Predicting the Invasion Risk of the Highly Invasive Acacia mearnsii in Asia under Global Climate Change
Abstract
1. Introduction
2. Results
2.1. Modeling Variables and Model Performance
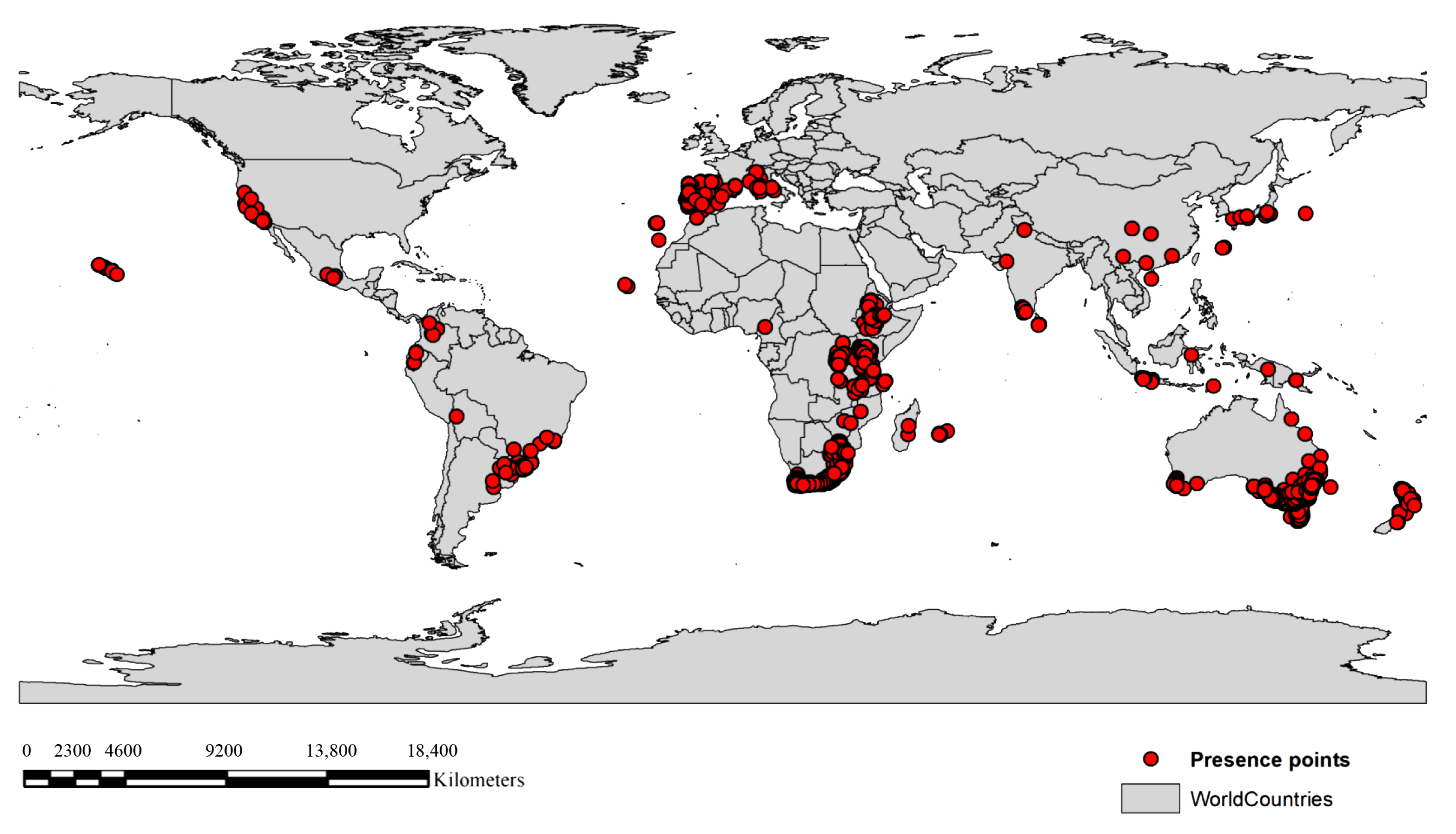
2.2. Distribution of A. mearnsii in Asia under Current Climate (1979–2013)
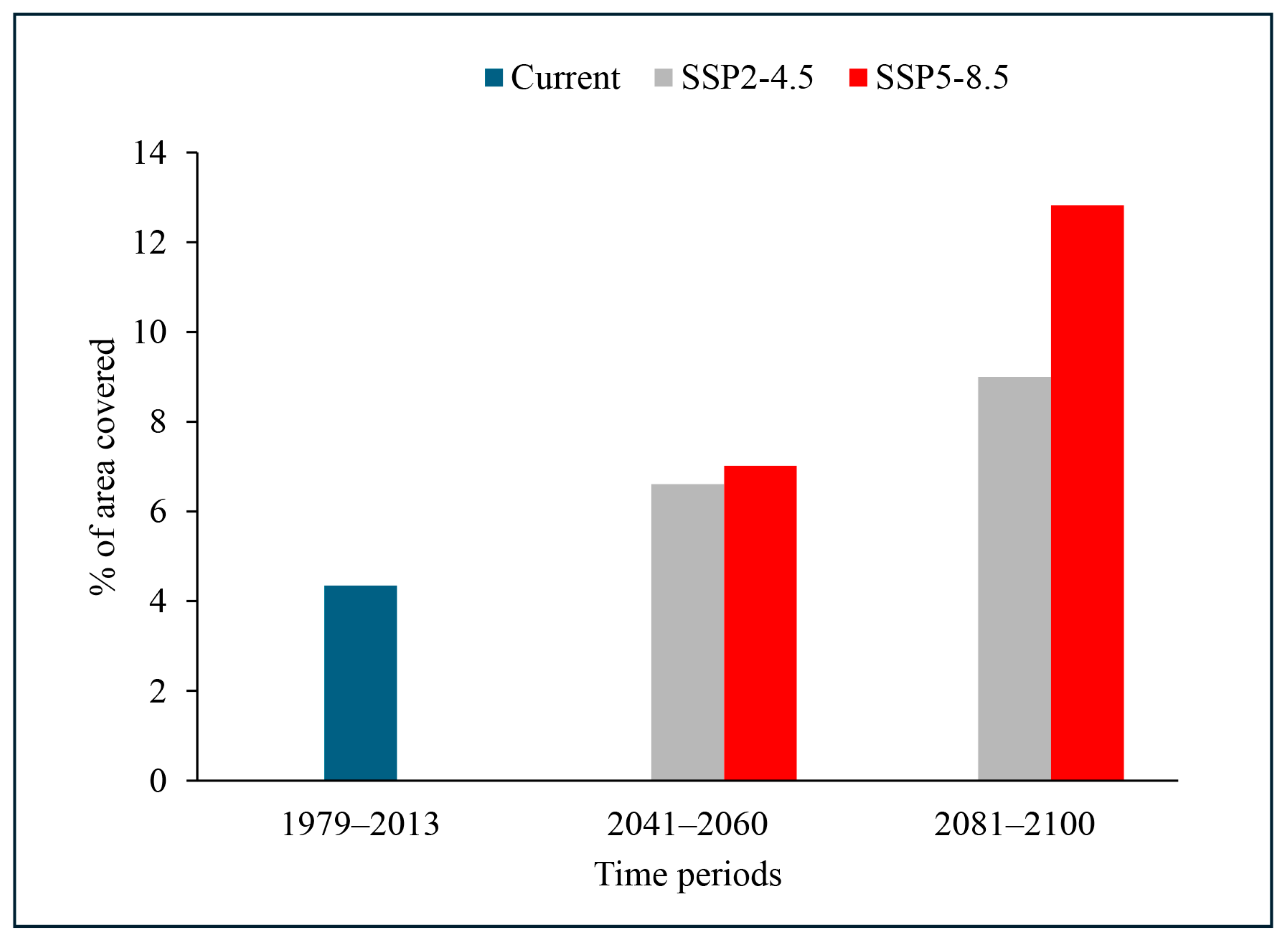
2.3. Potential Distribution of A. mearnsii under Future Climate Change Scenarios
2.4. Assessment of Mean Habitat Suitability of A. mearnsii across Various Countries in Asia
3. Discussion
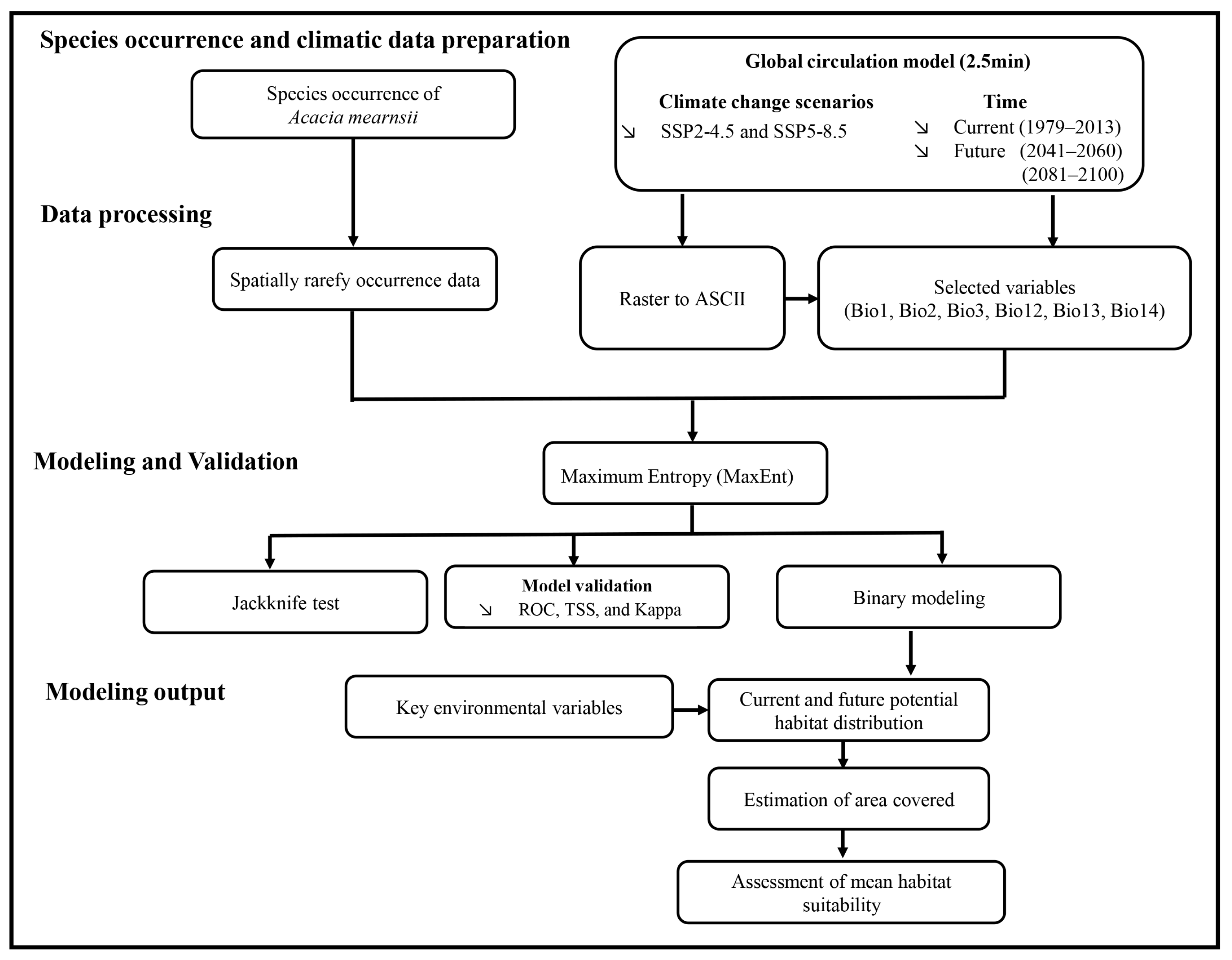
4. Materials and Methods
4.1. Occurrence Data
4.2. Environmental Variables
4.3. Model Development
4.4. Evaluating and Validating Model Results
4.5. Predicting Potential Habitat and Habitat Expansion of A. mearnsii in Asian Countries
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adhikari, P.; Jeon, J.-Y.; Kim, H.W.; Shin, M.-S.; Adhikari, P.; Seo, C. Potential impact of climate change on plant invasion in the Republic of Korea. J. Ecol. Environ. 2019, 43, 36. [Google Scholar] [CrossRef]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, A.; Kaur, S.; Kohli, R.K.; Batish, D.R. Plant Invasion and Climate Change: A Global Overview. In Plant Invasions and Global Climate Change; Springer: Berlin/Heidelberg, Germany, 2023; pp. 3–30. [Google Scholar]
- Liu, Y.; Oduor, A.M.; Zhang, Z.; Manea, A.; Tooth, I.M.; Leishman, M.R.; Xu, X.; Van Kleunen, M. Do invasive alien plants benefit more from global environmental change than native plants? Glob. Chang. Biol. 2017, 23, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- NOAA. Monthly Global Climate Report for Annual 2023. 2024. Available online: https://www.ncei.noaa.gov/access/monitoring/monthly-report/global/202313 (accessed on 5 May 2024).
- WMO. Climate Change and Extreme Weather Impacts Hit Asia Hard. 2024. Available online: https://wmo.int/news/media-centre/climate-change-and-extreme-weather-impacts-hit-asia-hard (accessed on 25 May 2024).
- Hughes, A.C. Understanding the drivers of Southeast Asian biodiversity loss. Ecosphere 2017, 8, e01624. [Google Scholar] [CrossRef]
- Dar, J.A.; Subashree, K.; Sundarapandian, S.; Saikia, P.; Kumar, A.; Khare, P.; Dayanandan, S.; Khan, M.L. Invasive species and their impact on tropical forests of Central India: A review. In Tropical Ecosystems: Structure, Functions and Challenges in the Face of Global Change; Springer: Berlin/Heidelberg, Germany, 2019; pp. 69–109. [Google Scholar]
- Qin, F.; Xue, T.T.; Liang, Y.F.; Zhang, W.D.; Liu, Q.; Chen, T.X.; Bussmann, R.W.; Han, B.C.; Yu, S.X. Present status, future trends, and control strategies of invasive alien plants in China affected by human activities and climate change. Ecography 2023, 2024, e06919. [Google Scholar] [CrossRef]
- Shrestha, B.B.; Witt, A.B.; Shen, S.; Khuroo, A.A.; Shrestha, U.B.; Naqinezhad, A. Plant invasions in Asia. In Global Plant Invasions; Springer: Berlin/Heidelberg, Germany, 2022; pp. 89–127. [Google Scholar]
- Peh, K.S.-H. Invasive species in Southeast Asia: The knowledge so far. Biodivers. Conserv. 2010, 19, 1083–1099. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, Y.H.; Poudel, A.; Hong, S.H.; Park, Y.-S. Global spatial distribution of Chromolaena odorata habitat under climate change: Random forest modeling of one of the 100 worst invasive alien species. Sci. Rep. 2023, 13, 9745. [Google Scholar] [CrossRef]
- Shrestha, S. Distribution, effect and utilization of Mikania micrantha on livelihood: Case study of Janakauli buffer zone community forest of Chitwan National Park. J. Agric. Nat. Resour. 2019, 2, 95–108. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, Y.H.; Adhikari, P.; Poudel, A.; Choi, S.H.; Yun, J.Y.; Hong, S.H. Global invasion risk assessment of Lantana camara, a highly invasive weed, under future environmental change. Glob. Ecol. Conserv. 2024, 55, e03212. [Google Scholar] [CrossRef]
- Wilgen, B.W.v.; Measey, J.; Richardson, D.M.; Wilson, J.R.; Zengeya, T.A. Biological Invasions in South Africa; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Kharivha, T.; Ruwanza, S.; Thondhlana, G. Effects of Elevated Temperature and High and Low Rainfall on the Germination and Growth of the Invasive Alien Plant Acacia mearnsii. Plants 2022, 11, 2633. [Google Scholar] [CrossRef] [PubMed]
- Bley Dalouman, H.; Broust, F.; Tran, A. Combining remote sensing and a geographic information system to map and assess the accessibility of invasive alien species forest stands: Case of Acacia mearnsii on Reunion Island. Forests 2023, 14, 2030. [Google Scholar] [CrossRef]
- Crous, C.J.; Jacobs, S.M.; Esler, K.J. Drought-tolerance of an invasive alien tree, Acacia mearnsii and two native competitors in fynbos riparian ecotones. Biol. Invasions 2012, 14, 619–631. [Google Scholar] [CrossRef]
- Moyo, H.; Fatunbi, A. Utilitarian perspective of the invasion of some South African biomes by Acacia mearnsii. Glob. J. Environ. Res. 2010, 4, 6–17. [Google Scholar]
- Pretorius, M.; Esler, K.; Holmes, P.; Prins, N. The effectiveness of active restoration following alien clearance in fynbos riparian zones and resilience of treatments to fire. S. Afr. J. Bot. 2008, 74, 517–525. [Google Scholar] [CrossRef]
- CABI. Acacia mearnsii (black wattle); CABI Compendium: Wallingford, Oxfordshire, UK, 2022. [Google Scholar] [CrossRef]
- Nyoka, B. Biosecurity in Forestry: A Case Study on the Status of Invasive Forest Trees Species in Southern Africa; FAO: Rome, Italy, 2003. [Google Scholar]
- Barbet-Massin, M.; Rome, Q.; Villemant, C.; Courchamp, F. Can species distribution models really predict the expansion of invasive species? PLoS ONE 2018, 13, e0193085. [Google Scholar] [CrossRef]
- Srivastava, V.; Lafond, V.; Griess, V.C. Species distribution models (SDM): Applications, benefits and challenges in invasive species management. CABI Rev. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Wu, Z.; Dong, H.; Li, L.; Zhao, L.; Song, N. Lineage-level species distribution model to assess the impact of climate change on the habitat suitability of Boleophthalmus pectinirostris. Front. Ecol. Evol. 2024, 12, 1364822. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.; Dudík, M.; Schapire, R. Maxent Software for Modeling Species Niches and Distributions; Version 3.4.1; American Museum of Natural History: New York, NY, USA, 2017. [Google Scholar]
- Masson-Delmotte, V.; Zhai, P.; Pirani, S.; Connors, C.; Péan, S.; Berger, N.; Caud, Y.; Chen, L.; Goldfarb, M.; Scheel Monteiro, P.M. Ipcc, 2021: Summary for policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group i to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Neukom, R.; Steiger, N.; Gómez-Navarro, J.J.; Wang, J.; Werner, J.P. No evidence for globally coherent warm and cold periods over the preindustrial Common Era. Nature 2019, 571, 550–554. [Google Scholar] [CrossRef]
- Gulev, S.K.; Thorne, P.W.; Ahn, J.; Dentener, F.J.; Domingues, C.M.; Gerland, S.; Gong, D.; Kaufman, D.S.; Nnamchi, H.C.; Quaas, J. Changing State of the Climate System; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.; Luo, Y.; Cheong, T.S.; Halim, S.A.; Chaturvedi, S.; Hashizume, M.; Insarov, G.E.; Ishikawa, Y.; Jafari, M.; Kitoh, A.; et al. Asia. In Climate Change 2022—Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2023; pp. 1457–1580. [Google Scholar]
- Rahman, M.A.; Afridi, S.; Hossain, M.B.; Rana, M.; Al Masum, A.; Rahman, M.M.; Al-Maruf, A. Nexus between heat wave, food security and human health (HFH): Developing a framework for livelihood resilience in Bangladesh. Environ. Chall. 2024, 14, 100802. [Google Scholar] [CrossRef]
- Diez, J.M.; D’Antonio, C.M.; Dukes, J.S.; Grosholz, E.D.; Olden, J.D.; Sorte, C.J.; Blumenthal, D.M.; Bradley, B.A.; Early, R.; Ibáñez, I. Will extreme climatic events facilitate biological invasions? Front. Ecol. Environ. 2012, 10, 249–257. [Google Scholar] [CrossRef]
- Gallagher, R.V.; Beaumont, L.J.; Hughes, L.; Leishman, M.R. Evidence for climatic niche and biome shifts between native and novel ranges in plant species introduced to Australia. J. Ecol. 2010, 98, 790–799. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, Y.H.; Adhikari, P.; Hong, S.H.; Park, Y.-S. Climate change-induced invasion risk of ecosystem disturbing alien plant species: An evaluation using species distribution modeling. Front. Ecol. Evol. 2022, 10, 880987. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, Y.H.; Lee, G.; Lee, D.-H.; Adhikari, P. Predicting impacts of climate change on northward range expansion of invasive weeds in South Korea. Plants 2021, 10, 1604. [Google Scholar] [CrossRef]
- Haag, I.; Jones, P.D.; Samimi, C. Central Asia’s changing climate: How temperature and precipitation have changed across time, space, and altitude. Climate 2019, 7, 123. [Google Scholar] [CrossRef]
- Ren, G.; Zhan, Y.; Ren, Y.; Wen, K.; Zhang, Y.; Sun, X.; Zhang, P.; Zheng, X.; Qin, Y.; Zhang, S. Observed changes in temperature and precipitation over Asia, 1901–2020. Clim. Res. 2023, 90, 31–43. [Google Scholar] [CrossRef]
- Geographic, N. Asia: Physical Geography; National Geographic Headquarters: Washington, DC, USA, 2024. [Google Scholar]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will climate change promote future invasions? Glob. Chang. Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef]
- Ziska, L. Invasive Species and Global Climate Change; CABI: Wallingford, UK, 2022. [Google Scholar]
- Wiersum, K. Acacia mearnsii De Wild. In Dye and Tannin-Producing Plants; PUDOC: San Vicente, Philippines, 1991; pp. 41–45. [Google Scholar]
- ISSG. Global Invasive Species Database (GISD); Invasive Species Specialist Group of the IUCN Species Survival Commission: Rome, Italy, 2007. [Google Scholar]
- Le Maitre, D.C.; Gaertner, M.; Marchante, E.; Ens, E.J.; Holmes, P.M.; Pauchard, A.; O’Farrell, P.J.; Rogers, A.M.; Blanchard, R.; Blignaut, J. Impacts of invasive Australian acacias: Implications for management and restoration. Divers. Distrib. 2011, 17, 1015–1029. [Google Scholar] [CrossRef]
- Crous, C.J.; Jacobs, S.M.; Esler, K.J. Wood anatomical traits as a measure of plant responses to water availability: Invasive Acacia mearnsii De Wild. compared with native tree species in fynbos riparian ecotones, South Africa. Trees 2012, 26, 1527–1536. [Google Scholar] [CrossRef]
- Witkowski, E. Effects of invasive alien acacias on nutrient cycling in the coastal lowlands of the Cape fynbos. J. Appl. Ecol. 1991, 28, 1–15. [Google Scholar] [CrossRef]
- HongYan; Booth, T.H.; Zuo, H. GREEN—A climatic mapping program for China and its use in forestry. In Proceedings of the international workshop, Bangkok, Thailand, 27–30 March 1995; pp. 24–29. [Google Scholar]
- Richardson, D.M.; Kluge, R.L. Seed banks of invasive Australian Acacia species in South Africa: Role in invasiveness and options for management. Perspect. Plant Ecol. Evol. Syst. 2008, 10, 161–177. [Google Scholar] [CrossRef]
- Olajuyigbe, O.O.; Afolayan, A.J. Pharmacological assessment of the medicinal potential of Acacia mearnsii De Wild.: Antimicrobial and Toxicity activities. Int. J. Mol. Sci. 2012, 13, 4255–4267. [Google Scholar] [CrossRef]
- Boudiaf, I.; Baudoin, E.; Sanguin, H.; Beddiar, A.; Thioulouse, J.; Galiana, A.; Prin, Y.; Le Roux, C.; Lebrun, M.; Duponnois, R. The exotic legume tree species, Acacia mearnsii, alters microbial soil functionalities and the early development of a native tree species, Quercus suber, in North Africa. Soil Biol. Biochem. 2013, 65, 172–179. [Google Scholar] [CrossRef]
- Olajuyigbe, O.O.; Afolayan, A.J. Synergistic interactions of methanolic extract of Acacia mearnsii De Wild. with antibiotics against bacteria of clinical relevance. Int. J. Mol. Sci. 2012, 13, 8915–8932. [Google Scholar] [CrossRef]
- Ntshanka, N.M.; Ejidike, I.P.; Mthunzi, F.M.; Moloto, M.J.; Mubiayi, K.P. Investigation into the phytochemical profile, antioxidant and antibacterial potentials of Combretum molle and Acacia mearnsii leaf parts. Biomed. Pharmacol. J. 2020, 13, 1683–1694. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Dyer, C.; Hoffmann, J.H.; Ivey, P.; Le Maitre, D.C.; Moore, J.L.; Richardson, D.M.; Rouget, M.; Wannenburgh, A.; Wilson, J.R. National-scale strategic approaches for managing introduced plants: Insights from Australian acacias in South Africa. Divers. Distrib. 2011, 17, 1060–1075. [Google Scholar] [CrossRef]
- Impson, F.; Kleinjan, C.; Hoffmann, J.; Post, J.; Wood, A. Biological control of Australian Acacia species and Paraserianthes lophantha (Willd.) Nielsen (Mimosaceae) in South Africa. Afr. Entomol. 2011, 19, 186–207. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Hill, D.J.; Dolan, A.M.; Carnaval, A.C.; Haywood, A.M. PaleoClim, high spatial resolution paleoclimate surfaces for global land areas. Sci. Data. 2018, 5, 180254. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L. The Beijing climate center climate system model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Zhao, H.; Li, M.; Han, W. Predicting the distribution of the invasive species Leptocybe invasa: Combining MaxEnt and geodetector models. Insects 2021, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Change 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Meinshausen, M.; Nicholls, Z.R.; Lewis, J.; Gidden, M.J.; Vogel, E.; Freund, M.; Beyerle, U.; Gessner, C.; Nauels, A.; Bauer, N. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Model Dev. 2020, 13, 3571–3605. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Araújo, M.B.; Peterson, A.T. Uses and misuses of bioclimatic envelope modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, Y.-H.; Park, Y.-S.; Hong, S.-H. Assessment of the spatial invasion risk of intentionally introduced alien plant species (IIAPS) under environmental change in South Korea. Biology 2021, 10, 1169. [Google Scholar] [CrossRef]
- Adhikari, P.; Shin, M.-S.; Jeon, J.-Y.; Kim, H.W.; Hong, S.; Seo, C. Potential impact of climate change on the species richness of subalpine plant species in the mountain national parks of South Korea. J. Ecol. Environ. 2018, 42, 36. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, Y.-H.; Poudel, A.; Lee, G.; Hong, S.-H.; Park, Y.-S. Predicting the impact of climate change on the habitat distribution of Parthenium hysterophorus around the world and in South Korea. Biology 2023, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Poudel, A.; Adhikari, P.; Na, C.S.; Wee, J.; Lee, D.-H.; Lee, Y.H.; Hong, S.H. Assessing the Potential Distribution of Oxalis latifolia, a Rapidly Spreading Weed, in East Asia under Global Climate Change. Plants 2023, 12, 3254. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Peterson, A.T.; Soberón, J.; Overton, J.; Aragón, P.; Lobo, J.M. Use of niche models in invasive species risk assessments. Biol. Invasions 2011, 13, 2785–2797. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Adhikari, P.; Jeon, J.-Y.; Kim, H.W.; Oh, H.-S.; Adhikari, P.; Seo, C. Northward range expansion of southern butterflies according to climate change in South Korea. J. Korean Soc. Clim. Change 2020, 11, 643–656. [Google Scholar] [CrossRef]
- Araujo, M.B.; Guisan, A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B. Niche properties and geographical extent as predictors of species sensitivity to climate change. Glob. Ecol. Biogeogr. 2005, 14, 347–357. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]



| Variables | Variable Descriptions | Units | Model Contribution (%) a |
|---|---|---|---|
| Bio1 | Annual mean temperature | °C | 19.64 |
| Bio2 | Mean diurnal temperature range | °C | 0.55 |
| Bio3 | Isothermality (BIO2/BIO7; * 100) | % | 51.72 |
| Bio12 | Mean annual precipitation | mm | 8.28 |
| Bio13 | Precipitation in wettest month | mm | 0.06 |
| Bio14 | Precipitation in driest month | mm | 19.74 |
| Risk to Countries | Current a (1979–2013) | Change in Risk Categories (%) | |||
|---|---|---|---|---|---|
| SSP2-4.5 | SSP5-8.5 | ||||
| 2041–2060 | 2081–2100 | 2041–2060 | 2081–2100 | ||
| Unsuitable | 417,059 | −1.25 | −73.31 | −71.51 | −73.31 |
| Low | 2,897,555 | −0.55 | 9.29 | 9.96 | 7.18 |
| Moderate | 108,204 | −5.41 | −29.16 | −47.13 | −37.47 |
| High | 8115 | 238.28 | 284.93 | 769.43 | 824.19 |
| Very high | 5009 | 155.60 | 896.51 | −36.57 | 1426.25 |
| S.N | Countries | Unsuitable Habitat | Low Suitability | Moderate Suitability | High Suitability | Very High Suitability |
|---|---|---|---|---|---|---|
| 1 | Armenia |  | ||||
| 2 | Azerbaijan |  | ||||
| 3 | China |  | ||||
| 4 | Georgia |  | ||||
| 5 | Indonesia |  | ||||
| 6 | Iraq |  | ||||
| 7 | Japan |  | ||||
| 8 | Kazakhstan |  | ||||
| 9 | Kyrgyzstan |  | ||||
| 10 | Laos |  | ||||
| 11 | Malaysia |  | ||||
| 12 | Myanmar |  | ||||
| 13 | Nepal |  | ||||
| 14 | North Korea |  | ||||
| 15 | Philippines |  | ||||
| 16 | Republic of Korea |  | ||||
| 17 | Tajikistan |  | ||||
| 18 | Turkey |  | ||||
| 19 | Turkmenistan |  | ||||
| 20 | Uzbekistan |  | ||||
| 21 | Vietnam |  | ||||
| S.N | Regions | Unsuitable Habitat | Low Suitability | Moderate Suitability | High Suitability | Very High Suitability |
|---|---|---|---|---|---|---|
| 1 | East Asia |  | ||||
| 2 | Southeast Asia |  | ||||
| 3 | West Asia |  | ||||
| 4 | Central Asia |  | ||||
| 5 | North Asia |  | ||||
| 6 | South Asia |  | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, A.; Adhikari, P.; Adhikari, P.; Choi, S.H.; Yun, J.Y.; Lee, Y.H.; Hong, S.H. Predicting the Invasion Risk of the Highly Invasive Acacia mearnsii in Asia under Global Climate Change. Plants 2024, 13, 2846. https://doi.org/10.3390/plants13202846
Poudel A, Adhikari P, Adhikari P, Choi SH, Yun JY, Lee YH, Hong SH. Predicting the Invasion Risk of the Highly Invasive Acacia mearnsii in Asia under Global Climate Change. Plants. 2024; 13(20):2846. https://doi.org/10.3390/plants13202846
Chicago/Turabian StylePoudel, Anil, Pradeep Adhikari, Prabhat Adhikari, Sue Hyuen Choi, Ji Yeon Yun, Yong Ho Lee, and Sun Hee Hong. 2024. "Predicting the Invasion Risk of the Highly Invasive Acacia mearnsii in Asia under Global Climate Change" Plants 13, no. 20: 2846. https://doi.org/10.3390/plants13202846
APA StylePoudel, A., Adhikari, P., Adhikari, P., Choi, S. H., Yun, J. Y., Lee, Y. H., & Hong, S. H. (2024). Predicting the Invasion Risk of the Highly Invasive Acacia mearnsii in Asia under Global Climate Change. Plants, 13(20), 2846. https://doi.org/10.3390/plants13202846








