; Figueroa, P.M.; Figueroa, C.R. Independent preharvest applications of methyl jasmonate and chitosan elicit differential upregulation of defense-related genes with reduced incidence of gray mold decay during postharvest storage of Fragaria chiloensis fruit. Int. J. Mol. Sci. 2017, 18, 1420. [Google Scholar] [CrossRef]
- Singh, U.B.; Malviya, D.; Singh, S.; Kumar, M.; Sahu, P.K.; Singh, H.V.; Kumar, S.; Roy, M.; Imran, M.; Rai, J.P.; et al. Trichoderma harzianum-and methyl jasmonate-induced resistance to Bipolaris sorokiniana through enhanced phenylpropanoid activities in bread wheat (Triticum aestivum L.). Front. Microbiol. 2019, 10, 1697. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yang, Y. Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol. Biochem. 2021, 165, 200–206. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Tian, D.D.; Guo, D.J.; Chen, Z.L.; Zhong, C.S.; Nikpay, A.; Singh, M.; Rajput, V.D.; Singh, R.K.; et al. Influence of silicon on biocontrol strategies to manage biotic stress for crop protection, performance, and improvement. Plants 2021, 10, 2163. [Google Scholar] [CrossRef]
- Polanco, L.R.; Rodrigues, F.A.; Nascimento, K.J.T.; Shulman, P.; Silva, L.C.; Neves, F.W.; Vale, F.X.R. Biochemical aspects of bean resistance to anthracnose mediated by silicon. Ann. Appl. Biol. 2012, 161, 140–150. [Google Scholar] [CrossRef]
- Dos Santos, C.; Franco, O.L. Pathogenesis-related proteins (PRs) with enzyme activity activating plant defense responses. Plants 2023, 12, 2226. [Google Scholar] [CrossRef]
- Palacıoğlu, G.; Özer, G.; Bayraktar, H. Expression comparisons of defense-related genes in resistant and susceptible chickpea cultivars in response to Ascochyta rabiei. Physiol. Mol. Plant Pathol. 2023, 127, 102107. [Google Scholar] [CrossRef]
- Alkan, M.; Bayraktar, H.; İmren, M.; Özdemir, F.; Lahlali, R.; Mokrini, F.; Özer, G. Monitoring of host suitability and defense-related genes in wheat to Bipolaris sorokiniana. J. Fungi 2022, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xiong, D.; Schneiter, R.; Tian, C. The function of plant PR1 and other members of the CAP protein superfamily in plant–pathogen interactions. Mol. Plant Pathol. 2023, 24, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Edwards, M.C. Molecular characterization and functional analysis of PR-1-like proteins identified from the wheat head blight fungus Fusarium graminearum. Phytopathology 2018, 108, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Silva, F.; Venancio, T.M. Pathogenesis-related protein 1 (PR-1) genes in soybean: Genome-wide identification, structural analysis and expression profiling under multiple biotic and abiotic stresses. Gene 2022, 809, 146013. [Google Scholar] [CrossRef]
- Borges, A.; Melotto, M.; Tsai, S.M.; Caldas, D.G.G. Changes in spatial and temporal gene expression during incompatible interaction between common bean and anthracnose pathogen. J. Plant Physiol. 2012, 169, 1216–1220. [Google Scholar] [CrossRef]
- Anisimova, O.K.; Shchennikova, A.V.; Kochieva, E.Z.; Filyushin, M.A. Pathogenesis-related genes of PR1, PR2, PR4, and PR5 families are involved in the response to Fusarium infection in garlic (Allium sativum L.). Int. J. Mol. Sci. 2021, 22, 6688. [Google Scholar] [CrossRef]
- Gulzar, N.; Ali, S.; Shah, M.A.; Kamili, A.N. Silicon supplementation improves early blight resistance in Lycopersicon esculentum Mill. by modulating the expression of defense-related genes and antioxidant enzymes. 3 Biotech 2021, 11, 232. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef]
- Dalalthakur, S.; Singh, P.; Singh, S.; Sasmal, A.; Deb, T.; Karmakar, S.; Dasgupta, T.; Sarkar, S.; Sarkar, S.; Ghosh, B.; et al. Role of Pathogenesis-Related (PR) Proteins in Plant Microbes Defence Mechanism. J. Surv. Fish. Sci. 2023, 10, 6344–6352. [Google Scholar] [CrossRef]
- Rahman, A.; Wallis, C.M.; Uddin, W. Silicon-induced systemic defense responses in perennial ryegrass against infection by Magnaporthe oryzae. Phytopathology 2015, 105, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Waewthongrak, W.; Pisuchpen, S.; Leelasuphakul, W. Effect of Bacillus subtilis and chitosan applications on green mold (Penicilium digitatum Sacc.) decay in citrus fruit. Postharvest Biol. Technol. 2015, 99, 44–49. [Google Scholar] [CrossRef]
- Sun, D.; Lu, X.; Hu, Y.; Li, W.; Hong, K.; Mo, Y.; Cahill, D.M.; Xie, J. Methyl jasmonate induced defense responses increase resistance to Fusarium oxysporum f. sp. cubense race 4 in banana. Sci. Hortic. 2013, 164, 484–491. [Google Scholar] [CrossRef]
- Liang, Y.C.; Sun, W.C.; Si, J.; Römheld, V. Effects of foliar-and root-applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathol. 2005, 54, 678–685. [Google Scholar] [CrossRef]
- El Hassni, M.; El Hadrami, A.; Daayf, F.; Barka, E.A.; El Hadrami, I. Chitosan, antifungal product against Fusarium oxysporum f. sp. albedinis and elicitor of defence reactions in date palm roots. Phytopathol. Mediterr. 2004, 43, 195–204. [Google Scholar]
- Obianom, C.; Romanazzi, G.; Sivakumar, D. Effects of chitosan treatment on avocado postharvest diseases and expression of phenylalanine ammonia-lyase, chitinase and lipoxygenase genes. Postharvest Biol. Technol. 2019, 147, 214–221. [Google Scholar] [CrossRef]
- Reglinski, T.; Elmer, P.A.G.; Taylor, J.T.; Wood, P.N.; Hoyte, S.M. Inhibition of Botrytis cinerea growth and suppression of botrytis bunch rot in grapes using chitosan. Plant Pathol. 2010, 59, 882–890. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, L.; Yan, H.; Kennedy, J.F.; Meng, X. Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr. Polym. 2013, 94, 272–277. [Google Scholar] [CrossRef]
- Schurt, D.A.; Cruz, M.F.; Nascimento, K.J.; Filippi, M.C.; Rodrigues, F.A. Silicon potentiates the activities of defense enzymes in the leaf sheaths of rice plants infected by Rhizoctonia solani. Trop. Plant Pathol. 2014, 39, 457–463. [Google Scholar] [CrossRef]
- Fortunato, A.A.; Debona, D.; Bernardeli, A.M.; Rodrigues, F.A. Defence-related enzymes in soybean resistance to target spot. J. Phytopathol. 2015, 163, 731–742. [Google Scholar] [CrossRef]
- Moosa, A.; Sahi, S.T.; Khan, S.A.; Malik, A.U. Salicylic acid and jasmonic acid can suppress green and blue moulds of citrus fruit and induce the activity of polyphenol oxidase and peroxidase. Folia Hortic. 2019, 31, 195–204. [Google Scholar] [CrossRef]

| Defense-Related Genes/Enzymes | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Peroxidase (POD) | TCC TTT TCA GCA CTT TCA CT | AGA AAG CAG TGT TCT TGT GG |
| Pathogenesis-related 1 (PR1) | AAA GCC AAG AGC GAT TCT CTT TTCA | GAA CAC TCT GAT TTG ATA ACA CTTC |
| β-1,3 endoglucanase (PR2) | GAA GAT GAG CTC AAA GCT GGT AA | CAA GGA TTG GCC AAA AGG TA |
| Chitinase class I (PR3) | ATT GTT GTG CCA ATC CCT TT | CAC CGC CAT ACA GTT CAA AA |
| Lipoxygenase (LOX) | AGC ACT GTG CCT GTT TTC AGT | AAC ACA CGA GAA GAT TCA ACCA |
| Phenylalanine ammonia-lyase (PAL) | GAC ACA CAA GTT GAA GCA CCA | TGC AGC TTC TTA GCA TCC TTC |
| Actin | TGC ATA CGT TGG TGA TGA GG | AGC CTT GGG GTT AAG AGG AG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacıoğlu, G. Chitosan, Methyl Jasmonate, and Silicon Induce Resistance to Angular Leaf Spot in Common Bean, Caused by Pseudocercospora griseola, with Expression of Defense-Related Genes and Enzyme Activities. Plants 2024, 13, 2915. https://doi.org/10.3390/plants13202915
Palacıoğlu G. Chitosan, Methyl Jasmonate, and Silicon Induce Resistance to Angular Leaf Spot in Common Bean, Caused by Pseudocercospora griseola, with Expression of Defense-Related Genes and Enzyme Activities. Plants. 2024; 13(20):2915. https://doi.org/10.3390/plants13202915
Chicago/Turabian StylePalacıoğlu, Gülsüm. 2024. "Chitosan, Methyl Jasmonate, and Silicon Induce Resistance to Angular Leaf Spot in Common Bean, Caused by Pseudocercospora griseola, with Expression of Defense-Related Genes and Enzyme Activities" Plants 13, no. 20: 2915. https://doi.org/10.3390/plants13202915
APA StylePalacıoğlu, G. (2024). Chitosan, Methyl Jasmonate, and Silicon Induce Resistance to Angular Leaf Spot in Common Bean, Caused by Pseudocercospora griseola, with Expression of Defense-Related Genes and Enzyme Activities. Plants, 13(20), 2915. https://doi.org/10.3390/plants13202915






