Variation in Nitrogen Utilization and Nutrient Composition across Various Organs under Different Strip Logging Management Models in Moso Bamboo (Phyllostachys edulis) Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Site
2.2. Methods
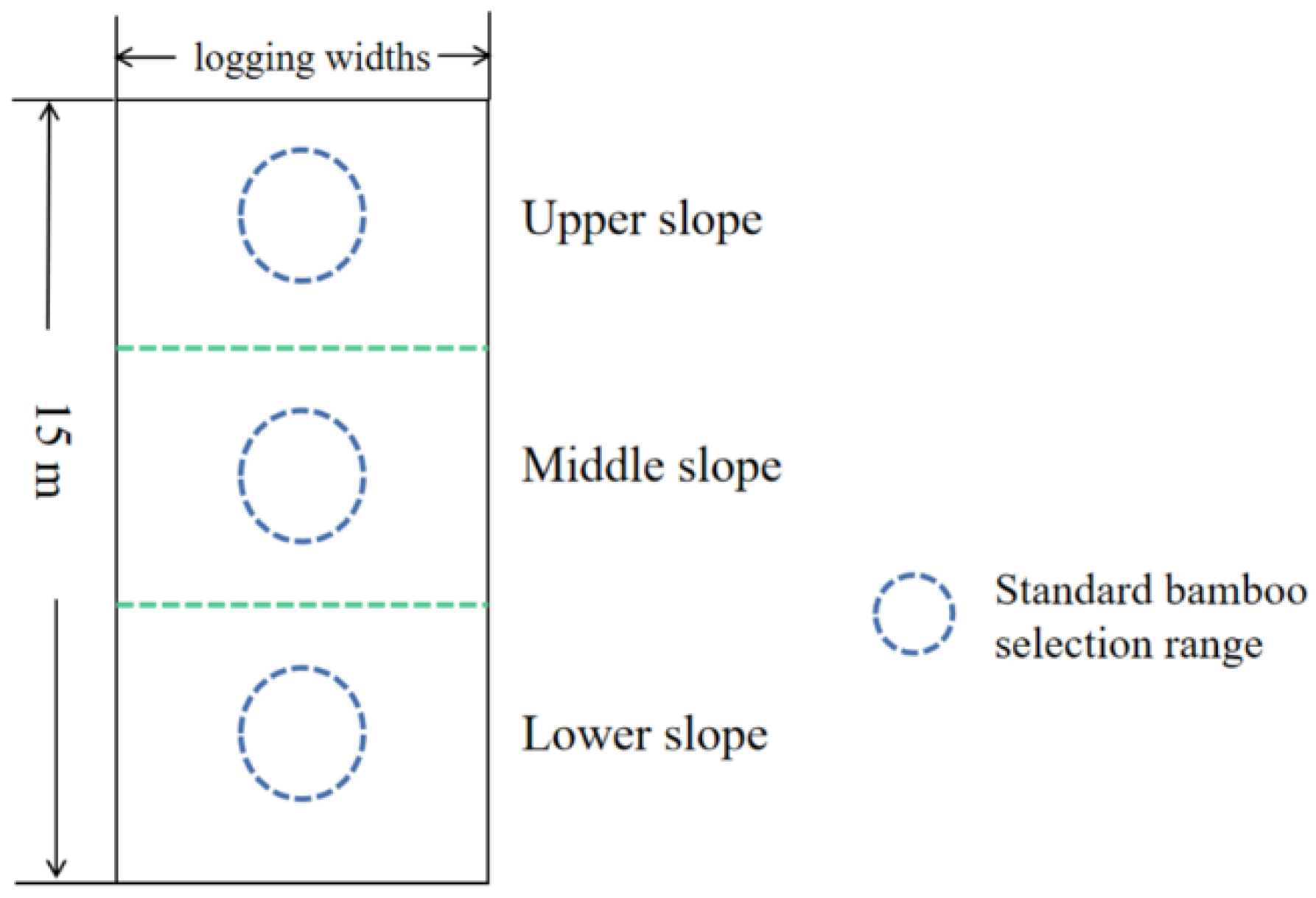
2.2.1. Plot Establishment and Experimental Design
2.2.2. Sample Collection and Analysis
2.3. Data Processing and Analysis
3. Results
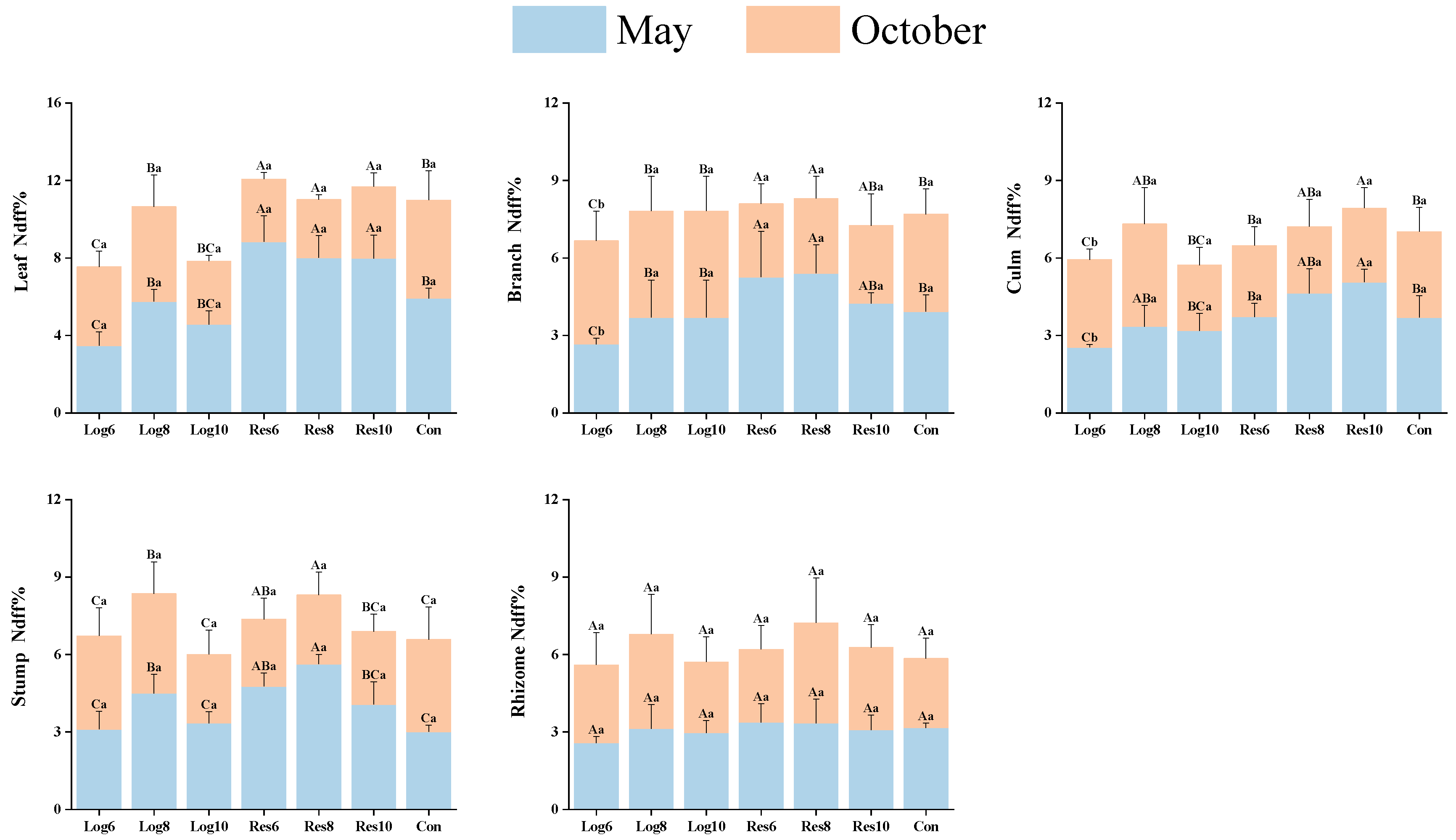
3.1. Various Strip Logging Treatments Exhibit Different Effects on the Nitrogen Accumulation Ability of Various Organs in Bamboo
3.2. Different Strip Logging Treatments Have Varying Effects on Nitrogen Utilization Efficiency in Various Bamboo Organs
3.3. Different Strip Logging Treatments Exhibit Varying Effects on the Contents of Key Elements in Various Bamboo Organs
3.3.1. Different Strip Logging Treatments Affect the Contents of Key Elements in Bamboo Leaves
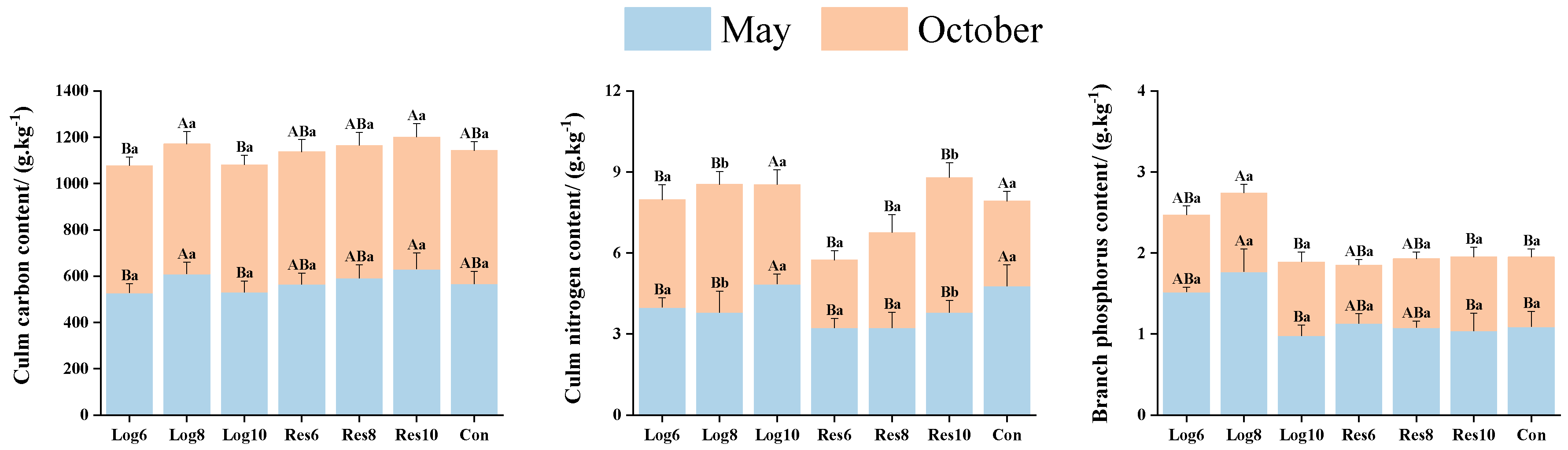
3.3.2. Different Strip Logging Treatments Have Varying Effects on the Elemental Composition of Bamboo Branches
3.3.3. Different Strip Logging Treatments Have Varying Effects on the Element Content in Bamboo Culms
3.3.4. Different Strip Logging Treatments Have Varying Effects on the Elemental Composition of Bamboo Stumps
3.3.5. Different Strip Logging Treatments Exhibit Varying Effects on the Elemental Composition of Bamboo Rhizomes
3.4. PCA of Nitrogen Utilization and Nutrient Content in Various Organs of Moso Bamboo under Different Strip Logging Treatments
Y2 = −0.483X1 − 0.559X2 − 0.037X3 − 0.206X4 − 0.236X5 − 0.504X6 − 0.711X7 + 0.685X8 + 0.633X9 + 0.516X10 − 0.322X11 + 0.813X12 + 0.900X13 − 0.079X14 + 0.859X15 + 0.464X16 − 0.119X17 + 0.899X18 + 0.595X19 − 0.029X20 + 0.667X21 + 0.727X22 − 0.471X23 + 0.761X24 + 0.379X25
Y3 = −0.135X1 + 0.290X2 − 0.292X3 + 0.578X4 + 0.262X5 − 0.174X6 + 0.372X7 − 0.642X8 + 0.182X9 + 0.142X10 − 0.123X11 − 0.051X12 + 0.284X13 − 0.107X14 − 0.084X15 + 0.648X16 − 0.236X17 − 0.365X18 + 0.663X19 − 0.231X20 − 0.213X21 + 0.566X22 + 0.425X23 − 0.268X24 − 0.306X25
Y4 = −0.266X1 − 0.262X2 − 0.005X3 + 0.145X4 + 0.224X5 − 0.496X6 − 0.297X7 + 0.072X8 + 0.513X9 − 0.035X10 + 0.417X11 − 0.075X12 + 0.104X13 − 0.056X14 − 0.209X15 − 0.494X16 + 0.006X17 + 0.134X18 + 0.108X19 + 0.184X20 + 0.639X21 + 0.226X22 + 0.077X23 − 0.188X24 − 0.452X25
Y5 = −0.030X1 + 0.190X2 + 0.263X3 + 0.017X4 − 0.183X5 − 0.018X6 + 0.301X7 + 0.097X8 − 0.041X9 − 0.105X10 + 0.146X11 + 0.087X12 − 0.262X13 − 0.473X14 + 0.433X15 − 0.237X16 + 0.107X17 + 0.194X18 + 0.418X19 + 0.090X20 − 0.148X21 − 0.283X22 + 0.273X23 − 0.123X24 − 0.262X25
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z.H. Bamboo and Rattan in the World; Liaoning Science and Technology Publishing House: Shenyang, China, 2002. [Google Scholar]
- Fan, S.H.; Liu, G.L.; Su, W.H.; Cai, C.J.; Guan, F.Y. Advances in research of bamboo forest cultivation. For. Res. 2018, 31, 137–144. [Google Scholar]
- Mao, F.; Zhou, G.; Li, P.; Du, H.; Xu, X.; Shi, Y.; Mo, L.; Zhou, Y.; Tu, G. Optimizing selective cutting strategies for maximum carbon stocks and yield of Moso bamboo forest using BIOME-BGC model. J. Environ. Manag. 2017, 191, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Vangansbeke, P.; Schrijver, A.; Frenne, P.; Verstraeten, A.; Gorissen, L.; Verheyen, K. Strong negative impacts of whole tree harvesting in pine stands on poor, sandy soils: A long-term nutrient budget modelling approach. For. Ecol. Manag. 2015, 356, 101–111. [Google Scholar] [CrossRef]
- Zeng, X.L. Research on the Characteristics and Influencing Factors of Band Logging Recovery of Moso Bamboo Forests in Southern Anhui. Master’s Thesis, Chinese Academy of Forestry Sciences, Beijing, China, 2019. [Google Scholar]
- Helmisaari, H.-S.; Hanssen, K.H.; Jacobson, S.; Kukkola, M.; Luiro, J.; Saarsalmi, A.; Tamminen, P.; Tveite, B. Logging residue removal after thinning in Nordic boreal forests: Long-term impact on tree growth. For. Ecol. Manag. 2011, 261, 1919–1927. [Google Scholar] [CrossRef]
- Kaarakka, L.; Tamminen; Saarsalmi, A.; Kukkola, M.; Helmisaari, H.S.; Burton, A.J. Effects of repeated whole-tree harvesting on soil properties and tree growth in a nor-way spruce (Picea abies (L.) Karst.) stand. For. Ecol. Manag. 2014, 313, 180–187. [Google Scholar] [CrossRef]
- Wang, S.M.; Fan, S.H.; Xiao, X.; Zheng, Y.X.; Zhou, Y.; Guan, F.Y. Effects of strip cutting on aboveground biomass accumulation and allocation, and allometric growth of Phyllostachys edulis. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2021, 45, 19–24. [Google Scholar]
- Zhang, M.C.; Guan, F.Y.; Yan, Y.J.; Zhang, M.M.; Zheng, Y.X. Effects of strip harvesting on species diversity of undergrowth in bamboo (Phyllostachys Edulis) forest. Chin. J. Ecol. 2020, 40, 4169–4179. [Google Scholar]
- Deng, S.J.; Gu, Y. Effects of different dosages of nitrogen, phosphorus and potassium fertilisers on rice yield and fertiliser utilisation. Shanghai Agric. Sci. Technol. 2022, 3, 98–100. [Google Scholar]
- Li, L.; Li, N.; Lu, D.; Chen, Y. Mapping Moso bamboo forest and its on-year and off-year distribution in a subtropical region using time-series Sentinel-2 and Landsat 8 data. Remote Sens. Environ. 2019, 231, 111265. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Fan, L.L.; Xu, W.D.; Rong, J.D.; Zheng, Y.S. Restoration characteristics and soil nutrient content of Phyllostachys edulis forests afters trip clear cutting. Acta Bot. Boreali Occident. Sin. 2020, 40, 1407–1413. [Google Scholar]
- Guo, B.H.; Liu, G.L.; Fan, S.H.; Du, M.Y.; Su, W.H. Distribution patterns and stoichiometry characteristics of C, N, P in Phyllostachys edulis forests of different productivity levels. Sci. Silvae Sin. 2014, 50, 1–9. [Google Scholar]
- Xiao, D.; Wang, X.J.; Zhang, K.; He, N.P.; Hou, J.H. Effects of nitrogen addition on leaf traits of major plants in a natural oil pine forest in Taiyue Mountain, Shanxi. Chin. J. Plant Ecol. 2016, 40, 686–701. [Google Scholar]
- Li, D.J.; Mo, J.N.; Fang, Y.T.; Li, Z.A. Effects of simulated nitrogen deposition on seedling biomass and its partitioning of two species of trees in the southern subtropics. Chin. J. Plant Ecol. 2005, 29, 543–549. [Google Scholar]
- Aber, J.; Mcdowell, W.; Nadelhiffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; Fernandez, I. Nitrogen saturation in temperate forest ecosystems: Hypotheses revisited. BioScience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Gordon, W.S.; Jackson, R.B. Nutrient concentrations in fine roots. Ecology 2000, 81, 275–280. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F. The world-wide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Wright, I.J. Leaf phosphorus influences the photosynthesis–nitrogen relation: A cross-biome analysis of 314 species. Oecologia 2009, 160, 207–212. [Google Scholar] [CrossRef]
- He, B.; Li, Q.; Li, W.J.; Zou, S.; Bai, X.L.; Xue, X.H. Ecological stoichiometry and reabsorption characteristics of nitrogen and phosphorus in Pinus armandii needles at different forest ages. Jiangsu Agric. Sci. 2023, 51, 137–144. [Google Scholar]
- Chen, Y.; Han, W.; Tang, L.; Tang, Z.; Fang, J. Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography 2011, 36, 178–184. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.; Ma, S.; Ding, Y.; Luo, Y.; Chen, Y.; Du, E.; Han, W.; Kovacs, E.D.; Shen, H.; et al. Family-level leaf nitrogen and phosphorus stoichiometry of global terrestrial plants. Sci. China Life Sci. 2019, 62, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Explanation of the principle and application of isotope tracer technology. Biol. Teach. 2018, 43, 79–80. [Google Scholar]
- Gu, J.T.; Wang, F.; Xu, Z.H.; Xu, J. Nitrogen utilisation of summer maize under different water and fertilisation conditions based on 15N tracer technology. Water Sav. Irrig. 2021, 11, 83–96. [Google Scholar]
- Wu, Y.L.; Zhou, L.L.; Zou, Z.G.; Zhang, M.X.; Lin, Y.; Li, S.B. 15N tracer technique was used to study the absorption, distribution and utilization of inorganic nitrogen in Chinese fir seedlings. J. Northwest For. Coll. 2023, 38, 28–33. [Google Scholar]
- Zhang, Y.Y.; Deng, Z.W.; Rong, J.D.; Chen, L.G.; Zheng, Y.S. Research progressin fertilization of Phyllostachyse dulis forest. World Bamboo Ratt. 2019, 17, 58–62. [Google Scholar]
- Liu, J.; Liu, G.Q.; Yang, Y.Z.; Ai, Y.; Zong, Q.Y.; Hao, B.B. Soil fertility evaluation in Sabina vulgaris ant. Community in the southern edge of the Muussandland. Chin. J. Soil Sci. 2021, 52, 129–138. [Google Scholar]
- Li, S.K. Responses of leaf nonstructural carbon and aboveground biomass allocation to fertilization in moso bamboo. Bamboo J. 2023, 42, 33–39. [Google Scholar]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.H.; Hong, Z.; Lei, X.H.; Wang, J.F.; Yan, D.L. Effect of nitrogen addition on C, N and P nutrient content and non-structural carbohydrate content of long sequential elm. Sci. Silvae Sin. 2020, 56, 186–192. [Google Scholar]
- Liu, Q.R. Research on Nitrogen Uptake Transformation and Utilisation in Moso Bamboo Forests. Master’s Thesis, China Academy of Forestry Sciences, Beijing, China, 2016. [Google Scholar]
- Mao, C. Study on Nitrogen Allocation and Utilisation in Moso Bamboo Forests. Master’s Thesis, China Academy of Forestry Sciences, Beijing, China, 2015. [Google Scholar]
- Wang, D.; Xu, C.; Yan, J.; Zhang, X.; Chen, S.; Chauhan, B.S.; Wang, L.; Zhang, X. 15 N tracer-based analysis of genotypic differences in the uptake and partitioning of N applied at different growth stages in transplanted rice. Field Crop. Res. 2017, 211, 27–36. [Google Scholar] [CrossRef]
- Wang, C.B.; Zheng, Y.M.; Shen, P.; Zheng, Y.P.; Wu, Z.F.; Sun, X.W.; Feng, H. Determining N supplied sources and N use efficiency for peanut under applications of four forms of N fertilizers labeled by iso-tope 15N. Integr Agric. 2016, 15, 432–439. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Fan, S.H.; Zhang, X.; Zhou, X.; Guan, F.Y. Patterns of change in productivity of strip logging moso bamboo forests. Sci.-Entia Silvae Sin. 2023, 59, 22–29. [Google Scholar]
- Zhang, Y.Y.; Fan, L.L.; Wang, M.; He, T.Y.; Rong, J.D. Effects of strip logging on soil physical and chemical properties and enzyme activities of moso bamboo forest. J. For. Environ. 2020, 40, 234–242. [Google Scholar]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.M.; Liu, S.R.; Wang, Y.; Nuan, J.W.; Cai, C.J. Effects of through fall reduction and nitrogen addition on stoichiometry of leaf and fine root in Phyllostachys edulis forests. Acta Ecol. Sin. 2021, 41, 1440–1450. [Google Scholar]
- Cai, X.; Wu, J.; Li, B.J.; Li, S.K.; Rong, J.D.; Chen, L.G.; Zheng, Y.S. Effects of bamboo density on rhizome characteristics and root activity of bamboo shoots under long-term mother bamboo retention mode. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2023, 52, 500–504. [Google Scholar]
- Su, W.H.; Zeng, X.L.; Fan, S.H.; Ni, H.J. Effects of strip clear-cutting on the allocation of non-structural carbohydrates and aboveground biomass of Phyllostachys edulis. Chin. J. Ecol. 2019, 38, 2934–2940. [Google Scholar]
- Wang, S.M.; Fan, S.H.; Guan, F.Y.; Xiao, X.; Zhang, M.M. Short-term influence of strip cutting on soil physical and chemical proper ties and dominant flora in Phyllostachys edulis stands. J. Northeast For. Univ. 2022, 50, 46–51. [Google Scholar]
- Song, Y.X.; Deng, J.F.; Li, J.H.; Wei, Y.W.; Chen, T.Y. Soil chemical properties and their relations with stand growth of typical afforestation tree species in northwest Liaoning Province. J. Northwest For. Univ. 2017, 32, 18–24. [Google Scholar]
- Fang, X.; Chen, C. Research progress on the effect of vegetation restoration on the transformation of soil N and P ac-cumulation and their coupling relationship. J. Cent. South For. Univ. Sci. Technol. 2022, 42, 84–97. [Google Scholar]
- Su, X.F.; Zheng, X.; Ma, L.; Ding, Y.L.; Liu, G.H. Relationship between productivity of moso bamboo and soil nutrients. J. Fujian Univ. Agric. For. (Nat. Sci. Ed.) 2020, 49, 192–198. [Google Scholar]
- Wang, S.M. Study on the Response Characteristics of Underground Rhizome Root System and Ground Bamboo Formation in Strip-Cutting Moso Bamboo Forest. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2021. [Google Scholar]
- Su, T.; Liang, K.; Wang, Z.G.; Zu, X.L.; Meng, S. Comparative study on leaf physiological characteristics and productivity of different bandwidths of “band coppice” light bamboo forests. J. Jiangxi Agric. Univ. 2023, 45, 1096–1105. [Google Scholar]


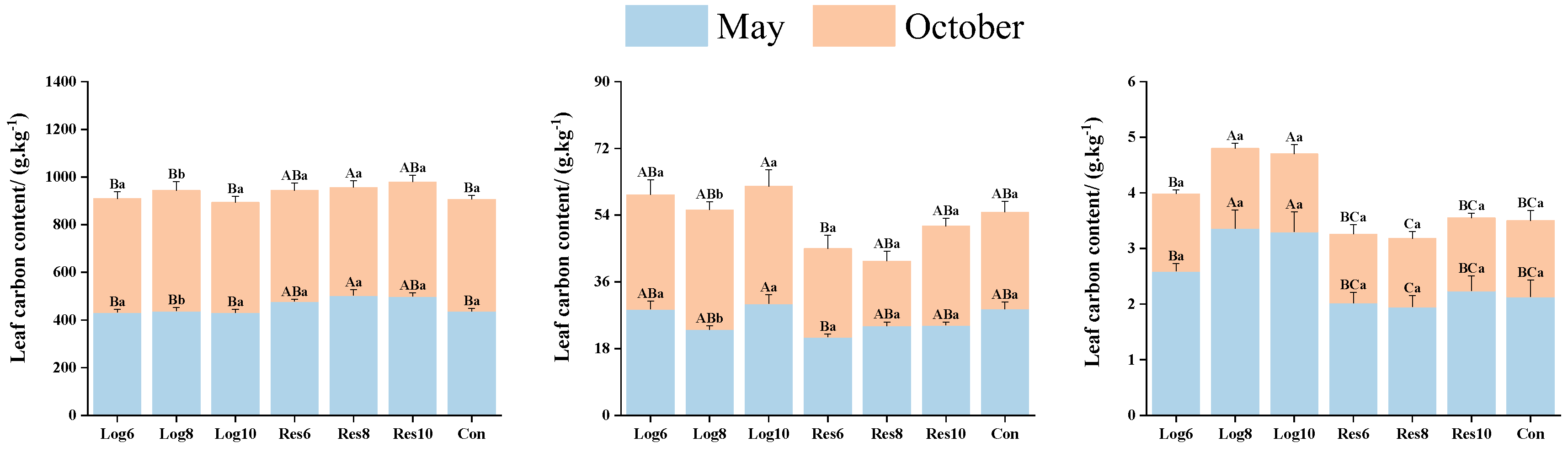
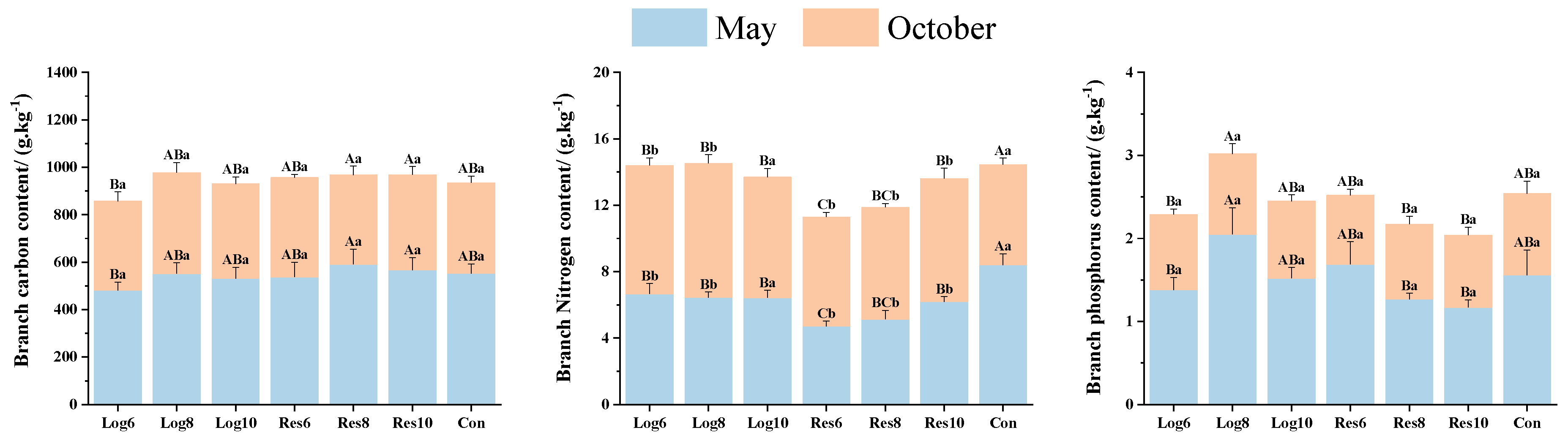
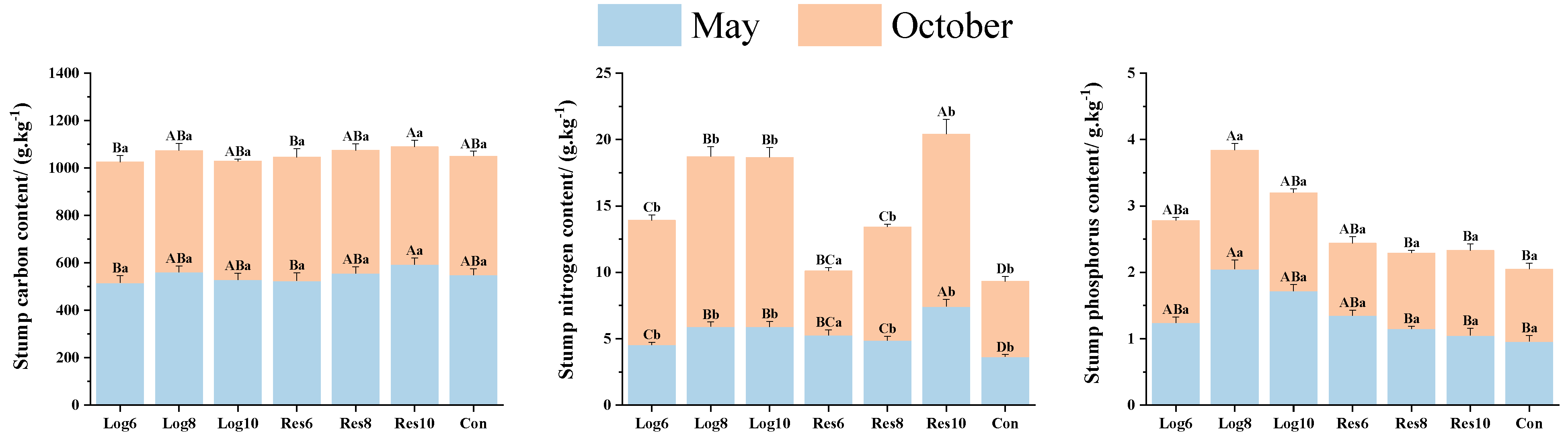

| Stoichiometric Characteristics | Period | Log6 | Log8 | Log10 | Res6 | Res8 | Res10 | Con |
|---|---|---|---|---|---|---|---|---|
| C/N | May | 15.18 ± 1.84 Ba | 18.87 ± 2.80 ABa | 14.34 ± 0.97 Ba | 22.93 ± 3.36 Aa | 20.83 ± 0.89 Ab | 20.54 ± 1.6 Aa | 15.22 ± 1.06 Ba |
| October | 15.42 ± 1.31 Ba | 15.99 ± 0.26 Ba | 14.32 ± 0.09 Ba | 19.5 ± 1.31 ABa | 26.35 ± 4.61 Aa | 18.32 ± 3.51 Ba | 18.01 ± 0.53 Ba | |
| N/P | May | 11.27 ± 2.44 ABb | 8.32 ± 3.74 Bb | 11.17 ± 6.92 ABb | 10.49 ± 1.34 ABb | 13.13 ± 3.52 Aa | 11.74 ± 3.61 ABb | 13.66 ± 1.79 Ab |
| October | 22.29 ± 0.20 Aa | 22.06 ± 0.64 Aa | 23.00 ± 0.41 Aa | 19.18 ± 0.37 ABa | 14.23 ± 2.16 Ba | 20.34 ± 3.04 Aa | 19.04 ± 0.77 ABa |
| Stoichiometric Characteristics | Period | Log6 | Log8 | Log10 | Res6 | Res8 | Res10 | Con |
|---|---|---|---|---|---|---|---|---|
| C/N | May | 72.31 ± 8.74 BCa | 85.57 ± 2.17 Ba | 82.8 ± 6.53 Ba | 113.71 ± 5.96 Aa | 115.68 ± 16.45 Aa | 91.69 ± 4.07 Aab | 65.88 ± 5.62 Ca |
| October | 48.46 ± 1.84 Bb | 58.81 ± 4.02 ABb | 49.36 ± 1.33 Bb | 63.78 ± 3.02 Ab | 56.01 ± 0.64 ABb | 54.12 ± 2.76 ABb | 63.03 ± 2.61 Aa | |
| N/P | May | 4.92 ± 0.61 Bb | 3.23 ± 0.60 BCb | 4.25 ± 0.37 Bb | 2.81 ± 0.14 Cb | 4.10 ± 0.72 Bb | 5.36 ± 0.82 ABb | 6.61 ± 2.97 Aa |
| October | 8.51 ± 0.22 Aa | 7.49 ± 0.31 ABa | 8.70 ± 0.05 Aa | 7.92 ± 0.40 ABa | 7.49 ± 0.11 ABa | 8.51 ± 0.26 Aa | 6.20 ± 0.28 Ba |
| Stoichiometric Characteristics | Period | Log6 | Log8 | Log10 | Res6 | Res8 | Res10 | Con |
|---|---|---|---|---|---|---|---|---|
| C/N | May | 132.52 ± 15.36 Ba | 160.52 ± 2.99 ABa | 109.44 ± 6.51 Ca | 174.99 ± 15.73 Ab | 187.70 ± 41.11 Aa | 165.84 ± 11.55 ABa | 118.48 ± 3.83 BCb |
| October | 137.98 ± 7.64 BCa | 153.8 ± 14.06 Ba | 116.18 ± 0.99 Ca | 228.84 ± 16.04 Aa | 170.19 ± 54.88 Ba | 114.36 ± 3.54 Cb | 183.97 ± 14.98 Ba | |
| N/P | May | 2.63 ± 0.20 Cb | 2.63 ± 1.21 Cb | 5.12 ± 1.10 Aa | 2.93 ± 0.51 Ca | 3.00 ± 0.35 Cb | 4.06 ± 1.39 Bb | 4.68 ± 1.32 ABa |
| October | 4.19 ± 0.15 Ba | 3.79 ± 0.6 BCa | 5.18 ± 0.12 Aa | 3.50 ± 0.30 Ca | 4.16 ± 0.86 Ba | 5.50 ± 0.24 Aa | 3.64 ± 0.14 BCb |
| Stoichiometric Characteristics | Period | Log6 | Log8 | Log10 | Res6 | Res8 | Res10 | Con |
|---|---|---|---|---|---|---|---|---|
| C/N | May | 113.44 ± 1.02 Ba | 94.85 ± 3.98 BCa | 89.69 ± 5.19 Ca | 99.49 ± 5.68 BCa | 114.35 ± 6.76 Ba | 79.73 ± 5.51 Ca | 150.45 ± 1.53 Aa |
| October | 39.73 ± 2.44 Cb | 54.72 ± 2.08 Bb | 39.13 ± 2.15 Cb | 97.91 ± 4.61 Aa | 60.61 ± 1.64 Bb | 38.31 ± 1.12 Cb | 87.97 ± 6.13 Ab | |
| N/P | May | 3.68 ± 0.30 BCa | 2.90 ± 0.24 Cb | 3.44 ± 0.14 BCb | 3.94 ± 0.42 Ba | 4.29 ± 0.61 Bb | 7.16 ± 0.74 Ab | 3.82 ± 0.32 BCb |
| October | 4.29 ± 0.19 Ca | 5.24 ± 0.08 Ca | 8.65 ± 0.10 Ba | 4.43 ± 0.08 Ca | 7.53 ± 0.03 Ba | 10.15 ± 0.08 Aa | 5.22 ± 0.13 Ca |
| Stoichiometric Characteristics | Period | Log6 | Log8 | Log10 | Res6 | Res8 | Res10 | Con |
|---|---|---|---|---|---|---|---|---|
| C/N | May | 120.17 ± 6.71 Aa | 95.91 ± 3.99 Ba | 79.86 ± 5.40 BCa | 103.45 ± 16.19 Ba | 71.75 ± 0.47 Ca | 80.91 ± 5.40 BCa | 58.47 ± 3.43 Da |
| October | 42.79 ± 6.23 Bb | 33.93 ± 4.06 Cb | 43.32 ± 3.59 Bb | 58.30 ± 6.94 Ab | 59.75 ± 3.89 Ab | 43.44 ± 6.48 Bb | 56.70 ± 3.40 Aa | |
| N/P | May | 5.80 ± 0.17 Ba | 4.29 ± 0.05 Cb | 5.52 ± 0.61 Bb | 5.09 ± 0.43 BCa | 6.54 ± 0.10 Aa | 6.48 ± 0.55 Aa | 6.80 ± 0.30 Aa |
| October | 6.71 ± 0.09 Ba | 8.06 ± 0.05 Aa | 6.87 ± 0.33 Ba | 5.46 ± 0.2 Ca | 4.80 ± 0.32 Db | 5.72 ± 0.07 Ca | 4.69 ± 0.99 Db |
| Parameters | Principal Components | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Leaf Ndff% | 0.781 | −0.483 | −0.135 | −0.266 | −0.03 |
| Branch Ndff% | 0.702 | −0.559 | 0.29 | −0.262 | 0.19 |
| Culm Ndff% | 0.919 | −0.037 | −0.292 | −0.005 | 0.263 |
| Stump Ndff% | 0.744 | −0.206 | 0.578 | 0.145 | 0.017 |
| Rhizome Ndff% | 0.813 | −0.236 | 0.262 | 0.224 | −0.183 |
| Leaf 15NUE% | 0.622 | 0.504 | −0.174 | −0.496 | −0.018 |
| Branch 15NUE% | 0.415 | 0.711 | 0.372 | −0.297 | 0.301 |
| Culm 15NUE% | 0.321 | 0.685 | −0.642 | 0.072 | 0.097 |
| Stump 15NUE% | 0.537 | 0.633 | 0.182 | 0.513 | −0.041 |
| Rhizome 15NUE% | 0.805 | 0.516 | 0.142 | −0.035 | −0.105 |
| Leaf C contents | 0.804 | −0.322 | −0.123 | 0.417 | 0.146 |
| Leaf N contents | −0.561 | 0.813 | −0.051 | −0.075 | 0.087 |
| Leaf P contents | −0.155 | 0.9 | 0.284 | 0.104 | −0.262 |
| Branch C contents | 0.865 | −0.079 | −0.107 | −0.056 | −0.473 |
| Branch N contents | −0.132 | 0.859 | −0.084 | −0.209 | 0.433 |
| Branch P contents | 0.182 | 0.464 | 0.648 | −0.494 | −0.237 |
| Culm C contents | 0.954 | −0.119 | −0.236 | 0.006 | 0.107 |
| Culm N contents | 0.036 | 0.899 | −0.365 | 0.134 | 0.194 |
| Culm P contents | 0.145 | 0.595 | 0.663 | 0.108 | 0.418 |
| Stump C contents | 0.95 | −0.029 | −0.231 | 0.184 | 0.09 |
| Stump N contents | 0.251 | 0.667 | −0.213 | 0.639 | −0.148 |
| Stump P contents | 0.049 | 0.727 | 0.566 | 0.226 | −0.283 |
| Rhizome C contents | 0.676 | −0.471 | 0.425 | 0.077 | 0.273 |
| Rhizome N contents | 0.518 | 0.761 | −0.268 | −0.188 | −0.123 |
| Rhizome P contents | 0.685 | 0.379 | −0.306 | −0.452 | −0.262 |
| Eigenvalue | 9.639 | 8.169 | 3.154 | 2.066 | 1.311 |
| Contribution rate/% | 38.557 | 32.678 | 12.618 | 8.264 | 5.242 |
| Accumulative contribution/% | 38.557 | 71.234 | 83.852 | 92.116 | 97.358 |
| Treatments | Comprehensive Scores | Comprehensive Rank |
|---|---|---|
| Log6 | −3.93 | 7 |
| Res6 | −3.67 | 6 |
| Log8 | 7.24 | 1 |
| Res8 | 0.37 | 3 |
| Log10 | −1.78 | 5 |
| Res10 | 3.13 | 2 |
| Con | −1.36 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Xu, L.; Chen, W.; Pan, Y.; He, T.; Chen, L.; Rong, J.; Zheng, Y. Variation in Nitrogen Utilization and Nutrient Composition across Various Organs under Different Strip Logging Management Models in Moso Bamboo (Phyllostachys edulis) Forest. Plants 2024, 13, 1448. https://doi.org/10.3390/plants13111448
Li B, Xu L, Chen W, Pan Y, He T, Chen L, Rong J, Zheng Y. Variation in Nitrogen Utilization and Nutrient Composition across Various Organs under Different Strip Logging Management Models in Moso Bamboo (Phyllostachys edulis) Forest. Plants. 2024; 13(11):1448. https://doi.org/10.3390/plants13111448
Chicago/Turabian StyleLi, Bingjun, Linzheng Xu, Wenchen Chen, Yanmei Pan, Tianyou He, Liguang Chen, Jundong Rong, and Yushan Zheng. 2024. "Variation in Nitrogen Utilization and Nutrient Composition across Various Organs under Different Strip Logging Management Models in Moso Bamboo (Phyllostachys edulis) Forest" Plants 13, no. 11: 1448. https://doi.org/10.3390/plants13111448
APA StyleLi, B., Xu, L., Chen, W., Pan, Y., He, T., Chen, L., Rong, J., & Zheng, Y. (2024). Variation in Nitrogen Utilization and Nutrient Composition across Various Organs under Different Strip Logging Management Models in Moso Bamboo (Phyllostachys edulis) Forest. Plants, 13(11), 1448. https://doi.org/10.3390/plants13111448






