Identification of a Promising Novel Genetic Source for Rice Root-Knot Nematode Resistance through Markers Associated with Trait-Specific Quantitative Trait Loci
Abstract
1. Introduction
2. Results
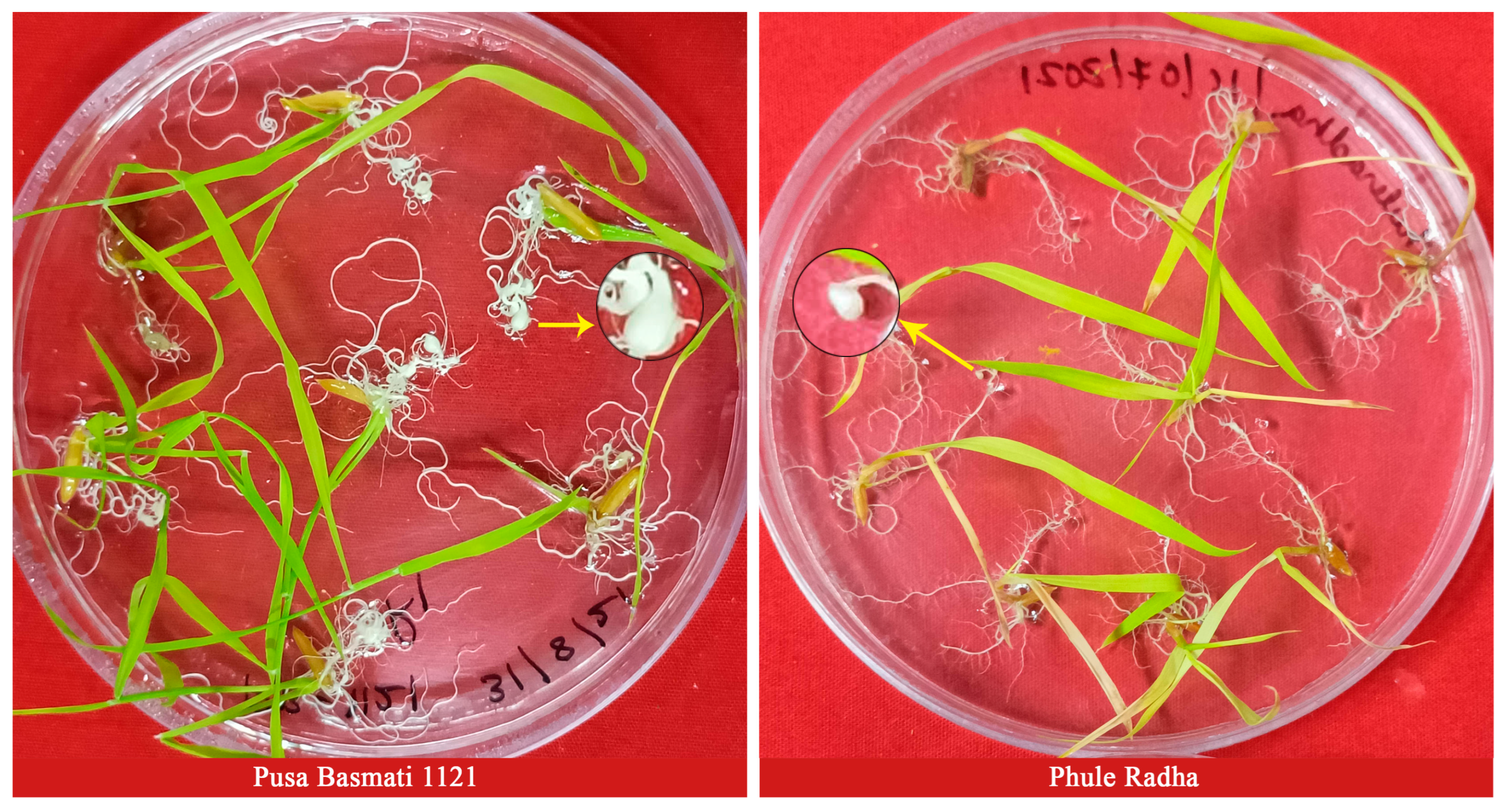
2.1. Characterization for RRKN Resistance-Related Traits
2.2. Identification of Markers Polymorphic between Parental Lines
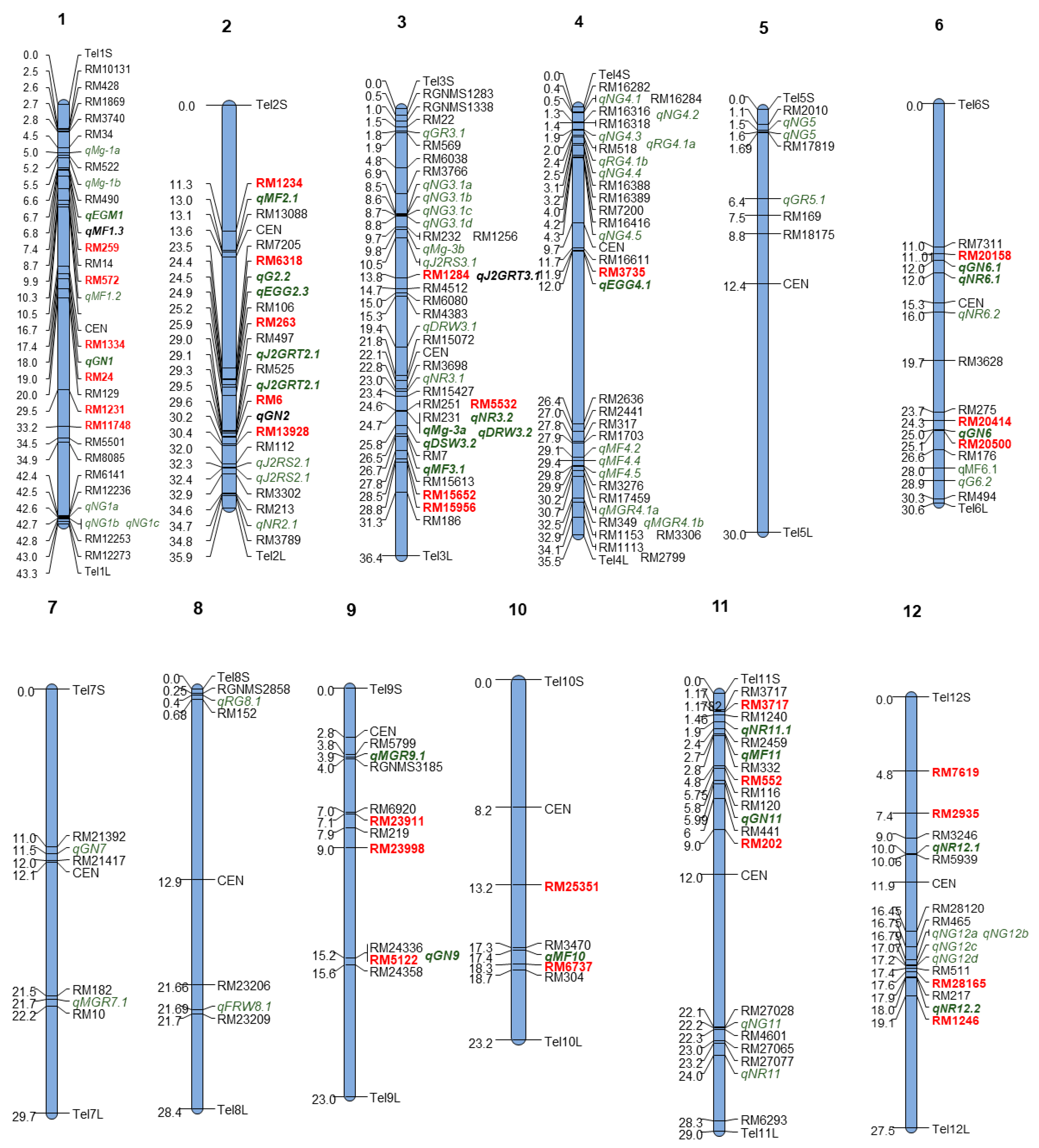
2.3. Single-Marker Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. RRKN Culture and In Vitro Screening
4.3. Molecular Marker Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, S.; Linquist, B.A.; Wilson, L.T.; Cassman, K.G.; Stuart, A.M.; Pede, V.; Miro, B.; Saito, K.; Agustiani, N.; Aristya, V.E.; et al. Sustainable intensification for a Larger Global Rice Bowl. Nat. Commun. 2021, 12, 7163. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Gheysen, G.; Fenoll, C. (Eds.) Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 Plant-Parasitic Nematodes in Molecular Plant Pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Mantelin, S.; Bellafiore, S.; Kyndt, T. Meloidogyne graminicola: A Major Threat to Rice Agriculture. Mol. Plant Pathol. 2017, 18, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.S.; Vijayakumar, C.H.M.; Sankar, M.; Varaprasad, K.S.; Prasad, M.S.; Rao, Y.K. Root-Knot Nematode Resistance in Advanced Back Cross Populations of Rice Developed for Water Stress Conditions. Nematol. Mediterr. 2006, 34, 1. [Google Scholar]
- Biswas, H.; Rao, Y.S. Studies on Nematodes of Rice and Rice Soils. II. Influence of Meloidogyne Graminicola Morgan and Birchfield Incidence on Yields of Rice. Oryza 1971, 1, 101–102. [Google Scholar]
- Hada, A.; Dutta, T.K.; Singh, N.; Singh, B.; Rai, V.; Singh, N.K.; Rao, U. A Genome-Wide Association Study in Indian Wild Rice Accessions for Resistance to the Root-Knot Nematode Meloidogyne graminicola. PLoS ONE 2020, 15, e0239085. [Google Scholar] [CrossRef] [PubMed]
- Cabasan, M.T.N.; Kumar, A.; De Waele, D. Host Response of Rice Genotypes from crosses of High-Yielding and Drought-tolerant Oryza Sativa Advanced Breeding Lines to the Rice Root-Knot Nematode Meloidogyne graminicola. Arch. Phytopathol. Plant Prot. 2018, 51, 81–94. [Google Scholar] [CrossRef]
- Kaur, G.; Yadav, I.S.; Bhatia, D.; Vikal, Y.; Neelam, K.; Dhillon, N.K.; Praba, U.P.; Mangat, G.S.; Singh, K. BSA-seq Identifies a Major Locus on Chromosome 6 for Root-Knot Nematode (Meloidogyne graminicola) Resistance from Oryza glaberrima. Front. Genet. 2022, 13, 871833. [Google Scholar] [CrossRef]
- Hada, A.; Raj, A.; Phani, V.; Hatzade, B.; Kumbhar, S.D.; Ellur, R.; Rao, U. Evaluation of Rice Genotypes against Varying Population Densities of Root-Knot Nematode Meloidogyne graminicola Identifies Potential Sources for Resistance Breeding. Indian Phytopathol. 2022, 75, 75–82. [Google Scholar] [CrossRef]
- Soriano, I.R.; Schmit, V.; Brar, D.S.; Prot, J.-C.; Reversat, G. Resistance to Rice Root-Knot Nematode Meloidogyne graminicola Identified in Oryza longistaminata and O. glaberrima. Nematology 1999, 1, 395–398. [Google Scholar] [CrossRef]
- Mattos, V.S.; Leite, R.R.; Cares, J.E.; Gomes, A.C.M.M.; Moita, A.W.; Lobo, V.L.S.; Carneiro, M.D.G. Oryza glumaepatula, a New Source of Resistance to Meloidogyne graminicola and Histological Characterization of Its Defense Mechanisms. Phytopathology 2019, 109, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Kumari, C.; Dutta, T.K.; Banakar, P.; Rao, U. Comparing the Defence-Related Gene Expression Changes upon Root-Knot Nematode Attack in Susceptible Versus Resistant Cultivars of Rice. Sci. Rep. 2016, 6, 22846. [Google Scholar] [CrossRef] [PubMed]
- Mhatre, P.H.; Pankaj; Malik, S.K.; Kaur, S.; Singh, A.K.; Mohan, S.; Sirohi, A. Histopathological changes and evaluation of resistance in Asian rice (Oryza sativa L.) against rice Root-Knot Nematode, Meloidogyne graminicola Golden & Birch. Indian J. Genet. Plant Breed. 2015, 75, 41. [Google Scholar] [CrossRef]
- Phan, N.T.; Orjuela, J.; Danchin, E.G.J.; Klopp, C.; Perfus-Barbeoch, L.; Kozlowski, D.K.; Koutsovoulos, G.D.; Lopez-Roques, C.; Bouchez, O.; Zahm, M.; et al. Genome Structure and Content of the Rice Root-Knot Nematode (Meloidogyne graminicola). Ecol. Evol. 2020, 10, 11006–11021. [Google Scholar] [CrossRef] [PubMed]
- Lahari, Z.; Ribeiro, A.; Talukdar, P.; Martin, B.; Heidari, Z.; Gheysen, G.; Price, A.H.; Shrestha, R. QTL-Seq Reveals a Major Root-Knot Nematode Resistance Locus on Chromosome 11 in Rice (Oryza sativa L.). Euphytica 2019, 215, 117. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.T.; De Waele, D.; Lorieux, M.; Xiong, L.; Bellafiore, S. A Hypersensitivity-like Response to Meloidogyne graminicola in Rice (Oryza sativa). Phytopathology 2018, 108, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, R.; Xu, D.; Huang, R.; Li, H.; Jin, L.; Wu, Y.; Tang, J.; Sun, C.; Peng, D.; et al. MG1 Interacts with a Protease Inhibitor and Confers Resistance to Rice Root-Knot Nematode. Nat. Commun. 2023, 14, 3354. [Google Scholar] [CrossRef] [PubMed]
- Jena, M.; Mohapatra, S.L.; Panda, R.S.; Mohanty, S.K.; Thatoi, H.N.; Sahu, S.C. Genetic Loci Associated with Root-Knot Nematode Resistance in Rice Cv. Ramakrishna. Oryza 2013, 50, 132–139. [Google Scholar]
- Shrestha, R.; Uzzo, F.; Wilson, M.J.; Price, A.H. Physiological and Genetic Mapping Study of Tolerance to Root-Knot Nematode in Rice. New Phytol. 2007, 176, 665–672. [Google Scholar] [CrossRef]
- Galeng-Lawilao, J.; Swamy, B.P.M.; Hore, T.K.; Kumar, A.; De Waele, D. Identification of Quantitative Trait Loci Underlying Resistance and Tolerance to the Rice Root-Knot Nematode, Meloidogyne graminicola, in Asian Rice (Oryza sativa). Mol. Breed. 2020, 40, 63. [Google Scholar] [CrossRef]
- Lawilao, J.G.; Swamy, B.M.; Kumar, A.; Cabasan, M.T.N.; De Waele, D. Mapping Quantitative Trait Loci of Meloidogyne graminicola Resistance and Tolerance in a Recombinant Inbred line Population of Oryza glaberrima × O. sativa. Nematology 2019, 21, 401–417. [Google Scholar] [CrossRef]
- Galeng-Lawilao, J.; Kumar, A.; De Waele, D. QTL Mapping for Resistance to and Tolerance for the Rice Root-Knot Nematode, Meloidogyne graminicola. BMC Genet. 2018, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, S.O.N.; Lahari, Z.; Shrestha, R.; Douglas, A.; Gheysen, G.; Price, A.H. A Genome-Wide Association Study of a Global Rice Panel Reveals Resistance in Oryza sativa to Root-Knot Nematodes. J. Exp. Bot. 2015, 67, 1191–1200. [Google Scholar] [CrossRef]
- Kyndt, T.; Denil, S.; Haegeman, A.; Trooskens, G.; Bauters, L.; Van Criekinge, W.; De Meyer, T.; Gheysen, G. Transcriptional Reprogramming by Root Knot and Migratory Nematode Infection in Rice. New Phytol. 2012, 196, 887–900. [Google Scholar] [CrossRef]
- Hatzade, B.; Singh, D.; Phani, V.; Kumbhar, S.; Rao, U. Profiling of Defense Responsive Pathway Regulatory Genes in Asian Rice (Oryza sativa) against Infection of Meloidogyne graminicola (Nematoda:Meloidogynidae). 3 Biotech 2020, 10, 60. [Google Scholar] [CrossRef]
- Singh, D.; Dutta, T.K.; Shivakumara, T.N.; Dash, M.; Bollinedi, H.; Rao, U. Suberin Biopolymer in Rice Root Exodermis Reinforces Preformed Barrier against Meloidogyne graminicola Infection. Rice Sci. 2021, 28, 301–312. [Google Scholar] [CrossRef]
- Rao, A.N.; Johnson, D.E.; Sivaprasad, B.; Ladha, J.K.; Mortimer, A.M. Weed Management in Direct-Seeded Rice. Adv. Agron. 2007, 93, 153–255. [Google Scholar] [CrossRef]
- Devi, L.J. Evaluation of some common rice varieties of manipur for resistance against rice root-knot nematode meloidogyne graminicola. J. Glob. Biosci. 2014, 3, 374–378. [Google Scholar]
- Milligan, S.B.; Bodeau, J.; Yaghoobi, J.; Kaloshian, I.; Zabel, P.; Williamson, V.M. The Root Knot Nematode Resistance Gene Mi from Tomato Is a Member of the Leucine Zipper, Nucleotide Binding, Leucine-Rich Repeat Family of Plant Genes. Plant Cell 1998, 10, 1307. [Google Scholar] [CrossRef]
- Claverie, M.; Dirlewanger, E.; Bosselut, N.; Van Ghelder, C.; Voisin, R.; Kleinhentz, M.; Lafargue, B.; Abad, P.; Rosso, M.-N.; Chalhoub, B.; et al. The Ma Gene for Complete-Spectrum Resistance to Meloidogyne Species in Prunus Is a TNL with a Huge Repeated C-Terminal Post-LRR Region. Plant Physiol. 2011, 156, 779–792. [Google Scholar] [CrossRef]
- Kumbhar, S.D.; Sarawate, C.D. Phule Radha (RDN 99-1) Early Duration, Fine Grained Paddy Variety for Maharashtra. J. Maharashtra Agric. Univ. 2010, 35, 225–228. [Google Scholar]
- Bybd, D.W.; K1rkpatrick, T.; Barker, K.R. An Improved Technique for Clearing and Staining Plant Tissues for Detection of Nematodes. J. Nematol. 1983, 15, 142–143. [Google Scholar] [PubMed]
- Murray, M.G.; Thompson, W.F. Nucleic Acids Research Rapid Isolation of High Molecular Weight Plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for Genetic Linkage Map Construction and Quantitative Trait Locus Mapping in Biparental Populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]

| Variable. | DF (Degrees of Freedom) | Sum of Squares | Mean Sum of Squares | F-Value | Pr (>F) |
|---|---|---|---|---|---|
| NG | 1 | 274.951 | 274.951 | 182.370 | 0 |
| Fm | 1 | 2634.830 | 2634.830 | 675.988 | 0 |
| EM | 1 | 2313.207 | 2313.207 | 613.281 | 0 |
| EEM | 1 | 74,220.810 | 74,220.810 | 74.860 | 0 |
| MF | 1 | 18,322.230 | 18,322.230 | 82.340 | 0 |
| Variables | DF | Sum of Squares | Mean Sum of Squares | F-Value | Pr (>F) |
|---|---|---|---|---|---|
| NG | 175 | 2103.85 | 12.02 | 9.32 | 0 |
| Fm | 175 | 31,626.87 | 180.73 | 16.26 | 0 |
| EM | 175 | 23,228.40 | 132.73 | 17.56 | 0 |
| EEM | 175 | 996,565.30 | 5694.66 | 8.55 | 0 |
| MF | 175 | 190,330.60 | 1087.60 | 10.25 | 0 |
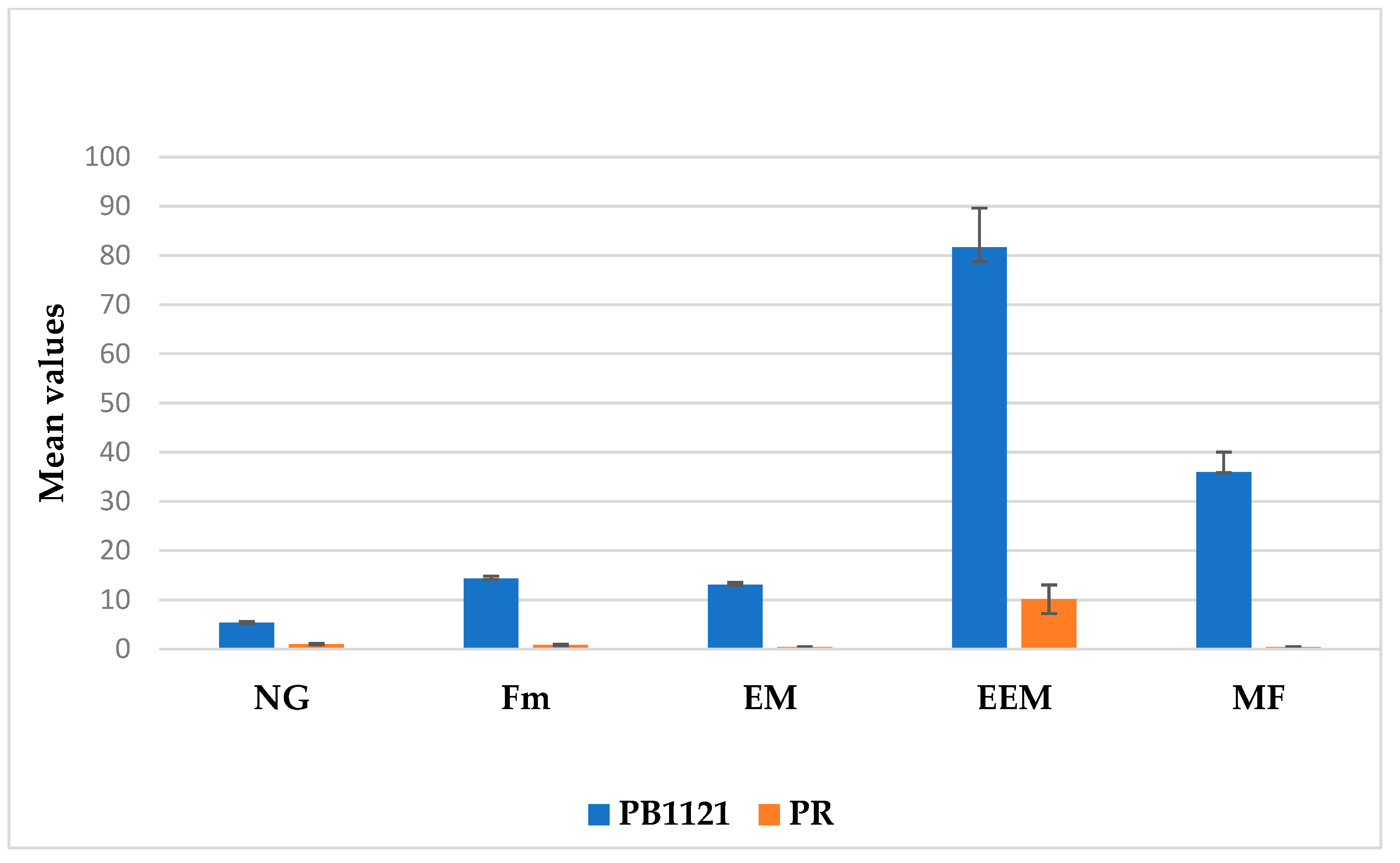
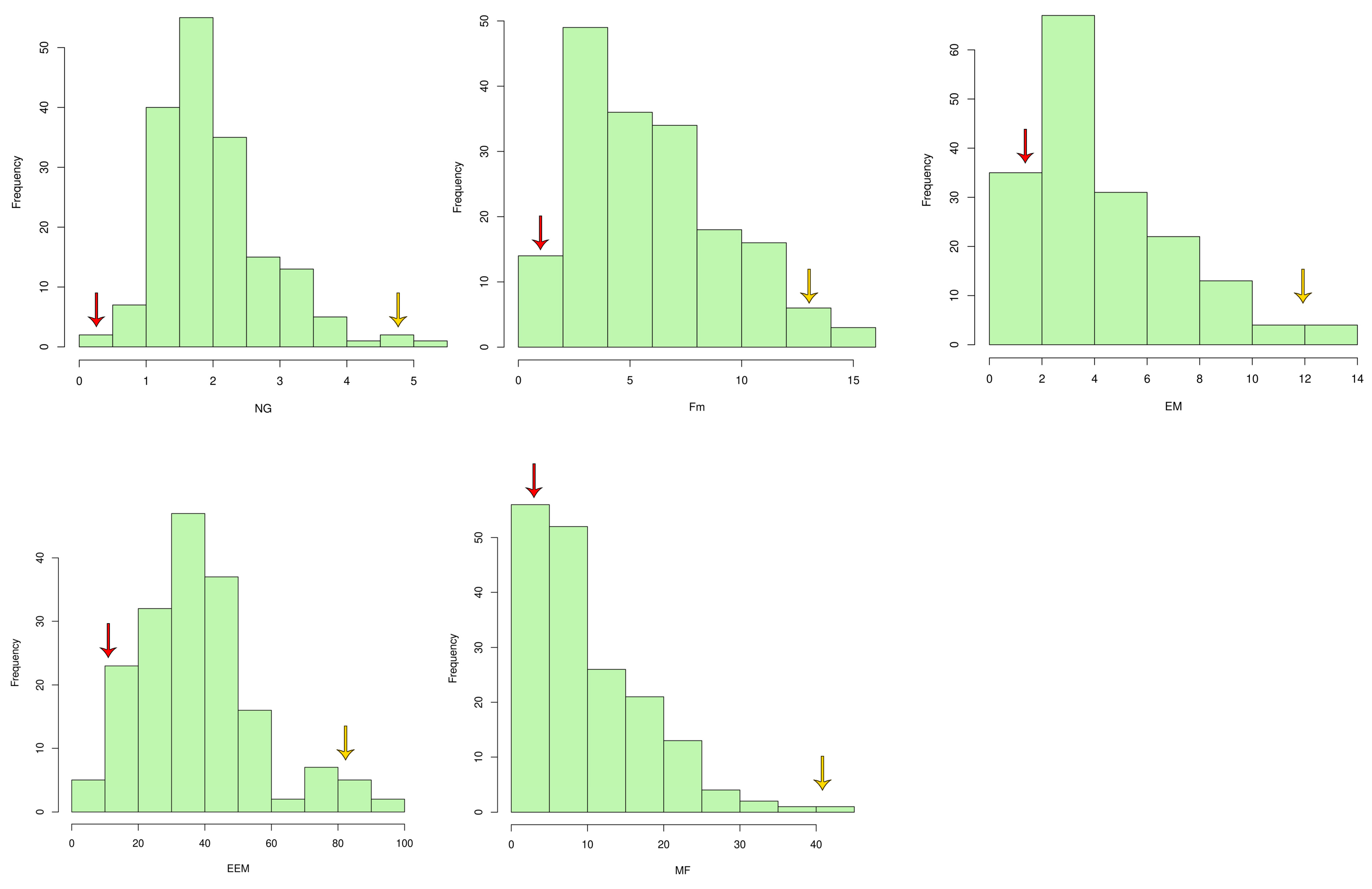
| Traits | Range | Mean | Standard Deviation | Skewness | PB1121 | PR |
|---|---|---|---|---|---|---|
| NG | 0.17–5.00 | 2.00 | 0.78 | 0.90 | 5.00 | 1.00 |
| Fm | 0.11–14.33 | 5.94 | 3.21 | 0.53 | 14.32 | 0.83 |
| EM | 0.00–14.00 | 4.26 | 2.74 | 1.05 | 13.07 | 0.43 |
| EEM | 0.00–96.60 | 37.44 | 17.85 | 0.76 | 81.70 | 10.00 |
| MF | 0.00–41.16 | 9.77 | 7.66 | 1.16 | 35.97 | 0.40 |
| Markers | NG | Fm | EM | EEM | MF | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 (%) | p-Value | R2 | p-Value | R2 | p-Value | R2 | p-Value | R2 | p-Value | |
| RM24 | 4 | 0.01 | 1 | 0.23 | 0 | 0.56 | 0 | 0.49 | 0 | 0.84 |
| RM1334 | 3 | 0.06 | 1 | 0.41 | 0 | 0.75 | 0 | 0.47 | 0 | 0.63 |
| RM6 | 1 | 0.30 | 0 | 0.44 | 0 | 0.58 | 1 | 0.35 | 0 | 0.62 |
| RM13928 | 0 | 0.84 | 0 | 0.40 | 0 | 0.80 | 0 | 0.42 | 0 | 0.85 |
| RM20158 | 0 | 0.94 | 3 | 0.04 | 1 | 0.13 | 1 | 0.15 | 2 | 0.08 |
| RM20414 | 0 | 0.54 | 1 | 0.24 | 1 | 0.19 | 0 | 0.68 | 1 | 0.18 |
| RM5122 | 1 | 0.13 | 3 | 0.03 | 3 | 0.02 | 5 | 0.00 | 5 | 0.00 |
| RM552 | 2 | 0.11 | 4 | 0.02 | 4 | 0.02 | 1 | 0.22 | 4 | 0.02 |
| RM202 | 1 | 0.23 | 0 | 0.94 | 0 | 0.83 | 0 | 0.88 | 0 | 1.00 |
| RM23911 | 1 | 0.13 | 1 | 0.19 | 1 | 0.21 | 4 | 0.01 | 3 | 0.02 |
| RM23998 | 2 | 0.07 | 1 | 0.14 | 1 | 0.12 | 1 | 0.30 | 2 | 0.06 |
| RM7619 | 0 | 0.57 | 0 | 0.80 | 0 | 0.57 | 1 | 0.29 | 0 | 0.74 |
| RM2935 | 0 | 0.59 | 0 | 0.47 | 1 | 0.27 | 0 | 0.90 | 0 | 0.83 |
| RM6293 | 0 | 0.39 | 2 | 0.11 | 3 | 0.02 | 0 | 0.77 | 1 | 0.12 |
| RM1231 | 0 | 0.66 | 0 | 0.86 | 0 | 0.79 | 0 | 0.57 | 0 | 0.96 |
| RM5532 | 0 | 0.73 | 1 | 0.35 | 1 | 0.30 | 1 | 0.12 | 1 | 0.21 |
| RM15652 | 0 | 0.53 | 0 | 0.46 | 0 | 0.43 | 3 | 0.03 | 2 | 0.11 |
| RM259 | 0 | 0.38 | 1 | 0.36 | 1 | 0.16 | 0 | 0.46 | 0 | 0.79 |
| RM28165 | 0 | 0.22 | 0 | 0.52 | 0 | 0.65 | 1 | 0.21 | 1 | 0.26 |
| RM1246 | 0 | 0.28 | 1 | 0.36 | 0 | 0.41 | 1 | 0.18 | 1 | 0.16 |
| RM1234 | 0 | 0.99 | 1 | 0.21 | 1 | 0.33 | 1 | 0.26 | 1 | 0.28 |
| RM6318 | 0 | 0.93 | 0 | 0.75 | 0 | 0.85 | 0 | 0.91 | 0 | 0.92 |
| RM263 | 0 | 0.91 | 0 | 0.76 | 0 | 0.52 | 0 | 0.73 | 1 | 0.38 |
| RM15956 | 0 | 0.62 | 1 | 0.28 | 0 | 0.38 | 1 | 0.29 | 1 | 0.15 |
| RM3735 | 0 | 0.70 | 0 | 0.70 | 1 | 0.18 | 1 | 0.13 | 0 | 0.58 |
| RM20500 | 0 | 0.78 | 1 | 0.20 | 1 | 0.25 | 0 | 0.38 | 0 | 0.82 |
| RM25351 | 0 | 0.60 | 0 | 0.46 | 0 | 0.79 | 2 | 0.10 | 0 | 0.41 |
| RM6737 | 0 | 0.99 | 2 | 0.11 | 3 | 0.04 | 1 | 0.30 | 0 | 0.03 |
| RM3717 | 0 | 0.38 | 2 | 0.15 | 2 | 0.11 | 1 | 0.39 | 0 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Premakumar; Mohanapure, P.; Chavhan, M.; Singh, D.; Yadav, J.; Somvanshi, V.S.; Gopala Krishnan, S.; Vinod, K.K.; Bhowmick, P.K.; Bollinedi, H.; et al. Identification of a Promising Novel Genetic Source for Rice Root-Knot Nematode Resistance through Markers Associated with Trait-Specific Quantitative Trait Loci. Plants 2024, 13, 2271. https://doi.org/10.3390/plants13162271
Premakumar, Mohanapure P, Chavhan M, Singh D, Yadav J, Somvanshi VS, Gopala Krishnan S, Vinod KK, Bhowmick PK, Bollinedi H, et al. Identification of a Promising Novel Genetic Source for Rice Root-Knot Nematode Resistance through Markers Associated with Trait-Specific Quantitative Trait Loci. Plants. 2024; 13(16):2271. https://doi.org/10.3390/plants13162271
Chicago/Turabian StylePremakumar, Pallavi Mohanapure, Meghraj Chavhan, Divya Singh, Jyoti Yadav, Vishal Singh Somvanshi, S. Gopala Krishnan, K. K. Vinod, Prolay K. Bhowmick, Haritha Bollinedi, and et al. 2024. "Identification of a Promising Novel Genetic Source for Rice Root-Knot Nematode Resistance through Markers Associated with Trait-Specific Quantitative Trait Loci" Plants 13, no. 16: 2271. https://doi.org/10.3390/plants13162271
APA StylePremakumar, Mohanapure, P., Chavhan, M., Singh, D., Yadav, J., Somvanshi, V. S., Gopala Krishnan, S., Vinod, K. K., Bhowmick, P. K., Bollinedi, H., Singh, A. K., Rao, U., & Ellur, R. K. (2024). Identification of a Promising Novel Genetic Source for Rice Root-Knot Nematode Resistance through Markers Associated with Trait-Specific Quantitative Trait Loci. Plants, 13(16), 2271. https://doi.org/10.3390/plants13162271







