Karyotype’s Rearrangement in Some Hybrids of the Orchidinae Subtribe
Abstract
1. Introduction
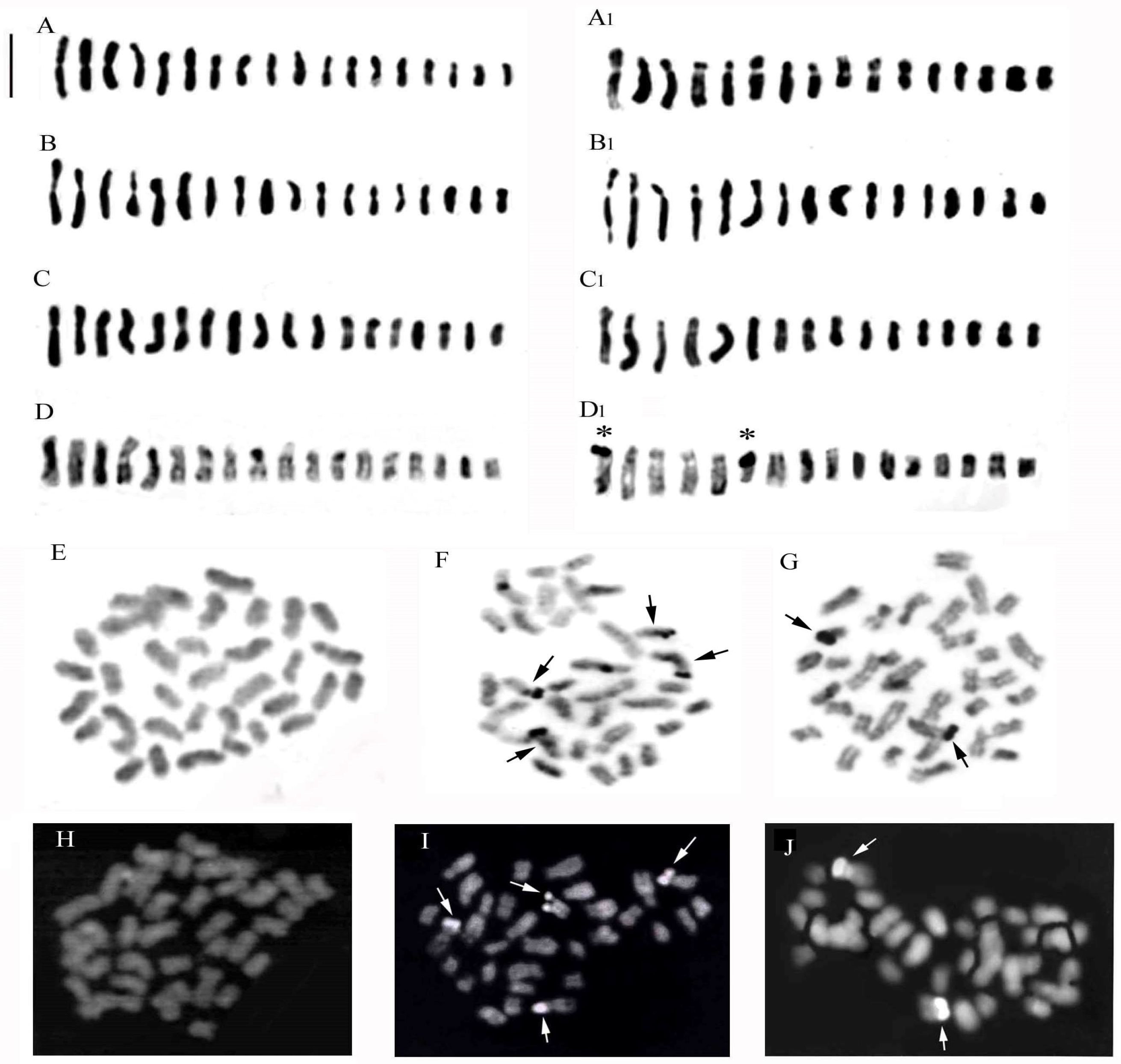
2. Results
2.1. Karyomorphological Analysis
2.1.1. Ophrys ×salentina O.Danesch & E.Danesch
2.1.2. Ophrys ×franciniae Bianco, Medagli, D’Emerico & Ruggiero
2.1.3. Ophrys ×sommieri E.G.Camus ex Cortesi
2.1.4. Anacamptis ×semisaccata nothosubsp. murgiana (Medagli, D’Emerico, Ruggiero & Bianco) H.Kretzschmar, Eccarius & H.Dietr.
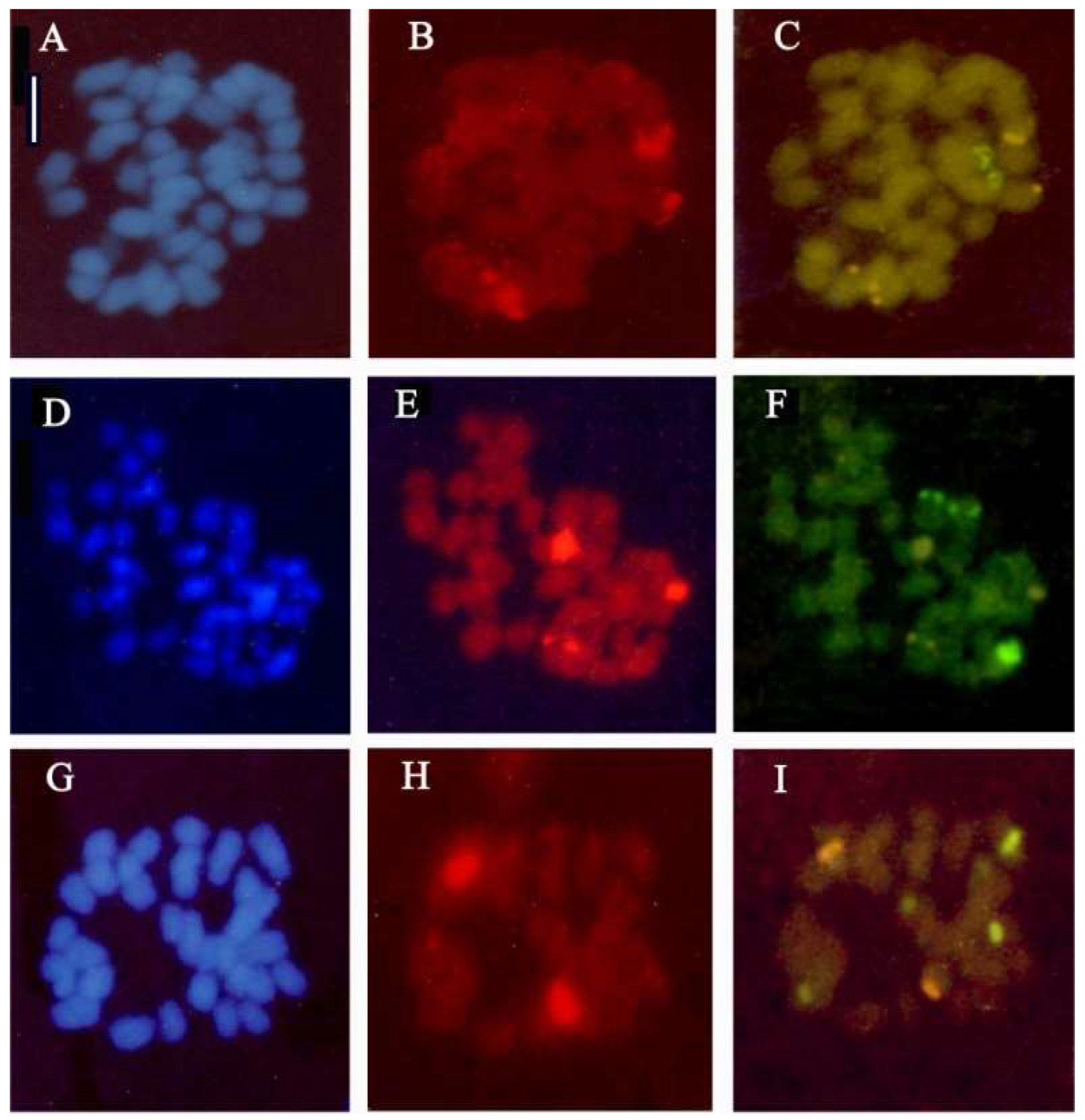
2.1.5. Anacamptis ×gennarii
2.1.6. ×Serapicamptis nelsoniana (Bianco, D’Emerico, Medagli & Ruggiero) J.M.H.Shaw
2.1.7. Ophrys tardans O.Danesch & E.Danesch
2.2. Fluorescence In Situ Hybridization (FISH) in Anacamptis ×gennarii and Its Parental Species
2.3. Diagram of the Morphometric Parameters
3. Discussion and Conclusions
3.1. Interspecific Hybrids in Ophrys
3.2. Interspecific Hybrids in Anacamptis
3.3. Intergeneric Hybrid between Anacamptis and Serapias
3.4. Homoploid Hybridization or Epigenetic Origin?
4. Materials and Methods
4.1. Natural Hybrids and Parental Species
4.2. Cytological Analysis
4.2.1. Feulgen Technique, Giemsa C-Banding, and Hoechst Fluorochrome
4.2.2. Fluorescence In Situ Hybridization
4.3. Chromosome Numbers and Karyotype Parameters
4.4. Nomenclature
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.L. Natural Hybridization and Evolution; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Gross, B.L.; Rieseberg, L.H. The ecological genetics of homoploid hybrid speciation. J. Hered. 2005, 96, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Paun, O.; Forest, F.; Fay, M.F.; Chase, M.W. Hybrid speciation in angiosperms: Parental divergence drives ploidy. New Phytol. 2009, 182, 507–518. [Google Scholar] [CrossRef]
- Mayr, E. Systematics and the Origin of Species; Columbia University Press: New York, NY, USA, 1942. [Google Scholar]
- Rieseberg, L.H.; Raymond, O.; Rosenthal, D.M.; Lai, Z.; Livingstone, K.; Nakazato, T.; Durphy, J.; Schwarzbach, A.E.; Donovan, L.A.; Lexer, C. Major ecological transitions in wild sunflowers facilitated by hybridization. Science 2003, 301, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Cronn, R.; Wendel, J.F. Cryptic trysts, genomic mergers, and plant speciation. New Phytol. 2004, 161, 133–142. [Google Scholar] [CrossRef]
- Gompert, Z.; Fordyce, J.A.; Forister, M.L.; Shapiro, A.M.; Nice, C.C. Homoploid hybrid speciation in an extreme habitat. Science 2006, 314, 1923–1925. [Google Scholar] [CrossRef]
- Yang, R.; Folk, R.; Zhang, N.; Gong, X. Homoploid hybridization of plants in the Hengduan mountains region. Ecol. Evol. 2019, 9, 8399–8410. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.R.; Waser, N.M. Evolutionary dynamics of an Ipomopsis hybrid zone: Confronting models with lifetime fitness data. Am. Nat. 2007, 169, 298–310. [Google Scholar] [CrossRef]
- Soltis, P.S.; Soltis, D.E. The role of hybridization in plant speciation. Ann. Rev. Plant Biol. 2009, 60, 561–588. [Google Scholar] [CrossRef]
- Yakimowski, S.B.; Rieseberg, L.H. The role of homoploid hybridization in evolution: A century of studies synthesizing genetics and ecology. Am. J. Bot. 2014, 101, 1247–1258. [Google Scholar] [CrossRef]
- Stebbins, G.L. The inviability, weakness and sterility of interspecific hybrids. Adv. Genet. 1958, 9, 147–215. [Google Scholar]
- Rieseberg, L.H.; Carney, S.E. Plant hybridization. New Phytol. 1998, 140, 599–624. [Google Scholar] [CrossRef]
- Bushell, C.; Spielman, M.; Scott, R.J. The basis of natural and artificial postzygotic hybridization barriers in Arabidopsis species. Plant Cell 2003, 15, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Cortis, P.; Vereecken, N.J.; Schiestl, F.P.; Lumaga, M.R.B.; Scrugli, A.; Cozzolino, S. Pollinator convergence and the nature of species’ boundaries in sympatric Sardinian Ophrys (Orchidaceae). Ann. Bot. 2009, 104, 497–506. [Google Scholar] [CrossRef]
- Scopece, G.; Cozzolino, S.; Johnson, S.D.; Schiestl, F.P. Pollination Efficiency and the Evolution of Specialized Deceptive Pollination Systems. Am. Nat. 2010, 175, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Rezende, L.; Suzigan, J.; Amorim, F.W.; Moraes, A.P. Can plant hybridization and polyploidy lead to pollinator shift? Acta Bot. Bras. 2020, 34, 229–242. [Google Scholar] [CrossRef]
- Bomblies, K.; Weigel, D. Arabidopsis and relatives as models for the study of genetic and genomic incompatibilities. Phil. Trans. R. Soc. B 2010, 365, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Arditti, J. An history of orchid hybridization, seed germination and tissue culture. Bot. J. Linn. Soc. 1984, 89, 359–381. [Google Scholar] [CrossRef]
- Grantham, M.A.; Ford, B.A.; Worley, A.C. Pollination and fruit set in two rewardless slipper orchids and their hybrids (Cypripedium, Orchidaceae): Large yellow flowers outperform small white flowers in the northern tall grass prairie. Plant Biol. 2019, 21, 997–1007. [Google Scholar] [CrossRef]
- Schiestl, F.P. On the success of a swindle: Pollination by deception in orchids. Naturwissenschaften 2005, 92, 255–264. [Google Scholar] [CrossRef]
- Delforge, P. Guide des Orchidées d’Europe, d’Afrique du Nord et du Proche-Orient [Guide to Orchids from Europe, North Africa and the Near East], 4th ed.; Delachaux et Niestlé: Lausanne, Switzerland, 2016; 544p. [Google Scholar]
- Kullenberg, B. Studies in Ophrys Pollination; Almquist & Wiksells Boktrykeri AB: Uppsala, Sweden, 1961. [Google Scholar]
- Schiestl, F.P.; Ayasse, M.; Paulus, H.F.; Erdmann, D.; Francke, W. Variation of floral scent emission and post pollination changes in individual flowers of Ophrys sphegodes subsp. sphegodes. J. Chem. Ecol. 1997, 23, 2881–2895. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Ayasse, M.; Paulus, H.F.; Löfstedt, C.; Hansson, B.S.; Ibarra, F.; Francke, W. Orchid pollination by sexual swindle. Nature 1999, 399, 421–422. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Peakall, R.; Mant, J.; Ibarra, F.; Schulz, C.; Francke, S.; Francke, W. The chemistry of sexual deception in an orchid-wasp pollination system. Science 2003, 302, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Ayasse, M.; Schiestl, F.P.; Paulus, H.F.; Ibarra, F.; Francke, W. Pollinator attraction in a sexually deceptive orchid by means of unconventional chemicals. Proc. R. Soc. Lond. B 2003, 270, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Vereecken, N.J.; Cozzolino, S.; Schiestl, F.P. Hybrid floral scent drives pollinator shift in sexually deceptive orchids. BMC Evol. Biol. 2010, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Paulus, H.F.; Gack, C. Pollinators as prepollinating isolation factors: Evolution and speciation in Ophrys (Orchidaceae). Isr. J. Bot. 1990, 39, 43–79. [Google Scholar]
- Scopece, G.; Musacchio, A.; Widmer, A.; Cozzolino, S. Patterns of reproductive isolation in Mediterranean orchids. Evolution 2007, 61, 2623–2642. [Google Scholar] [CrossRef]
- Cozzolino, S.; Scopece, G. Specificity in pollination and consequences for postmating reproductive isolation in deceptive Mediterranean orchids. Phil. Trans. R. Soc. B 2008, 263, 3037–3046. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Cozzolino, S. Evolution of sexual mimicry in the Orchidinae: The role of preadaptations in the attraction of male bees as pollinators. BMC Evol. Biol. 2008, 8, 27. [Google Scholar] [CrossRef]
- Stace, C.A. Cytology and cytogenetics as a fundamental taxonomic resource for the 20th and 21st centuries. Taxon 2000, 49, 451–479. [Google Scholar] [CrossRef]
- D’Emerico, S. Orchideae, Cytogenetics. In Genera Orchidacearum 2: Orchidoideae; Pridgeon, A.M., Cribb, P.J., Chase, M.C., Rasmussen, F.N., Eds.; Oxford University Press: Oxford, UK, 2001; Part 1; p. 335. [Google Scholar]
- Baltisberger, M.; Widmer, A. Karyological data of angiosperms from Romania. Willdenowia 2009, 39, 353–363. [Google Scholar] [CrossRef]
- Baltisberger, M.; Hörandl, E. Karyotype evolution supports the molecular phylogeny in the genus Ranunculus (Ranunculaceae). Perspect. Plant Ecol. Evol. Syst. 2016, 18, 1–14. [Google Scholar] [CrossRef][Green Version]
- Felix, L.P.; Guerra, M. Variation in chromosome number and the basic number of subfamily Epidendroideae (Orchidaceae). Bot. J. Linn. Soc. 2010, 163, 234–278. [Google Scholar] [CrossRef]
- D’Emerico, S. Cytogenetic diversity in Orchis s.l. and allied genera (Orchidinae, Orchidaceae). In Plant Genome, Biodiversity, and Evolution, Phanerogams (Higher Groups); Sharma, A.K., Sharma, A., Eds.; Science, Inc.: Enfield, NH, USA, 2005; Volume 1 (Part B), pp. 61–87. [Google Scholar]
- Turco, A.; Albano, A.; Medagli, P.; Wagensommer, R.P.; D’Emerico, S. Comparative Cytogenetic of the 36-Chromosomes Genera of Orchidinae Subtribe (Orchidaceae) in the Mediterranean Region: A Summary and New Data. Plants 2023, 12, 2798. [Google Scholar] [CrossRef] [PubMed]
- Turco, A.; Wagensommer, R.P.; Medagli, P.; Albano, A.; D’Emerico, S. Advances in the Study of Orchidinae Subtribe (Orchidaceae) Species with 40,42-Chromosomes in the Mediterranean Region. Diversity 2024, 16, 41. [Google Scholar] [CrossRef]
- Turco, A.; Albano, A.; Medagli, P.; Wagensommer, R.P.; D’Emerico, S. Comparative chromosome studies in species of subtribe Orchidinae (Orchidaceae). Comp. Cytogen. 2021, 15, 507–525. [Google Scholar] [CrossRef]
- Peruzzi, L.; Eroğlu, H.E. Karyotype asymmetry: Again, how to measure and what to measure? Comp. Cytogen. 2013, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peruzzi, L.; Altınordu, F. A proposal for a multivariate quantitative approach to infer karyological relationships among taxa. Comp. Cytogen. 2014, 8, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Greilhuber, J.; Ehrendorfer, F. Chromosome numbers and evolution in Ophrys (Orchidaceae). Plant Syst. Evol. 1975, 124, 125–138. [Google Scholar] [CrossRef]
- Del Prete, C. Contributi alla conoscenza delle orchidaceae d’Italia. VI. Tavole cromosomiche delle orchidaceae italiane con alcune considerazioni citosistematiche sui generi Ophrys, Orchis e Serapias. Inform. Bot. Ital. 1978, 10, 379–389. [Google Scholar]
- Bianco, P.; D’Emerico, S.; Medagli, P.; Ruggiero, L. Karyological studies of some taxa of the genus Ophrys (Orchidaceae) from Apulia (Italy). Caryologia 1989, 42, 57–63. [Google Scholar] [CrossRef]
- Bianco, P.; D’Emerico, S.; Medagli, P.; Ruggiero, L. Polyploidy and aneuploidy in Ophrys, Orchis and Anacamptis (Orchidaceae). Plant Syst. Evol. 1991, 178, 235–245. [Google Scholar] [CrossRef]
- D’Emerico, S.; Pignone, D.; Bartolo, G.; Pulvirenti, S.; Terrasi, C.; Stuto, S.; Scrugli, A. Karyomorphology, heterochromatic patterns and evolution in the genus Ophrys (Orchidaceae). Bot. J. Linn. Soc. 2005, 148, 87–99. [Google Scholar] [CrossRef][Green Version]
- Turco, A.; D’Emerico, S.; Medagli, P.; Albano, A. A cytological study on Ophrys (Orchidaceae) in Italy: New evidence and the importance of polyploidy. Plant Biosyst. 2013, 149, 24–30. [Google Scholar] [CrossRef]
- Turco, A.; Albano, A.; Medagli, P.; Pulvirenti, S.; D’Emerico, S. New cytological data in Ophrys sect. Pseudophrys Godfery and comparative karyomorphological studies in Ophrys L. (Orchidaceae). Plant Biosyst. 2018, 152, 901–910. [Google Scholar] [CrossRef]
- Deniz, I.G.; Genç, I.; Yücel, G.; Sümbül, H.; Sezik, E.; Tuna, M. Karyomorphology and nuclear DNA content for sixteen Ophrys L. taxa from Turkey. Plant Biosyst. 2017, 152, 711–719. [Google Scholar] [CrossRef]
- Soliva, M.; Kocyan, A.; Widmer, A. Molecular phylogenetics of the sexually deceptive orchid genus Ophrys (Orchidaceae) based on nuclear and chloroplast DNA sequences. Mol. Phylogenet. Evol. 2001, 20, 78–88. [Google Scholar] [CrossRef]
- Devey, D.S.; Bateman, M.F.; Hawkins, J.A. Genetic structure and systematic relationships within the Ophrys fuciflora aggregate (Orchidaceae: Orchidinae): High diversity in Kent and a wind-induced discontinuity bisecting the Adriatic. Ann. Bot. 2009, 104, 483–495. [Google Scholar] [CrossRef]
- Breitkopf, H.; Onstein, R.E.; Cafasso, D.; Schluter, P.M.; Cozzolino, S. Multiple shifts to different pollinators fuelled rapid diversification in sexually deceptive Ophrys orchids. New Phytol. 2015, 207, 377–386. [Google Scholar] [CrossRef]
- D’Emerico, S.; Pignone, D.; Bianco, P. Karyomorphological analyses and heterochromatin characteristic disclose phyletic relationships among 2n = 32 and 2n = 36 species of Orchis (Orchidaceae). Plant Syst. Evol. 1996, 200, 111–124. [Google Scholar] [CrossRef]
- D’Emerico, S.; Bianco, P.; Medagli, P. Cytological and karyological studies on Orchidaceae. Caryologia 1993, 46, 309–319. [Google Scholar] [CrossRef]
- Moccia, M.D.; Widmer, A.; Cozzolino, S. The strength of reproductive isolation in two hybridizing food-deceptive orchid species. Mol. Ecol. 2007, 16, 2855–2866. [Google Scholar] [CrossRef] [PubMed]
- D’Emerico, S.; Galasso, I.; Pignone, D.; Scrugli, A. Localization of rDNA loci by Fluorescent In situ Hybridization in some wild orchids from Italy (Orchidaceae). Caryologia 2001, 54, 31–36. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D.; et al. A second update to the checklist of the vascular flora native to Italy. Plant Biosyst. 2024, 158, 219–296. [Google Scholar] [CrossRef]
- Battaglia, E. A simplified Feulgen method using cold hydrolysis. Caryologia 1957, 9, 372–373. [Google Scholar] [CrossRef]
- Perrino, R.; Pignone, D. Contribution to the taxonomy of Vicia species belonging to the section Faba. Kulturpflanze 1981, 29, 311–319. [Google Scholar] [CrossRef]
- Gerlach, W.L.; Bedbrook, J.R. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucl. Acids Res. 1979, 7, 1869–1885. [Google Scholar] [CrossRef]
- Gerlach, W.L.; Dyer, T.A. Sequence organization of the repeating units in the nucleus of wheat which contain 5S rDNA genes. Nucl. Acids Res. 1980, 8, 4851–4865. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.S. The molecular cytogenetics of plants. J. Cell Sci. 1991, 100, 15–21. [Google Scholar] [CrossRef]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Paszko, B. A critical review and a new proposal of karyotype asymmetry indices. Plant Syst. Evol. 2006, 258, 39–48. [Google Scholar] [CrossRef]
- Zuo, L.; Yuan, Q. The difference between the heterogeneity of the centromeric index and intrachromosomal symmetry. Plant Syst. Evol. 2011, 297, 141–145. [Google Scholar] [CrossRef]
- GIROS Orchidee d’Italia. Guida Alle Orchidee Spontanee; Il Castello: Cornaredo, Italy, 2024. [Google Scholar]




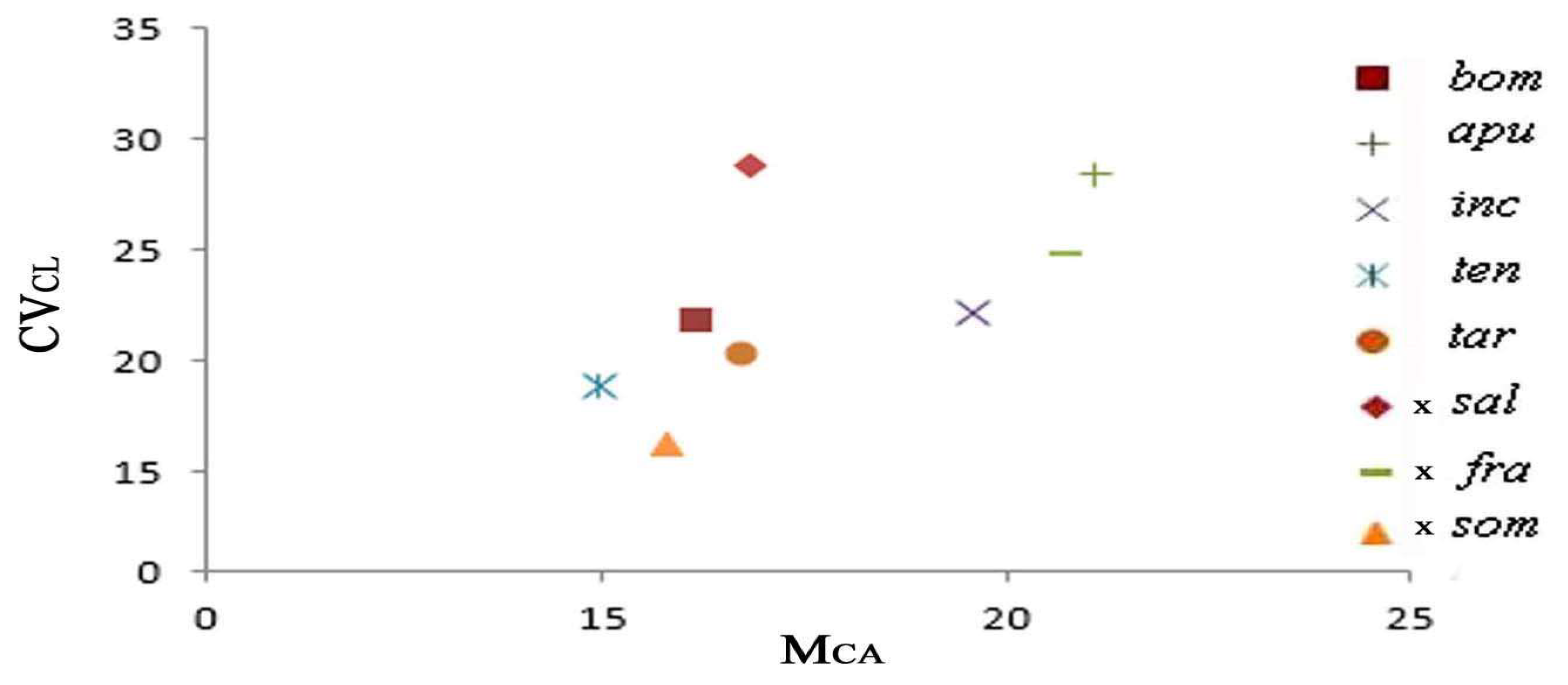
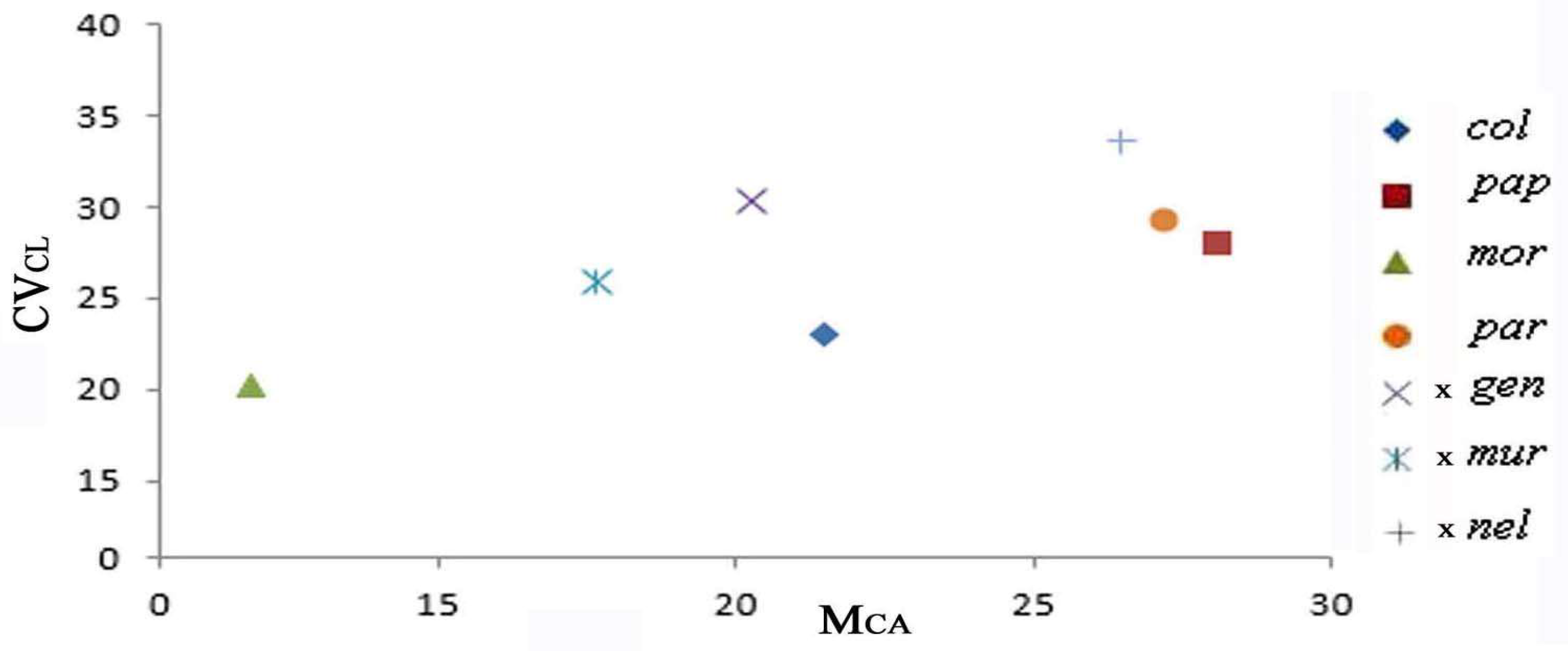
| Taxon | Code | Chromosome Number | Formula | THL | MCA | CVCL | CVCI |
|---|---|---|---|---|---|---|---|
| Ophrys bombyliflora Link | bom | 36 | 32 m + 4 sm | 43.61 | 16.20 | 21.72 | 10.13 |
| O. apulica (O.Danesch & E.Danesch) O.Danesch & E.Danesch | apu | 36 | 20 m + 16 sm | 46.86 | 21.08 | 28.46 | 15.67 |
| O. incubacea Bianca | inc | 36 | 26 m + 10 sm | 42.91 | 19.55 | 22.24 | 12.42 |
| O. tenthredinifera Willd. | ten | 36 | 30 m + 6 sm | 50.96 | 14.95 | 18.94 | 10.58 |
| O. tardans O.Danesch & E.Danesch | tar | 36 | 32 m + 4 sm | 41.64 | 16.72 | 20.26 | 10.17 |
| Ophrys ×salentina O.Danesch & E.Danesch | sal | 36 | 26 m + 10 sm | 45.71 | 16.84 | 28.87 | 14.70 |
| Ophrys ×franciniae Bianco, Medagli, D’Emerico & Ruggiero | fra | 36 | 22 m + 14 sm | 51.51 | 20.78 | 24.76 | 19.03 |
| Ophrys ×sommieri E.G.Camus ex Cortesi | som | 36 | 32 m + 4 sm | 44.80 | 15.81 | 16.29 | 13.82 |
| Anacamptis collina (Banks & Sol. ex Russell) R.M.Bateman, Pridgeon & M.W.Chase | col | 36 | 18 m + 18 sm | 48.47 | 21.43 | 22.85 | 19.86 |
| A. papilionacea (L.) R.M.Bateman, Pridgeon & M.W.Chase | pap | 32 | 16 m + 12 sm + 4 st | 40.70 | 28.11 | 27.77 | 25.68 |
| A. morio (L.) R.M.Bateman, Pridgeon & M.W.Chase | mor | 36 | 30 m + 6 sm | 44.94 | 11.86 | 20.12 | 10.61 |
| Anacamptis ×gennarii (Rchb.f.) H.Kretzschmar, Eccarius & Dietr. | gen | 34 | 22 m + 10 sm + 2 st | 44.52 | 20.21 | 30.24 | 21.60 |
| Anacamptis ×semisaccata nothosubsp. murgiana (Medagli, D’Emerico, Ruggiero & Bianco) H.Kretzschmar, Eccarius & H.Dietr. | sem | 36 | 26 m + 8 sm + 2 st | 54.58 | 17.61 | 25.91 | 21.27 |
| Serapias parviflora Parl. | par | 36 | 16 m + 18 sm + 2 st | 40.87 | 27.21 | 29.12 | 20.07 |
| ×Serapicamptis nelsoniana (Bianco, D’Emerico, Medagli & Ruggiero) J.M.H.Shaw | nel | 36 | 16 m + 14 sm + 6 st | 48.05 | 26.44 | 33.49 | 26.29 |
| Taxon | Code | Number of Specimens | Growing Sites (Collecting Sites of the Ovaries) |
|---|---|---|---|
| O. bombyliflora Link | Bom | 5 | S. Cataldo, LE; Cassano Murge, BA; Santeramo in Colle, BA (Apulia, Italy) |
| O. apulica (O.Danesch & E.Danesch) O.Danesch & E.Danesch | Apu | 5 | S. Cataldo, LE; Cassano Murge, BA (Apulia, Italy) |
| O. incubacea Bianca | Inc | 4 | Cassano Murge, BA (Apulia, Italy) |
| O. tenthredinifera Willd. | Ten | 8 | S. Cataldo, LE; Le Cesine, LE; Porto Selvaggio, LE; Cassano Murge, BA (Apulia, Italy) |
| O. tardans O.Danesch & E.Danesch | Tar | 6 | S. Cataldo, LE; Le Cesine, LE; Otranto, LE (Apulia, Italy) |
| Ophrys ×salentina O.Danesch & E.Danesch | Sal | 4 | S. Cataldo, LE; Le Cesine, LE; Porto Selvaggio, LE; Cassano Murge, BA (Apulia, Italy) |
| Ophrys ×franciniae Bianco, Medagli, D’Emerico & Ruggiero | Fra | 2 | Cassano Murge, BA (Apulia, Italy) |
| Ophrys ×sommieri E.G.Camus ex Cortesi | Som | 3 | Bosco Rauccio, LE; Cassano Murge, BA; Santeramo in Colle, BA (Apulia, Italy) |
| Anacamptis collina (Banks & Sol. ex Russell) R.M.Bateman, Pridgeon & M.W.Chase | Col | 4 | Nardò, LE; Cassano Murge, BA (Apulia, Italy) |
| A. papilionacea (L.) R.M.Bateman, Pridgeon & M.W.Chase | Pap | 6 | Adelfia, BA; Cassano Murge, BA; Conversano, BA (Apulia, Italy) |
| A. morio (L.) R.M.Bateman, Pridgeon & M.W.Chase | Mor | 5 | Adelfia, BA; Cassano Murge, BA; Conversano, BA (Apulia, Italy) |
| Anacamptis ×gennarii (Rchb.f.) H.Kretzschmar, Eccarius & Dietr. | Gen | 5 | Adelfia, BA; Cassano Murge, BA; Conversano, BA (Apulia, Italy) |
| Anacamptis ×semisaccata nothosubsp. murgiana (Medagli, D’Emerico, Ruggiero & Bianco) H.Kretzschmar, Eccarius & H.Dietr. | Sem | 2 | Cassano Murge, BA (Apulia, Italy) |
| Serapias parviflora Parl. | Par | 4 | Nardò, LE; Cassano Murge, BA (Apulia, Italy) |
| ×Serapicamptis nelsoniana (Bianco, D’Emerico, Medagli & Ruggiero) J.M.H.Shaw | Nel | 1 | Nardò, LE (Apulia, Italy) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turco, A.; Wagensommer, R.P.; Albano, A.; Medagli, P.; D’Emerico, S. Karyotype’s Rearrangement in Some Hybrids of the Orchidinae Subtribe. Plants 2024, 13, 2838. https://doi.org/10.3390/plants13202838
Turco A, Wagensommer RP, Albano A, Medagli P, D’Emerico S. Karyotype’s Rearrangement in Some Hybrids of the Orchidinae Subtribe. Plants. 2024; 13(20):2838. https://doi.org/10.3390/plants13202838
Chicago/Turabian StyleTurco, Alessio, Robert Philipp Wagensommer, Antonella Albano, Pietro Medagli, and Saverio D’Emerico. 2024. "Karyotype’s Rearrangement in Some Hybrids of the Orchidinae Subtribe" Plants 13, no. 20: 2838. https://doi.org/10.3390/plants13202838
APA StyleTurco, A., Wagensommer, R. P., Albano, A., Medagli, P., & D’Emerico, S. (2024). Karyotype’s Rearrangement in Some Hybrids of the Orchidinae Subtribe. Plants, 13(20), 2838. https://doi.org/10.3390/plants13202838








