New Cytogenetic Data for the Neottieae Tribe (Orchidaceae) in the Mediterranean Region
Abstract
:1. Introduction
2. Results
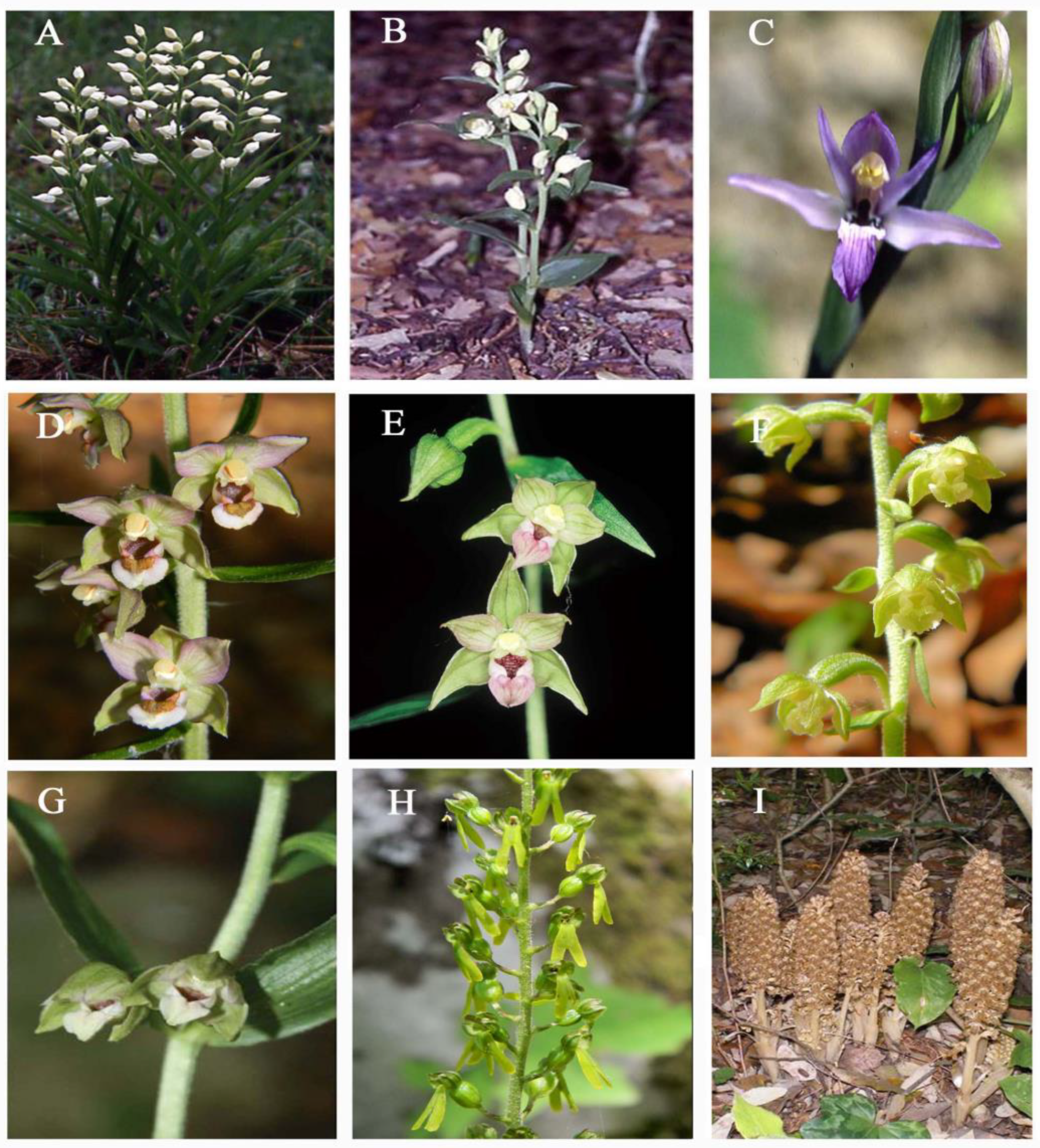
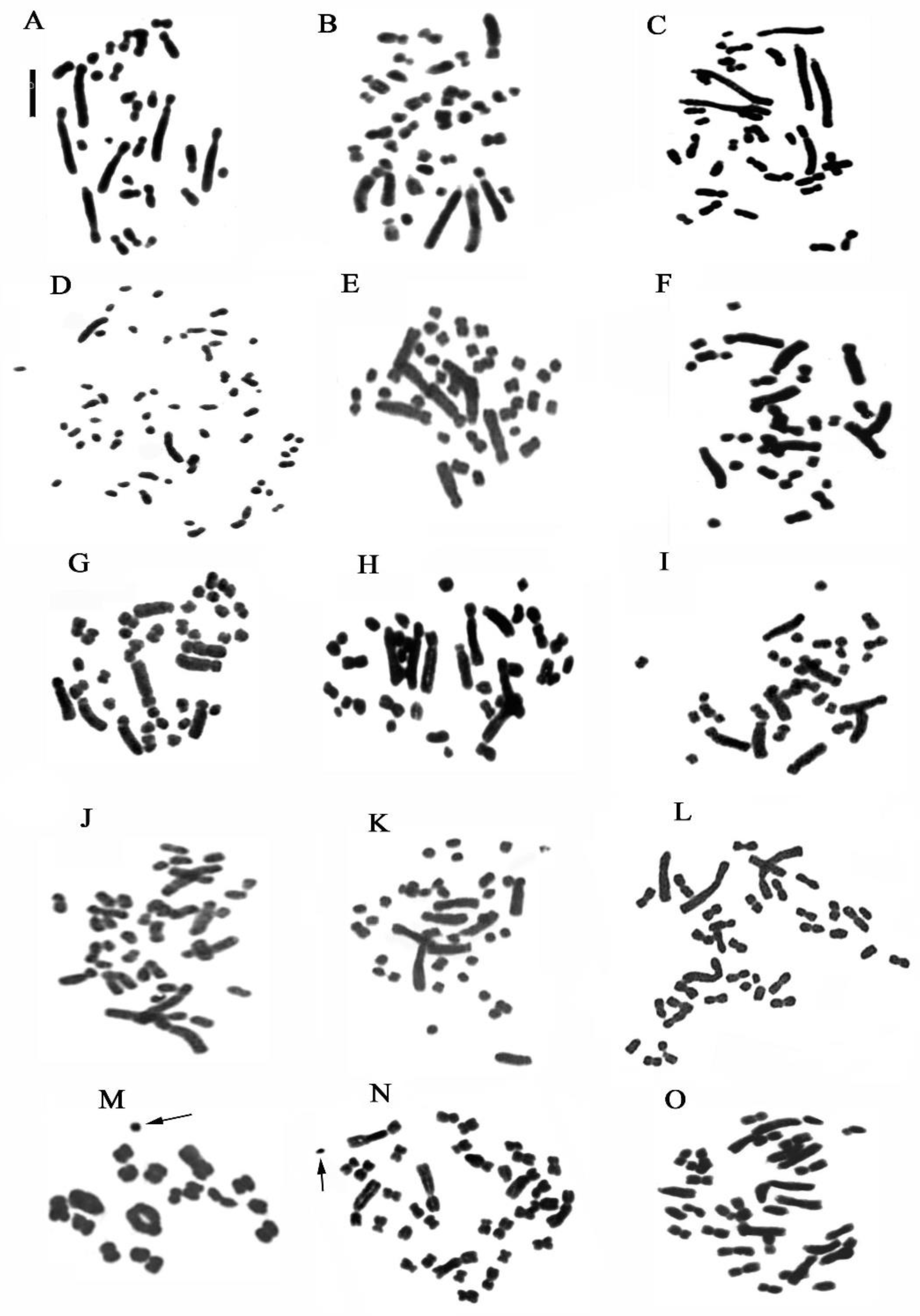
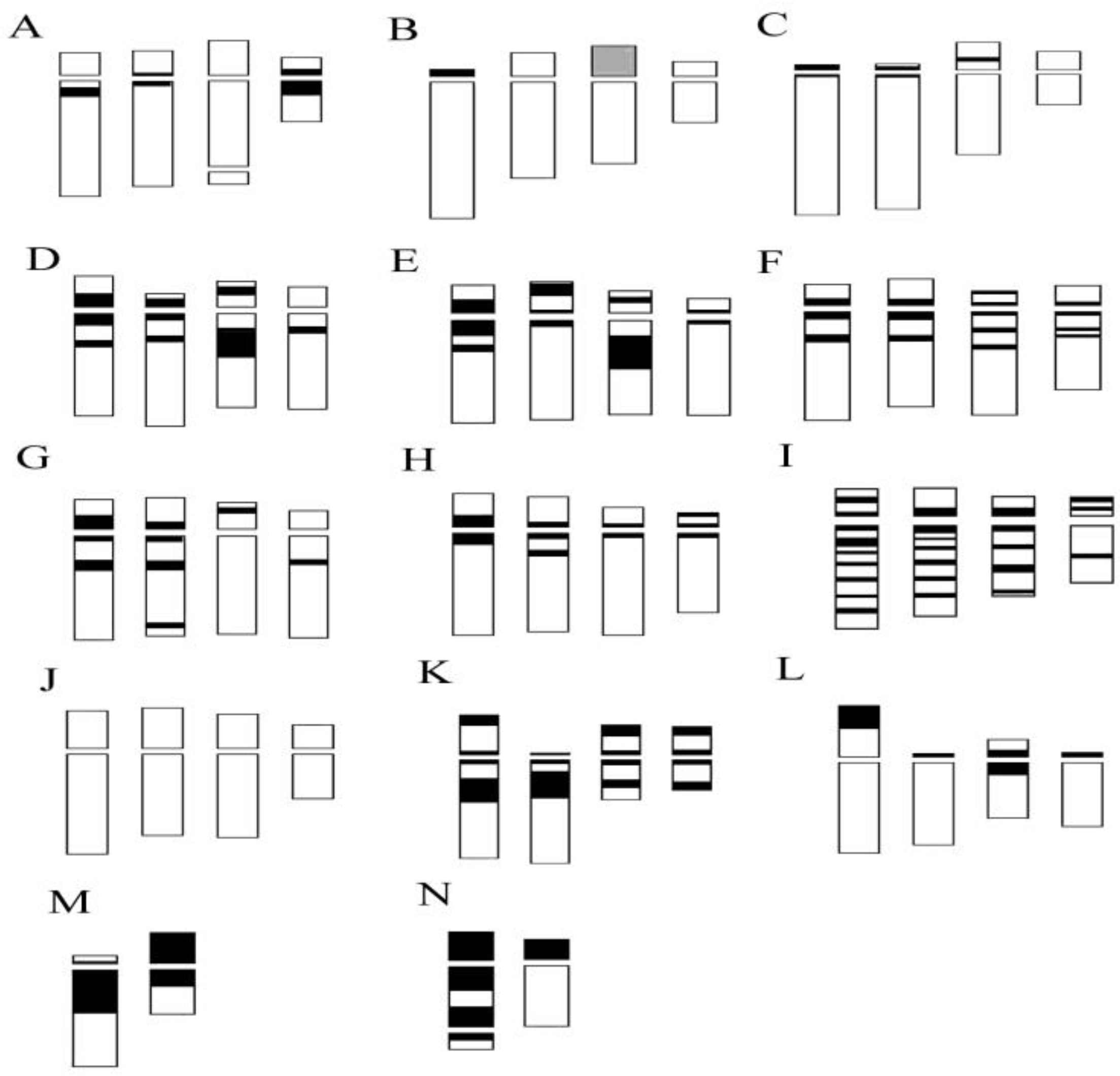
2.1. Genus Cephalanthera Rich
2.2. Genus Limodorum Boehm. in Ludwig
2.3. Genus Epipactis Zinn
2.4. Genus Neottia Guett. (Including Listera)
2.5. Plot of the Morphometric Parameters Mca (Mean Centromeric Asymmetry) and CVcl (Coefficient of Variation of Chromosome Length)
3. Discussion
3.1. Cytotaxonomy between Groups and Species
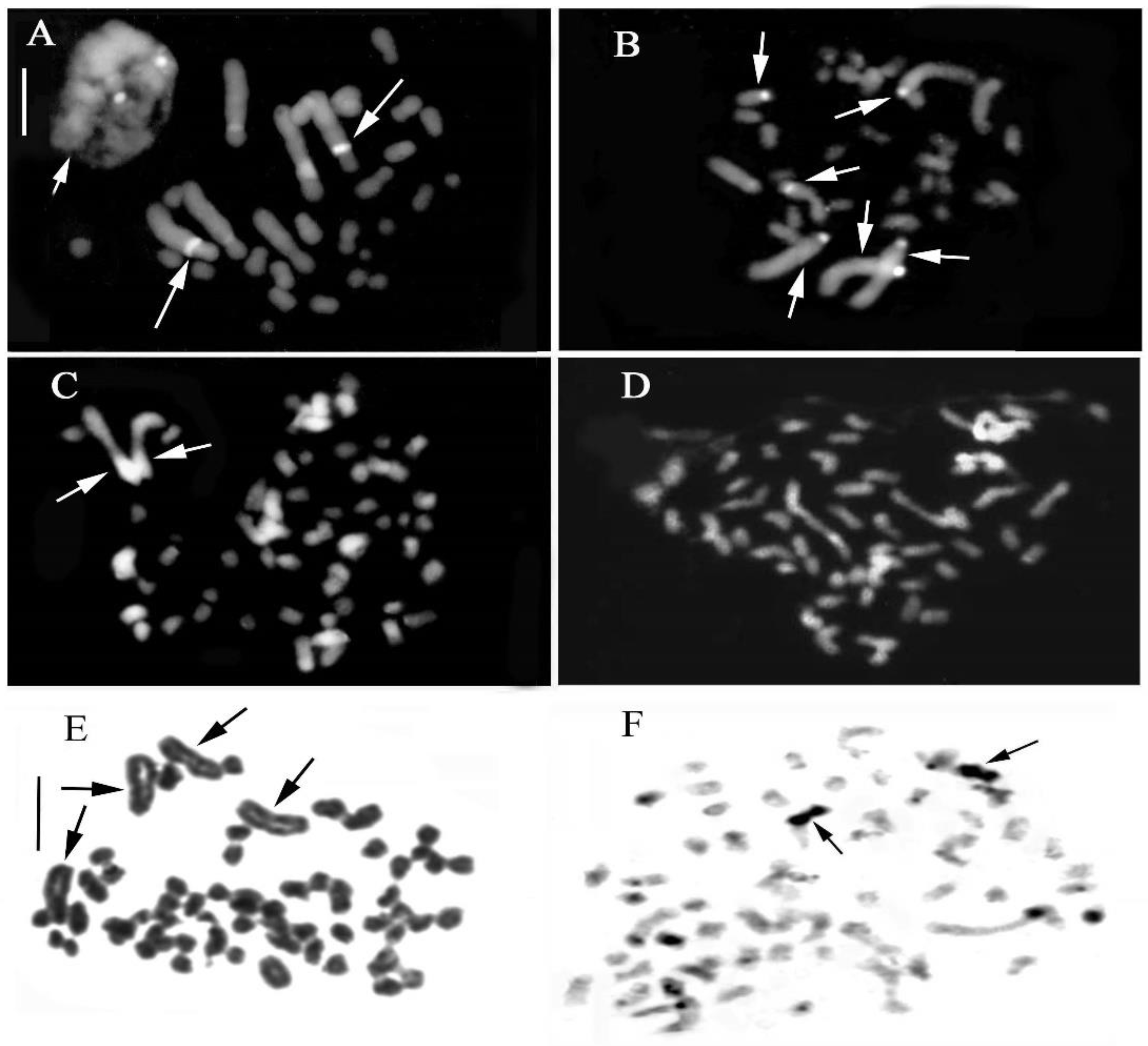
3.2. Chromosome Alterations and Heterochromatin Distribution
4. Materials and Methods
4.1. Cytological Analysis
4.2. Chromosome Numbers and Karyotype Parameters
4.3. Nomenclature
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quentin, P. Synopsis des Orchidées Européennes, 2nd ed.; Cahiers de la Société Française d’Orchidophilie: Paris, France, 1995. [Google Scholar]
- Plants of the World Online (POWO). Facilitated by the Royal Botanic Gardens, Kew. 2024. Available online: http://www.plantsoftheworldonline.org/ (accessed on 12 March 2024).
- Dressler, R.L. Phylogeny and Classification of the Orchid Family; Dioscorides Press: Portland, OR, USA, 1993; 330p. [Google Scholar]
- Bateman, R.M.; Hollingsworth, P.M.; Squirrel, J.; Hollingsworth, M. Tribe Neottieae. In Genera Orchidacearum, Vol. 4. Epidendroideae, Pt. 1; Pridgeon, A., Cribb, P.J., Chase, M.W., Rasmussen, F.N., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 487–515. [Google Scholar]
- Zhou, T.; Jin, X.-H. Molecular systematics and the evolution of mycoheterotrophy of tribe Neottieae (Orchidaceae, Epidendroideae). PhytoKeys 2018, 94, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Delforge, P. Orchidés d’Europe, d’Afrique du Nord et do Proche-Orient, 4th ed.; Delachaux et Niestle: Paris, France, 2016. [Google Scholar]
- GIROS. Orchidee D’Italia, 2nd ed.; Il Castello: Milano, Italy, 2024. [Google Scholar]
- Sramkó, G.; Paun, O.; Brandrud, M.K.; Laczkó, L.; Molnár, A.V.; Bateman, R.M. Iterative allogamy–autogamy transitions drive actual and incipient speciation during the ongoing evolutionary radiation within the orchid genus Epipactis (Orchidaceae). Ann. Bot. 2019, 124, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, F.; Logacheva, M.; Le Clainche, I.; Berard, A.; Zheleznaia, E.; May, M.; Jakalski, M.; Delannoy, E.; Le Paslier, M.-C.; Selosse, M.-A. Thirteen New Plastid Genomes from Mixotrophic and Autotrophic Species Provide Insights into Heterotrophy Evolution in Neottieae Orchids. Genome Biol. Evol. 2019, 11, 2457–2467. [Google Scholar] [CrossRef]
- Kirca, S.; Kreutz, K.; Çolak, A.H. A biogeographical and ecological classification of orchids in Turkey. Phytocoenologia 2020, 50, 65–77. [Google Scholar] [CrossRef]
- Bateman, R.M. Implications of next-generation sequencing for the systematics and evolution of the terrestrial orchid genus Epipactis, with particular reference to the British Isles. Kew Bull. 2020, 75, 4. [Google Scholar] [CrossRef]
- So, J.H.; Lee, N.-S. Phylogenetic analysis of Neottia japonica(Orchidaceae) based on ITS and matK regions. Korean J. Plant Taxon. 2020, 50, 385–394. [Google Scholar] [CrossRef]
- Wagensommer, R.P.; Medagli, P.; Turco, A.; Perrino, E.V. IUCN Red List evaluation of the Orchidaceae endemic to Apulia (Italy) and considerations on the application of the IUCN protocol to rare species. Nat. Conserv. Res. 2020, 5 (Suppl. 1), 90–101. [Google Scholar] [CrossRef]
- Shao, B.Y.; Wang, M.Z.; Chen, S.S.; Ya, J.D.; Jin, X.H. Habitat-related plastome evolution in the mycoheterotrophic Neottia listeroides complex (Orchidaceae, Neottieae). BMC Plant Biol. 2023, 23, 282. [Google Scholar] [CrossRef]
- Turco, A.; Albano, A.; Medagli, P.; D’Emerico, S.; Wagensommer, R.P. Orchidaceae in Puglia (Italy): Consistency, distribution, and conservation. Plants 2023, 12, 2223. [Google Scholar] [CrossRef]
- Mehra, P.N.; Bawa, K.S. Cytological Observations on some Northwest Himalayan Orchids. Caryologia 1970, 23, 273–282. [Google Scholar] [CrossRef]
- Mehra, P.N.; Vij, S.P. Cytological Studies in the East Himalayan Orchidaceae—1: Neottieae. Caryologia 1972, 25, 237–251. [Google Scholar] [CrossRef]
- Vosa, C.G.; Barlow, P.W. Meiosis and B-Chromosomes in Listera ovata (Orchidaceae). Caryologia 1972, 25, 1–8. [Google Scholar] [CrossRef]
- Vij, S.P.; Gupta, G.C. Cytological Investigations into W. Himalayan Orchidaceae I. Chromosome numbers and karyotypes of taxa from Kashmir. Cytologia 1975, 40, 613–621. [Google Scholar] [CrossRef]
- Mehra, P.N.; Kashyap, S.K. Cytological Studies in Some West Himalayan Orchids. Tribe: Neottieae. II. Sub-Tribe: Neottinae. Caryologia 1983, 36, 47–55. [Google Scholar] [CrossRef]
- Vosa, C.G. The Ecology of B-Chromosomes in Listera ovata (L.) R. Br. (Orchidaceae). Caryologia 1983, 36, 113–120. [Google Scholar] [CrossRef]
- D’Emerico, S.; Bianco, P.; Medagli, P. Cytological and karyological studies on Orchidaceae. Caryologia 1993, 46, 309–319. [Google Scholar] [CrossRef]
- Bernardos, S.; Tyteca, D.; Revuelta, J.L.; Amich, F. A new endemic species of Epipactis (Orchidaceae) from north-east Portugal. Bot. J. Linn. Soc. 2004, 145, 239–249. [Google Scholar] [CrossRef]
- D’Emerico, S.; Grünanger, P.; Scrugli, A.; Pignone, D. Karyomorphological parameters and C-bands distribution suggest phyletic relationship within the subtribe Limodorinae Bentham (Orchidaceae). Plant Syst. Evol. 1999, 217, 147–161. [Google Scholar] [CrossRef]
- D’Emerico, S.; Pignone, D.; Scrugli, A. Karyomorphology and Evolution in Italian Populations of Three Neottieae Species (Orchidaceae). Cytologia 2000, 65, 189–195. [Google Scholar] [CrossRef]
- Bartolo, G.; D’Emerico, S.; Pulvirenti, S.; Terrasi, M.C.; Stuto, S. Cytotaxonomical considerations on Epipactis robatschiana (Orchidaceae), new species from Calabria (S Italy). Caryologia 2003, 56, 439–445. [Google Scholar] [CrossRef]
- Moscone, E.A.; Samuel, R.; Schwarzacher, T.; Schweizer, D.; Pedrosa-Harand, A. Complex rearrangements are involved in Cephalanthera (Orchidaceae) chromosome evolution. Chromosome Res. 2007, 15, 931. [Google Scholar] [CrossRef] [PubMed]
- Bartolo, G.; Brullo, C.; Pulvirenti, S.; Scrugli, A.; Terrasi, M.C.; D’Emerico, S. Advances in chromosomal studies in Neottieae (Orchidaceae): Constitutive heterochromatin, chromosomal rearrangements and speciation. Caryologia 2010, 63, 184–191. [Google Scholar]
- Felix, L.P.; Guerra, M. Variation in chromosome number and the basic number of subfamily Epidendroideae (Orchidaceae). Bot. J. Linn. Soc. 2010, 163, 234–278. [Google Scholar] [CrossRef]
- Cauwet-Marc, A.M.; Balayer, M. Les Orchidées du Bassin méditerranéen Contribution à l’étude caryologique des espèces des Pyrénées orientales (France) et contrées limitrophes—Tribu des Neottiae Lindl. Bull. Société Bot. France. Lett. Bot. 1984, 131, 121–137. [Google Scholar] [CrossRef]
- Stebbins, G.L. Chromosomal Evolution in Higher Plants; Arnold: London, UK, 1971. [Google Scholar]
- Goldblatt, P. Cytological Variability in the African Genus Lapeirousia (Iridaceae- Ixioideae). Ann. Mo. Bot. Gard. 1990, 77, 375–382. [Google Scholar] [CrossRef]
- Pazy, B.; Plitma, U.; Cohen, O. Bimodal karyotype in Cynomorium L. and its systematic implications. Bot. J. Linn. Soc. 1996, 120, 279–281. [Google Scholar] [CrossRef]
- Forrest, L.L.; Jong, K. Karyotype asymmetry in Galtonia and Pseudogaltonia (Hyacinthaceae). Edinb. J. Bot. 2004, 60, 569–579. [Google Scholar] [CrossRef]
- Mckain, M.R.; Wickett, N.; Zhang, Y.; Ayyampalayam, S.; Mccombie, W.R.; Chase, M.W.; Pires, J.C.; Depamphilis, C.W.; Leebens-Mack, J. Phylogenomic analysis of transcriptome data elucidates Co-occurrence of a paleopolyploid event and the origin of bimodal karyotypes in Agavoideae (Asparagaceae). Am. J. Bot. 2012, 99, 397–406. [Google Scholar] [CrossRef]
- Medeiros-Neto, E.; Nollet, F.; Moraes, A.P.; Felix, L.P. Intrachromosomal karyotype asymmetry in Orchidaceae. Genet. Mol. Biol. 2017, 40, 610–619. [Google Scholar] [CrossRef]
- Báez, M.; Vaio, M.; Dreissig, S.; Schubert, V.; Houben, A.; Pedrosa-Harand, A. Together but Different: The Subgenomes of the Bimodal Eleutherine Karyotypes Are Differentially Organized. Front. Plant Sci. 2019, 7, 1170. [Google Scholar] [CrossRef]
- Ibiapino, A.; Báez, M.; García, M.A.; Costea, M.; Stefanović, S.; Pedrosa-Harand, A. Karyotype asymmetry in Cuscuta L. subgenus Pachystigma refects its repeat DNA composition. Chromosome Res. 2022, 30, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.V.; Shrirang, R.; Yadav, R.; Lekhak, M.M. Karyomorphological Study in Ledebouria botryoides (Asparagaceae). Cytologia 2023, 88, 15–19. [Google Scholar]
- Vosa, C.G.; Perry, P.L. On the cytotaxonomy of the genus Eriospermum Jacq. ex Willd. (Eriospermaceae). Caryologia 1999, 52, 117–125. [Google Scholar] [CrossRef]
- Yin, G.-S.; Yang, Z.-Y.; Chiang, T.-Y.; Cong, X. Tracing the origin of the bimodal karyotypes of the Tribe Lilieae (Liliaceae) based on comparative karyotype analyses. Plant Divers. Resour. 2014, 36, 737–746. [Google Scholar]
- Tapia-Pastrana, F.; Tapia-Aguirre, F. Evidence of autopolyploidy and translocations in the karyotype of Tigridia pavonia (Iridaceae, Iridoideae) from the Reserva Ecológica del Pedregal de San Ángel, Mexico. Acta Bot. Mex. 2017, 121, 151–158. [Google Scholar] [CrossRef]
- Lisachov, A.P.; Tishakova, K.V.; Romanenko, S.A.; Molodtseva, A.S.; Prokopov, D.Y.; Pereira, J.C.; Ferguson-Smith, M.A.; Borodin, P.M.; Trifonov, V.A. Whole-chromosome fusions in the karyotype evolution of Sceloporus (Iguania, Reptilia) are more frequent in sex chromosomes than autosomes. Phil. Trans. R. Soc. B 2021, 376, 20200099. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.J. Chromosome abbreviations. In Plant Cytogenetics, 3rd ed.; Singh, R.J., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 161–163. [Google Scholar]
- Yu, Y. A Novel Role of Ring Chromosomes as Evolutionary Drivers of Herbicide Resistance. Plant Physiol. 2018, 176, 1892–1893. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Rodriguez, V.M.; Rodriguez-Garay, B.; Palomino, G.; Martínez, J.; Barba-Gonzalez, R. Physical mapping of 5S and 18S ribosomal DNA in three species of Agave (Asparagales, Asparagaceae). Comp. Cytogenet. 2013, 7, 191–203. [Google Scholar] [CrossRef]
- Ramos, L.C.; Báez, M.A.; Houben, A.; Cavalcanti, J.J.V.; Carvalho, R.; Pedrosa-Harand, A. Repetitive DNA distribution in Agave (Asparagaceae) bimodal karyotype. Ciências Biológicas Saúde Londrina 2017, 38 (Suppl. 1), 233. [Google Scholar]
- Felix, L.P.; Guerra, M. Cytogenetics and cytotaxonomy of some Brazilian species of Cymbidioid orchids. Genet. Mol. Biol. 2000, 23, 957–978. [Google Scholar]
- Brullo, C.; D’Emerico, S.; Pulvirenti, S. Karyological and taxonomical considerations on Epipactis cupaniana sp. nov. (Orchidaceae) from Sicily. Nord. J. Bot. 2013, 30, 001–013. [Google Scholar] [CrossRef]
- Cameron, K.M.; Chase, M.W.; Whitten, W.M.; Keres, P.J.; Jarrel, D.C.; Albert, V.A.; Yukawa, T.; Hills, H.G.; Goldman, D.H. A phylogenetic analysis of the Orchidaceae: Evidence from rbcl nucleotide sequences. Am. J. Bot. 1999, 86, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Brandham, P. Cytogenetics. In Genera Orchidacearum. 1. General introduction, Apostasioideae, Cypripedioideae; Pridgeon, A.M., Cribb, P.J., Chase, M.W., Rasmussen, F.N., Eds.; Oxford University Press: Oxford, UK, 1999; pp. 67–80. [Google Scholar]
- Schwarzacher, T. Karyotype and heterochromatin in Cephalanthera (Orchidaceae). In Kew Chromosome Conference II; Brandham, P.E., Bennett, M.D., Eds.; George Allen & Unwin: London, UK, 1983; p. 364. [Google Scholar]
- Yang, D.-Q.; Zhu, X.-F. Studies on Karyotype of Cephalanthera erecta and C. falcata. J. Syst. Evol. 1989, 27, 114–117. [Google Scholar]
- Sera, T.; Aoyama, M. Chromosomes of Cephalanthera longifolia (L) Fritsch from Hiroshima Prefecture. Bull. Hiroshima Bot. Gard. 2019, 34, 9–11. [Google Scholar]
- Bartolo, G.; D’Emerico, S.; Pulvirenti, S.; Scrugli, A.; Terrasi, M.C. Karyotype structure and chromosome banding in Limodorum brulloi Bartolo e Pulvirenti (Orchidaceae). J. Eur. Orchid. 2002, 34, 87–96. [Google Scholar]
- Bartolo, G.; Pulvirenti, S. Limodorum brulloi (Orchidaceae) a new species from Calabria (S. Italy). Candollea 1993, 48, 485–491. [Google Scholar]
- Flavell, R.B. Repetitive DNA and chromosome evolution in plants. Philos. Trans. 1986, 312, 227–242. [Google Scholar]
- Bartolo, G.; Pulvirenti, S.; Robatsch, K. Epipactis aspromontana (Orchidaceae): Una nuova specie dalla Calabria (Italia meridionale). Caesiana 1996, 6, 41–47. [Google Scholar]
- Bateman, R.M. Systematics and conservation of British and Irish orchids: A “state of the union” assessment to accompany Atlas 2020. Kew Bull. 2022, 77, 355–402. [Google Scholar] [CrossRef]
- Tranchida-Lombardo, V.; Cafasso, D.; Cristaudo, A.; Cozzolino, S. Phylogeographic patterns, genetic affinities and morphological differentiation between Epipactis helleborine and related lineages in a Mediterranean glacial refugium. Ann. Bot. 2011, 107, 427–436. [Google Scholar] [CrossRef]
- Ramos, L.C.; Báez, M.; Fuchs, J.; Houben, A.; Carvalho, R.; Pedrosa-Harand, A. Differential Repeat Accumulation in the Bimodal Karyotype of Agave L. Genes 2023, 14, 491. [Google Scholar] [CrossRef]
- Schwarzacher, T.; Schweizer, D. Karyotype analysis and heterochromatin differentiation with Giemsa C-banding and fluorescent counterstaining in Cephalanthera (Orchidaceae). Plant Syst. Evol. 1982, 141, 91Y113. [Google Scholar] [CrossRef]
- Rewicz, A.; Rewers, M.; Jędrzejczyk, I.; Rewicz, T.; Kołodziejek, J.; Jakubska-Busse, A. Morphology and genome size of Epipactis helleborine (L.) Crantz (Orchidaceae) growing in anthropogenic and natural habitats. PeerJ 2018, 6, e5992. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, R.; Sasaki, T.; Ishikawa, R.; Osabe, K.; Kawanabe, T.; Dennis, E.S. Molecular Mechanisms of Epigenetic Variation in Plants. Int. J. Mol. Sci. 2012, 13, 9900–9922. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Mukai, Y. Chromosome research in orchids: Current status and future prospects with special emphasis from molecular and epigenetic perspective. Nucleus 2015, 58, 173–184. [Google Scholar] [CrossRef]
- McClintock, B. A correlation of ring-shaped chromosomes with variegation in Zea mays. Proc. nat. Acad. Sci. 1932, 18, 677–681. [Google Scholar] [CrossRef]
- McClintock, B. Spontaneous alterations in chromosome size and form in Zea mays. Cold Spr. Harb. Symp. Quant. Biol. 1941, 9, 72–81. [Google Scholar] [CrossRef]
- Singh, R.J.; Tsuchiya, T. Ring Chromosomes in Barley (Hordeum vulgare L.). Chromosoma 1981, 82, 133–141. [Google Scholar] [CrossRef]
- Palomino, G.; Martínez, J.; Méndez, I. Karyotype studies in cultivars of Agave tequilana Weber. Caryologia 2008, 61, 144–153. [Google Scholar] [CrossRef]
- Weiss-Schneeweiss, H.; Schneeweiss, G.M. Karyotype Diversity and Evolutionary Trends in Angiosperms. In Plant Genome Diversity; Greilhuber, J., Dolezel, J., Wendel, J., Eds.; Springer: Vienna, Austria, 2013; Volume 2. [Google Scholar] [CrossRef]
- Deanna, R.; Acosta, M.C.; Scaldaferro, M.; Chiarini, F. Chromosome Evolution in the Family Solanaceae. Front. Plant Sci. 2022, 12, 787590. [Google Scholar] [CrossRef]
- Hughes, S.E.; Hawley, R.S. Heterochromatin: A Rapidly Evolving Species Barrier. PLoS Biol. 2009, 7, e1000233. [Google Scholar] [CrossRef]
- Jones, K. Aspects of chromosome evolution in higher plants. Adv. Bot. Res. 1978, 6, 119–194. [Google Scholar]
- Battaglia, E. A simplified Feulgen method using cold hydrolysis. Caryologia 1957, 9, 372–373. [Google Scholar] [CrossRef]
- Perrino, R.; Pignone, D. Contribution to the taxonomy of Vicia species belonging to the section Faba. Kulturpflanze 1981, 29, 311–319. [Google Scholar] [CrossRef]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Paszko, B. A critical review and a new proposal of karyotype asymmetry indices. Plant Syst. Evol. 2006, 258, 39–48. [Google Scholar] [CrossRef]
- Zuo, L.; Yuan, Q. The difference between the heterogeneity of the centromeric index and intrachromosomal symmetry. Plant Syst. Evol. 2011, 297, 141–145. [Google Scholar] [CrossRef]
- Peruzzi, L.; Eroğlu, H.E. Karyotype asymmetry: Again, how to measure and what to measure? Comp. Cytogenet. 2013, 7, 1–9. [Google Scholar] [CrossRef]



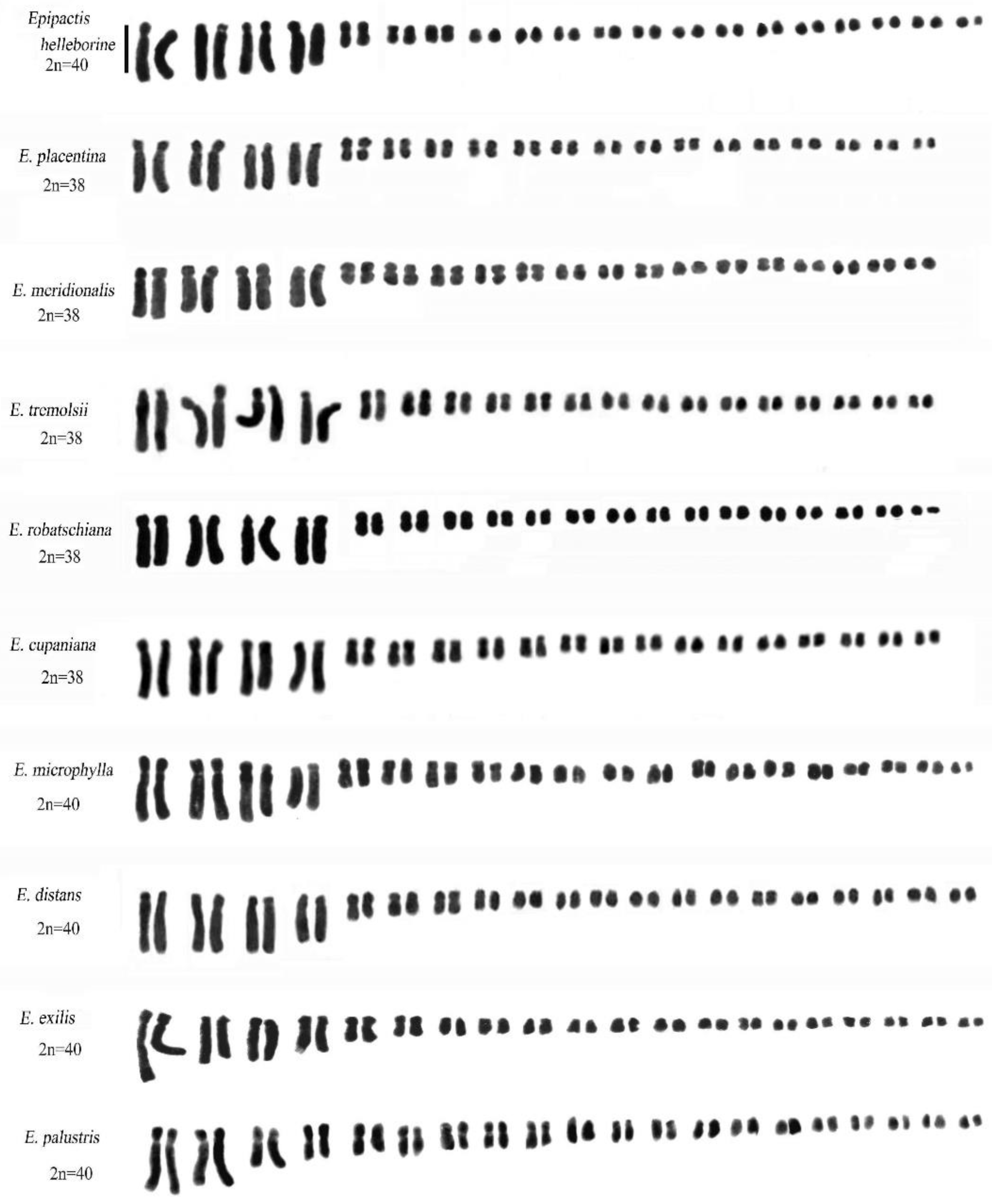
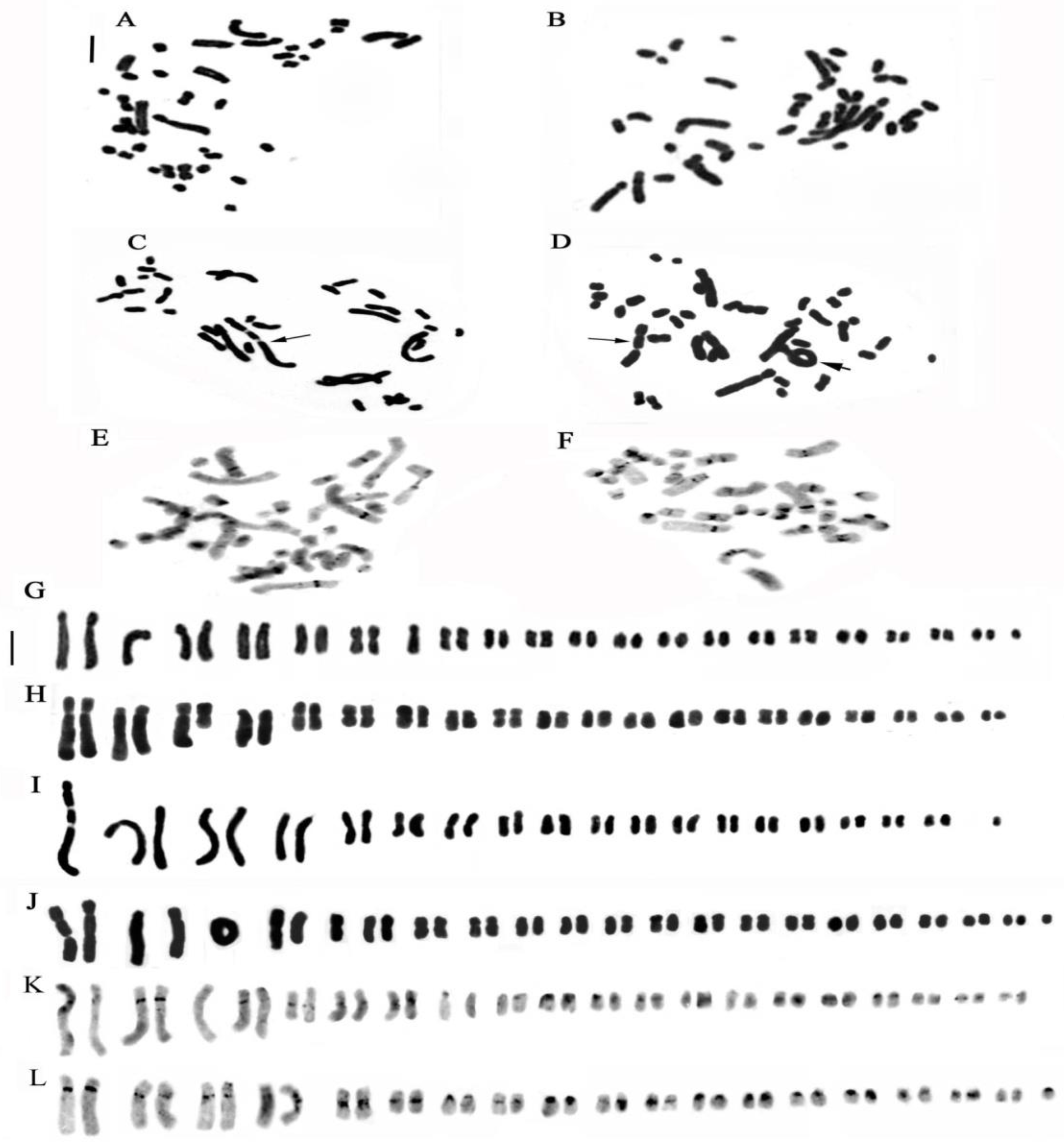

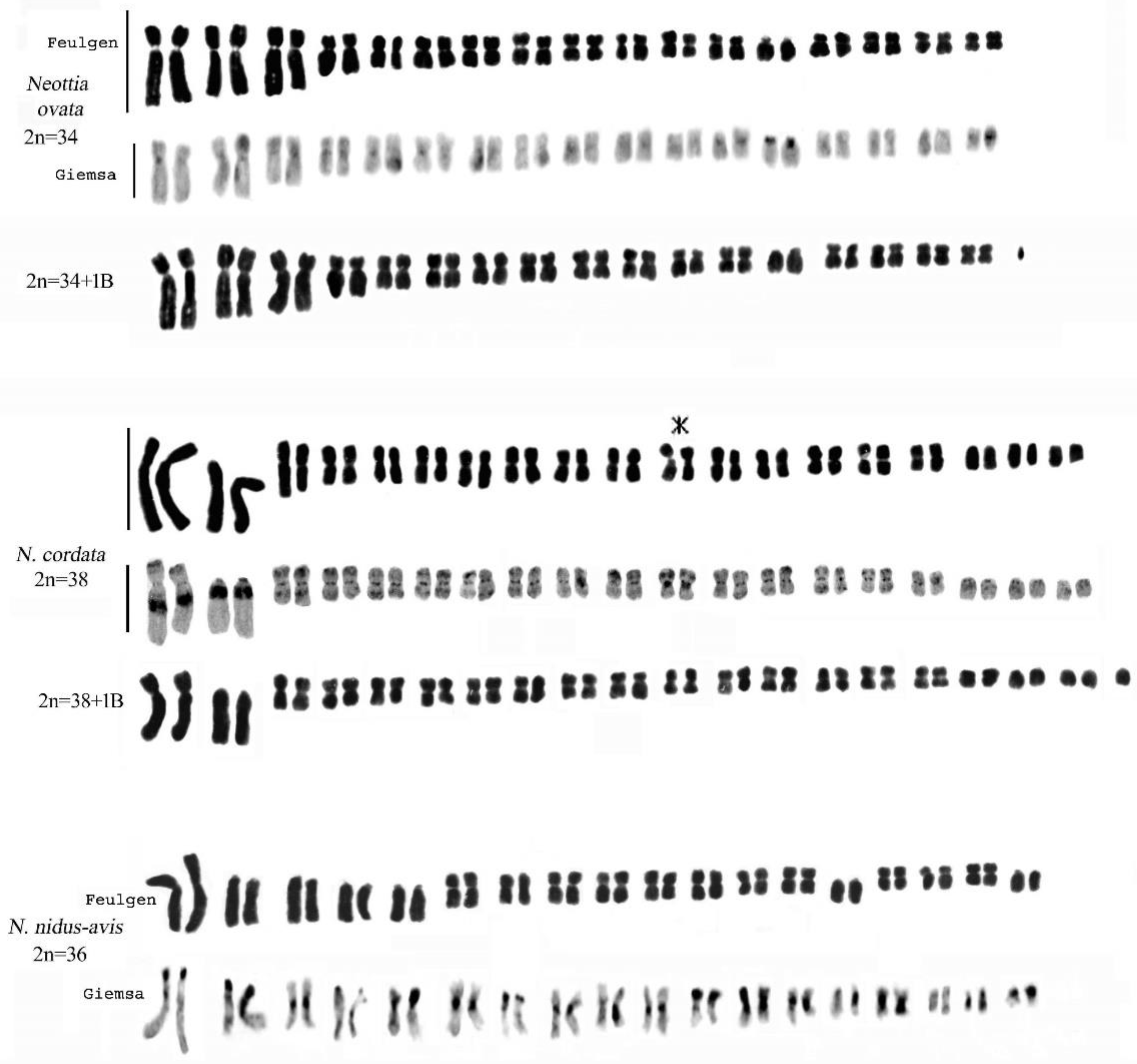
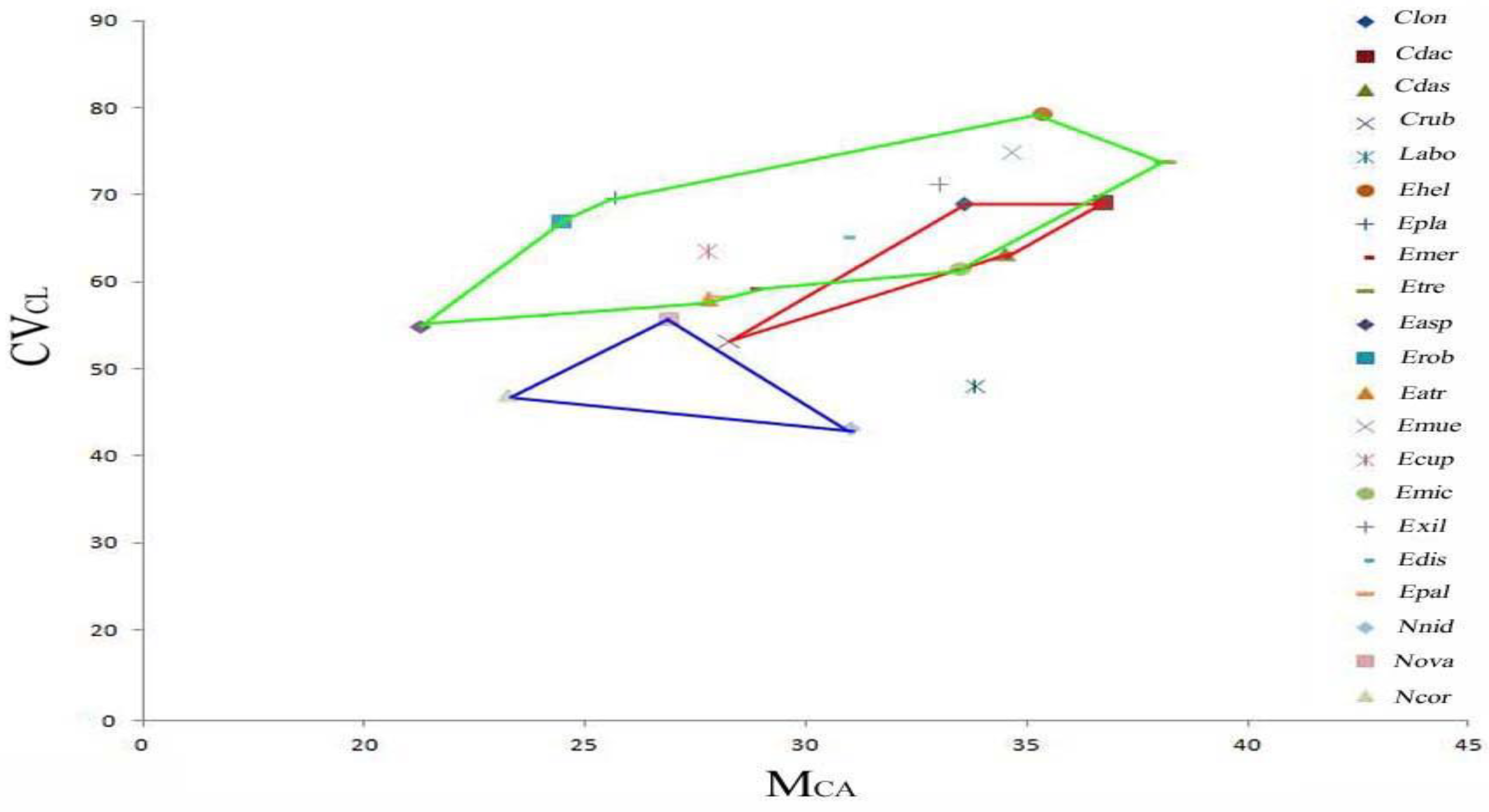
| Taxon | Code | Provenance | 2n | Formula | THL | MCA | CVCL | CVCI |
|---|---|---|---|---|---|---|---|---|
| Cephalanthera damasonium (Mill.) Druce | Cdac | Continental Italy, Sardinia | 36 | 12 m + 16 sm + 4 st + 4 t | 68.96 | 36.73 | 69.08 | 36.08 |
| C. damasonium (Mill.) Druce | Cdas | Sardinia | 36 | 16 m + 12 sm + 4 st + 4 t | 51.88 | 34.52 | 63.17 | 38.64 |
| C. longifolia (L.) Fritsch | Clon | Italy | 32 | 10 m + 16 sm + 6 st | 60.00 | 33.57 | 68.86 | 25.40 |
| C. rubra (L.) Rich. | Crub | Italy | 44 | 22 m + 16 sm + 6 st | 66.28 | 28.26 | 53.09 | 24.64 |
| Limodorum abortivum (L.) Sw. | Labo | Italy | 56 | 18 m + 26 sm + 12 st | 59.57 | 33.83 | 47.98 | 26.46 |
| Epipactis aspromontana Bartolo, Pulv. & Robatsch | Easp | Italy | 38 | 30 m + 4 sm + 4 st | 50.67 | 21.30 | 54.82 | 22.71 |
| E. atrorubens (Hoffm.) Besser | Eatr | Italy | 38 | 20 m + 10 sm + 8 st | 48.70 | 27.80 | 57.97 | 30.29 |
| E. cupaniana C. Brullo, D’Emerico & Pulv. | Ecup | Italy | 38 | 24 m + 4 sm + 10 st | 52.77 | 27.79 | 63.50 | 30.37 |
| E. distans Arv.-Touv. | Edis | Italy, France | 40 | 20 m + 10 sm + 10 st | 52.88 | 30.90 | 65.09 | 30.66 |
| E. exilis P. Delforge | Exil | Italy | 40 | 16 m + 18 sm + 6 st | 57.15 | 33.02 | 71.20 | 26.48 |
| E. helleborine (L.) Crantz | Ehel | Italy | 40 | 16 m + 14 sm + 10 st | 50.90 | 35.35 | 79.28 | 29.90 |
| E. meridionalis H. Baumann & R. Lorenz | Emer | Italy | 38 | 20 m + 8 sm + 10 st | 44.76 | 28.78 | 59.21 | 30.12 |
| E. microphylla (Ehrh.) Sw. | Emic | Italy | 40 | 16 m + 12 sm + 12 st | 63.02 | 33.50 | 61.46 | 34.63 |
| E. muelleri Godfery | Emue | Italy | 38 | 16 m + 12 sm + 10 st | 47.43 | 34.67 | 74.89 | 28.56 |
| E. placentina Bongiorni & Grünanger | Epla | Italy | 38 | 26 m + 4 sm + 8 st | 45.25 | 25.71 | 69.64 | 27.72 |
| E. palustris (L.) Crantz | Epal | Italy | 40 | 24 m + 8 sm + 8 st | 63.60 | 28.05 | 58.28 | 24.36 |
| E. robatschiana Bartolo, D’Emerico, Pulv., Terrasi & Stuto | Erob | Italy | 38 | 26 m + 4 sm + 8 st | 51.08 | 24.48 | 66.98 | 29.13 |
| E. tremolsii Pau | Etre | Italy | 38 | 16 m + 8 sm + 10 st + 4 t | 52.83 | 38.14 | 73.74 | 38.99 |
| Neottia cordata (L.) Rich. (Listera) | Ncor | Italy | 38 | 28 m + 4 sm + 4 st + 2 t | 38.61 | 23.28 | 47.04 | 36.06 |
| N. nidus-avis (L.) Rich. | Nnid | Italy | 36 | 24 m + 4 sm + 2 st + 6 t | 66.26 | 31.03 | 43.07 | 42.53 |
| N. ovata (L.) Hartm. (Listera) | Nova | Italy | 34 | 22 m + 10 sm + 2 st | 58.59 | 26.92 | 55.69 | 22.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turco, A.; Wagensommer, R.P.; Albano, A.; Medagli, P.; D’Emerico, S. New Cytogenetic Data for the Neottieae Tribe (Orchidaceae) in the Mediterranean Region. Plants 2024, 13, 1776. https://doi.org/10.3390/plants13131776
Turco A, Wagensommer RP, Albano A, Medagli P, D’Emerico S. New Cytogenetic Data for the Neottieae Tribe (Orchidaceae) in the Mediterranean Region. Plants. 2024; 13(13):1776. https://doi.org/10.3390/plants13131776
Chicago/Turabian StyleTurco, Alessio, Robert Philipp Wagensommer, Antonella Albano, Pietro Medagli, and Saverio D’Emerico. 2024. "New Cytogenetic Data for the Neottieae Tribe (Orchidaceae) in the Mediterranean Region" Plants 13, no. 13: 1776. https://doi.org/10.3390/plants13131776
APA StyleTurco, A., Wagensommer, R. P., Albano, A., Medagli, P., & D’Emerico, S. (2024). New Cytogenetic Data for the Neottieae Tribe (Orchidaceae) in the Mediterranean Region. Plants, 13(13), 1776. https://doi.org/10.3390/plants13131776









