Exploring the Anticancer Potential of Traditional Thai Medicinal Plants: A Focus on Dracaena loureiri and Its Effects on Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Results
2.1. The Total Phenolic and Total Flavonoid Contents in DLEE, HPEE, and FREE
2.2. HPLC Analysis of Phytochemical Constituents in DLEE
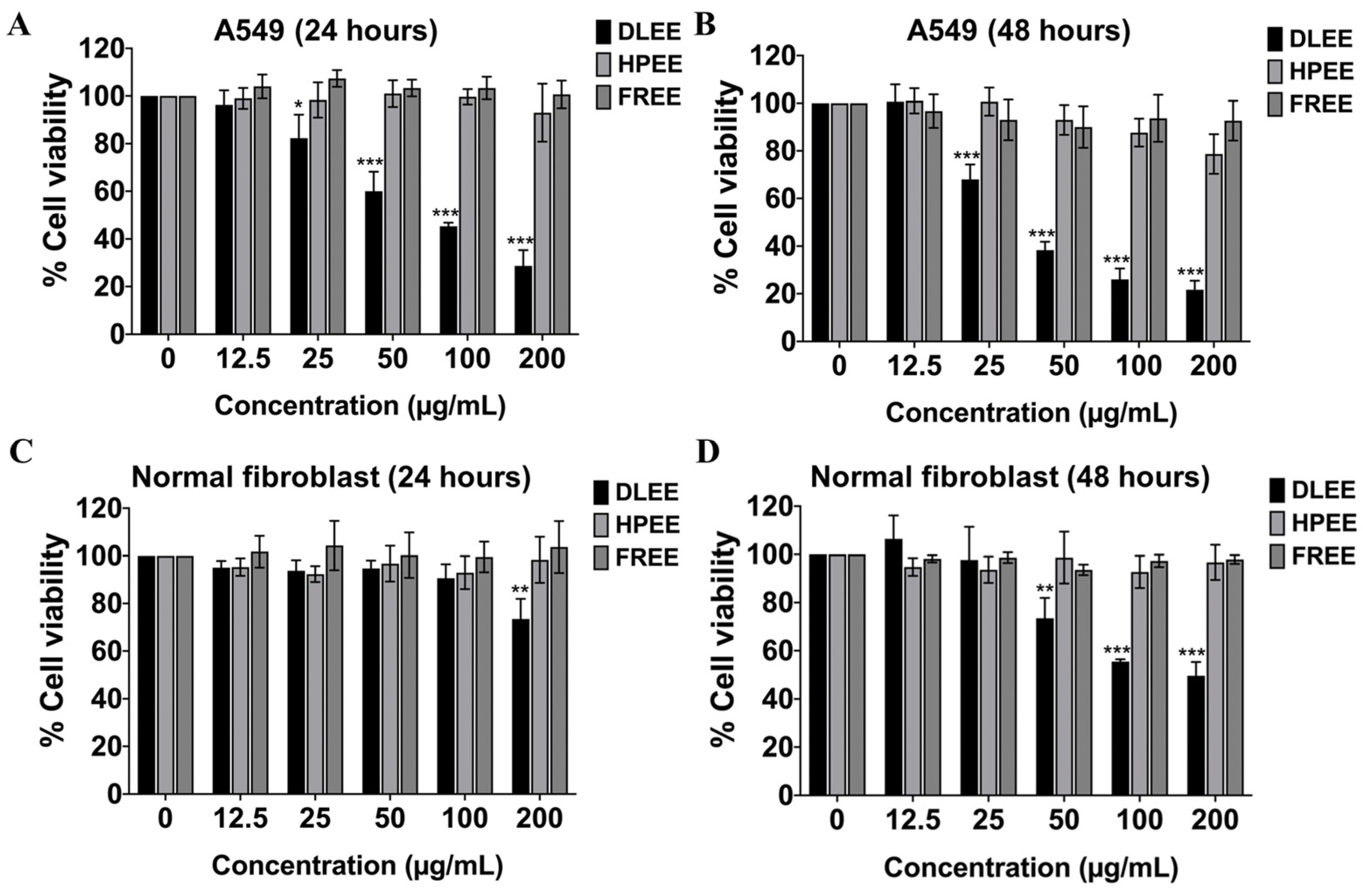
2.3. Evaluation of the Anti-Cancer Potential of DLEE, HPEE, and FREE against A549 Cells
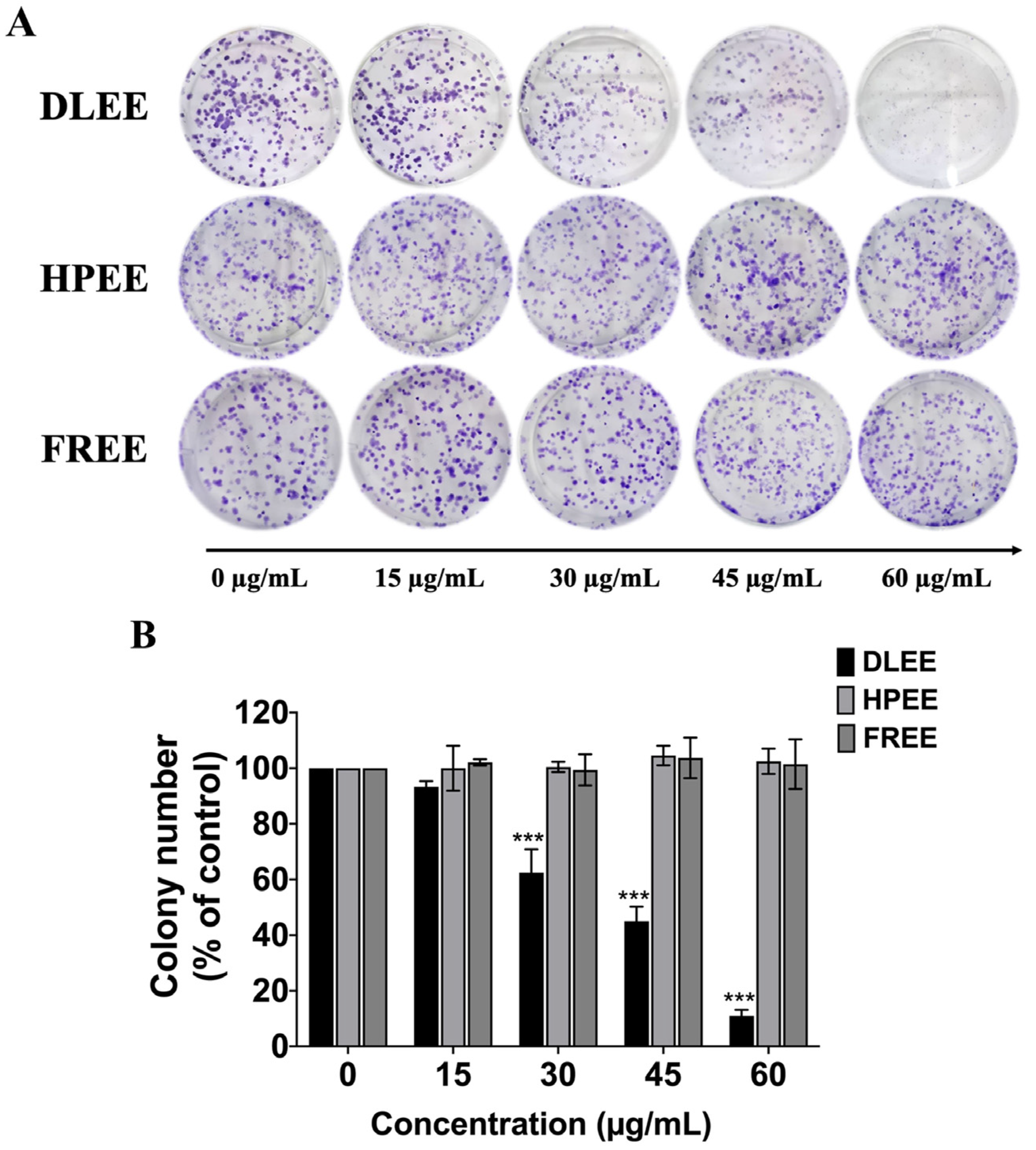
2.4. Reduction of Colony Formation by DLEE in A549 Cells
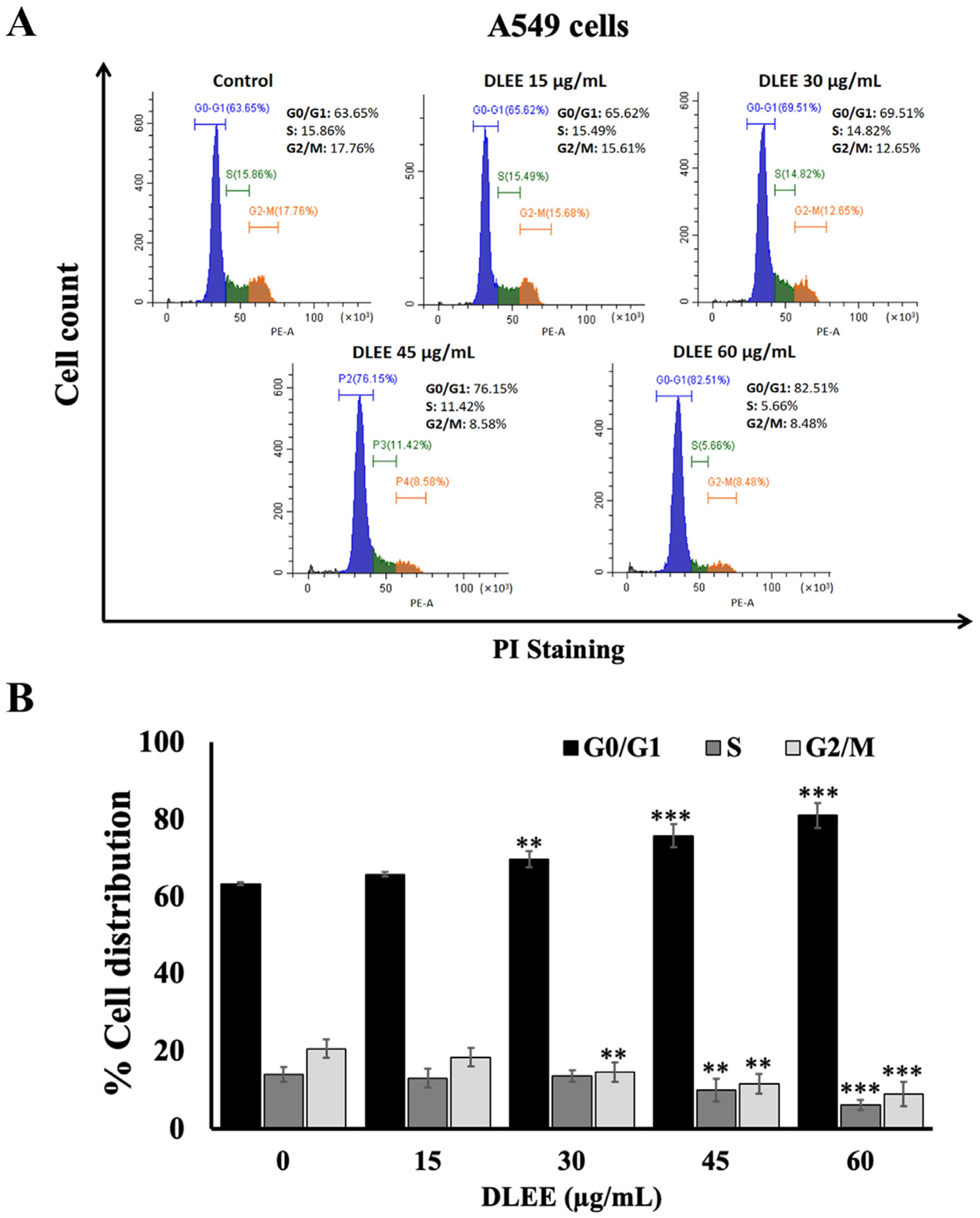
2.5. Effect of DLEE on Cell Cycle Arrest in A549 Cells
2.6. Effects of DLEE on Proteins Involved in G0/G1 Phase Arrest in A549 Cells
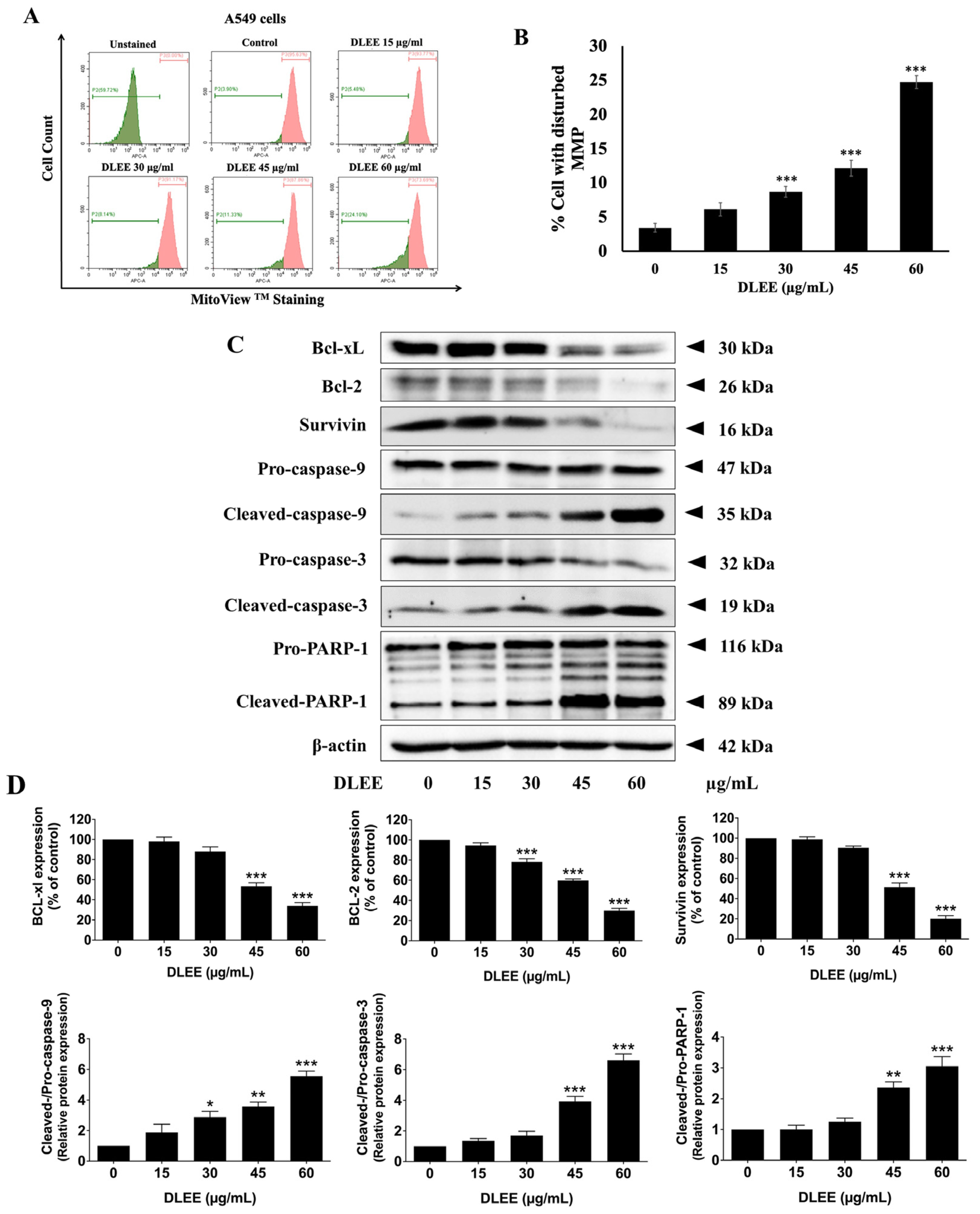
2.7. Evaluation of DLEE’s Impact on Apoptosis in A549 Cells
2.8. Impact of DLEE on Pro- and Anti-Apoptotic Protein Expression in A549 Cells
3. Discussion
4. Materials and Methods
4.1. Herb Materials
4.2. Reagents and Chemicals
4.3. Preparation of Herbal Extracts
4.4. Total Phenolic Content
4.5. Total Flavonoid Content
4.6. Phytochemicals Content Determination Using HPLC
4.7. Cell Line and Cell Culture
4.8. Cell Viability Assay
4.9. Colony Formation Assay
4.10. Cell Cycle Assay
4.11. Apoptosis Assay
4.12. Mitochondrial Membrane Potential
4.13. Western Blot Analysis
4.14. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Wen, Y.; Zhou, C. Non-small cell lung cancer in China. Cancer Commun. 2022, 42, 937–970. [Google Scholar]
- Huang, C.-Y.; Ju, D.-T.; Chang, C.-F.; Reddy, P.M.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine 2017, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sarkar, A. Current therapeutic strategies and challenges in NSCLC treatment: A comprehensive review. Exp. Oncol. 2022, 44, 7–16. [Google Scholar] [CrossRef]
- Fennell, D.; Summers, Y.; Cadranel, J.; Benepal, T.; Christoph, D.; Lal, R.; Das, M.; Maxwell, F.; Visseren-Grul, C.; Ferry, D. Cisplatin in the modern era: The backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat. Rev. 2016, 44, 42–50. [Google Scholar] [CrossRef]
- Group, I.A.L.C.T.C. Cisplatin-based adjuvant chemotherapy in patients with completely resected non–small-cell lung cancer. N. Engl. J. Med. 2004, 350, 351–360. [Google Scholar]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [PubMed]
- Wu, J.; Li, Y.; He, Q.; Yang, X. Exploration of the Use of Natural Compounds in Combination with Chemotherapy Drugs for Tumor Treatment. Molecules 2023, 28, 1022. [Google Scholar] [CrossRef]
- Sofi, M.S.; Nabi, S.; Mohammed, C.; Sofi, S. The role of phytocompounds in cancer treatment: A current review. J. Med. Plant Stud. 2018, 6, 83–93. [Google Scholar]
- Sukhramani, P.S.; Vidyasagar, G.; Patel, P.M. In-vitro screening of Ficus racemosa for Anticancer activity. Res. J. Pharmacogn. Phytochem. 2013, 5, 119–122. [Google Scholar]
- Dharmadeva, S.; Galgamuwa, L.S.; Prasadinie, C.; Kumarasinghe, N. In vitro anti-inflammatory activity of Ficus racemosa L. bark using albumin denaturation method. Ayu 2018, 39, 239. [Google Scholar] [PubMed]
- Ahmed, F.; Urooj, A. Traditional uses, medicinal properties, and phytopharmacology of Ficus racemosa: A review. Pharm. Biol. 2010, 48, 672–681. [Google Scholar] [CrossRef]
- Juckmeta, T.; Pipatrattanaseree, W.; Jaidee, W.; Dechayont, B.; Chunthorng-Orn, J.; Andersen, R.J.; Itharat, A. Cytotoxicity to five cancer cell lines of the respiratory tract system and anti-inflammatory activity of Thai traditional remedy. Nat. Prod. Commun. 2019, 14, 1934578X19845815. [Google Scholar] [CrossRef]
- Nguyen-Pouplin, J.; Tran, H.; Tran, H.; Phan, T.A.; Dolecek, C.; Farrar, J.; Tran, T.H.; Caron, P.; Bodo, B.; Grellier, P. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J. Ethnopharmacol. 2007, 109, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Somsil, P.; Ruangrungsi, N.; Limpanasitikul, W.; Itthipanichpong, C. In vivo and in vitro anti-inflammatory activity of Harrisonia perforata root extract. Pharmacogn. J. 2012, 4, 38–44. [Google Scholar] [CrossRef]
- Choodej, S.; Sommit, D.; Pudhom, K. Rearranged limonoids and chromones from Harrisonia perforata and their anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2013, 23, 3896–3900. [Google Scholar]
- Cheenpracha, S.; Chokchaisiri, R.; Ganranoo, L.; Maneerat, T.; Rujanapun, N.; Charoensup, R.; Laphookhieo, S.; Injan, N.; Nokbin, S. Isoprenylated chromones from the stems of Harrisonia perforata. Phytochem. Lett. 2022, 49, 192–196. [Google Scholar] [CrossRef]
- Chea, A.; Jonville, M.-C.; Bun, S.-S.; Laget, M.; Elias, R.; Duménil, G.; Balansard, G. In vitro antimicrobial activity of plants used in Cambodian traditional medicine. Am. J. Chin. Med. 2007, 35, 867–873. [Google Scholar] [CrossRef]
- Thu, Z.M.; Myo, K.K.; Aung, H.T.; Armijos, C.; Vidari, G. Flavonoids and stilbenoids of the genera Dracaena and Sansevieria: Structures and bioactivities. Molecules 2020, 25, 2608. [Google Scholar] [CrossRef]
- Ichikawa, K.; Kitaoka, M.; Taki, M.; Takaishi, S.; Boriboon, M.; Akiyama, T. Retrodihydrochalcones and homoisoflavones isolated from Thai medicinal plant Dracaena loureiri and their estrogen agonist activity. Planta Med. 1997, 63, 540–543. [Google Scholar] [CrossRef]
- Reanmongkol, W.; Subhadhirasakul, S.; Bouking, P. Antinociceptive and antipyretic activities of extracts and fractions from Dracaena loureiri in experimental animals. Songklanakarin J. Sci. Technol. 2003, 25, 467–476. [Google Scholar]
- Sun, J.; Liu, J.-N.; Fan, B.; Chen, X.-N.; Pang, D.-R.; Zheng, J.; Zhang, Q.; Zhao, Y.-F.; Xiao, W.; Tu, P.-F. Phenolic constituents, pharmacological activities, quality control, and metabolism of Dracaena species: A review. J. Ethnopharmacol. 2019, 244, 112138. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wen, K.; Xu, Z.; He, Z.; Wu, B.; Yang, X.; Wang, X. Effect of Loureirin B on Crohn’s disease rat model induced by TNBS via IL-6/STAT3/NF-κB signaling pathway. Chin. Med. 2020, 15, 2. [Google Scholar] [PubMed]
- Chen, M.J.; Cheng, A.C.; Lee, M.F.; Hsu, Y.C. Simvastatin induces G1 arrest by up-regulating GSK3β and down-regulating CDK4/cyclin D1 and CDK2/cyclin E1 in human primary colorectal cancer cells. J. Cell. Physiol. 2018, 233, 4618–4625. [Google Scholar] [CrossRef]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal plants and cancer chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef]
- Khan, M.I.; Bouyahya, A.; Hachlafi, N.E.; Menyiy, N.E.; Akram, M.; Sultana, S.; Zengin, G.; Ponomareva, L.; Shariati, M.A.; Ojo, O.A. Anticancer properties of medicinal plants and their bioactive compounds against breast cancer: A review on recent investigations. Environ. Sci. Pollut. Res. 2022, 29, 24411–24444. [Google Scholar] [CrossRef]
- Jain, R.; Venkatasubramanian, P. Proposed correlation of modern processing principles for Ayurvedic herbal drug manufacturing: A systematic review. Anc. Sci. Life 2014, 34, 8. [Google Scholar]
- Sánchez-Martínez, C.; Lallena, M.J.; Sanfeliciano, S.G.; de Dios, A. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs: Recent advances (2015–2019). Bioorg. Med. Chem. Lett. 2019, 29, 126637. [Google Scholar]
- Maddika, S.; Ande, S.R.; Panigrahi, S.; Paranjothy, T.; Weglarczyk, K.; Zuse, A.; Eshraghi, M.; Manda, K.D.; Wiechec, E.; Los, M. Cell survival, cell death and cell cycle pathways are interconnected: Implications for cancer therapy. Drug Resist. Updates 2007, 10, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.I.; Brown, L.; Whyte, M.; Harrington, E. Apoptosis and the cell cycle. Curr. Opin. Cell Biol. 1995, 7, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Vaux, D.L.; Korsmeyer, S.J. Cell death in development. Cell 1999, 96, 245–254. [Google Scholar]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (Δψ m) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, E.; Armour, S.; Harris, M.; Thompson, C. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003, 10, 709–717. [Google Scholar] [PubMed]
- Niu, S.; Liu, T.; Deng, Y.; Wang, W.; Zhang, Y.; Hong, W.; Zhang, D.; Hua, J.; Luo, S. Production and evaluation of antifungal stilbenoids in Dracaena cochinchinensis elicited by fungal inoculation. Ind. Crops Prod. 2020, 145, 112148. [Google Scholar] [CrossRef]
- Ouncharoen, K.; Itharat, A.; Chaiyawatthanananthn, P. In vitro free radical scavenging and cell-based antioxidant activities of Kheaw-Hom remedy extracts and its plant ingredients. J. Med. Assoc. Thail. 2017, 100, 241. [Google Scholar]
- Dechayont, B.; Phuaklee, P.; Chunthorng-Orn, J.; Juckmeta, T.; Prajuabjinda, O.; Jiraratsatit, K. Antibacterial, anti-inflammatory and antioxidant activities of Mahanintangtong and its constituent herbs, a formula used in Thai traditional medicine for treating pharyngitis. BMC Complement. Med. Ther. 2021, 21, 105. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K.; Sawasdee, K.; Kirtikara, K. Flavonoids and stilbenoids with COX-1 and COX-2 inhibitory activity from Dracaena loureiri. Planta Med. 2002, 68, 841–843. [Google Scholar] [CrossRef]
- Meksuriyen, D.; Cordell, G.A. Traditional medicinal plants of Thailand XIII. Flavonoid derivatives from Dracaena loureiri (Agavaceae). Sci. Asia 1988, 14, 3–24. [Google Scholar] [CrossRef]
- Hao, Q.; Saito, Y.; Matsuo, Y.; Li, H.-Z.; Tanaka, T. Chalcane–stilbene conjugates and oligomeric flavonoids from Chinese dragon’s blood produced from Dracaena cochinchinensis. Phytochemistry 2015, 119, 76–82. [Google Scholar] [PubMed]
- Hu, S.-L.; Wang, K.; Shi, Y.-F.; Shao, Z.-X.; Zhang, C.-X.; Sheng, K.-W.; Ge, Z.-D.; Chen, J.-X.; Wang, X.-Y. Downregulating Akt/NF-κB signaling and its antioxidant activity with Loureirin A for alleviating the progression of osteoarthritis: In vitro and vivo studies. Int. Immunopharmacol. 2020, 78, 105953. [Google Scholar] [PubMed]
- Zheng, S.-Y.; Li, Y.; Jiang, D.; Zhao, J.; Ge, J.-F. Anticancer effect and apoptosis induction by quercetin in the human lung cancer cell line A-549. Mol. Med. Rep. 2012, 5, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, Y.; Xia, J.; Liu, B.; Zhang, Q.; Liu, J.; Luo, L.; Peng, Z.; Song, Z.; Zhu, R. Resveratrol induces cell cycle arrest via a p53-independent pathway in A549 cells. Mol. Med. Rep. 2015, 11, 2459–2464. [Google Scholar]
- Takashina, M.; Inoue, S.; Tomihara, K.; Tomita, K.; Hattori, K.; Zhao, Q.-L.; Suzuki, T.; Noguchi, M.; Ohashi, W.; Hattori, Y. Different effect of resveratrol to induction of apoptosis depending on the type of human cancer cells. Int. J. Oncol. 2017, 50, 787–797. [Google Scholar] [CrossRef]
- Gosslau, A.; Pabbaraja, S.; Knapp, S.; Chen, K.Y. Trans-and cis-stilbene polyphenols induced rapid perinuclear mitochondrial clustering and p53-independent apoptosis in cancer cells but not normal cells. Eur. J. Pharmacol. 2008, 587, 25–34. [Google Scholar]
- Wu, F.; Chen, J.; Fan, L.M.; Liu, K.; Zhang, N.; Li, S.W.; Zhu, H.; Gao, H.C. Analysis of the effect of rutin on GSK-3β and TNF-α expression in lung cancer. Exp. Ther. Med. 2017, 14, 127–130. [Google Scholar] [CrossRef]
- Birsu Cincin, Z.; Unlu, M.; Kiran, B.; Sinem Bireller, E.; Baran, Y.; Cakmakoglu, B. Anti-proliferative, apoptotic and signal transduction effects of hesperidin in non-small cell lung cancer cells. Cell. Oncol. 2015, 38, 195–204. [Google Scholar]
- Xia, R.; Sheng, X.; Xu, X.; Yu, C.; Lu, H. Hesperidin induces apoptosis and G0/G1 arrest in human non-small cell lung cancer A549 cells. Int. J. Mol. Med. 2018, 41, 464–472. [Google Scholar] [CrossRef]
- Noreen, H.; Semmar, N.; Farman, M.; McCullagh, J.S. Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus didymus. Asian Pac. J. Trop. Med. 2017, 10, 792–801. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Wu, J.; Xing, H.; Tang, D.; Gao, Y.; Yin, X.; Du, Q.; Jiang, X.; Yang, D. Simultaneous determination of nine flavonoids in beagle dog by HPLC with DAD and application of Ginkgo biloba extracts on the pharmacokinetic. Acta Chromatogr. 2012, 24, 627–642. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Liu, M.; Tai, Z.; Li, G. Quantitative evaluation of loureirin A and loureirin B in Dragon’s blood capsules from different manufacturers by HPLC. J. Chem. Pharm. Res. 2014, 6, 2243–2248. [Google Scholar]
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) assay in cell culture to investigate cell proliferation. Bio Protoc. 2016, 6, e1984. [Google Scholar]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]


| Herb Extracts | Total Phenolic Content (mg of GAE/g of Extract) | Total Flavonoid Content (mg of CE/g of Extract) |
|---|---|---|
| DLEE | 251.49 ± 23.13 *** | 85.83 ± 4.80 *** |
| HPEE | 193.53 ± 3.21 | 62.13 ± 1.22 |
| FREE | 89.05 ± 3.42 | 46.64 ± 1.59 |
| Standard Compounds | mg/g of Extract (Mean ± S.D.) |
|---|---|
| Loureirin A | 28.11 ± 0.34 |
| Quercetin | 25.81 ± 1.12 |
| Quercitrin | 6.48 ± 0.51 |
| Loureirin B | 6.27 ± 0.15 |
| Hesperetin | 6.14 ± 0.18 |
| Rutin | 5.86 ± 0.27 |
| Catechin | 3.32 ± 0.46 |
| Resveratrol | 2.65 ± 0.03 |
| Hesperidin | 1.98 ± 0.26 |
| Luteolin | ND |
| Apigenin | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Arjsri, P.; Srisawad, K.; Yodkeeree, S.; Dejkriengkraikul, P. Exploring the Anticancer Potential of Traditional Thai Medicinal Plants: A Focus on Dracaena loureiri and Its Effects on Non-Small-Cell Lung Cancer. Plants 2024, 13, 290. https://doi.org/10.3390/plants13020290
Huang X, Arjsri P, Srisawad K, Yodkeeree S, Dejkriengkraikul P. Exploring the Anticancer Potential of Traditional Thai Medicinal Plants: A Focus on Dracaena loureiri and Its Effects on Non-Small-Cell Lung Cancer. Plants. 2024; 13(2):290. https://doi.org/10.3390/plants13020290
Chicago/Turabian StyleHuang, Xiaomin, Punnida Arjsri, Kamonwan Srisawad, Supachai Yodkeeree, and Pornngarm Dejkriengkraikul. 2024. "Exploring the Anticancer Potential of Traditional Thai Medicinal Plants: A Focus on Dracaena loureiri and Its Effects on Non-Small-Cell Lung Cancer" Plants 13, no. 2: 290. https://doi.org/10.3390/plants13020290
APA StyleHuang, X., Arjsri, P., Srisawad, K., Yodkeeree, S., & Dejkriengkraikul, P. (2024). Exploring the Anticancer Potential of Traditional Thai Medicinal Plants: A Focus on Dracaena loureiri and Its Effects on Non-Small-Cell Lung Cancer. Plants, 13(2), 290. https://doi.org/10.3390/plants13020290








