Cytological Observation and RNA-Seq Analyses Reveal miR9564 and Its Target Associated with Pollen Sterility in Autotetraploid Rice
Abstract
1. Introduction
2. Results
2.1. The Low-Fertility Tetraploid Line (LF) Exhibited Defects in Pollen Development and Fertilization
2.2. LF Showed Severe Abnormal Male Meiotic Process
2.3. Comparative miRNA Expression Profiles in Meiotic Anthers of H1, HF, and LF
2.4. Identification of Negative Regulative miRNA-Target Pairs during Meiosis in Neo-Tetraploid Rice
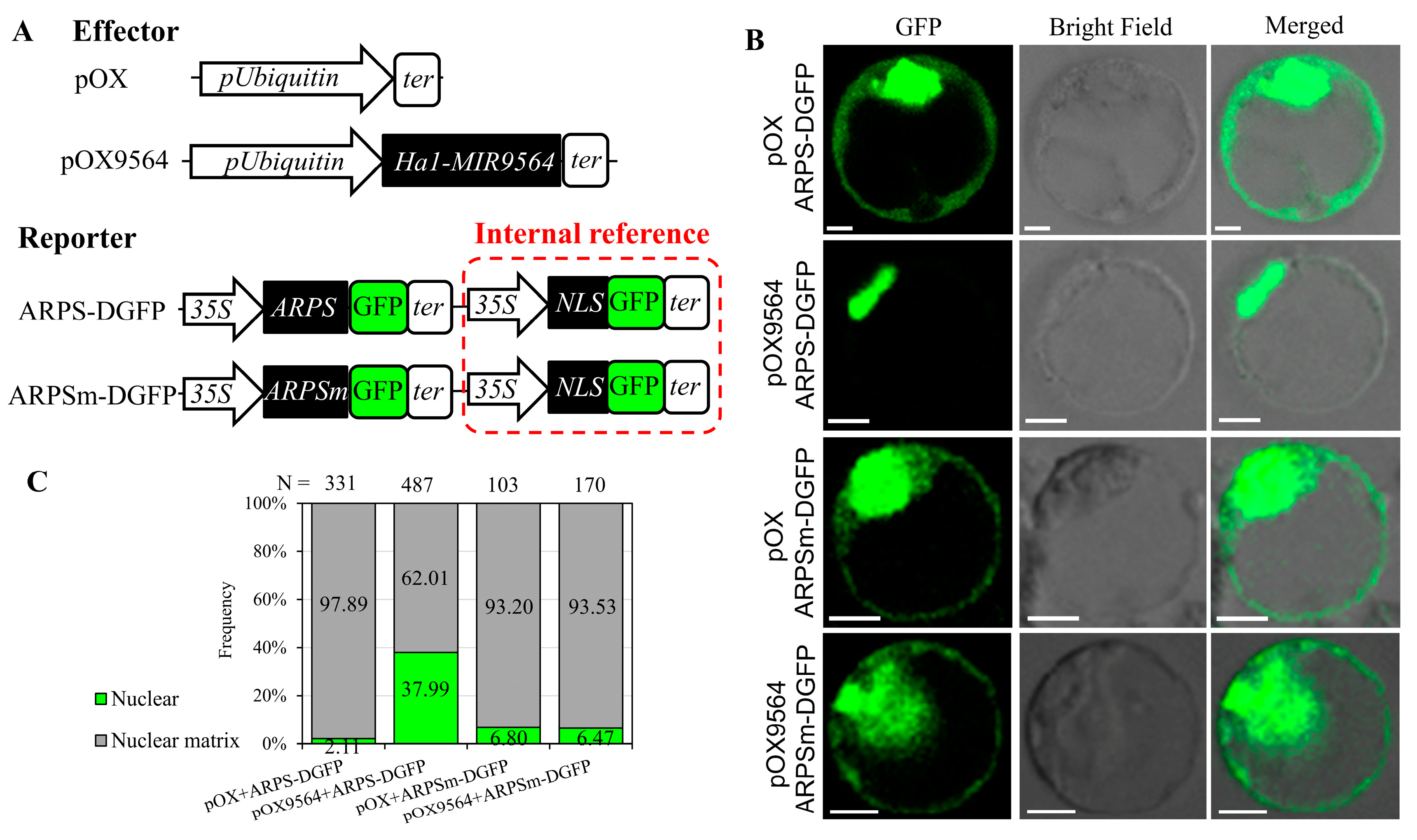
2.5. miR9564 Could Negatively Regulate the Expression Level of ARPS
2.6. Over-Expression of ARPS Reduced Pollen Fertility and Seed Set in Neo-Tetraploid Rice
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Cytological Observation
4.3. miRNA Analysis
4.4. Bioinformatics Analysis Tools
4.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.6. Dual-GFP Assay
4.7. Over-Expression Line of ARPS in Neo-Tetraploid Rice
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chao, D.; Dilkes, B.; Luo, H.; Douglas, A.; Yakubova, E.; Lahner, B.; Salt, D. Polyploids exhibit higher potassium uptake and salinity tolerance in Arabidopsis. Science 2013, 341, 658–659. [Google Scholar] [CrossRef]
- Yu, H.; Lin, T.; Meng, X.; Du, H.; Zhang, J.; Liu, G.; Chen, M.; Jing, Y.; Kou, L.; Li, X.; et al. A route to de novo domestication of wild allotetraploid rice. Cell 2021, 184, 1156–1170. [Google Scholar] [CrossRef] [PubMed]
- International Wheat Genome Sequencing Consortium (IWGSC). A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 2014, 345, 1251788. [Google Scholar] [CrossRef]
- Morgan, C.; White, M.A.; Franklin, F.C.H.; Zickler, D.; Kleckner, N.; Bomblies, K. Evolution of crossover interference enables stable autopolyploidy by ensuring pairwise partner connections in Arabidopsis arenosa. Curr. Biol. 2021, 31, 4713–4726. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Zhang, H.; Henry, C.E.; Franklin, F.C.H.; Bomblies, K. Derived alleles of two axis proteins affect meiotic traits in autotetraploid Arabidopsis arenosa. Proc. Nat. Acad. Sci. USA 2020, 117, 8980–8988. [Google Scholar] [CrossRef]
- Westermann, J.; Srikant, T.; Gonzalo, A.; Tan, H.S.; Bomblies, K. Defective pollen tube tip growth induces neo-polyploid infertility. Science 2024, 383, h755. [Google Scholar] [CrossRef]
- Corneillie, S.; De Storme, N.; Van Acker, R.; Fangel, J.U.; De Bruyne, M.; De Rycke, R.; Geelen, D.; Willats, W.; Vanholme, B.; Boerjan, W. Polyploidy affects plant growth and alters cell wall composition. Plant Physiol. 2019, 179, 74–87. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Wang, Y.; Wang, S.; Wang, X.; Li, G.; Zhang, X.; Liang, Z.; Li, J.; Gong, L.; et al. Homoploid F1 hybrids and segmental allotetraploids of japonica and indica rice subspecies show similar and enhanced tolerance to nitrogen deficiency than parental lines. J. Exp. Bot. 2021, 72, 5612–5624. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, F.; Zhou, Y.; Wang, J.; Sun, S.; Wang, B.; Zhang, Z.; Li, G.; Lin, X.; Wang, X.; et al. Genomic mosaicism due to homoeologous exchange generates extensive phenotypic diversity in nascent allopolyploids. Natl. Sci. Rev. 2020, 8, nwaa277. [Google Scholar] [CrossRef]
- Wang, L.; Cao, S.; Wang, P.; Lu, K.; Song, Q.; Zhao, F.; Chen, Z. DNA hypomethylation in tetraploid rice potentiates stress-responsive gene expression for salt tolerance. Proc. Natl. Acad. Sci. USA 2021, 118, e2023981118. [Google Scholar] [CrossRef]
- Wang, N.; Fan, X.; Lin, Y.; Li, Z.; Wang, Y.; Zhou, Y.; Meng, W.; Peng, Z.; Zhang, C.; Ma, J. Alkaline stress induces different physiological, hormonal and gene expression responses in diploid and autotetraploid rice. Int. J. Mol. Sci. 2022, 23, 5561. [Google Scholar] [CrossRef]
- Li, X.; Shahid, M.Q.; Wu, J.; Wang, L.; Liu, X.; Lu, Y. Comparative small RNA analysis of pollen development in autotetraploid and diploid rice. Int. J. Mol. Sci. 2016, 17, 499. [Google Scholar] [CrossRef]
- Li, X.; Shahid, M.Q.; Xia, J.; Lu, Z.; Fang, N.; Wang, L.; Wu, J.; Chen, Z.; Liu, X. Analysis of small RNAs revealed differential expressions during pollen and embryo sac development in autotetraploid rice. BMC Genom. 2017, 18, 129. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.; Gu, H.; Li, Z.; Tian, B.; Xie, Z.; Shi, G.; Chen, W.; Wei, F.; Cao, G. Developmental differences between anthers of diploid and autotetraploid rice at meiosis. Plants 2022, 11, 1647. [Google Scholar] [CrossRef]
- He, Y.; Ge, J.; Jiang, A.; Gan, L.; Song, Z.; Cai, D. Using a polyploid meiosis stability (PMeS) line as a parent improves embryo development and the seed set rate of a tetraploid rice hybrid. Can. J. Plant Sci. 2011, 91, 325–335. [Google Scholar] [CrossRef]
- Guo, H.; Mendrikahy, J.N.; Xie, L.; Deng, J.; Lu, Z.; Wu, J.; Li, X.; Shahid, M.Q.; Liu, X. Transcriptome analysis of neo-tetraploid rice reveals specific differential gene expressions associated with fertility and heterosis. Sci. Rep. 2017, 7, 40139. [Google Scholar] [CrossRef]
- Koide, Y.; Kuniyoshi, D.; Kishima, Y. Fertile tetraploids: New resources for future rice breeding? Front. Plant Sci. 2020, 11, 1231. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, Y.; Wu, J.; Chen, Z.; Wang, L.; Shahid, M.Q.; Liu, X. Carbohydrate metabolism and fertility related genes high expression levels promote heterosis in autotetraploid rice harboring double neutral genes. Rice 2019, 12, 34. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, J.; Ma, Y.; Jiao, W.; Ye, W.; Yang, D.; Yi, C.; Chen, Z. Rice interploidy crosses disrupt epigenetic regulation, gene expression, and seed development. Mol. Plant 2018, 11, 300–314. [Google Scholar] [CrossRef]
- Iwakawa, H.O.; Tomari, Y. The functions of microRNAs: mRNA decay and translational repression. Trends Cell Biol. 2015, 25, 651–665. [Google Scholar] [CrossRef]
- Zhang, Y.; He, R.; Lian, J.; Zhou, Y.; Li, Q.; Yu, Y.; Feng, Y.; Yang, Y.; Lei, M.; He, H.; et al. OsmiR528 regulates rice pollen intine formation by targeting an uclacyanin to influence flavonoid metabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, P.; Mei, H.; Wang, D.; Sun, J.; Yang, C.; Hao, L.; Cao, S.; Chu, C.; Hu, S.; et al. Fine-tuning of miR528 accumulation modulates flowering time in rice. Mol. Plant 2019, 12, 1103–1113. [Google Scholar] [CrossRef]
- Yao, S.; Yang, Z.; Yang, R.; Huang, Y.; Guo, G.; Kong, X.; Lan, Y.; Zhou, T.; Wang, H.; Wang, W.; et al. Transcriptional regulation of miR528 by OsSPL9 orchestrates antiviral response in rice. Mol. Plant 2019, 12, 1114–1122. [Google Scholar] [CrossRef]
- Araki, S.; Le, N.T.; Koizumi, K.; Villar-Briones, A.; Nonomura, K.; Endo, M.; Inoue, H.; Saze, H.; Komiya, R. miR2118-dependent U-rich phasiRNA production in rice anther wall development. Nat. Commun. 2020, 11, 3115. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, X.L.; Zhu, Y.; Yang, X.M.; Zhang, K.N.; Xiao, Z.Y.; Wang, H.; Zhao, J.; Zhang, L.; Li, G.; et al. Osa-miR398b boosts H2O2 production and rice blast disease-resistance via multiple superoxide dismutases. New Phytol. 2019, 222, 1507–1522. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanuy, F.; Peris-Peris, C.; Tomiyama, S.; Okada, K.; Hsing, Y.I.; San Segundo, B.; Campo, S. Osa-miR7695 enhances transcriptional priming in defense responses against the rice blast fungus. BMC Plant Biol. 2019, 19, 563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Feng, Q.; Cao, X.; Zhu, Y.; Wang, H.; Chandran, V.; Fan, J.; Zhao, J.; Pu, M.; Li, Y.; et al. Osa-miR167d facilitates infection of Magnaporthe oryzae in rice. J. Integr. Plant Biol. 2020, 62, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhu, Y.; Wang, L.; Zheng, Y.; Chen, J.; Li, T.; Yang, X.; Wang, H.; Li, X.; Ma, X.; et al. Osa-miR1873 fine-tunes rice immunity against Magnaporthe oryzae and yield traits. J. Integr. Plant Biol. 2020, 62, 1213–1226. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, Y.; Xue, D.; Wang, J.; Yan, M.; Liu, G.; Dong, G.; Zeng, D.; Lu, Z.; Zhu, X.; et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 2010, 42, 541–544. [Google Scholar] [CrossRef]
- Miao, C.; Wang, Z.; Zhang, L.; Yao, J.; Hua, K.; Liu, X.; Shi, H.; Zhu, J. The grain yield modulator miR156 regulates seed dormancy through the gibberellin pathway in rice. Nat. Commun. 2019, 10, 3822. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, T.; Sun, H.; Teotia, S.; Wen, H.; Du, Y.; Zhang, J.; Li, J.; Tang, G.; Xue, H.; et al. miR1432-OsACOT (Acyl-CoA thioesterase) module determines grain yield via enhancing grain filling rate in rice. Plant Biotechnol. J. 2019, 17, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xu, X.; Li, Y.; Xie, L.; He, Y.; Li, W.; Lu, X.; Sun, H.; Xie, X. OsmiR530 acts downstream of OsPIL15 to regulate grain yield in rice. New Phytol. 2020, 226, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Guo, X.; Huang, Z.; Xia, J.; Li, X.; Wu, J.; Yu, H.; Shahid, M.Q.; Liu, X. Transcriptome and gene editing analyses reveal MOF1a defect alters the expression of genes associated with tapetum development and chromosome behavior at meiosis stage resulting in low pollen fertility of tetraploid rice. Int. J. Mol. Sci. 2020, 21, 7489. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, X.; Wen, M.; Yin, W.; Chen, Y.; Liu, Y.; Liu, X. Cytological observation and RNA-seq analysis reveal novel miRNAs high expression associated with the pollen fertility of neo-tetraploid rice. BMC Plant Biol. 2023, 23, 434. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, S.; Qin, Q.; Shi, W.; Longfeng, Y.; Hou, S. Rice Protein Phosphatase 1 regulatory subunits OsINH2 and OsINH3 participate actively in growth and adaptive responses under abscisic acid. Front. Plant Sci. 2022, 13, 990575. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.N.; Yamagata, Y.; Shigematsu, Y.; Watanabe, M.; Miyazaki, Y.; Doi, K.; Tashiro, K.; Kuhara, S.; Kanamori, H.; Wu, J.; et al. Duplication and loss of function of genes encoding RNA Polymerase III Subunit C4 causes hybrid incompatibility in rice. G3 2017, 7, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Hou, Q.; Si, L.; Huang, X.; Luo, J.; Lu, D.; Zhu, J.; Shangguan, Y.; Miao, J.; Xie, Y.; et al. The PLATZ transcription factor GL6 affects grain length and number in rice. Plant Physiol. 2019, 180, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Zou, T.; He, Z.; Xiao, Q.; Li, G.; Liu, S.; Xiong, P.; Chen, H.; Peng, K.; Zhang, X.; et al. SWOLLEN TAPETUM AND STERILITY 1 is required for tapetum degeneration and pollen wall formation in rice. Plant Physiol. 2022, 190, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Lv, M.; Liang, Y.; Ma, Z.; Cao, J. Identification of novel and conserved miRNAs involved in pollen development in Brassica campestris ssp. chinensis by high-throughput sequencing and degradome analysis. BMC Genom. 2014, 15, 146. [Google Scholar] [CrossRef]
- Hermkes, R.; Fu, Y.; Nürrenberg, K.; Budhiraja, R.; Schmelzer, E.; Elrouby, N.; Dohmen, R.J.; Bachmair, A.; Coupland, G. Distinct roles for Arabidopsis SUMO protease ESD4 and its closest homolog ELS1. Planta 2011, 233, 63–73. [Google Scholar] [CrossRef]
- Pei, W.; Jain, A.; Ai, H.; Liu, X.; Feng, B.; Wang, X.; Sun, Y.; Xu, G.; Sun, S. OsSIZ2 regulates nitrogen homeostasis and some of the reproductive traits in rice. J. Plant Physiol. 2019, 232, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Zhang, C.; Caine, R.S.; Gray, J.; Sadanandom, A. Rice SUMO protease Overly Tolerant to Salt 1 targets the transcription factor, OsbZIP23 to promote drought tolerance in rice. Plant J. 2017, 92, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhuang, Z.; Zhao, P. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
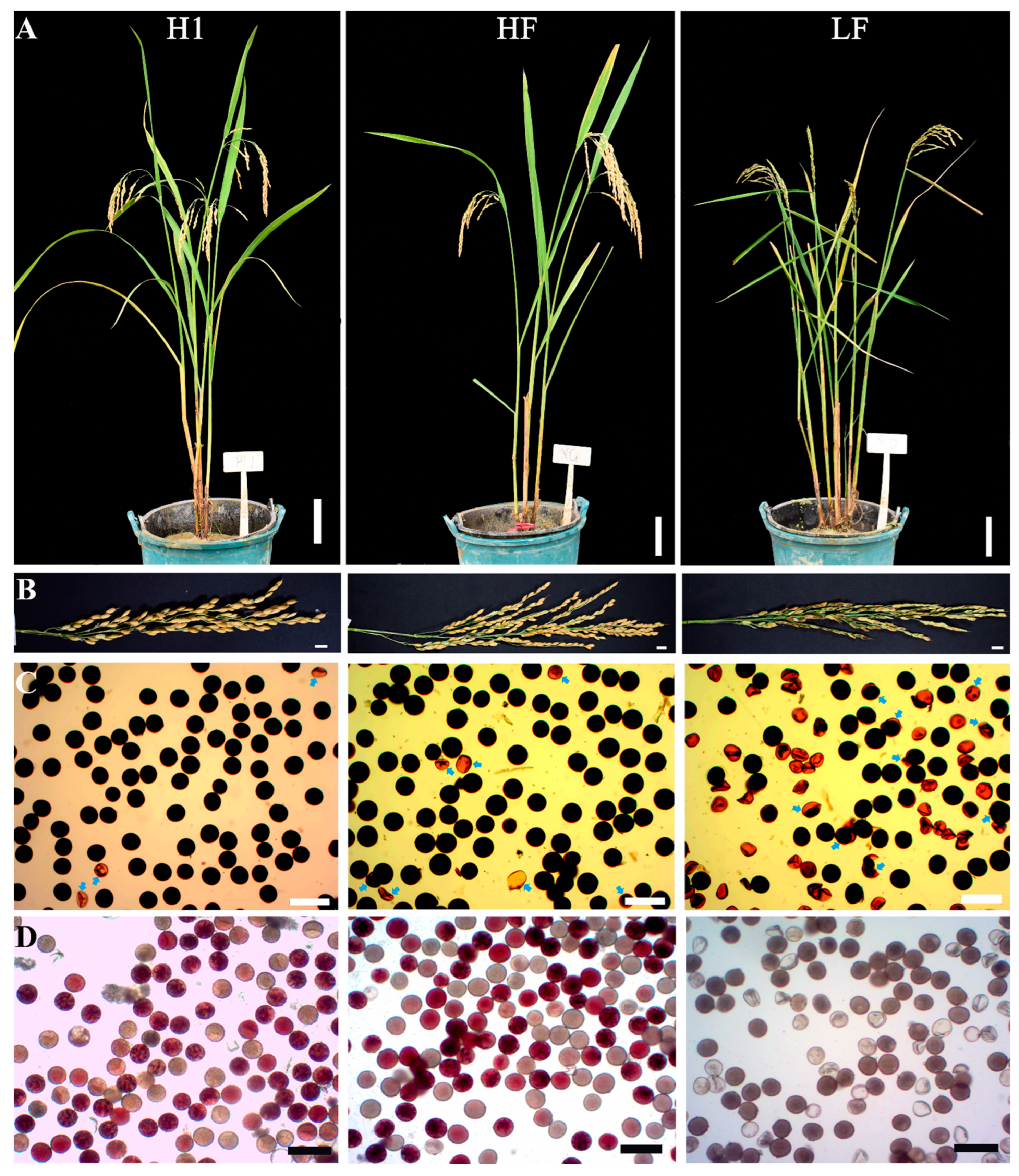
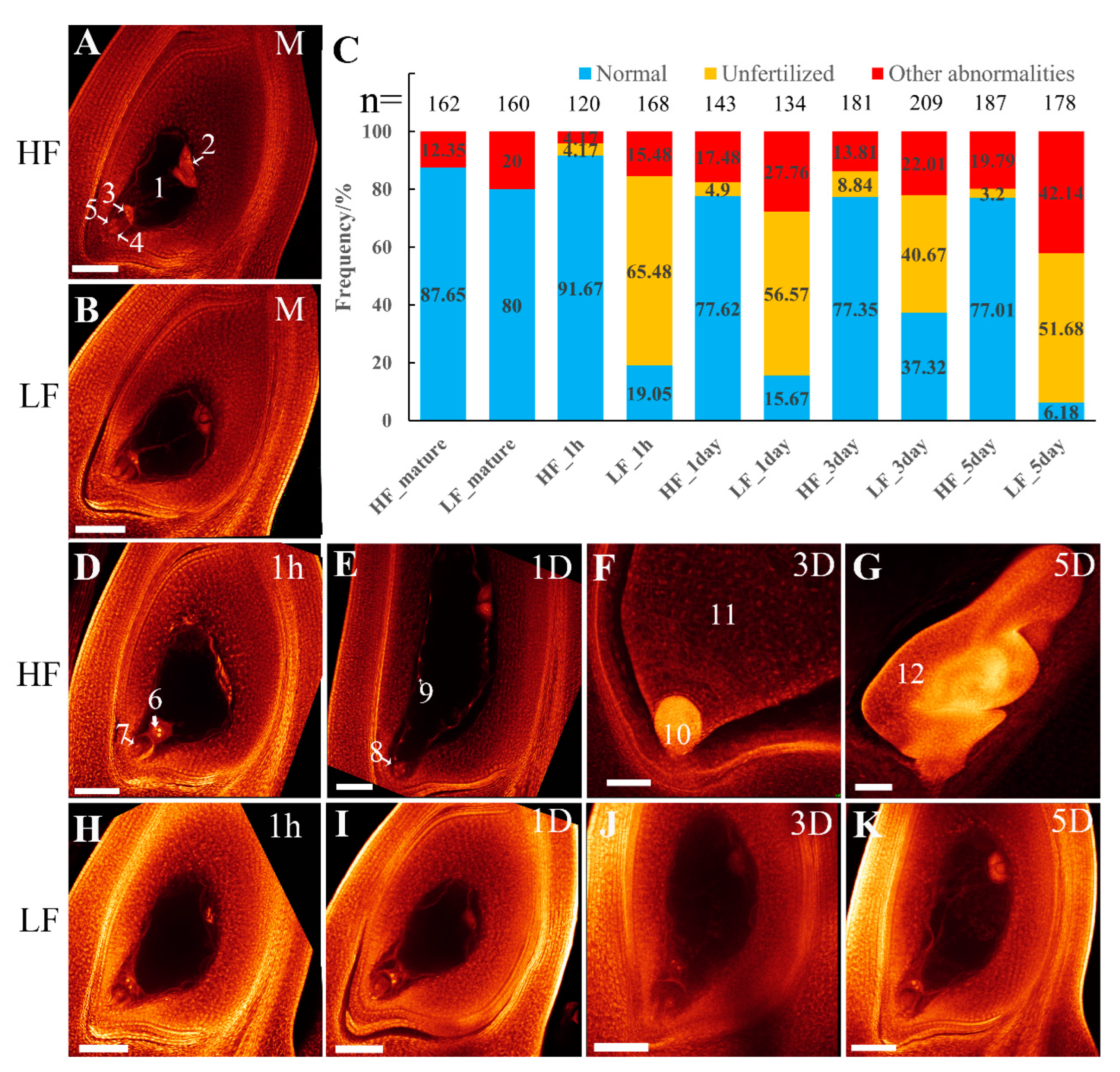
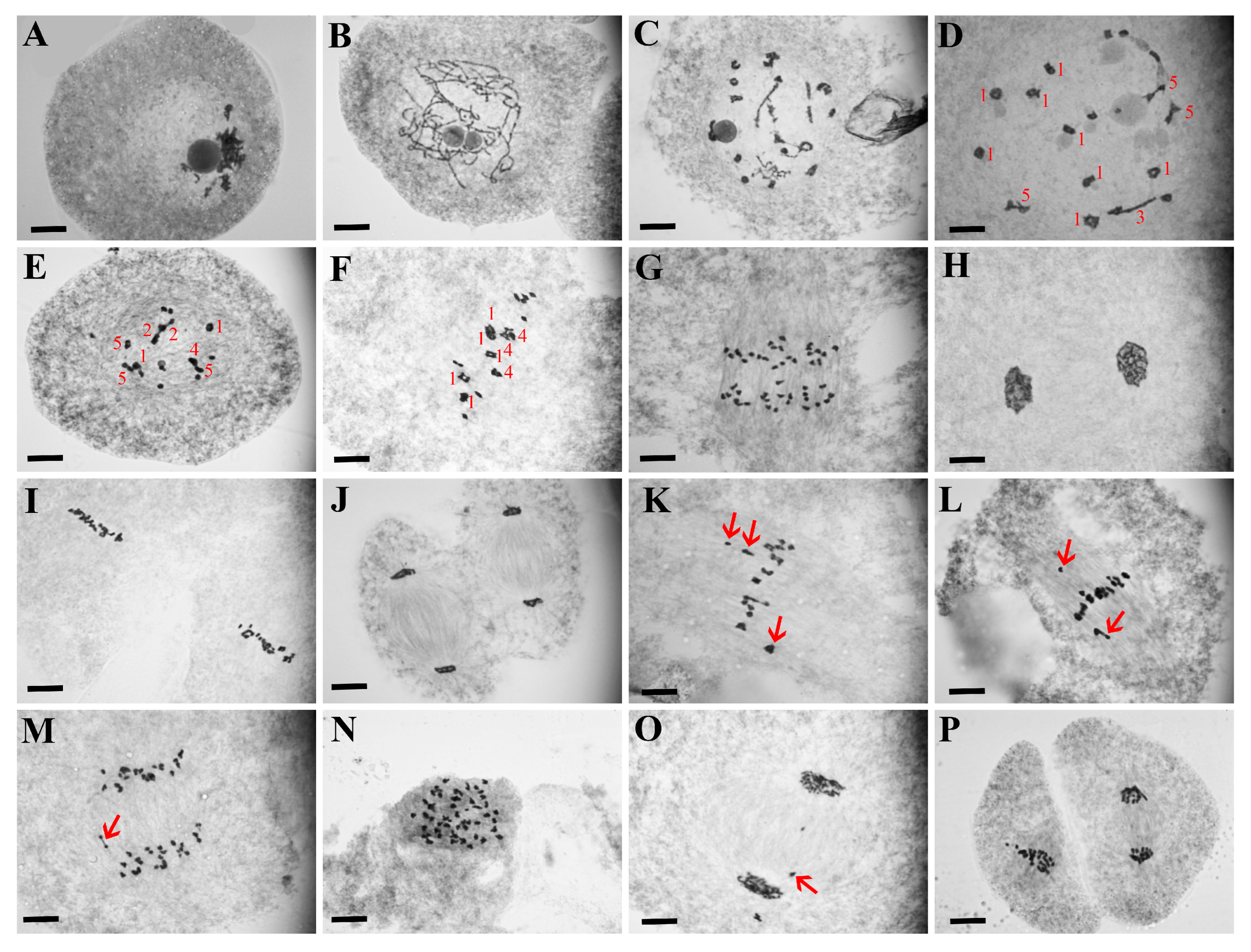


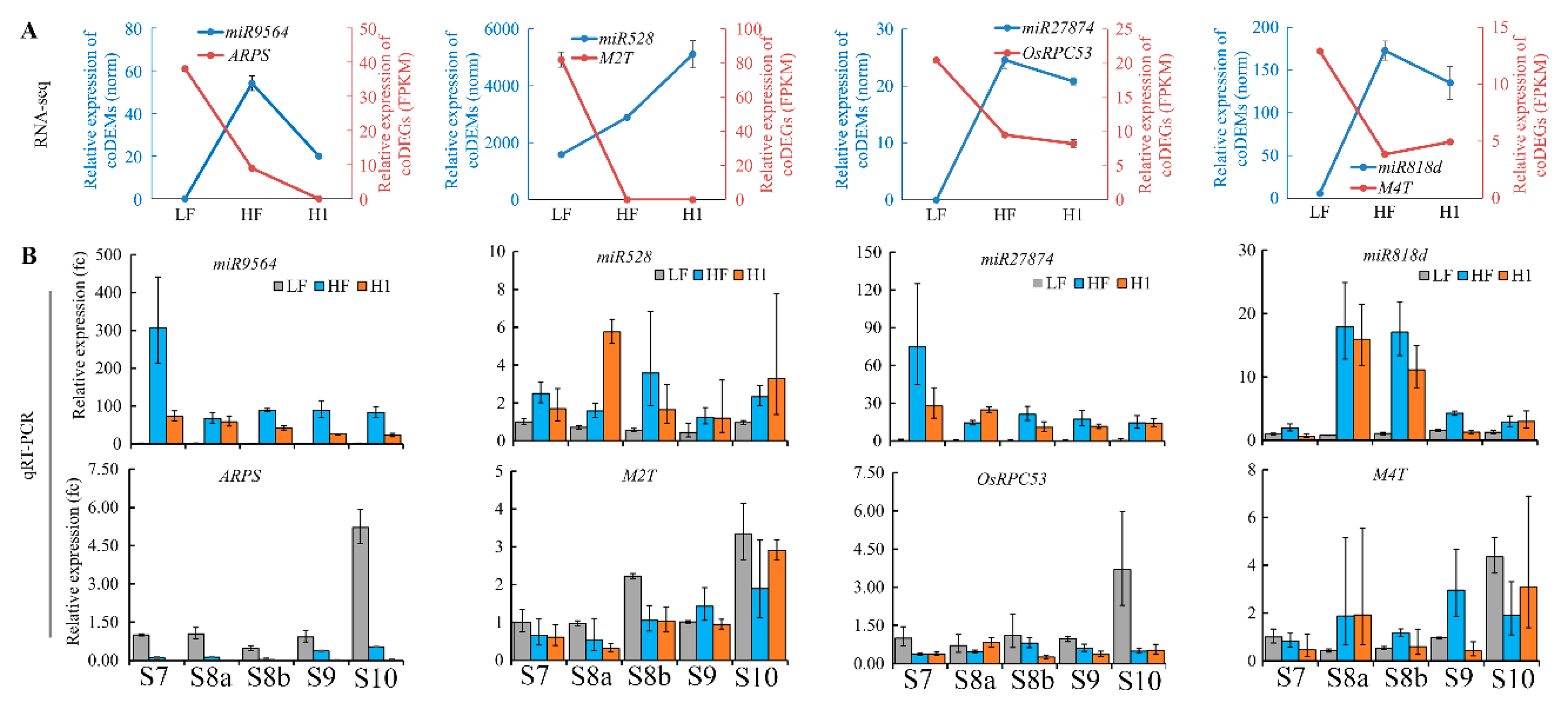


| Materials | Seed Setting (%) | I2/KI-Stained Pollen Grains | TTC-Stained Pollen Grains | ||
|---|---|---|---|---|---|
| Normal (%) | Number | Viable (%) | Number | ||
| H1 | 74.38 ± 1.90 A | 97.10 ± 0.26 A | 2770 | 46.93 ± 4.26 A | 5697 |
| HF | 71.39 ± 1.91 A | 90.31 ± 1.58 A | 3741 | 65.75 ± 2.78 A | 6452 |
| LF | 5.17 ± 0.80 C | 26.02 ± 8.06 B | 3560 | 17.95 ± 6.27 B | 3907 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Huang, W.; Zhu, L.; Liang, G.; Huang, Y.; Wu, J.; Chen, R.; Li, X.; Liu, X. Cytological Observation and RNA-Seq Analyses Reveal miR9564 and Its Target Associated with Pollen Sterility in Autotetraploid Rice. Plants 2024, 13, 1461. https://doi.org/10.3390/plants13111461
Lu Z, Huang W, Zhu L, Liang G, Huang Y, Wu J, Chen R, Li X, Liu X. Cytological Observation and RNA-Seq Analyses Reveal miR9564 and Its Target Associated with Pollen Sterility in Autotetraploid Rice. Plants. 2024; 13(11):1461. https://doi.org/10.3390/plants13111461
Chicago/Turabian StyleLu, Zijun, Weicong Huang, Lianjun Zhu, Guobin Liang, Yu Huang, Jinwen Wu, Rou Chen, Xiang Li, and Xiangdong Liu. 2024. "Cytological Observation and RNA-Seq Analyses Reveal miR9564 and Its Target Associated with Pollen Sterility in Autotetraploid Rice" Plants 13, no. 11: 1461. https://doi.org/10.3390/plants13111461
APA StyleLu, Z., Huang, W., Zhu, L., Liang, G., Huang, Y., Wu, J., Chen, R., Li, X., & Liu, X. (2024). Cytological Observation and RNA-Seq Analyses Reveal miR9564 and Its Target Associated with Pollen Sterility in Autotetraploid Rice. Plants, 13(11), 1461. https://doi.org/10.3390/plants13111461







