Dissection of the Genetic Basis of Genotype by Environment Interactions for Morphological Traits and Protein Content in Winter Wheat Panel Grown in Morocco and Spain
Abstract
1. Introduction
2. Results
2.1. Characterization of Environmental Field Trials
2.2. Grain Morphological Traits and Protein Content of the WWAGI Genotypes in Multiple Environments
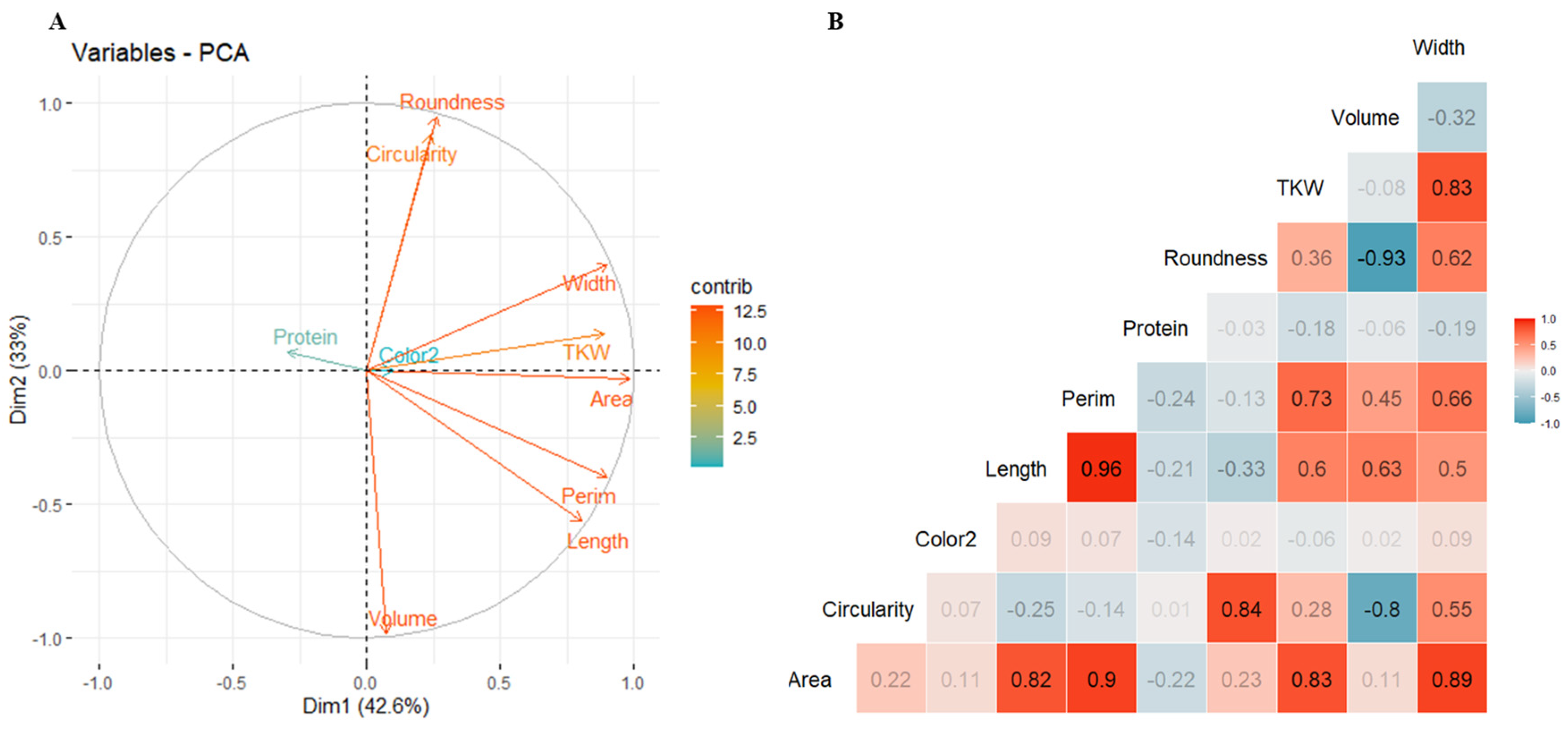
2.3. Relationship and Correlation between Studied Traits
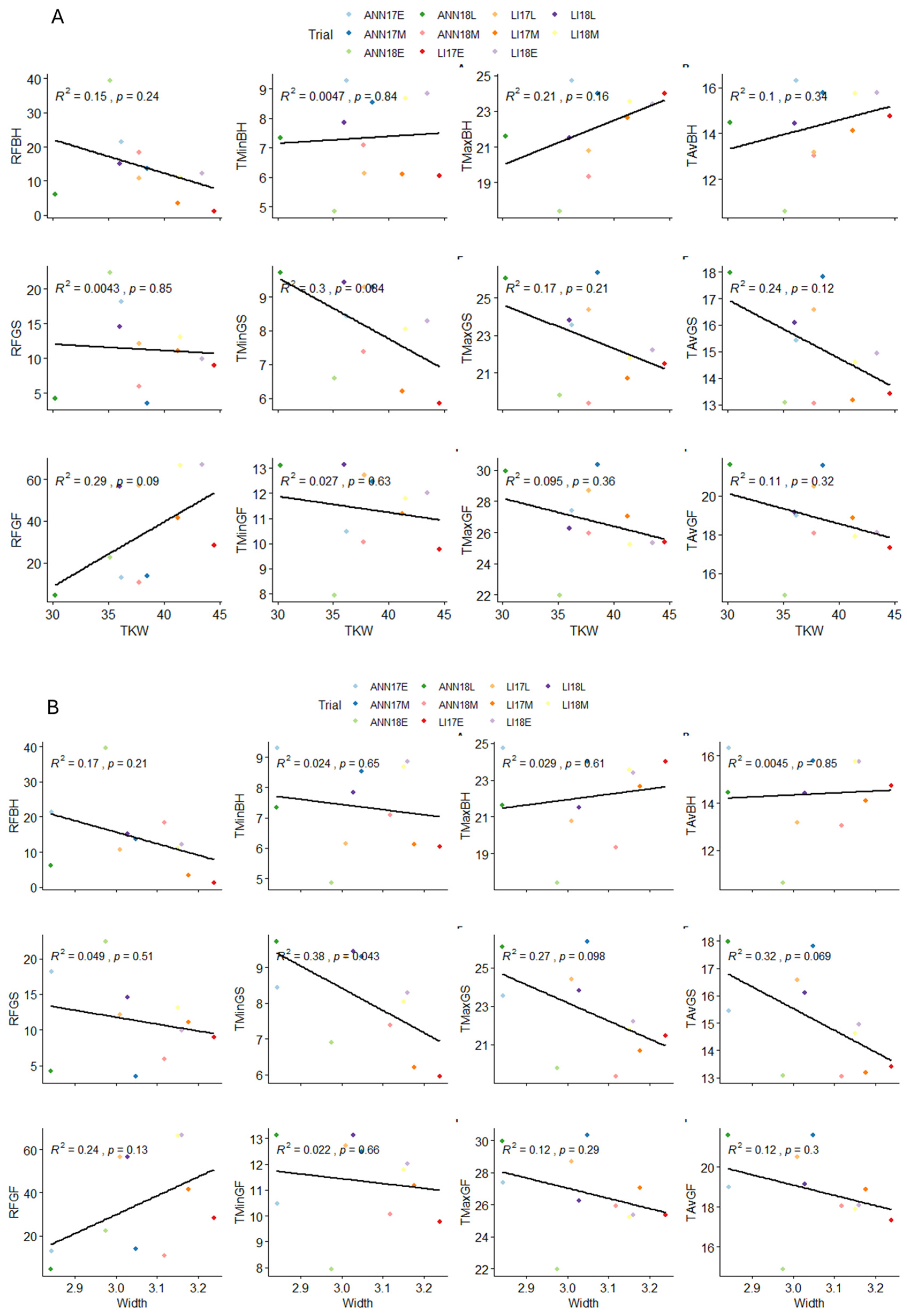
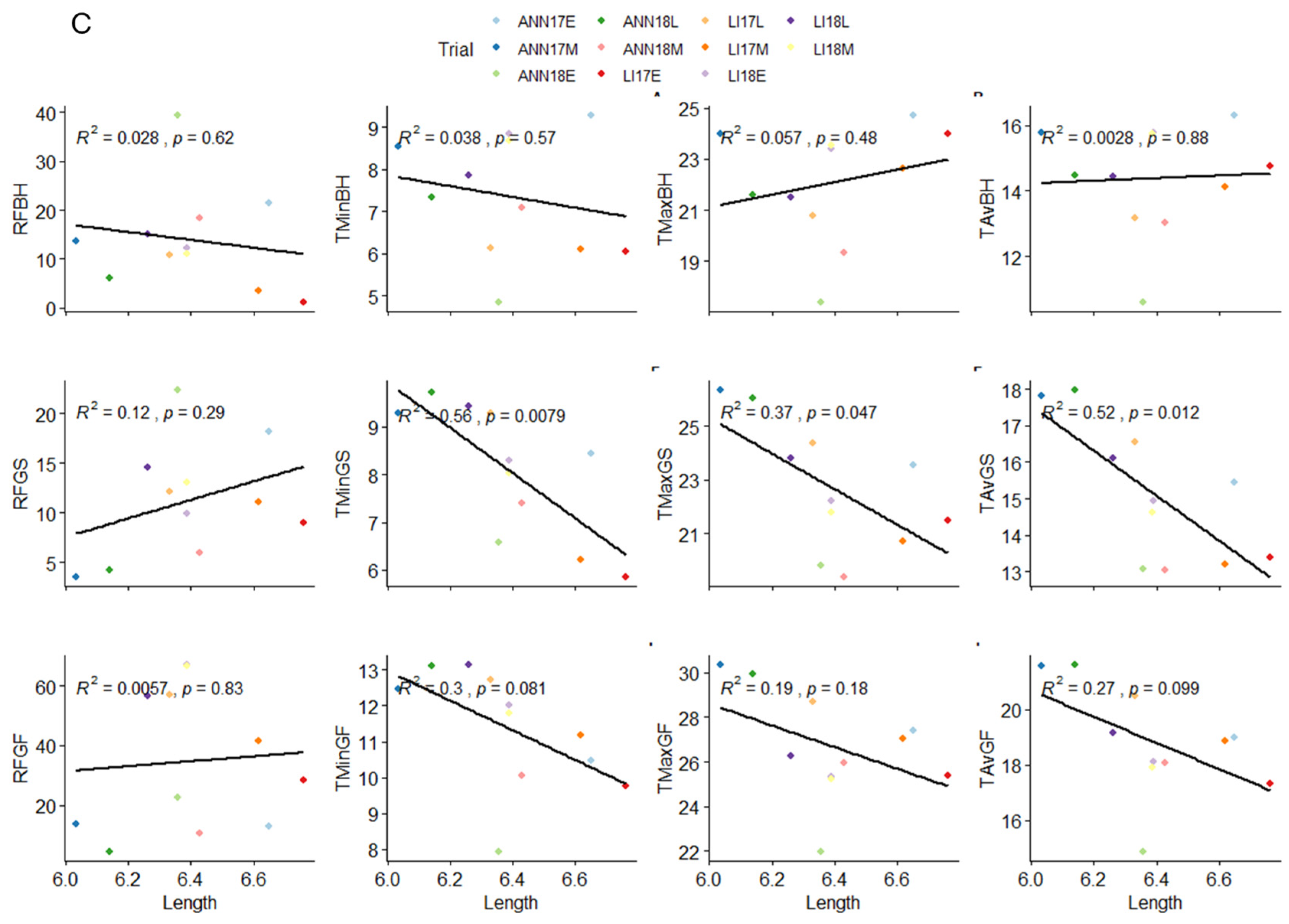
2.4. Influence of Climatic Variables on Grain Morphological Traits
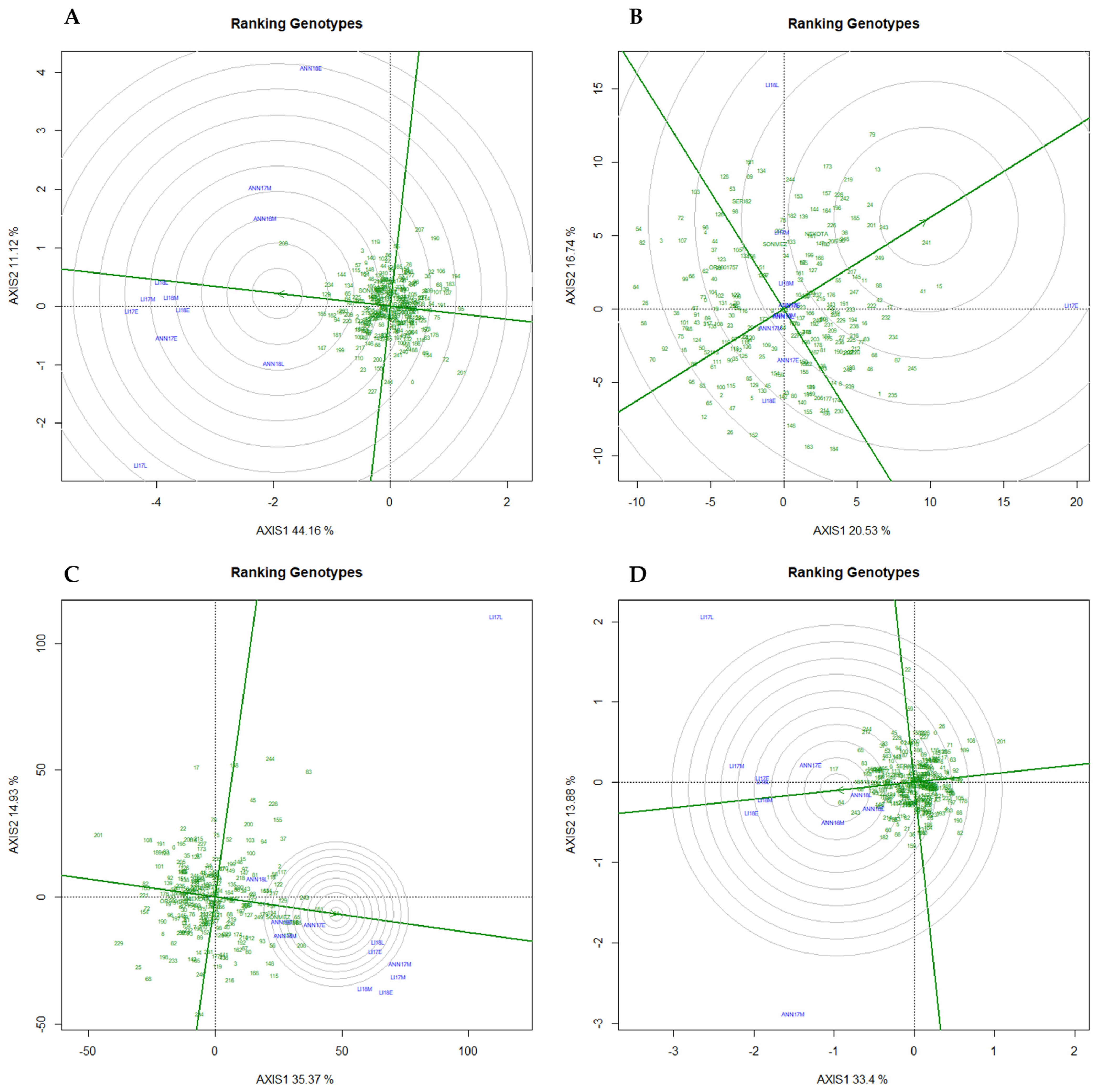
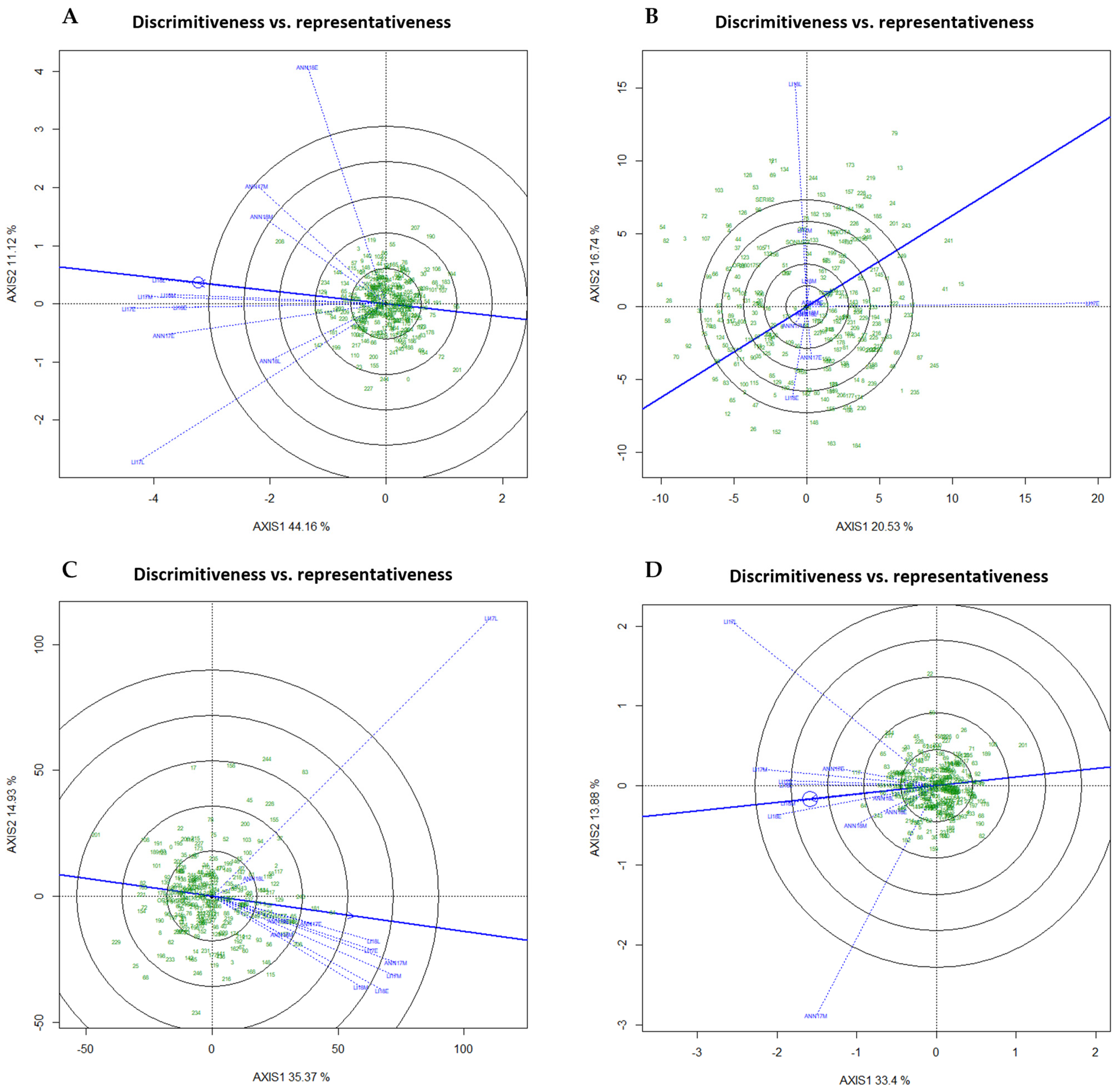
2.5. GGE Biplots for Environment and Genotype Evaluation
2.6. Promising Genotypes in Diverse Mega-Environments
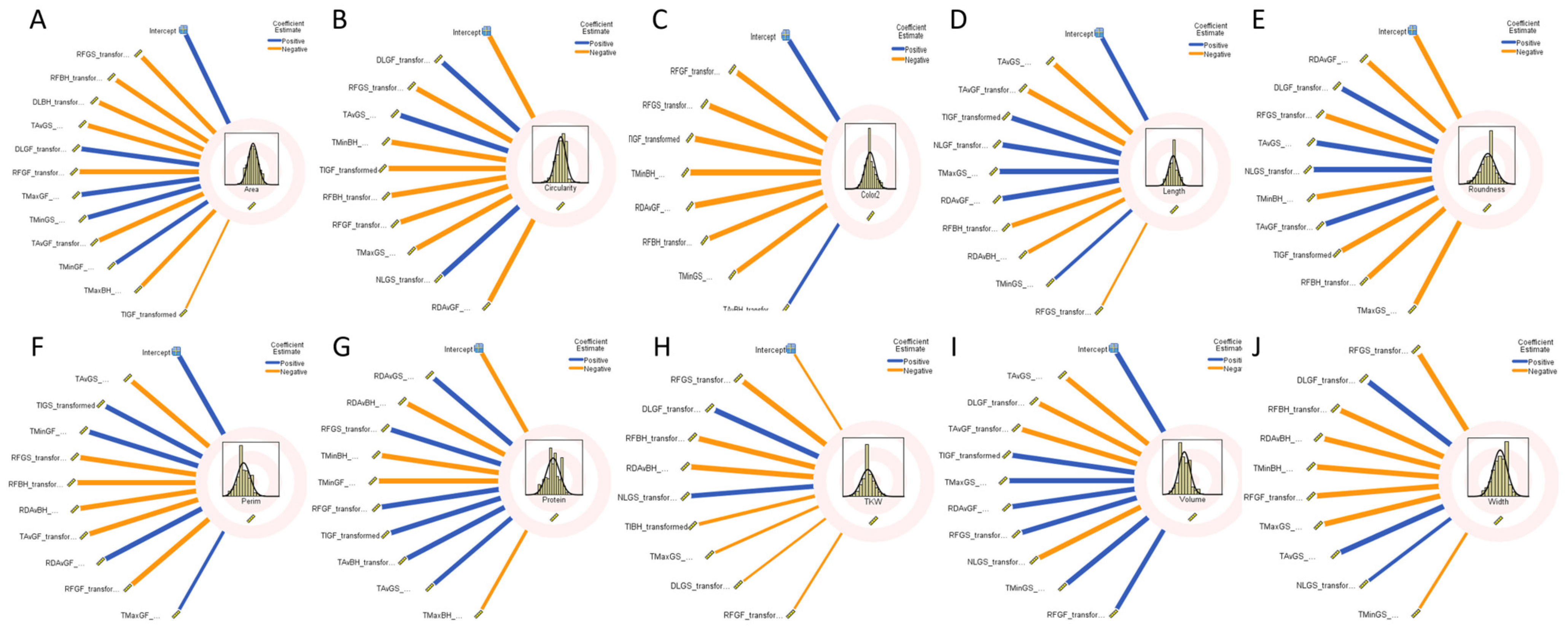
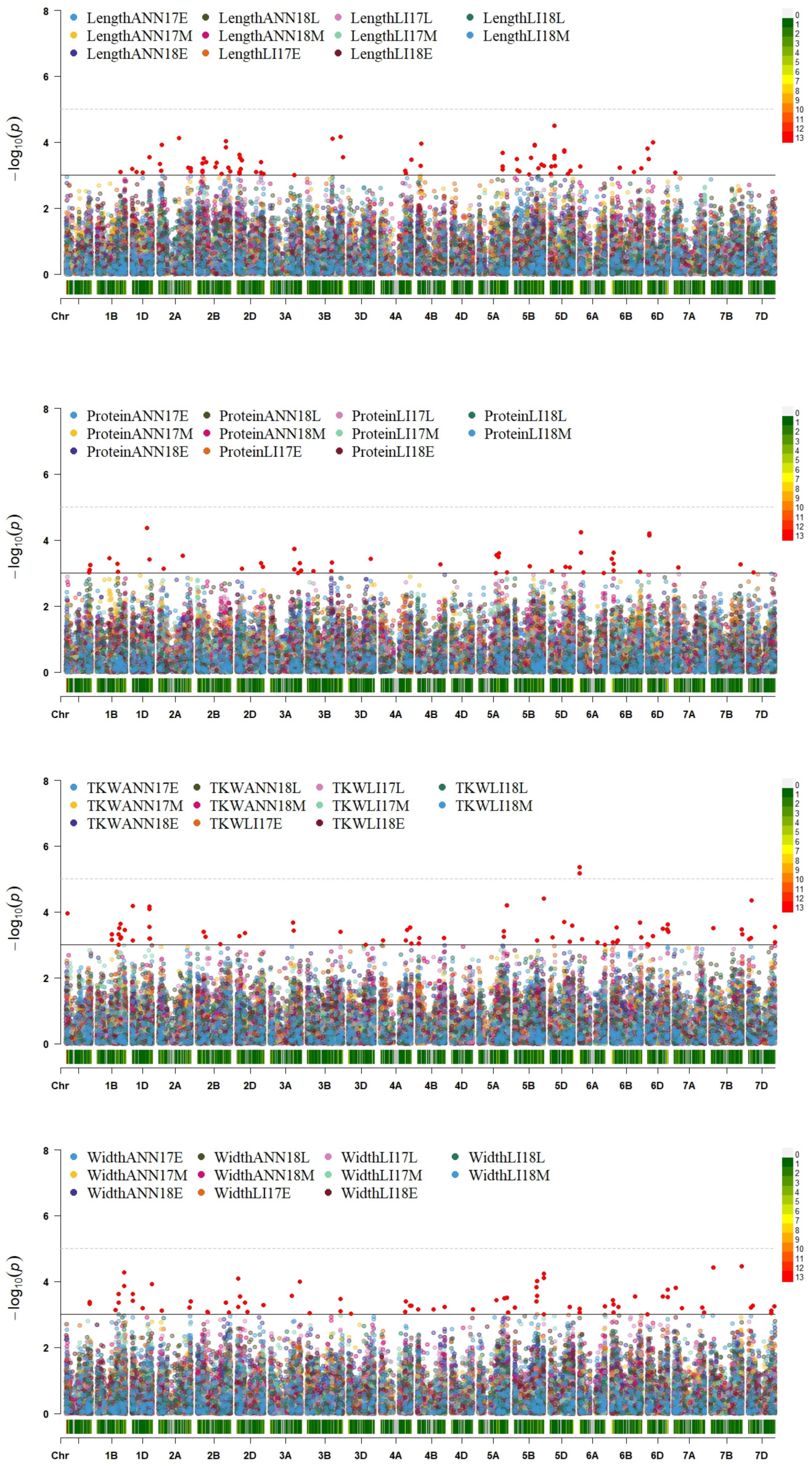
2.7. Genome-Wide Association Study
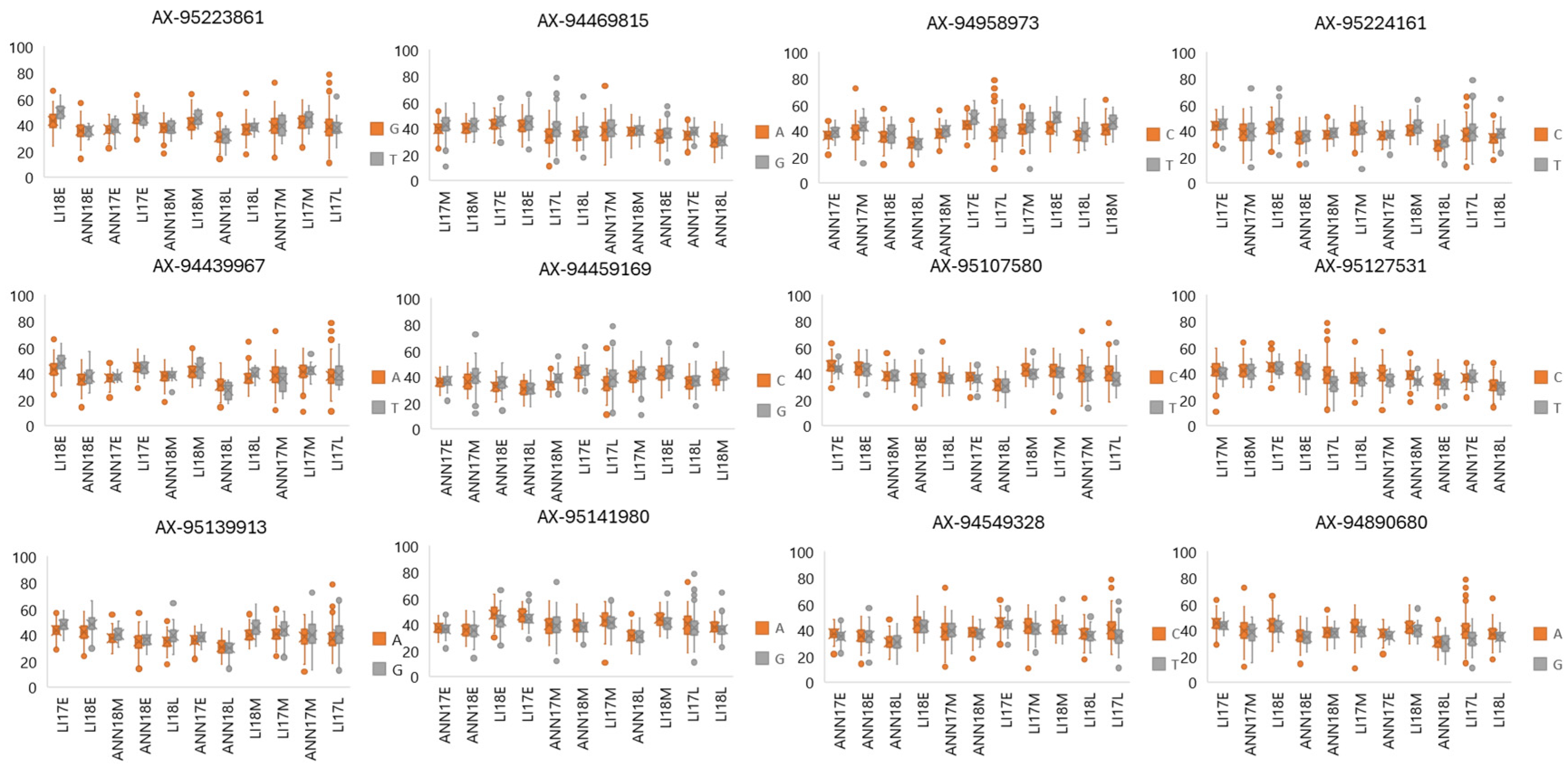
2.8. QTL–Environment Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design and Area of Study
4.3. Environment Characterization
4.4. Grain Morphology and Protein Content
4.5. Genotyping
4.6. Statistical Analysis
4.7. Genome-Wide Association Study (GWAS)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Crop Prospects and Food Situation; FAO: Rome, Italy, 2019; ISBN 9789251358580. [Google Scholar]
- Tester, M. Breeding Technologies to Increase Crop Production in a Changing World Breeding Technologies to Increase Crop Production in a Changing World. Science 2010, 327, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Semenov, M.A.; Stratonovitch, P.; Alghabari, F.; Gooding, M.J. Adapting Wheat in Europe for Climate Change. J. Cereal Sci. 2014, 59, 245–256. [Google Scholar] [CrossRef]
- Mohammadi, R.; Armion, M.; Zadhasan, E.; Ahmadi, M.M.; Amri, A. THE USE of AMMI MODEL for INTERPRETING GENOTYPE × ENVIRONMENT INTERACTION in DURUM WHEAT. Exp. Agric. 2018, 54, 670–683. [Google Scholar] [CrossRef]
- Bergman, C.J.; Gualberto, D.G.; Campbell, K.G.; Sorrells, M.E.; Finney, P.L. Genotype and Environment Effects on Wheat Quality Traits in a Population Derived from a Soft by Hard Cross. Cereal Chem. 1998, 75, 729–737. [Google Scholar] [CrossRef]
- Yabwalo, D.N.; Berzonsky, W.A.; Brabec, D.; Pearson, T.; Glover, K.D.; Kleinjan, J.L. Impact of Grain Morphology and the Genotype by Environment Interactions on Test Weight of Spring and Winter Wheat (Triticum aestivum L.). Euphytica 2018, 214, 125. [Google Scholar] [CrossRef]
- Aguirre, A.; Badiali, O.; Cantarero, M.; León, A.; Ribotta, P.; Rubiolo, O. Relationship of Test Weight and Kernel Properties to Milling and Baking Quality in Argentine Triticales. Cereal Res. Commun. 2002, 30, 203–208. [Google Scholar] [CrossRef]
- Griffiths, S.; Wingen, L.; Pietragalla, J.; Garcia, G.; Hasan, A.; Miralles, D.; Calderini, D.F.; Ankleshwaria, J.B.; Waite, M.L.; Simmonds, J.; et al. Genetic Dissection of Grain Size and Grain Number Trade-Offs in CIMMYT Wheat Germplasm. PLoS ONE 2015, 10, e0118847. [Google Scholar] [CrossRef]
- Giura, A. Chromosomal Location of Genes Controlling Grain Size in a Large Grained Selection of Wheat (Triticum aestivum L.). Euphytica 1996, 89, 77–80. [Google Scholar] [CrossRef]
- Xie, Q.; Mayes, S.; Sparkes, D.L. Carpel Size, Grain Filling, and Morphology Determine Individual Grain Weight in Wheat. J. Exp. Bot. 2015, 66, 6715–6730. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kajimura, T.; Ikeda, T.M.; Takumi, S. Evidence from Principal Component Analysis for Improvement of Grain Shape- and Spikelet Morphology-Related Traits after Hexaploid Wheat Speciation. Genes Genet. Syst. 2012, 87, 299–310. [Google Scholar] [CrossRef]
- Cristina, D.; Ciuca, M.; Cornea, P.C. Genetic Control of Grain Size and Weight in Wheat-Where Are We Now? Sci. Bull. Ser. F. Biotechnol. 2016, XX, 27–34. [Google Scholar]
- Marza, F.; Bai, G.H.; Carver, B.F.; Zhou, W.C. Quantitative Trait Loci for Yield and Related Traits in the Wheat Population. Theor. Appl. Genet. 2006, 112, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Gasparis, S.; Miłoszewski, M.M. Genetic Basis of Grain Size and Weight in Rice, Wheat, and Barley. Int. J. Mol. Sci. 2023, 24, 16921. [Google Scholar] [CrossRef] [PubMed]
- El Hassouni, K.; Alahmad, S.; Belkadi, B.; Filali-Maltouf, A.; Hickey, L.T.; Bassi, F.M. Root System Architecture and Its Association with Yield under Different Water Regimes in Durum Wheat. Crop Sci. 2018, 58, 2331–2346. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, C.; Guan, B.; Yang, R.; Liu, K.; Wang, Z.; Li, X.; Xue, K.; Yin, L.; Wang, X. The Effect of Different Sowing Dates on Dry Matter and Nitrogen Dynamics for Winter Wheat: An Experimental Simulation Study. PeerJ 2021, 9, e11700. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Fayyaz-Ul-Hassan Response of Spring Wheat (Triticum aestivum L.) Quality Traits and Yield to Sowing Date. PLoS ONE 2015, 10, e0126097. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, V.; Khajoie-Nejad, G.; Mohammadi-Nejad, G.; Yousefi, K. The Effect of Different Sowing Dates on Yield and Yield Components of Wheat (Triticum aestivum L.) Cultivars. Intl. J. Agron. Plant Prod. 2010, 1, 77–82. [Google Scholar]
- Ale, A.; Bacchetta, C.; Cazenave, J. Feb-Fresenius Environmental Bulletin. Fresenius Environ. Bull. 2017, 26, 4082–4087. [Google Scholar]
- Telfer, P.; Edwards, J.; Bennett, D.; Ganesalingam, D.; Able, J.; Kuchel, H. A Field and Controlled Environment Evaluation of Wheat (Triticum aestivum) Adaptation to Heat Stress. Field Crops Res. 2018, 229, 55–65. [Google Scholar] [CrossRef]
- Rodrigues, P.C. An Overview of Statistical Methods to Detect and Understand Genotype-by-Environment Interaction and QTL-by-Environment Interaction. Biom. Lett. 2018, 55, 123–138. [Google Scholar] [CrossRef]
- Peterson, C.J.; Graybosch, R.A.; Baenziger, P.S.; Grombacher, A.W. Genotype and Environment Effects on Quality Characteristics of Hard Red Winter Wheat. Crop Sci. 1992, 32, 98–103. [Google Scholar] [CrossRef]
- Hasan, M.J.; Kulsum, M.U.; Sarker, U.; Matin, M.Q.I.; Shahin, N.H.; Kabir, M.S.; Ercisli, S.; Marc, R.A. Assessment of GGE, AMMI, Regression, and Its Deviation Model to Identify Stable Rice Hybrids in Bangladesh. Plants 2022, 11, 2336. [Google Scholar] [CrossRef] [PubMed]
- Danakumara, T.; Kumar, T.; Kumar, N.; Patil, B.S.; Bharadwaj, C.; Patel, U.; Joshi, N.; Bindra, S.; Tripathi, S.; Varshney, R.K.; et al. A Multi-Model Based Stability Analysis Employing Multi-Environmental Trials (METs) Data for Discerning Heat Tolerance in Chickpea (Cicer arietinum L.) Landraces. Plants 2023, 12, 3691. [Google Scholar] [CrossRef] [PubMed]
- Amelework, A.B.; Bairu, M.W.; Marx, R.; Laing, M.; Venter, S.L. Genotype × Environment Interaction and Stability Analysis of Selected Cassava Cultivars in South Africa. Plants 2023, 12, 2490. [Google Scholar] [CrossRef] [PubMed]
- Wodebo, K.Y.; Tolemariam, T.; Demeke, S.; Garedew, W.; Tesfaye, T.; Zeleke, M.; Gemiyu, D.; Bedeke, W.; Wamatu, J.; Sharma, M. AMMI and GGE Biplot Analyses for Mega-Environment Identification and Selection of Some High-Yielding Oat (Avena sativa L.) Genotypes for Multiple Environments. Plants 2023, 12, 3064. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Xia, X.; Ogbonnaya, F.; Mahmood, T.; Zhang, Z.; Mujeeb-Kazi, A.; He, Z. Genome-Wide Association for Grain Morphology in Synthetic Hexaploid Wheats Using Digital Imaging Analysis. BMC Plant Biol. 2014, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Mangini, G.; Giancaspro, A.; Giove, S.; Colasuonno, P.; Simeone, R.; Signorile, A.; De Vita, P.; Mastrangelo, A.M.; Cattivelli, L.; et al. Relationships between Grain Protein Content and Grain Yield Components through Quantitative Trait Locus Analyses in a Recombinant Inbred Line Population Derived from Two Elite Durum Wheat Cultivars. Mol. Breed. 2012, 30, 79–92. [Google Scholar] [CrossRef]
- Oury, F.X.; Godin, C. Yield and Grain Protein Concentration in Bread Wheat: How to Use the Negative Relationship between the Two Characters to Identify Favourable Genotypes? Euphytica 2007, 157, 45–57. [Google Scholar] [CrossRef]
- Cui, F.; Fan, X.; Chen, M.; Zhang, N.; Zhao, C.; Zhang, W.; Han, J.; Ji, J.; Zhao, X.; Yang, L.; et al. QTL Detection for Wheat Kernel Size and Quality and the Responses of These Traits to Low Nitrogen Stress. Theor. Appl. Genet. 2016, 129, 469–484. [Google Scholar] [CrossRef]
- Battenfield, S.D.; Guzmán, C.; Gaynor, R.C.; Singh, R.P.; Peña, R.J.; Dreisigacker, S.; Fritz, A.K.; Poland, J.A. Genomic Selection for Processing and End-Use Quality Traits in the CIMMYT Spring Bread Wheat Breeding Program. Plant Genome 2016, 9, plantgenome2016.01.0005. [Google Scholar] [CrossRef]
- Groos, C.; Robert, N.; Bervas, E.; Charmet, G. Genetic Analysis of Grain Protein-Content, Grain Yield and Thousand-Kernel Weight in Bread Wheat. Theor. Appl. Genet. 2003, 106, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, X.; Jin, J.; Duan, S.; Zhen, W. Dissecting the Genetic Basis of Grain Morphology Traits in Chinese Wheat by Genome Wide Association Study. Euphytica 2021, 217, 56. [Google Scholar] [CrossRef]
- Simmonds, J.; Scott, P.; Brinton, J.; Mestre, T.C.; Bush, M.; del Blanco, A.; Dubcovsky, J.; Uauy, C. A Splice Acceptor Site Mutation in TaGW2-A1 Increases Thousand Grain Weight in Tetraploid and Hexaploid Wheat through Wider and Longer Grains. Theor. Appl. Genet. 2016, 129, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Kenzhebayeva, S.S.; Doktyrbay, G.; Capstaff, N.M.; Sarsu, F.; Omirbekova, N.Z.; Eilam, T.; Tashenev, D.K.; Miller, A.J. Searching a Spring Wheat Mutation Resource for Correlations between Yield, Grain Size, and Quality Parameters. J. Crop Improv. 2017, 31, 209–228. [Google Scholar] [CrossRef]
- Matus-Cádiz, M.A.; Hucl, P.; Perron, C.E.; Tyler, R.T. Genotype X Environment Interaction for Grain Color in Hard White Spring Wheat. Crop Sci. 2003, 43, 219–226. [Google Scholar] [CrossRef]
- Mohammadi, R.; Jafarzadeh, J.; Armion, M.; Poursiahbidi, M.M.; Hatamzadeh, H.; Khalilzadeh, G.R. Mega-Environment Investigation in Durum Wheat Yield Trials in Iran. Euphytica 2023, 219, 18. [Google Scholar] [CrossRef]
- Huang, X.; Jang, S.; Kim, B.; Piao, Z.; Redona, E.; Koh, H.J. Evaluating Genotype × Environment Interactions of Yield Traits and Adaptability in Rice Cultivars Grown under Temperate, Subtropical and Tropical Environments. Agriculture 2021, 11, 558. [Google Scholar] [CrossRef]
- Khan, M.M.H.; Rafii, M.Y.; Ramlee, S.I.; Jusoh, M.; Al Mamun, M. AMMI and GGE Biplot Analysis for Yield Performance and Stability Assessment of Selected Bambara Groundnut (Vigna subterranea L. Verdc.) Genotypes under the Multi-Environmental Trails (METs). Sci. Rep. 2021, 11, 22791. [Google Scholar] [CrossRef]
- Gauch, H.G.; Zobel, R.W. Identifying Mega-Environments and Targeting Genotypes. Crop Sci. 1997, 37, 311–326. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar Evaluation and Mega-Environment Investigation Based on the GGE Biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Roostaei, M.; Jafarzadeh, J.; Roohi, E.; Nazary, H.; Rajabi, R.; Haghparast, R.; Mohammadi, R.; Abediasl, G.R.; Khalilzadeh, G.R.; Seif, F.; et al. Grouping Patterns of Rainfed Winter Wheat Test Locations and the Role of Climatic Variables. Euphytica 2021, 217, 183. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Borlaug, N.E. Impacts of Breeding on International Collaborative Wheat Improvement. J. Agric. Sci. 2006, 144, 3–17. [Google Scholar] [CrossRef]
- Lopes, M.S.; Royo, C.; Alvaro, F.; Sanchez-Garcia, M.; Ozer, E.; Ozdemir, F.; Karaman, M.; Roustaii, M.; Jalal-Kamali, M.R.; Pequeno, D. Optimizing Winter Wheat Resilience to Climate Change in Rain Fed Crop Systems of Turkey and Iran. Front. Plant Sci. 2018, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, M.; Bentley, A. Global Journeys of Adaptive Wheat Genes; Woodhead Publishing: Amsterdam, The Netherlands, 2019; ISBN 9780081021637. [Google Scholar]
- Loss, S.P.; Siddique, K.H.M. Morphological and Physiological Traits Associated with Wheat Yield Increases in Mediterranean Environments. Adv. Agron. 1994, 52, 229–276. [Google Scholar] [CrossRef]
- Araki, H.; Hamada, A.; Hossain, M.A.; Takahashi, T. Waterlogging at Jointing and/or after Anthesis in Wheat Induces Early Leaf Senescence and Impairs Grain Filling. Field Crops Res. 2012, 137, 27–36. [Google Scholar] [CrossRef]
- de San Celedonio, R.P.; Abeledo, L.G.; Miralles, D.J. Identifying the Critical Period for Waterlogging on Yield and Its Components in Wheat and Barley. Plant Soil 2014, 378, 265–277. [Google Scholar] [CrossRef]
- Pais, I.P.; Moreira, R.; Semedo, J.N.; Ramalho, J.C.; Lidon, F.C.; Coutinho, J.; Maçãs, B.; Scotti-Campos, P. Wheat Crop under Waterlogging: Potential Soil and Plant Effects. Plants 2023, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Gevrek, M.N.; Atasoy, G.D. Effect of Post Anthesis Drought on Certain Agronomical Characteristics of Wheat under Two Different Nitrogen Application Conditions. Turkish J. Field Crops 2012, 17, 19–23. [Google Scholar]
- Asseng, S.; Foster, I.; Turner, N.C. The Impact of Temperature Variability on Wheat Yields. Glob. Change Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.; Álvaro, F.; Martín-Sánchez, J.A.; Sillero, J.C.; Escribano, J.; Royo, C. Breeding Effects on the Genotype×environment Interaction for Yield of Bread Wheat Grown in Spain during the 20th Century. Field Crops Res. 2012, 126, 79–86. [Google Scholar] [CrossRef]
- Bruckner, P.L.; Frohberg, R.C. Rate and Duration of Grain Fill in Spring Wheat 1. Crop Sci. 1987, 27, 451–455. [Google Scholar] [CrossRef]
- Calderini, D.F.; Abeledo, L.G.; Savin, R.; Slafer, G.A. Final Grain Weight in Wheat as Affected by Short Periods of High Temperature during Pre- and Post-Anthesis under Field Conditions. Aust. J. Plant Physiol. 1999, 26, 453–458. [Google Scholar] [CrossRef]
- Okamoto, Y.; Nguyen, A.T.; Yoshioka, M.; Iehisa, J.C.M.; Takumi, S. Identification of Quantitative Trait Loci Controlling Grain Size and Shape in the D Genome of Synthetic Hexaploid Wheat Lines. Breed. Sci. 2013, 63, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Wang, H.W.; Zhang, X.C.; Du, X.Y.; Li, A.F.; Kong, L.R. Association Analysis of Grain Traits with SSR Markers between Aegilops Tauschii and Hexaploid Wheat (Triticum aestivum L.). J. Integr. Agric. 2015, 14, 1936–1948. [Google Scholar] [CrossRef]
- Arora, S.; Singh, N.; Kaur, S.; Bains, N.S.; Uauy, C.; Poland, J.; Chhuneja, P. Genome-Wide Association Study of Grain Architecture in Wild Wheat Aegilops tauschii. Front. Plant Sci. 2017, 8, 270244. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Ding, A.; Li, J.; Zhao, C.; Li, X.; Feng, D.; Wang, X.; Wang, L.; Gao, J.; Wang, H. Wheat Kernel Dimensions: How Do They Contribute to Kernel Weight at an Individual QTL Level? J. Genet. 2011, 90, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Liu, D.C.; Guo, X.L.; Yang, W.L.; Sun, J.Z.; Wang, D.W.; Zhang, A. Genomic Distribution of Quantitative Trait Loci for Yield and Yield-Related Traits in Common Wheat. J. Integr. Plant Biol. 2010, 52, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.G.; Bergman, C.J.; Gualberto, D.G.; Anderson, J.A.; Giroux, M.J.; Hareland, G.; Fulcher, R.G.; Sorrells, M.E.; Finney, P.L. Quantitative Trait Loci Associated with Kernel Traits in a Soft x Hard Wheat Cross. Crop Sci. 1999, 39, 1184–1195. [Google Scholar] [CrossRef]
- Xiao, Y.; He, S.; Yan, J.; Zhang, Y.; Zhang, Y.; Wu, Y.; Xia, X.; Tian, J.; Ji, W.; He, Z. Molecular Mapping of Quantitative Trait Loci for Kernel Morphology Traits in a Non-1BL.1RS1BL.1RS Wheat Cross. Crop Pasture Sci. 2011, 62, 625–638. [Google Scholar] [CrossRef]
- Himi, E.; Maekawa, M.; Miura, H.; Noda, K. Development of PCR Markers for Tamyb10 Related to R-1, Red Grain Color Gene in Wheat. Theor. Appl. Genet. 2011, 122, 1561–1576. [Google Scholar] [CrossRef]
- El-Maghraby, O.; Fayez, K.; Abdo, F.; Sabra, H. Effect of Sowing Date on Yield and Yield Components of Bread Wheat Cultivars under Environmental Conditions of Sohag Region. J. Environ. Stud. 2016, 15, 19–30. [Google Scholar] [CrossRef]
- Liu, B.; Asseng, S.; Liu, L.; Tang, L.; Cao, W.; Zhu, Y. Testing the Responses of Four Wheat Crop Models to Heat Stress at Anthesis and Grain Filling. Glob. Chang. Biol. 2016, 22, 1890–1903. [Google Scholar] [CrossRef]
- Ottman, M.J.; Kimball, B.A.; White, J.W.; Wall, G.W. Wheat Growth Response to Increased Temperature from Varied Planting Dates and Supplemental Infrared Heating. Agron. J. 2012, 104, 7–16. [Google Scholar] [CrossRef]
- Allison, J.C.S.; Daynard, T.B. Effect of Photoperiod on Development and Number of Spikelets of a Temperate and Some Low-latitude Wheats. Ann. Appl. Biol. 1976, 83, 93–102. [Google Scholar] [CrossRef]
- Arjona, J.M.; Royo, C.; Dreisigacker, S.; Ammar, K.; Villegas, D. Effect of Ppd-A1 and Ppd-B1 Allelic Variants on Grain Number and Thousand Kernel Weight of Durum Wheat and Their Impact on Final Grain Yield. Front. Plant Sci. 2018, 9, 371213. [Google Scholar] [CrossRef]
- Bustos, D.V.; Hasan, A.K.; Reynolds, M.P.; Calderini, D.F. Combining High Grain Number and Weight through a DH-Population to Improve Grain Yield Potential of Wheat in High-Yielding Environments. Field Crops Res. 2013, 145, 106–115. [Google Scholar] [CrossRef]
- García, G.A.; Serrago, R.A.; Dreccer, M.F.; Miralles, D.J. Post-Anthesis Warm Nights Reduce Grain Weight in Field-Grown Wheat and Barley. Field Crops Res. 2016, 195, 50–59. [Google Scholar] [CrossRef]
- Mursalova, J.; Akparov, Z.; Ojaghi, J.; Eldarov, M.; Belen, S.; Gummadov, N.; Morgounov, A. Evaluation of Drought Tolerance of Winter Bread Wheat Genotypes under Drip Irrigation and Rain-Fed Conditions. Turkish J. Agric. For. 2015, 39, 817–824. [Google Scholar] [CrossRef]
- El Baouchi, A.; Ibriz, M.; Dreisigacker, S.; Lopes, M.S.; Garcia, M.S. Genetic Diversity and Genome-Wide Association Study for the Phenology Response of Winter Wheats of North America, Western Asia, and Europe. Plants 2023, 12, 4053. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Djanaguiraman, M. Response of Floret Fertility and Individual Grain Weight of Wheat to High Temperature Stress: Sensitive Stages and Thresholds for Temperature and Duration. Funct. Plant Biol. 2014, 41, 1261–1269. [Google Scholar] [CrossRef]
- Gooding, M.J.; Ellis, R.H.; Shewry, P.R.; Schofield, J.D. Effects of Restricted Water Availability and Increased Temperature on the Grain Filling, Drying and Quality of Winter Wheat. J. Cereal Sci. 2003, 37, 295–309. [Google Scholar] [CrossRef]
- Whan, A.P.; Smith, A.B.; Cavanagh, C.R.; Ral, J.P.F.; Shaw, L.M.; Howitt, C.A.; Bischof, L. GrainScan: A Low Cost, Fast Method for Grain Size and Colour Measurements. Plant Methods 2014, 10, 23. [Google Scholar] [CrossRef]
- Allen, A.M.; Winfield, M.O.; Burridge, A.J.; Downie, R.C.; Benbow, H.R.; Barker, G.L.A.; Wilkinson, P.A.; Coghill, J.; Waterfall, C.; Davassi, A.; et al. Characterization of a Wheat Breeders’ Array Suitable for High-Throughput SNP Genotyping of Global Accessions of Hexaploid Bread Wheat (Triticum aestivum). Plant Biotechnol. J. 2017, 15, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z. GAPIT Version 3: Boosting Power and Accuracy for Genomic Association and Prediction. Genomics, Proteomics Bioinform. 2021, 19, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal Components Analysis Corrects for Stratification in Genome-Wide Association Studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ersoz, E.; Lai, C.Q.; Todhunter, R.J.; Tiwari, H.K.; Gore, M.A.; Bradbury, P.J.; Yu, J.; Arnett, D.K.; Ordovas, J.M.; et al. Mixed Linear Model Approach Adapted for Genome-Wide Association Studies. Nat. Genet. 2010, 42, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Bradbury, P.; Yu, J.; Zhang, Y.M.; Todhunter, R.J.; Buckler, E.S.; Zhang, Z. Enrichment of Statistical Power for Genome-Wide Association Studies. BMC Biol. 2014, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- VanRaden, P.M. Efficient Methods to Compute Genomic Predictions. J. Dairy Sci. 2008, 91, 4414–4423. [Google Scholar] [CrossRef]
- Lipka, A.E.; Tian, F.; Wang, Q.; Peiffer, J.; Li, M.; Bradbury, P.J.; Gore, M.A.; Buckler, E.S.; Zhang, Z. GAPIT: Genome Association and Prediction Integrated Tool. Bioinformatics 2012, 28, 2397–2399. [Google Scholar] [CrossRef]



| Site | ANN17 | ANN18 | LI17 | LI18 |
|---|---|---|---|---|
| Trial identification | 33°41′05.2″ N, 4°51′19.9″ W | 41°40′ N, 00°20′ E | ||
| Environmental conditions for Early planting (EP) | ||||
| Sum ET0 (mm) | 633.7 | - | 580.08 | 694.71 |
| Average Tmin (°C) | 6.88 | 2.94 | 5.82 | 4.64 |
| Average Tmax (°C) | 21.9 | 16.47 | 17.99 | 17.74 |
| Average Rhmin (%) | 48.2 | 59.58 | - | 72.62 |
| Average Rhmax (%) | 55.98 | 66.6 | - | 94.12 |
| Sum Rainfall (mm) | 1060.7 | 713.4 | 295.3 | 263.5 |
| Average Daylength (H) | 12.03 | 11.72 | 11.72 | 11.69 |
| Average Nightlength (H) | 11.96 | 12.27 | 12.28 | 12.03 |
| Environmental conditions for Medium planting (MP) | ||||
| Sum ET0 (mm) | 600.1 | - | 565.3 | 664.8 |
| Average Tmin (°C) | 5.06 | 3.05 | 6.51 | 7.37 |
| Average Tmax (°C) | 21.18 | 16.52 | 19.3 | 20.27 |
| Average Rhmin (%) | 52.79 | 68.1 | - | 72 |
| Average Rhmax (%) | 61.08 | 75.05 | - | 93.96 |
| Sum Rainfall (mm) | 184.8 | 628 | 231 | 260.9 |
| Average Daylength (H) | 12.08 | 12.2 | 12.05 | 12.12 |
| Average Nightlength (H) | 11.92 | 11.8 | 11.95 | 11.88 |
| Environmental conditions for Late planting (LP) | ||||
| Sum ET0 (mm) | - | - | 553.05 | 643.88 |
| Average Tmin (°C) | - | 5.25 | 7.48 | 8.26 |
| Average Tmax (°C) | - | 18.32 | 21.5 | 21.84 |
| Average Rhmin (%) | - | 69.36 | - | 69.96 |
| Average Rhmax (%) | - | 76.18 | - | 93.68 |
| Sum Rainfall (mm) | - | 494.2 | 223.4 | 237 |
| Average Daylength (H) | - | 12.89 | 12.63 | 12.98 |
| Average Nightlength (H) | - | 11.1 | 11.37 | 11.02 |
| Trial | ANN17 | ANN18 | LI17 | LI18 | ||||
|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Early planting (E) | ||||||||
| Area | 14.83 | 1.22 | 15.05 | 1.74 | 17.28 | 1.44 | 15.89 | 1.5 |
| Circularity | 0.45 | 0.02 | 0.5 | 0.04 | 0.51 | 0.02 | 0.51 | 0.02 |
| Color | 126.51 | 9.95 | 133.85 | 9.59 | 136.89 | 9.67 | 127.32 | 8.04 |
| Length | 6.65 | 0.35 | 6.36 | 0.38 | 6.76 | 0.31 | 6.39 | 0.31 |
| Perimeter | 20.28 | 0.95 | 19.34 | 1.03 | 20.69 | 0.91 | 19.88 | 0.93 |
| Protein | 12.19 | 0.81 | 12.82 | 0.39 | 12.19 | 1.25 | 12.29 | 0.97 |
| Roundness | 0.43 | 0.03 | 0.47 | 0.03 | 0.48 | 0.02 | 0.5 | 0.03 |
| TKW | 36.19 | 4.65 | 35.14 | 6.8 | 44.55 | 5.58 | 43.45 | 6.9 |
| Volume | 6.06 | 0.56 | 5.3 | 0.51 | 5.31 | 0.44 | 4.93 | 0.45 |
| Width | 2.84 | 0.15 | 2.98 | 0.19 | 3.24 | 0.16 | 3.16 | 0.19 |
| Medium planting (M) | ||||||||
| Area | 14.58 | 1.82 | 15.98 | 1.48 | 16.59 | 1.64 | 15.89 | 1.46 |
| Circularity | 0.52 | 0.02 | 0.51 | 0.02 | 0.5 | 0.02 | 0.51 | 0.02 |
| Color | 126.55 | 9.37 | 132.64 | 9.63 | 131.81 | 8.39 | 129.28 | 7.01 |
| Length | 6.03 | 0.35 | 6.43 | 0.35 | 6.62 | 0.32 | 6.39 | 0.31 |
| Perimeter | 18.93 | 1.03 | 19.72 | 0.98 | 20.32 | 0.95 | 19.72 | 0.94 |
| Protein | 12.94 | 0.99 | 13.24 | 0.21 | 11.57 | 1.06 | 12.52 | 1.08 |
| Roundness | 0.51 | 0.04 | 0.49 | 0.03 | 0.48 | 0.03 | 0.5 | 0.03 |
| TKW | 38.52 | 9.12 | 37.75 | 5.27 | 41.23 | 7.27 | 41.47 | 6.57 |
| Volume | 4.59 | 0.5 | 5.05 | 0.45 | 5.24 | 0.44 | 4.93 | 0.42 |
| Width | 3.05 | 0.26 | 3.12 | 0.16 | 3.18 | 0.2 | 3.15 | 0.18 |
| Late planting (L) | ||||||||
| Area | - | - | 13.77 | 1.54 | 15.06 | 2.08 | 14.95 | 1.45 |
| Circularity | - | - | 0.49 | 0.02 | 0.5 | 0.02 | 0.51 | 0.02 |
| Color | - | - | 128.62 | 9.72 | 121.88 | 11 | 125.82 | 8.15 |
| Length | - | - | 6.14 | 0.31 | 6.33 | 0.4 | 6.26 | 0.32 |
| Perimeter | - | - | 18.63 | 0.92 | 19.4 | 1.22 | 19.28 | 0.92 |
| Protein | - | - | 14.28 | 0.41 | 14.25 | 0.44 | 12.81 | 1.13 |
| Roundness | - | - | 0.46 | 0.03 | 0.48 | 0.03 | 0.49 | 0.02 |
| TKW | - | - | 30.23 | 6.09 | 37.61 | 9.67 | 36.02 | 6.11 |
| Volume | - | - | 5.23 | 0.44 | 5.16 | 0.43 | 4.98 | 0.41 |
| Width | - | - | 2.84 | 0.2 | 3.01 | 0.25 | 3.03 | 0.18 |
| Trait | Fixed Term | d.f | Sum of Squares | Chi-Sq Prob | %TSS |
|---|---|---|---|---|---|
| Area | Environment (E) | 10 | 2259.76 | <0.001 | 29% |
| Genotype (G) | 252 | 2138.46 | <0.001 | 28% | |
| GE interaction | 2299 | 3303.75 | <0.001 | 43% | |
| Circularity | Environment (E) | 10 | 7288.65 | <0.001 | 53% |
| Genotype (G) | 252 | 2318.66 | <0.001 | 17% | |
| GE interaction | 2299 | 4089.96 | <0.001 | 30% | |
| Color2 | Environment (E) | 10 | 870 | <0.001 | 17% |
| Genotype (G) | 252 | 2082.64 | <0.001 | 40% | |
| GE interaction | 2299 | 2243.19 | 0.794 | 43% | |
| Length | Environment (E) | 10 | 3325.37 | <0.001 | 30% |
| Genotype (G) | 252 | 3599.8 | <0.001 | 33% | |
| GE interaction | 2299 | 3990.61 | <0.001 | 37% | |
| Perim | Environment (E) | 10 | 2728.26 | <0.001 | 29% |
| Genotype (G) | 252 | 3115.05 | <0.001 | 33% | |
| GE interaction | 2299 | 3565.56 | <0.001 | 38% | |
| Protein | Environment (E) | 10 | 2711.6 | <0.001 | 34% |
| Genotype (G) | 252 | 508.02 | <0.001 | 6% | |
| GE interaction | 2297 | 4841.68 | <0.001 | 60% | |
| Roundness | Environment (E) | 10 | 6210.9 | <0.001 | 44% |
| Genotype (G) | 252 | 3219.73 | <0.001 | 23% | |
| GE interaction | 2299 | 4701.13 | <0.001 | 33% | |
| TKW | Environment (E) | 10 | 1597.97 | <0.001 | 27% |
| Genotype (G) | 252 | 1644.63 | <0.001 | 28% | |
| GE interaction | 2287 | 2679.47 | <0.001 | 45% | |
| Volume | Environment (E) | 10 | 8402.18 | <0.001 | 43% |
| Genotype (G) | 252 | 5409.19 | <0.001 | 28% | |
| GE interaction | 2299 | 5790.41 | <0.001 | 30% | |
| Width | Environment (E) | 10 | 2470.96 | <0.001 | 34% |
| Genotype (G) | 252 | 1597.06 | <0.001 | 22% | |
| GE interaction | 2299 | 3182.64 | <0.001 | 44% |
| Environment | Trait | Marker | Chromosome | Position | −log10(p) | R2 |
|---|---|---|---|---|---|---|
| LI17E | Color | AX-94824017 | 3D | 572188094 | 6.399121 | 0.40 |
| LI17E | Color | AX-94910107 | 3D | 572478517 | 6.058349 | 0.39 |
| LI17E | Color | AX-94748732 | 3D | 572154318 | 5.949095 | 0.39 |
| LI18L | Color | AX-94824017 | 3D | 572188094 | 5.727154 | 0.38 |
| LI17E | Color | AX-94415259 | 3D | 572708647 | 5.716138 | 0.39 |
| LI17E | Color | AX-95120609 | 3A | 705206286 | 5.470853 | 0.38 |
| LI17E | Color | AX-94721213 | 3D | 572837198 | 5.386912 | 0.38 |
| ANN18M | TKW | AX-94394209 | 6A | 1717618 | 5.357748 | 0.13 |
| LI18L | Color | AX-94721213 | 3D | 572837198 | 5.355953 | 0.37 |
| LI18E | Roundness | AX-95156025 | 2B | 148607565 | 5.322127 | 0.19 |
| LI17E | Color | AX-95124645 | 3D | 572704056 | 5.233392 | 0.38 |
| LI17M | Color | AX-94824017 | 3D | 572188094 | 5.20292 | 0.43 |
| Environment | Trait | Marker | Chromosome | Position | −log10(p) |
|---|---|---|---|---|---|
| LI18E | Length | AX-94407122 | 1B | 564644617 | 3.09 |
| LI17M | TKW | AX-94407122 | 1B | 564644617 | 3.63 |
| LI17E | TKW | AX-94804372 | 1B | 353759591 | 3.16 |
| ANN18M | TKW | AX-94804372 | 1B | 353759591 | 3.32 |
| LI18L | TKW | AX-95224161 | 1B | 518659990 | 3.32 |
| LI18M | Width | AX-95224161 | 1B | 518659990 | 3.36 |
| LI18L | Width | AX-95224161 | 1B | 518659990 | 3.62 |
| LI17M | Width | AX-94596631 | 1D | 17533473 | 3.62 |
| LI17M | TKW | AX-94596631 | 1D | 17533473 | 4.18 |
| LI18L | Length | AX-94766246 | 1D | 418872302 | 3.54 |
| LI17M | TKW | AX-94766246 | 1D | 418872302 | 4.08 |
| LI17M | TKW | AX-94865808 | 1D | 17709714 | 3.13 |
| LI17M | Width | AX-94865808 | 1D | 17709714 | 3.43 |
| ANN18M | Width | AX-94818538 | 2A | 74116149 | 3.12 |
| ANN18M | Length | AX-94818538 | 2A | 74116149 | 3.91 |
| LI18E | TKW | AX-95141980 | 2B | 158603336 | 3.41 |
| LI18E | Length | AX-95141980 | 2B | 158603336 | 3.51 |
| ANN18M | Length | AX-94610074 | 2D | 81446063 | 3.53 |
| ANN18M | Width | AX-94610074 | 2D | 81446063 | 3.54 |
| LI17L | TKW | AX-95107580 | 2D | 189198358 | 3.35 |
| LI17L | Width | AX-95107580 | 2D | 189198358 | 3.36 |
| LI17L | Width | AX-94986528 | 3B | 795484711 | 3.10 |
| LI17L | TKW | AX-94986528 | 3B | 795484711 | 3.39 |
| LI17L | TKW | AX-94469815 | 4A | 616106716 | 3.14 |
| LI17L | Width | AX-94469815 | 4A | 616106716 | 3.40 |
| ANN17E | TKW | AX-94426780 | 5A | 597363315 | 3.26 |
| LI17L | TKW | AX-94426780 | 5A | 597363315 | 3.42 |
| LI18E | Width | AX-94958973 | 5A | 683270025 | 3.50 |
| LI18E | TKW | AX-94958973 | 5A | 683270025 | 4.21 |
| LI17M | Length | AX-94476475 | 5B | 714516404 | 3.28 |
| LI17M | Width | AX-94476475 | 5B | 714516404 | 4.24 |
| LI17M | Width | AX-94890680 | 5B | 714134727 | 3.01 |
| LI17L | Width | AX-94890680 | 5B | 714134727 | 4.10 |
| LI17L | TKW | AX-94890680 | 5B | 714134727 | 4.40 |
| ANN18M | Width | AX-94394209 | 6A | 1717618 | 3.17 |
| ANN18M | TKW | AX-94394209 | 6A | 1717618 | 5.36 |
| ANN18M | Width | AX-94459169 | 6A | 1719973 | 3.06 |
| ANN18M | TKW | AX-94459169 | 6A | 1719973 | 5.17 |
| ANN18M | Width | AX-94484443 | 6B | 54673673 | 3.06 |
| LI17L | Width | AX-94484443 | 6B | 54673673 | 3.31 |
| LI17L | TKW | AX-94549328 | 6B | 36319220 | 3.08 |
| LI17L | Width | AX-94549328 | 6B | 36319220 | 3.44 |
| LI18E | TKW | AX-94439967 | 6D | 23560832 | 3.01 |
| LI18E | Length | AX-94439967 | 6D | 23560832 | 3.49 |
| LI17L | TKW | AX-95127531 | 6D | 2385467 | 3.03 |
| LI17L | Length | AX-95127531 | 6D | 2385467 | 3.80 |
| LI17L | TKW | AX-95132187 | 6D | 488482849 | 3.44 |
| LI17L | Width | AX-95132187 | 6D | 488482849 | 3.52 |
| LI18L | TKW | AX-95139913 | 6D | 364760471 | 3.48 |
| LI18L | Width | AX-95139913 | 6D | 364760471 | 3.54 |
| LI17L | TKW | AX-95157078 | 6D | 488537244 | 3.63 |
| LI17L | Width | AX-95157078 | 6D | 488537244 | 3.75 |
| LI18E | TKW | AX-95223861 | 7B | 66518147 | 3.51 |
| LI18E | Width | AX-95223861 | 7B | 66518147 | 4.43 |
| LI18L | TKW | AX-94435697 | 7D | 56637758 | 3.21 |
| LI18L | Width | AX-94435697 | 7D | 56637758 | 3.21 |
| LI18E | Width | AX-94463985 | 7D | 551895334 | 3.05 |
| ANN17E | Width | AX-94463985 | 7D | 551895334 | 3.12 |
| Marker | Chromosome | Position | Allelic Effect TKW | Significant Climatic Variable for TKW | Allelic Effect Width | Significant Climatic Variable for Width | Allelic Effect Length | Significant Climatic Variable for Length |
|---|---|---|---|---|---|---|---|---|
| AX-95224161 | 1B | 518659990 | −0.827 | DiffDaylength (30 days after sowing) | −0.606 | Average MaxTemperature (135 days after sowing) | ||
| AX-95141980 | 2B | 158603336 | −0.773 | DiffDaylength (150 days after sowing) | 0.813 | Average MinTemperature (30 days after sowing) | ||
| AX-95107580 | 2D | 189198358 | −0.669 | ThermalInversion (90 days after sowing) | −0.774 | Average MinTemperature (60 days after sowing) | ||
| AX-94469815 | 4A | 616106716 | 0.855 | Daylength (15 days after sowing) | 0.748 | Average MaxTemperature (15 days after sowing) | ||
| AX-94958973 | 5A | 683270025 | −0.706 | Average MinTemperature (Heading Day) | 0.667 | Nightlength (15 days after sowing) | ||
| AX-94890680 | 5B | 714134727 | 0.767 | DiffDaylength (30 days after sowing) | 0.837 | DiffDaylength (30 days after sowing) | ||
| AX-94459169 | 6A | 1719973 | −0.876 | Rainfall (Heading Day) | −0.750 | Rainfall (Heading Day) | ||
| AX-94549328 | 6B | 36319220 | −0.755 | AccumDaylength (150 days after sowing) | −0.782 | Average MinTemperature (135 days after sowing) | ||
| AX-94439967 | 6D | 23560832 | 0.739 | AccumDaylength (135 days after sowing) | −0.725 | ThermalInversion (30 days after sowing) | ||
| AX-95127531 | 6D | 2385467 | 0.674 | ThermalInversion (105 days after sowing) | −0.814 | Average Temperature (Heading Day) | ||
| AX-95139913 | 6D | 364760471 | −0.830 | ThermalInversion (90 days after sowing) | −0.870 | Average MaxTemperature (60 days after sowing) | ||
| AX-95223861 | 7B | 66518147 | 0.798 | Average MaxTemperature (90 days after sowing) | 0.757 | Average MinTemperature (90 days after sowing) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Baouchi, A.; Ibriz, M.; Dreisigacker, S.; Lopes, M.S.; Sanchez-Garcia, M. Dissection of the Genetic Basis of Genotype by Environment Interactions for Morphological Traits and Protein Content in Winter Wheat Panel Grown in Morocco and Spain. Plants 2024, 13, 1477. https://doi.org/10.3390/plants13111477
El Baouchi A, Ibriz M, Dreisigacker S, Lopes MS, Sanchez-Garcia M. Dissection of the Genetic Basis of Genotype by Environment Interactions for Morphological Traits and Protein Content in Winter Wheat Panel Grown in Morocco and Spain. Plants. 2024; 13(11):1477. https://doi.org/10.3390/plants13111477
Chicago/Turabian StyleEl Baouchi, Adil, Mohammed Ibriz, Susanne Dreisigacker, Marta S. Lopes, and Miguel Sanchez-Garcia. 2024. "Dissection of the Genetic Basis of Genotype by Environment Interactions for Morphological Traits and Protein Content in Winter Wheat Panel Grown in Morocco and Spain" Plants 13, no. 11: 1477. https://doi.org/10.3390/plants13111477
APA StyleEl Baouchi, A., Ibriz, M., Dreisigacker, S., Lopes, M. S., & Sanchez-Garcia, M. (2024). Dissection of the Genetic Basis of Genotype by Environment Interactions for Morphological Traits and Protein Content in Winter Wheat Panel Grown in Morocco and Spain. Plants, 13(11), 1477. https://doi.org/10.3390/plants13111477









