Influence of β-Ionone in the Phytotoxicity of the Rhizome of Iris pallida Lam
Abstract
1. Introduction
2. Results and Discussions
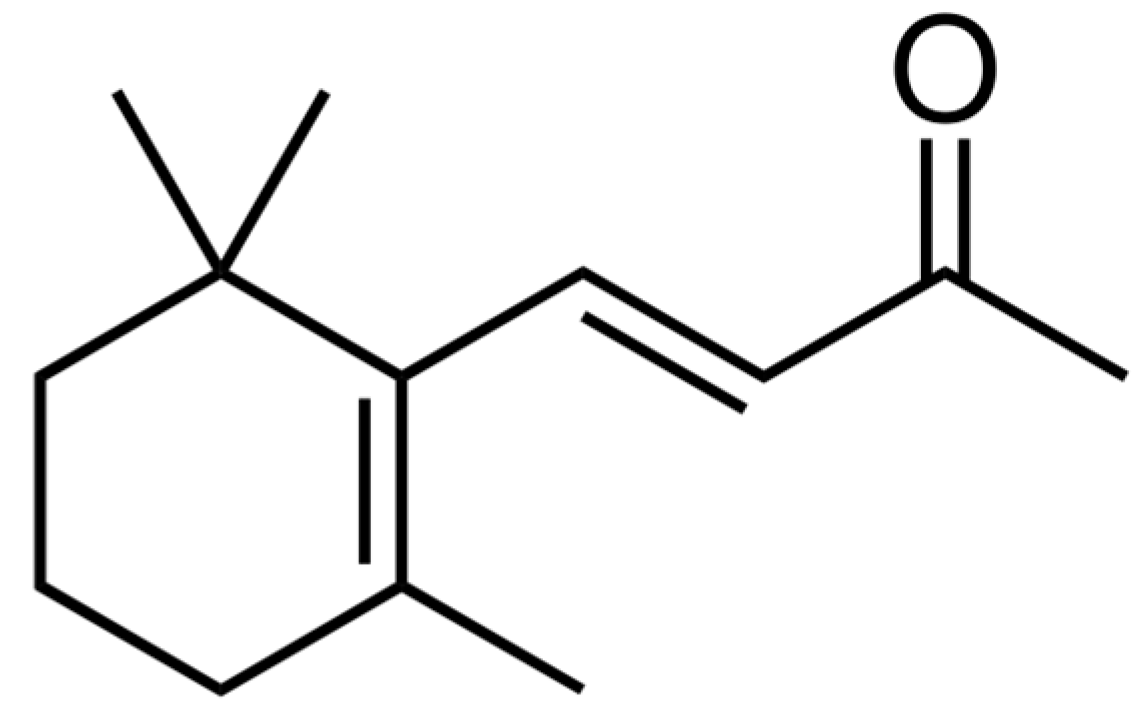
2.1. The Content of β-Ionone in Sweetie Iris
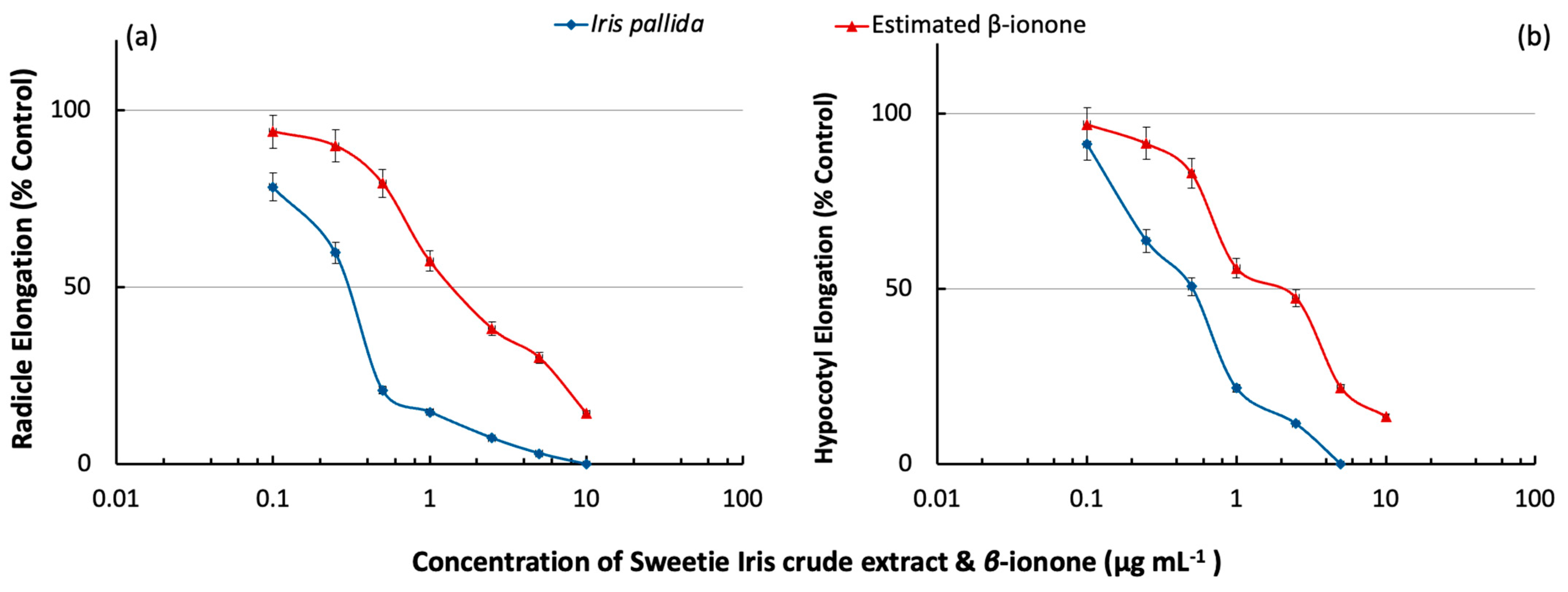
2.2. Inhibitory Effects of the Crude Extract and Estimated Contribution of β-Ionone in the Phytotoxicity of Sweetie Iris
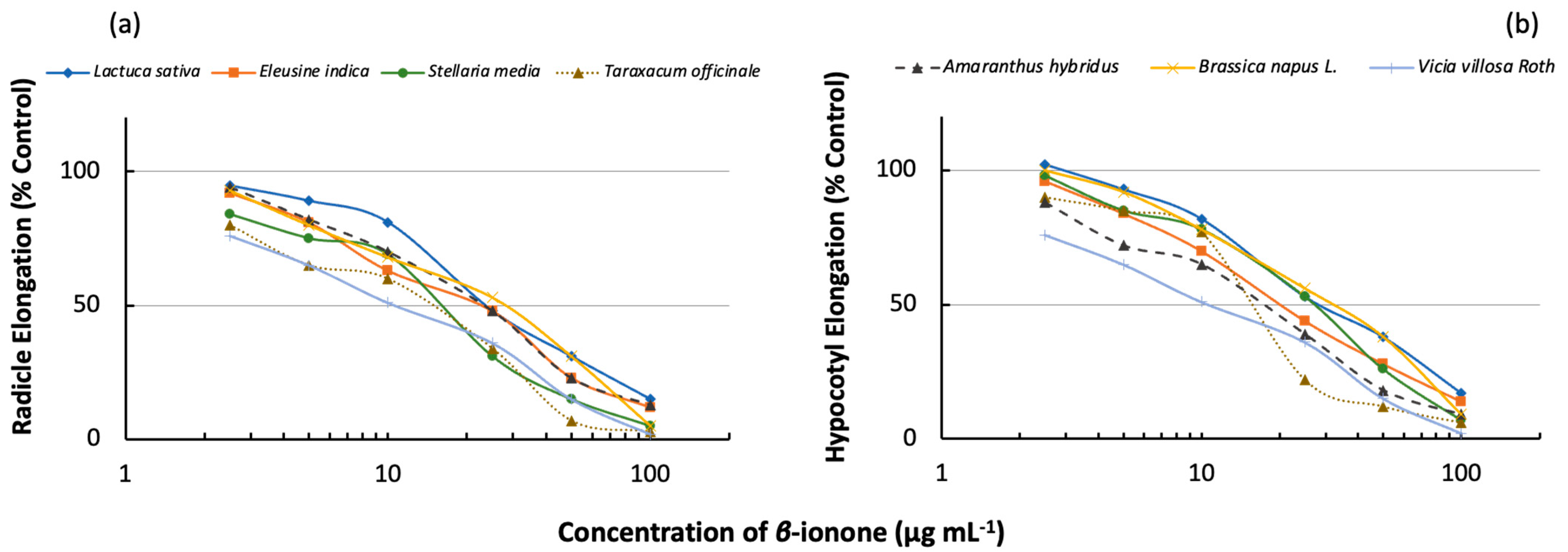
2.3. Inhibitory Effects of β-Ionone on the Radicle and Hypocotyl Elongation of Other Plant Species
3. Conclusions
4. Materials and Methods
4.1. Plant Samples and Chemicals for the Bioassay
4.2. Bioassay for the Phytotoxic Activity of Sweetie Iris and β-Ionone
4.3. High-Performance Liquid Chromatography Analysis (HPLC)
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Allelopathy Society (IAS). Constitution and Bylaws. Drawn Up during the First World Congress on Allelopathy; A Science for the Future; International Allelopathic Society: Cádiz, Spain, 1996. [Google Scholar]
- Weir, T.L.; Park, S.W.; Vivanco, J.M. Biochemical and Physiological Mechanisms Mediated by Allelochemicals. Curr. Opin. Plant Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Barazani, O.; Friedman, J. Allelopathic Bacteria and Their Impact on Higher Plants. Crit. Rev. Microbiol. 2001, 27, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Chiapusio, G.; Weston, L.A. Allelopathy and the Role of Allelochemicals in Plant Defence. Adv. Bot. Res. 2017, 82, 19–54. [Google Scholar]
- Fujii, Y. Screening and future exploitation of allelopathic plants as alternative herbicides with special reference to hairy vetch. J. Crop Prod. 2001, 4, 257–275. [Google Scholar] [CrossRef]
- Xuan, T.D.; Tsuzuki, E. Effects of application of alfalfa pellet on germination and growth of weeds. J. Crop Prod. 2001, 4, 303–312. [Google Scholar] [CrossRef]
- Felix, D.D. Plant flavonoids: Biological molecules for useful exploitation. Aust. J. Plant Physiol. 1995, 22, 87–99. [Google Scholar]
- Kropff, M.J.; Walter, H. EWRS and the challenges for weed research at the start of a new millennium. Weed Res. 2000, 40, 7–10. [Google Scholar] [CrossRef]
- Willer, H.; Klicher, L. The World of Organic Agriculture. Statistics and Emerging Trends 2009; FIBL-IFOAM Report; IFOAM: Bonn, Germany; FiBL: Frick, Switzerland; ITC: Geneva, Switzerland, 2009. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 1984. [Google Scholar]
- Appiah, K.S.; Mardani, H.K.; Osivand, A.; Kpabitey, S.; Amoatey, C.A.; Oikawa, Y.; Fujii, Y. Exploring Alternative Use of Medicinal Plants for Sustainable Weed Management. Sustainability 2017, 9, 1468. [Google Scholar] [CrossRef]
- Wink, M. Introduction: Biochemistry, Role and Biotechnology of Secondary Metabolites. Annu. Plant Rev. 1999, 3, 1–16. [Google Scholar]
- Mominul, I.A.K.M.; Sabina, Y.; Jamal, R.S.Q.; Abdul, S.J.; Parvez, M.D.A. Allelopathy of Medicinal Plants: Current Status and Future Prospects in Weed Management. Agric. Sci. 2018, 9, 12. [Google Scholar] [CrossRef]
- Sharma, S.; Devkota, A. Allelopathic Potential and Phytochemical Screening of Four Medicinal Plants of Nepal. Sci. World 2014, 12, 56–61. [Google Scholar] [CrossRef][Green Version]
- Fujii, Y.; Azizi, M. Allelopathic effect of some medicinal plant substances on seed germination of Amaranthus retroflexus and Portulaca oleraceae. Acta Hortic. 2006, 699, 61–68. [Google Scholar]
- Duke, S.O.; Vaughn, K.C.; Croom, E.M., Jr.; Elsohly, H.N. Artemisinin, a constituent of annual wormwood Artemisia annua is a selective phytotoxin. Weed Sci. 1987, 35, 499–505. [Google Scholar] [CrossRef]
- Li, F.M.; Hu, H.Y. Isolation and Characterization of a Novel Antialgal Allelochemical from Phragmites communis. Appl. Environ. Microbiol. 2002, 71, 6545–6553. [Google Scholar] [CrossRef] [PubMed]
- Mardani, H.; Sekine, T.; Azizi, M.; Mishyna, M.; Fujii, Y. Identification of Safranal as the Main Allelochemical from Saffron (Crocus sativus). Nat. Prod. Commun. 2015, 10, 775–777. [Google Scholar] [CrossRef]
- Fujii, Y.; Shibuya, T.; Tamaki, Y. Allelopathy of Velvetbean: Determination and Identification of L-DOPA as a Candidate of Allelopathic Substances. Japan Agric. Res. Q. 1992, 25, 238–247. [Google Scholar]
- Golisz, A.; Lata, B.; Gawronski, S.W.; Fujii, Y. Specific and Total Activities of the Allelochemicals Identified in Buckwheat. Weed Biol. Manag. 2007, 7, 164–171. [Google Scholar] [CrossRef]
- Appiah, K.S.; Mardani, H.K.; Omari, R.A.; Eziah, V.Y.; Ofosu-Anim, J.; Onwona-Agyeman, S.; Amoatey, C.A.; Kawada, K.; Katsura, K.; Oikawa, Y.; et al. Involvement of Carnosic Acid in the Phytotoxicity of Rosmarinus officinalis Leaves. Toxins 2018, 10, 498. [Google Scholar] [CrossRef]
- Kamo, T.; Hiradate, S.; Fujii, Y. First Isolation of Natural Cyanamide as a Possible Allelochemical from Hairy Vetch Vicia villosa. J. Chem. Ecol. 2003, 29, 275–283. [Google Scholar] [CrossRef]
- Temesgen, B.; Workissa, Y. Review on the role of Allelopathy in pest management and crop production. Int. J. Adv. Res. Biol. Sci. 2021, 8, 88–100. [Google Scholar]
- Swarbrick, J.T.; Mercado, B.L. Weed Science and Weed Control in Southeast Asia; FAO: Rome, Italy, 1987; p. 81. [Google Scholar]
- Oerke, E.C.; Dehne, H.W. Global crop production and the efficacy of crop protection—Current situation and future trends. Eur. J. Plant Pathol. 1997, 103, 203–215. [Google Scholar] [CrossRef]
- Karim, S.M.R. Relative yields of crops and crop losses due to weed competition in Bangladesh. Pak. J. Sci. Ind. Res. 1998, 41, 318–324. [Google Scholar]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H. The role of allelopathy in agricultural pest Muhammad Farooq. Pest Manag. Sci. 2011, 67, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.J.; Rathinasabapathi, B.; Chase, C.A. Allelopathy: How Plants Suppress Other Plants. Edis 2013, 3, hs186. [Google Scholar] [CrossRef]
- Hanawa, F.; Tahara, S.; Mizutani, J. Isoflavonoids produced by Iris pseudacorus leaves treated with cupric chloride. Phytochemistry 1991, 30, 157–163. [Google Scholar] [CrossRef]
- Wang, H.; Cui, Y.; Zhao, C. Flavonoids of the genus Iris (Iridaceae). Mini Rev. Med. Chem. 2010, 10, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T.; Ootani, S. Flavonoids of the genus Iris; structures, distribution and function: Review. Ann. Tsukuba Bot. Gard. 1998, 17, 147–183. [Google Scholar]
- Kassak, P. Secondary metabolites of the chosen genus Iris species. Acta Univ. Agric. Silv. Mendel. Brun. 2012, 60, 269–280. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Sieniawska, E.; Widelski, J.; Urjin, O.; Głowniak, P.; Skalicka-Woźniak, K. Major secondary metabolites of Iris spp. Phytochem. Rev. 2015, 14, 51–80. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants: Modified Stems, Roots and Bulbs; Springer International Publishing AG: Cham, Switzerland, 2016; Volume 11, pp. 3–28. [Google Scholar]
- DeBaggio, T.; Tucker, A.O. The Encyclopedia of Herbs: A Comprehensive Reference to Herbs of Flavor and Fragrance; Timber Press Inc.: Portland, OR, USA, 2009; pp. 266–267. [Google Scholar]
- Yuan, Y.; Sun, Y.; Zhao, Y.; Liu, C.; Chen, X.; Li, F. Identification of Floral Scent Profiles in Bearded Irises. Molecules 2019, 24, 1773. [Google Scholar] [CrossRef]
- Mykhailenko, O. Composition of Volatile Oil of Iris pallida Lam. From Ukraine. Turk. J. Pharm. Sci. 2018, 15, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Roger, B.; Jeannot, V.; Fernandez, X.; Cerantola, S.; Chahboun, J. Characterisation and Quantification of Flavonoids in Iris germanica L. and Iris pallida Lam. Resinoids from Morocco. Phytochem. Anal. 2012, 23, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Sothearith, Y.; Appiah, K.S.; Motobayashi, T.; Watanabe, I.; Somaly, C.; Sugiyama, A.; Fujii, Y. Evaluation of Allelopathic Potentials from Medicinal Plant Species in Phnom Kulen National Park, Cambodia by the Sandwich Method. Sustainability 2021, 13, 264. [Google Scholar] [CrossRef]
- Sothearith, Y.; Appiah, K.S.; Mardani, H.; Motobayashi, T.; Yoko, S.; Eang Hourt, K.; Sugiyama, A.; Fujii, Y. Determination of the Allelopathic Potential of Cambodia’s Medicinal Plants Using the Dish Pack Method. Sustainability 2021, 13, 9062. [Google Scholar] [CrossRef]
- Silva, I.; Coimbra, M.A.; Barros, A.; Marriott, P.; Rocha, S.M. Can volatile organic compounds be markers of sea salt. Food Chem. 2015, 169, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Li, M.T.; Jin, W.W.; Zhang, S.Q.; Yu, L.J. Variations in the components of Osmanthus fragrans Lour. essential oil at different stages of flowering. Food Chem. 2009, 114, 233–236. [Google Scholar] [CrossRef]
- Ibdah, M.; Azulay, Y.; Portnoy, V.; Wasserman, B.; Bar, E.; Meir, A.; Burger, Y.; Hirschberg, J.; Schaffer, A.A.; Katzir, N. Functional characterization of CmCCD1, a carotenoid cleavage dioxygenase from melon. Phytochemistry 2006, 67, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, A.O.; Ekundayo, O.; Koenig, W.A. Essential oil composition of Lawsonia inermis L. leaves from Nigeria. J. Essent. Oil Res. 2005, 17, 403–404. [Google Scholar] [CrossRef]
- Honarvar, M.; Javidnia, K.; Khosh-Khui, M. Essential oil composition of fresh and dried flowers of Rosa moschata from Iran. Chem. Nat. Compd. 2011, 47, 826–828. [Google Scholar] [CrossRef]
- Anca, T.; Philippe, V.; Ilioara, O.; Mircea, T. Composition of essential oils of Viola tricolor and V. arvensis from Romania. Chem. Nat. Compd. 2009, 45, 91–92. [Google Scholar] [CrossRef]
- Flamini, G.; Luigi Cioni, P.; Morelli, I.; Ceccarini, L.; Andolfi, L.; Macchia, M. Composition of the essential oil of Medicago marina L. from the coastal dunes of Tuscany, Italy. Flavour Fragr. J. 2003, 18, 460–462. [Google Scholar] [CrossRef]
- Tellez, M.R.; Khan, I.A.; Kobaisy, M.; Schrader, K.K.; Dayan, F.E.; Osbrink, W. Composition of the essential oil of Lepidium meyenii (Walp.). Phytochemistry 2002, 61, 149–155. [Google Scholar] [CrossRef]
- Dionísio, A.P.; Molina, G.; Souza, C.D.; Santos, R.D.; Bicas, J.L.; Pastore, G.M. 11—Natural flavorings from biotechnology for foods and beverages. In Natural Food Additives, Ingredients and Flavourings; Baines, D., Seal, R., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2012; pp. 231–259. ISBN 9781845698119. [Google Scholar] [CrossRef]
- Winterhalter, P.; Rouseff, R. Carotenoid-Derived Aroma Compounds: An Introduction. Am. Chem. Soc. 2001, 7, 1–17. [Google Scholar]
- Lalko, J.; Lapczynski, A.; McGinty, D.; Bhatia, S.; Letizia, C.S.; Api, A.M. Fragrance material review on beta-ionone. Food Chem. Toxicol. 2007, 45, 241–247. [Google Scholar] [CrossRef]
- Elizabeth, A.B.; John, W.S.; Christine, K.S.; Wolfgang, W.S. Flavor Trivia, and Tomato Aroma: Biochemistry and Possible Mechanisms for Control of Important Aroma Components. Hortscience 2000, 35, 1013–1022. [Google Scholar]
- Gomes, C.M.R.; Daniela, M.M.D.; Francisco, J.R.P. Study on the mutagenicity and antimutagenicity of beta-ionone in the Salmonella/microsome assay. Food Chem. Toxicol. 2006, 44, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Cooma, I.; Mohammed, A.; Steele, V.E. Beta-ionone inhibits colonic aberrant crypt foci formation in rats, suppresses cell growth, and induces retinoid X receptor-alpha in human colon cancer cells. Mol. Cancer Ther. 2008, 7, 181–190. [Google Scholar] [CrossRef]
- Shi, J.; Cao, C.; Xu, J.; Zhou, C. Research advances on biosynthesis, regulation, and biological activities of apocarotenoid aroma in horticultural plants. J. Chem. 2020, 11, 2526956. [Google Scholar] [CrossRef]
- Aloum, L.; Alefishat, E.; Adem, A.; Petroianu, G. Ionone Is More than a Violet’s Fragrance: A Review. Molecules 2020, 25, 5822. [Google Scholar] [CrossRef]
- Giuliano, G.; Al-Babili, S.; Lintig, J.V. Carotenoid oxygenases: Cleave it or leave it. Trends Plant Sci. 2003, 8, 145–149. [Google Scholar] [CrossRef]
- Schiltz, P. Action inhibitrice de la β-ionone au cours du développement de Peronospora tabacina. Ann. Tabac. 1974, 11, 207–216. [Google Scholar]
- Mikhlin, E.D.; Radina, V.P.; Dmitrovskii, A.A.; Blinkova, L.P.; Butova, L.G. Antifungal and antimicrobic activity of β-ionone and vitamin A derivatives. Prikl. Biokhim. Mikrobiol. 1983, 19, 795–803. (In Russian) [Google Scholar]
- Utama, I.M.S.; Wills, R.B.H.; Ben-Yehoshua, S.; Kuek, C. In vitro efficacy of plant volatiles for inhibiting the growth of fruit and vegetable decay microorganisms. J. Agric. Food Chem. 2002, 50, 6371–6637. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.B.; Gao, Y.N.; Wang, J.; Liu, B.Y.; Zhou, Q.H. Allelopathic effects of phenolic compounds present in submerged macrophytes on Microcystis aeruginosa. Allelopathy J. 2009, 23, 403–410. [Google Scholar]
- Hiradate, S. Strategies for searching bioactive compounds: Total activity vs. specific activity. In Proceedings of the 227th ACS National Meeting, Anaheim, CA, USA, 28 March–1 April 2004; No. AGFD7. American Chemical Society: Washington, DC, USA, 2004. [Google Scholar]
- Fujii, Y.; Hiradate, S. A critical survey of allelochemicals in action–the importance of total activity and the weed suppression equation. In Proceedings of the 4th World Congress of Allelopathy “Establishing the Scientific Base”, Wagga Wagga, NSW, Australia, 21–26 August 2005; The Regional Institute: Wagga Wagga, NSW, Australia, 2005; pp. 73–76. [Google Scholar]
- Hiradate, S. Isolation strategies for finding bioactive compounds: Specific activity vs. total activity. In Natural Products for Pest Management; Rimando, A.M., Duke, S.O., Eds.; ACS Symposium Series 927; ACS Publications: Washington, DC, USA, 2016; pp. 113–126. [Google Scholar]
- Hiradate, S.; Ohse, K.; Furubayashi, A.; Fujii, Y. Quantitative Evaluation of Allelopathic Potentials in Soils: Total Activity Approach. Weed Biol. Manag. 2010, 58, 258–264. [Google Scholar] [CrossRef]
- Morikawa, C.I.O.; Miyaura, R.; Kamo, T.; Hiradate, S.; Chávez Pérez, J.A.; Fujii, Y. Isolation of Umbelliferone as a Principal Allelochemical from the Peruvian Medicinal Plant Diplostephium foliosissimum (Asteraceae). Rev. Soc. Quim. Peru. 2011, 77, 285–291. [Google Scholar]
- Mishyna, M.; Laman, N.; Prokhorov, V.; Fujii, Y. Angelicin as the Principal Allelochemical in Heracleum sosnowskyi. Nat. Commun. 2015, 10, 1–5. [Google Scholar] [CrossRef]
- Holm, L.; Doll, L.; Holm, E. World Weeds. Natural Histories and Distributions; John Wiley and Sons, Inc.: New York, NY, USA, 1997. [Google Scholar]
- Stewart, W.S.M.; Neumann, S.; Collins, L.L. The biology of Canadian weeds. 117. Taraxacum officinale G. H. Weber ex Wiggers. Can. J. Plant Sci. 2002, 82, 825–853. [Google Scholar]
- Breuste, L.H. Investigations of the urban street tree forest of Mendoza, Argentina. Urban Ecosyst. 2013, 16, 801–818. [Google Scholar] [CrossRef]
- Rice, E.L. Biological Control of Weeds and Plant Diseases: Advances in Applied Allelopathy; University of Oklahoma Press: Norman, OK, USA, 1995. [Google Scholar]
- Mann, H.H.; Barnes, T.W. The competition between barley and certain weeds under controlled conditions. Ann. Appl. Biol. 1950, 37, 139–148. [Google Scholar] [CrossRef]
- Wilen, C.A. Chickweeds: Integrated Pest Management for Home Gardeners and Landscape Professionals. Pest Notes; University of California: Los Angeles, CA, USA, 2006; pp. 1–4. [Google Scholar]
- Chin, H.F. Weed seed—A potential source of danger. In Proceedings of the Plant Protection Seminar, Kuala Lumpur, Malaysia, 22–23 September 1979; Kwee, L.T., Ed.; Malaysian Plant Protection Society: Kuala Lumpur, Malaysia, 1979; pp. 115–119. [Google Scholar]
- Holm, L.G.; Pancho, J.V.; Herberger, J.P.; Plucknett, D.L. The World’s Worst Weeds—Distribution and Biology, 1st ed.; Hawaii University Press: Honolulu, HI, USA, 1977; pp. 52–53. [Google Scholar]
- Sauer, J.D. Revision of the dioecious amaranths. Madroño 1955, 13, 5–46. [Google Scholar]
- Weave, S.E.; McWilliams, E.L. The Biology of Canadian Weeds. 44. Amaranthus retroflexus L., A. powellii S. Wats. and A. hybridus L. Can. J. Plant Sci. 1980, 60, 1215–1234. [Google Scholar]
- Moolani, M.K.; Knake, E.L.; Slife, F.W. Competition of smooth pigweed with corn and soybeans. Weeds 1954, 12, 126–128. [Google Scholar] [CrossRef]
- Massinga, R.A.; Currie, R.S.; Horak, M.J.; Boyer, J. Interference of Palmer amaranth in corn. Weed Sci. 2001, 49, 202–208. [Google Scholar] [CrossRef]
- USDA Forest Service. Weed of the Week—Hairy Vetch. 4 August 2016. Available online: https://www.na.fs.fed.us/fhp/invasive_plants/weeds/hairy-vetch.pdf (accessed on 10 September 2017).
- Halde, C.; Gulden, G.H.; Entz, M.H. Selecting Cover Crop Mulches for Organic Rotational No-Till Systems in Manitoba, Canada. Agron. J. 2014, 106, 1193–1204. [Google Scholar] [CrossRef]
- Teasdale, J.R.; Devine, T.E.; Mosjidis, J.A.; Bellinder, R.R.; Beste, C.E. Growth and development of hairy vetch cultivars in the Northeastern United States as influenced by planting and harvesting date. Agron. J. 2004, 96, 1266–1271. [Google Scholar] [CrossRef]
- Wang, H.Z.; Wang, L.P.; Bai, S.; Guo, W.L.; Wang, J.X.; Liu, W.T. Germination ecology of giant chickweed (Myosoton aquaticum). Weed Sci. 2020, 68, 619–626. [Google Scholar] [CrossRef]
- Lv, X.S.; Zhang, L.L.; Li, Q.; Zhao, K.P.; Wang, J.X. Influence of environmental factors on seed germination and seedling emergence of Capsella bursa-pastoris. Chin. Agron. Sci. Bull. 2017, 33, 68–72. [Google Scholar]
- Fernandez-Quinantilla, C.; Andujar, J.L.G.; Appleby, A.P. Characterization of the germination and emergence response to temperature and soil moisture of Avena fatua and A. sterilis. Weed Res. 1990, 30, 289–295. [Google Scholar] [CrossRef]
- Yang, C.; Sun, R.; Lu, X.; Jin, T.; Peng, X.; Zhang, N.; Wang, J.; Wang, H.; Liu, W. Seed-Germination Ecology of Vicia villosa Roth, a Cover Crop in Orchards. Agronomy 2022, 12, 2488. [Google Scholar] [CrossRef]
- Downey, R.K.; Rimmer, S.R. Agronomic Improvements in Oilseed Brassicas. Adv. Agron. 1993, 50, 1–66. [Google Scholar]
- Al-Shehbaz, I.A.; Beilstein, M.A.; Kellogg, E.A. Systematics and phylogeny of the Brassicaceae (Cruciferae): An overview. Plant Syst. Evol. 2006, 259, 89–120. [Google Scholar] [CrossRef]
- Meyer, R.S.; Purugganan, M.D. Evolution of crop species: Domestication and diversification. Nat. Rev. Genet. 2013, 14, 840–852. [Google Scholar] [CrossRef]
- Gulden, R.H.; Warwick, S.I.; Thomas, A.G. The biology of Canadian weeds. 137. Brassica napus L. and B. rapa L. Can. J. Plant Sci. 2008, 88, 951–996. [Google Scholar] [CrossRef]
- Alford, D.V. “Rapeseed”. Agricultural Marketing Resource Center. AgMRC. 2018. Available online: https://handwiki.org/wiki/Biology:Rapeseed (accessed on 20 March 2019).
- Harker, K.N.; Clayton, G.W.; Blakshaw, R.E.; O’Donovan, J.T.; Johnson, E.N.; Gan, Y.; Holm, F.A.; Sapford, K.L.; Irvine, R.B.; Van Acker, R.C. Persistence of glyphosate-resistant canola in western Canadian cropping systems. Agron. J. 2006, 98, 107–119. [Google Scholar] [CrossRef]
- Légère, A.; Simard, M.J.; Thomas, A.; Pageau, D.; Lajeunesse, J.; Warwick, S.I.; Derksen, D. Presence and persistence of volunteer canola in Canadian cropping systems. In Proceedings of the The BCPC Conference, Weeds, Brighton, UK, 13–15 November 2001; pp. 1–2. [Google Scholar]
- Thomas, P. Canola Growers Manual; Canola Council of Canada: Winnipeg, MB, Canada, 2003. [Google Scholar]
- Naila, S.; Haq, Z.U.; Abdulllah; Salam, A. Allelopathic Effect of Taraxacum officinale L. on Germination and Physiology of Wheat. In ustainable Intensification for Agroecosystem Services and Management; Springer: Singapore, 2021; pp. 711–741. [Google Scholar] [CrossRef]
- Inderjit; Dakshini, K.M.M. Allelopathic interference of chickweed, Stellaria media with seedling growth of wheat (Triticum aestivum). Can. J. Bot. 2011, 76, 1317–1321. [Google Scholar] [CrossRef]
- Jihai, S.; Yao, X.; Zhongjie, W.; Yongguang, J.; Gongliang, Y.; Xin, P.; Renhui, L. Elucidating the toxicity targets of beta-ionone on photosynthetic system of Microcystis aeruginosa NIES-843 (Cyanobacteri). Aquat. Toxicol. 2011, 104, 48–55. [Google Scholar]
- Levizio, E.; Karageorgou, P.; Psaras, G.K.; Manesta, Y. Inhibitory effects of water-soluble leaf leachates from Dittrichia viscosa on lettuce root growth, statocyte development, and graviperception. Flora Morphol. Distrib. Funct. Ecol. Plants 2002, 197, 152–157. [Google Scholar] [CrossRef]
- Stachon, W.J.; Zimdahl, R.L. Allelopathic activity of Canada thistle (Cirsium arvense) in Colorado. Weed Sci. 1980, 28, 83–86. [Google Scholar] [CrossRef]
- Aliotta, G.; Cafiero, G.; Fiorentino, A.; Strumia, S. Inhibition of radish germination and root growth by coumarin and phenylpropanoids. J. Chem. Ecol. 1993, 19, 175–183. [Google Scholar] [CrossRef]
- Salam, A.; Kato, N.H. Evaluation of Allelopathic Potential of Neem (Azadirachta indica A. Juss) Against Seed Germination and Seedling Growth of Different Test Plant Species. Int. J. Sustain. Agric. 1020, 2, 20–25. [Google Scholar]
- Pukclari, P.; Kato, N.H. Allelopathic activity of Piper sarmentosum Roxb. Asian J. Plant Sci. 2011, 10, 147–152. [Google Scholar] [CrossRef]
- Salam, M.A.; Kato, N.H. Allelopathic potential of methanol extract of Bangladeshi rice seedlings. Asian J. Crop Sci. 2010, 2, 70–77. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoides produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Hayes, B.; Mould, A.; Khou, E.H.; Hartmann, T.; Hoa, K.; Calame, T.; Boughey, K.; Yon, T. A Biodiversity Assessment of Phnom Kulen National Park, with Recommendations for Management. Assessment Survey; Ministry of Environment: Phnom Penh, Cambodia, 2013. [Google Scholar]
- Fujii, Y.; Shibuya, T.; Yasuda, T. Survey of Japanese weed and crops for the detection of water-extractable allelopathic chemicals using Richards’ function fitted to lettuce germination test. Weed Res. Jpn. 1990, 35, 362–370. [Google Scholar]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Allelopathic Effect of Ashwagandha against the Germination and Radicle Growth of Cicer Arietinum and Triticum Aestivum. Pharmacogn. Res. 2012, 4, 166. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sothearith, Y.; Appiah, K.S.; Sophea, C.; Smith, J.; Samal, S.; Motobayashi, T.; Fujii, Y. Influence of β-Ionone in the Phytotoxicity of the Rhizome of Iris pallida Lam. Plants 2024, 13, 326. https://doi.org/10.3390/plants13020326
Sothearith Y, Appiah KS, Sophea C, Smith J, Samal S, Motobayashi T, Fujii Y. Influence of β-Ionone in the Phytotoxicity of the Rhizome of Iris pallida Lam. Plants. 2024; 13(2):326. https://doi.org/10.3390/plants13020326
Chicago/Turabian StyleSothearith, Yourk, Kwame Sarpong Appiah, Chhin Sophea, Jady Smith, Say Samal, Takashi Motobayashi, and Yoshiharu Fujii. 2024. "Influence of β-Ionone in the Phytotoxicity of the Rhizome of Iris pallida Lam" Plants 13, no. 2: 326. https://doi.org/10.3390/plants13020326
APA StyleSothearith, Y., Appiah, K. S., Sophea, C., Smith, J., Samal, S., Motobayashi, T., & Fujii, Y. (2024). Influence of β-Ionone in the Phytotoxicity of the Rhizome of Iris pallida Lam. Plants, 13(2), 326. https://doi.org/10.3390/plants13020326








