Inhibitory Effects on Staphylococcus aureus Sortase A by Aesculus sp. Extracts and Their Toxicity Evaluation
Abstract
1. Introduction
2. Results
2.1. Extracts Preparation
2.2. Quantitative Determination of the Phenolic Compounds
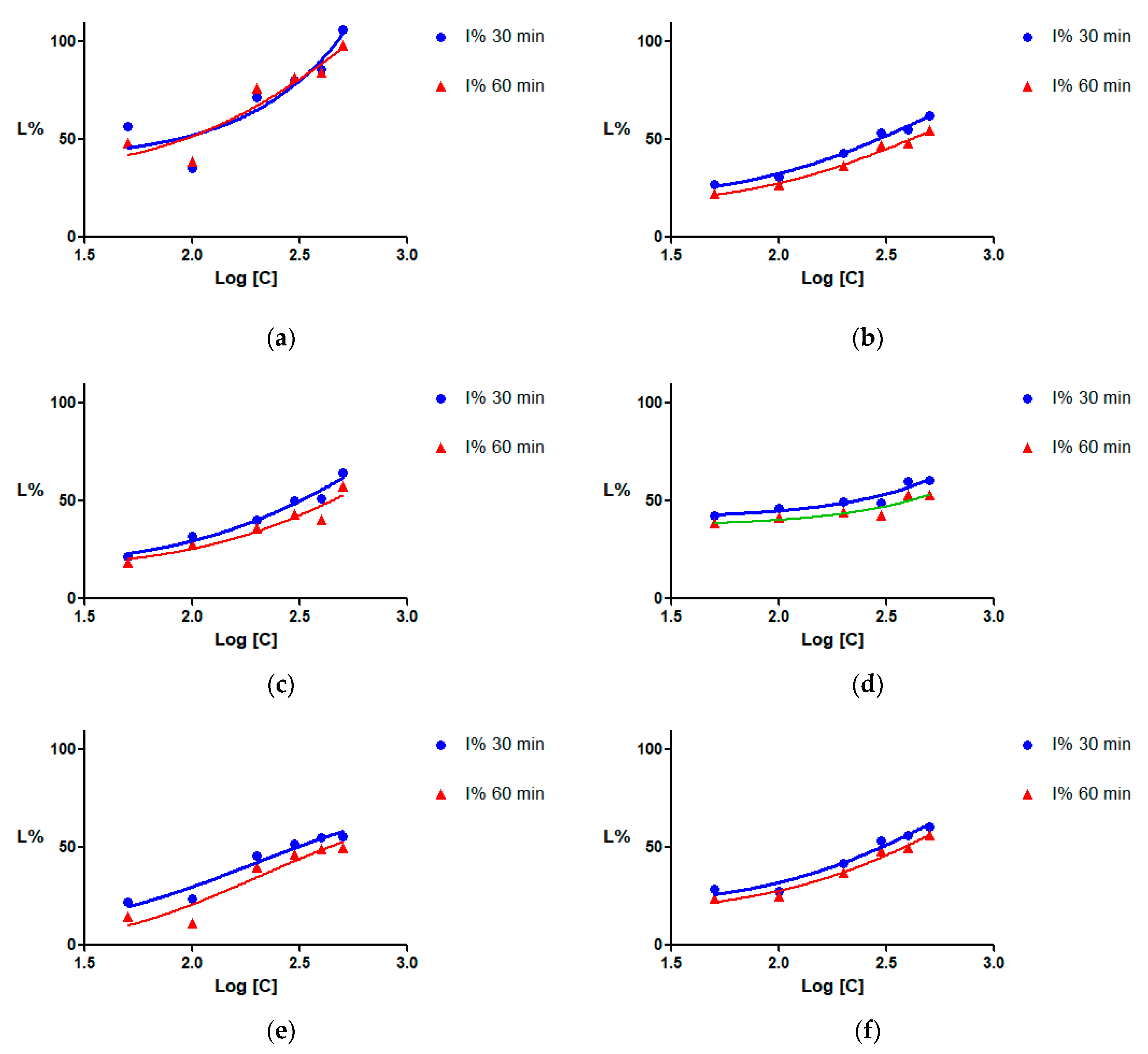
2.3. Sortase Inhibition
2.4. UHPLC-HRMS Analysis
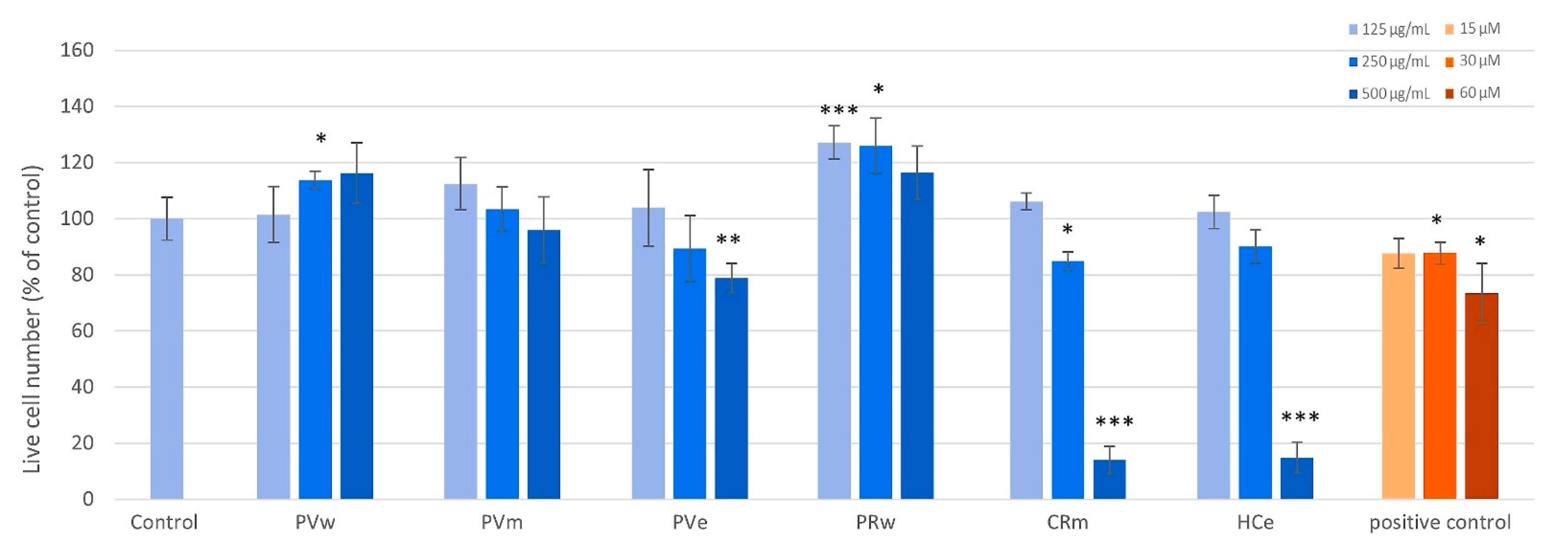
2.5. Screening on Human Fibroblasts
2.6. Acute Toxicity Assessment Using Daphnia magna
3. Discussion
4. Materials and Methods
4.1. Preparation of the Extracts
4.2. Quantitative Determination of the Phenolic Compounds
4.3. UHPLC-HRMS Analysis
4.4. Inhibition of Sortase A
4.5. Screening on Human Fibroblasts
4.6. Acute Toxicity Assessment Using Daphnia magna
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Nina, P.B.; JP, D.; Kumar, S.; Singh, B.; Tiwari, R.R. Futuristic Non-Antibiotic Therapies to Combat Antibiotic Resistance: A Review. Front. Microbiol. 2021, 12, 609459. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, G. Strategies to Overcome Antimicrobial Resistance (AMR) Making Use of Non-Essential Target Inhibitors: A Review. Int. J. Mol. Sci. 2019, 20, 5844. [Google Scholar] [CrossRef] [PubMed]
- Roncarati, D.; Scarlato, V.; Vannini, A. Targeting of Regulators as a Promising Approach in the Search for Novel Antimicrobial Agents. Microorganisms 2022, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Dehbanipour, R.; Ghalavand, Z. Anti-Virulence Therapeutic Strategies against Bacterial Infections: Recent Advances. Germs 2022, 12, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Calvert, M.B.; Jumde, V.R.; Titz, A. Pathoblockers or Antivirulence Drugs as a New Option for the Treatment of Bacterial Infections. Beilstein J. Org. Chem. 2018, 14, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Martínez, O.F.; Cardoso, M.H.; Ribeiro, S.M.; Franco, O.L. Recent Advances in Anti-Virulence Therapeutic Strategies with a Focus on Dismantling Bacterial Membrane Microdomains, Toxin Neutralization, Quorum-Sensing Interference and Biofilm Inhibition. Front. Cell. Infect. Microbiol. 2019, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U.; Piddock, L.J.V. Non-Traditional Antibacterial Therapeutic Options and Challenges. Cell Host Microbe 2019, 26, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Fernandez Lynch, H.; Cohen, I.G.; Darrow, J.J.; Outterson, K. Designing Development Programs for Non-Traditional Antibacterial Agents. Nat. Commun. 2019, 10, 3416. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.; Koch, G. Exploring Functional Membrane Microdomains in Bacteria: An Overview. Curr. Opin. Microbiol. 2017, 36, 76–84. [Google Scholar] [CrossRef]
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different Drugs for Bad Bugs: Antivirulence Strategies in the Age of Antibiotic Resistance. Nat. Rev. Drug Discov. 2017, 16, 457–471. [Google Scholar] [CrossRef]
- Suree, N.; Liew, C.K.; Villareal, V.A.; Thieu, W.; Fadeev, E.A.; Clemens, J.J.; Jung, M.E.; Clubb, R.T. The Structure of the Staphylococcus Aureus Sortase-Substrate Complex Reveals How the Universally Conserved LPXTG Sorting Signal Is Recognized. J. Biol. Chem. 2009, 284, 24465–24477. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cai, S.; Gu, G.; Guo, Z.; Long, Z. Recent Progress in the Development of Sortase A Inhibitors as Novel Anti-Bacterial Virulence Agents. RSC Adv. 2015, 5, 49880–49889. [Google Scholar] [CrossRef]
- Nitulescu, G.; Margina, D.; Zanfirescu, A.; Olaru, O.T.; Nitulescu, G.M. Targeting Bacterial Sortases in Search of Anti-Virulence Therapies with Low Risk of Resistance Development. Pharmaceuticals 2021, 14, 415. [Google Scholar] [CrossRef]
- Alharthi, S.; Alavi, S.E.; Moyle, P.M.; Ziora, Z.M. Sortase A (SrtA) Inhibitors as an Alternative Treatment for Superbug Infections. Drug Discov. Today 2021, 26, 2164–2172. [Google Scholar] [CrossRef]
- Cascioferro, S.; Totsika, M.; Schillaci, D. Sortase A: An Ideal Target for Anti-Virulence Drug Development. Microb. Pathog. 2014, 77, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bi, C.; Cai, H.; Liu, B.; Zhong, X.; Deng, X.; Wang, T.; Xiang, H.; Niu, X.; Wang, D. The Therapeutic Effect of Chlorogenic Acid against Staphylococcus Aureus Infection through Sortase A Inhibition. Front. Microbiol. 2015, 6, 1031. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Liu, X.Y.; Bai, J.R.; Xie, H.C.; Ye, S.L.; Zhong, K.; Huang, Y.N.; Gao, H. Inhibitory Effect of a Natural Phenolic Compound, 3-: P-Trans -Coumaroyl-2-Hydroxyquinic Acid against the Attachment Phase of Biofilm Formation of Staphylococcus Aureus through Targeting Sortase A. RSC Adv. 2019, 9, 32453–32461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Lian, X.-Y. An Overview of Genus aesculus L.: Ethnobotany, Phytochemistry, and Pharmacological Activities. Pharm. Crop. 2014, 1, 24–51. [Google Scholar] [CrossRef]
- Dridi, A.; Reis, F.S.; Pires, T.C.S.P.; Calhelha, R.C.; Pereira, C.; Zaghdoudi, K.; Ferreira, I.C.F.R.; Barros, L.; Barreira, J.C.M. Aesculus hippocastanum L.: A Simple Ornamental Plant or a Source of Compelling Molecules for Industry? Separations 2023, 10, 160. [Google Scholar] [CrossRef]
- Gözcü, S. Aesculus hippocastanum L. In Novel Drug Targets with Traditional Herbal Medicines: Scientific and Clinical Evidence; Springer: Cham, Switzerland, 2022; ISBN 9783031077531. [Google Scholar]
- Margină, D.; Olaru, O.T.; Ilie, M.; Gradinaru, D.; Gutu, C.; Voicu, S.; Dinischiotu, A.; Spandidos, D.A.; Tsatsakis, A.M. Assessment of the Potential Health Benefits of Certain Total Extracts from Vitis Vinifera, Aesculus Hyppocastanum and Curcuma Longa. Exp. Ther. Med. 2015, 10, 1681–1688. [Google Scholar] [CrossRef]
- Mihai, D.P.; Seremet, O.C.; Nitulescu, G.; Ivopol, M.; Sevastre, A.S.; Negres, S.; Ivopol, G.; Nitulescu, G.M.; Olaru, O.T. Evaluation of Natural Extracts in Animal Models of Pain and Inflammation for a Potential Therapy of Hemorrhoidal Disease. Sci. Pharm. 2019, 87, 14. [Google Scholar] [CrossRef]
- Vasile, D.; Iancu, G.; Iancu, R.C.; Davitoiu, D.V. Main Characteristics for Materials Used as Synthetic Surgical Meshes. Mater. Plast. 2017, 54, 229. [Google Scholar] [CrossRef]
- Wei, F.; Ma, L.Y.; Jin, W.T.; Ma, S.C.; Han, G.Z.; Khan, I.A.; Lin, R.C. Antiinflammatory Triterpenoid Saponins from the Seeds of Aesculus Chinensis. Chem. Pharm. Bull. 2004, 52, 1246–1248. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Ogawa, S.; Jisaka, M.; Kimura, Y.; Katsube, T.; Yokota, K. Identification of Novel Saponins from Edible Seeds of Japanese Horse Chestnut (Aesculus Turbinata Blume) after Treatment with Wooden Ashes and Their Nutraceutical Activity. J. Pharm. Biomed. Anal. 2006, 41, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.N.; Zhu, X.M.; Han, L.K.; Saito, M.; Sun, Y.S.; Yoshikawa, M.; Kimura, Y.; Zheng, Y.N. Anti-Obesity Effects of Escins Extracted from the Seeds of Aesculus Turbinata Blume (Hippocastanaceae). Chem. Pharm. Bull. 2008, 56, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Küp, F.Ö.; Çoşkunçay, S.; Duman, F. Biosynthesis of Silver Nanoparticles Using Leaf Extract of Aesculus Hippocastanum (Horse Chestnut): Evaluation of Their Antibacterial, Antioxidant and Drug Release System Activities. Mater. Sci. Eng. C 2020, 107, 110207. [Google Scholar] [CrossRef] [PubMed]
- Paterska, M.; Bandurska, H.; Wysłouch, J.; Molińska-Glura, M.; Moliński, K. Chemical Composition of Horse-Chestnut (Aesculus) Leaves and Their Susceptibility to Chestnut Leaf Miner Cameraria Ohridella Deschka & Dimić. Acta Physiol. Plant. 2017, 39, 105. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Li, G.; Lou, J.; Chen, X.; He, Y.; Peng, W.X. Pyrolysis of Aesculus Chinensis Bunge Leaves as for Extracted Bio-Oil Material. Polymers 2022, 14, 5003. [Google Scholar] [CrossRef] [PubMed]
- Hanaka, A.; Dresler, S.; Mułenko, W.; Wójciak, M.; Sowa, I.; Sawic, M.; Stanisławek, K.; Strzemski, M. Phenolic-Based Discrimination between Non-Symptomatic and Symptomatic Leaves of Aesculus Hippocastanum Infested by Cameraria Ohridella and Erysiphe Flexuosa. Int. J. Mol. Sci. 2023, 24, 14071. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kolniak-Ostek, J.; Biernat, A. The Content of Phenolic Compounds in Leaf Tissues of Aesculus Glabra and Aesculus Parviflora Walt. Molecules 2015, 20, 2176–2189. [Google Scholar] [CrossRef]
- Dahash, S.L.; Abass, O.K.; Abdul-Razaq, M.M.; Al-Kuraishy, H.M.; Al-Gareeb, A.I. Aesculus Hippocastanum-Derived Extract β-Aescin and In Vitro Antibacterial Activity. J. Microsc. Ultrastruct. 2021, 9, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.; Mishra, A.; Khushtar, M. Phytochemical, Ethanomedicinal and Pharmacological Applications of Escin from Aesculus hippocastanum L. towards Future Medicine. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190115. [Google Scholar] [CrossRef] [PubMed]
- Ćalić-Dragosavac, D.; Zdravković-Korać, S.; Šavikin-Fodulović, K.; Radojevič, L.; Vinterhalter, B. Determination of Escin Content in Androgenic Embryos and Hairy Root Culture of Aesculus Hippocastanum. Pharm. Biol. 2010, 48, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luan, Y.; Hou, J.; Jiang, T.; Zhao, Y.; Song, W.; Wang, L.; Kong, X.; Guan, J.; Song, D.; et al. The Protection Effect of Rhodionin against Methicillin-Resistant Staphylococcus Aureus-Induced Pneumonia through Sortase A Inhibition. World J. Microbiol. Biotechnol. 2023, 39, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jing, S.; Qu, H.; Wang, K.; Jin, Y.; Ding, Y.; Yang, L.; Yu, H.; Shi, Y.; Li, Q.; et al. Orientin Mediates Protection against MRSA-Induced Pneumonia by Inhibiting Sortase A. Virulence 2021, 12, 2149–2161. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, F.; Bi, C.; Wang, L.; Zhong, X.; Cai, H.; Deng, X.; Niu, X.; Wang, D. Quercitrin, an Inhibitor of Sortase A, Interferes with the Adhesion of Staphylococcal Aureus. Molecules 2015, 20, 6533–6543. [Google Scholar] [CrossRef] [PubMed]
- Evangelina, I.A.; Herdiyati, Y.; Laviana, A.; Rikmasari, R.; Zubaedah, C.; Anisah; Kurnia, D. Bio-Mechanism Inhibitory Prediction of β-Sitosterol from Kemangi (Ocimum basilicum L.) as an Inhibitor of MurA Enzyme of Oral Bacteria: In Vitro and in Silico Study. Adv. Appl. Bioinform. Chem. 2021, 14, 103–115. [Google Scholar] [CrossRef]
- Park, J.S.; Chung, B.; Lee, W.H.; Lee, J.; Suh, Y.; Oh, D.C.; Oh, K.B.; Shin, J. Sortase A-Inhibitory Coumarins from the Folk Medicinal Plant Poncirus Trifoliata. J. Nat. Prod. 2020, 83, 3004–3011. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Kim, J.G.; Kim, M.R.; Lee, S.E.; Takeoka, G.R.; Oh, K.B.; Kim, J.H. Curcuma longa L. Constituents Inhibit Sortase A and Staphylococcus Aureus Cell Adhesion to Fibronectin. J. Agric. Food Chem. 2005, 53, 9005–9009. [Google Scholar] [CrossRef]
- Kang, S.S.; Kim, J.-G.; Lee, T.-H.; Oh, K.-B. Flavonols Inhibit Sortases and Sortase-Mediated Staphylococcus aureus Clumping to Fibrinogen. Biol. Pharm. Bull. 2006, 29, 1751–1755. [Google Scholar] [CrossRef]
- Huang, P.; Hu, P.; Zhou, S.; Li, Q.; Chen, W. Morin Inhibits Sortase A and Subsequent Biofilm Formation in Streptococcus Mutans. Curr. Microbiol. 2014, 68, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Lawag, I.L.; Nolden, E.S.; Schaper, A.A.M.; Lim, L.Y.; Locher, C. A Modified Folin-Ciocalteu Assay for the Determination of Total Phenolics Content in Honey. Appl. Sci. 2023, 13, 2135. [Google Scholar] [CrossRef]
- Dias, M.I.; Albiston, C.; Añibarro-Ortega, M.; Ferreira, I.C.F.R.; Pinela, J.; Barros, L. Sonoextraction of Phenolic Compounds and Saponins from Aesculus Hippocastanum Seed Kernels: Modeling and Optimization. Ind. Crops Prod. 2022, 185, 115142. [Google Scholar] [CrossRef]
- Yadav, N.; Singh, A.P.; Rana, A.C.; Kumar, S.; Kaur, P.; Singh, J.; Jangra, A.; Kumar, D. Aesculus Indica: An Updated Review on Its Pharmacognosy, Phytochemistry and Pharmacological Profile. Egypt. J. Basic Appl. Sci. 2022, 9, 125–135. [Google Scholar] [CrossRef]
- Curir, P.; Galeotti, F.; Dolci, M.; Barile, E.; Lanzotti, V. Pavietin, a Coumarin from Aesculus Pavia with Antifungal Activity. J. Nat. Prod. 2007, 70, 1668–1671. [Google Scholar] [CrossRef] [PubMed]
- Srijayanta, S.; Raman, A.; Goodwin, B.L. A Comparative Study of the Constituents of Aesculus Hippocastanum and Aesculus Indica. J. Med. Food 1999, 2, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, A.R.; Chițescu, C.L.; Luță, E.A.; Moroșan, A.; Mihaiescu, D.E.; Mihai, D.P.; Costea, L.; Ozon, E.A.; Fița, A.C.; Balaci, T.D.; et al. Outlook on Chronic Venous Disease Treatment: Phytochemical Screening, In Vitro Antioxidant Activity and In Silico Studies for Three Vegetal Extracts. Molecules 2023, 28, 3668. [Google Scholar] [CrossRef]
- Parrino, B.; Carbone, D.; Cascioferro, S.; Pecoraro, C.; Giovannetti, E.; Deng, D.; Di Sarno, V.; Musella, S.; Auriemma, G.; Cusimano, M.G.; et al. 1,2,4-Oxadiazole Topsentin Analogs as Staphylococcal Biofilm Inhibitors Targeting the Bacterial Transpeptidase Sortase A. Eur. J. Med. Chem. 2021, 209, 112892. [Google Scholar] [CrossRef]
- Volynets, G.; Vyshniakova, H.; Nitulescu, G.; Nitulescu, G.M.; Ungurianu, A.; Margina, D.; Moshynets, O.; Bdzhola, V.; Koleiev, I.; Iungin, O.; et al. Identification of Novel Antistaphylococcal Hit Compounds Targeting Sortase A. Molecules 2021, 26, 7095. [Google Scholar] [CrossRef]
- Nitulescu, G.; Mihai, D.P.; Nicorescu, I.M.; Olaru, O.T.; Ungurianu, A.; Zanfirescu, A.; Nitulescu, G.M.; Margina, D. Discovery of Natural Naphthoquinones as Sortase A Inhibitors and Potential Anti-Infective Solutions against Staphylococcus Aureus. Drug Dev. Res. 2019, 80, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.; Nicorescu, I.M.; Olaru, O.T.; Ungurianu, A.; Mihai, D.P.; Zanfirescu, A.; Nitulescu, G.M.; Margina, D. Molecular Docking and Screening Studies of New Natural Sortase A Inhibitors. Int. J. Mol. Sci. 2017, 18, 2217. [Google Scholar] [CrossRef] [PubMed]
- Olkova, A. Control of Suitability of the Culture Daphnia Magna Straus for Bioassays of Aquatic Environments, Taking into Account Demographic Indicators of Model Populations. Water 2021, 13, 47. [Google Scholar] [CrossRef]
| Code | Plant | Solvent | Yield (% w/w) |
|---|---|---|---|
| PVw | Aesculus pavia | Water | 22.38 |
| PVm | Aesculus pavia | 50% ethanol | 29.98 |
| PVe | Aesculus pavia | Ethanol | 13.63 |
| PRw | Aesculus parviflora | Water | 22.75 |
| CRm | Aesculus x carnea | 50% ethanol | 28.76 |
| HCe | Aesculus hippocastanum | Ethanol | 7.99 |
| Code | Plant | Solvent | TPC (mgGAE/g) | CI95% (mgGAE/g) |
|---|---|---|---|---|
| PVw | Aesculus pavia | Water | 425.7 ± 50.31 | 300.7~550.7 |
| PVm | Aesculus pavia | 50% ethanol | 427.4 ± 40.43 | 286.3~327.0 |
| PVe | Aesculus pavia | Ethanol | 451.9 ± 66.68 | 286.3~617.6 |
| PRw | Aesculus parviflora | Water | 79.62 ± 11.27 | 51.62~107.6 |
| CRm | Aesculus x carnea | 50% ethanol | 244.8 ± 5.267 | 231.7~257.9 |
| HCe | Aesculus hippocastanum | Ethanol | 270.7 ± 23.40 | 212.6~328.8 |
| Code | Plant | Solvent | IC50 ± SD (µg/mL) | CI95% (µg/mL) | ||
|---|---|---|---|---|---|---|
| 30 min | 60 min | 30 min | 60 min | |||
| PVw | Aesculus pavia | Water | 82.70 ± 2.6994 | 90.89 ± 2.2634 | NC * | NC *~233.60 |
| PVm | Aesculus pavia | 50% ethanol | 286.42 ± 1.8633 | 407.36 ± 1.8697 | 222.88~345.19 | NC *~341.78 |
| PVe | Aesculus pavia | Ethanol | 315.11 ± 1.8917 | 441.93 ± 2.0554 | 220.23~412.65 | 258.56~NC * |
| PRw | Aesculus parviflora | Water | 224.47 ± 2.1019 | 399.34 ± 2.3527 | NC * | NC * |
| CRm | Aesculus x carnea | 50% ethanol | 296.80 ± 1.9330 | 379.65 ± 2.0387 | 198.92~395.02 | NC *~290.03 |
| HCe | Aesculus hippocastanum | Ethanol | 304.31 ± 1.9312 | 426.95 ± 1.9197 | NC *~168.70 | NC *~188.19 |
| Code | Concentration (μg/g Dry Extract) | Concentration (mg/g Dry Extract) | |||||
|---|---|---|---|---|---|---|---|
| Esculin | Caffeic Acid | Chlorogenic Acid | Syringic Acid | Epigallocatechin Gallate | Ferulic Acid | Rutin | |
| PVw | - | 46.67 | 40.61 | - | 0.30 | 0.16 | 1.21 |
| PVm | - | 23.81 | 49.86 | 0.50 | 0.36 | 0.16 | 2.19 |
| PVe | - | 21.15 | 42.58 | 0.39 | 0.30 | 0.13 | 2.16 |
| PRw | 114.15 | 94.48 | 2472.87 | - | 0.30 | 0.12 | 12.57 |
| CRm | - | - | 61.09 | 5.52 | 0.37 | 0.17 | 1.52 |
| HCe | 14.17 | 29.24 | 78.46 | 0.27 | 0.35 | 0.15 | 2.39 |
| Code | Plant | Solvent | LC50 ± SD (µg/mL) | CI95% |
|---|---|---|---|---|
| PVw | Aesculus pavia | Water | 743.29 ± 1.0735 | NC * |
| PVm | Aesculus pavia | 50% ethanol | 371.17 ± 1.3579 | NC *~741.78 |
| PVe | Aesculus pavia | 96% ethanol | 569.44 ± 0.6281 | 398.35~780.00 |
| PRw | Aesculus parviflora | Water | 1049.81 ± 0.3042 | NC * |
| CRm | Aesculus x carnea | 50% ethanol | 190.26 ± 0.5159 | NC *~488.23 |
| HCe | Aesculus hippocastanum | 96% ethanol | 131.15 ± 0.1957 | NC * |
| Compound | Chemical Formula | Retention Time (min) | Adduct | m/z | Fragments |
|---|---|---|---|---|---|
| Esculin | C15H16O9 | 4.4 | -H | 339.0722 | 177.0195 |
| Caffeic acid | C9H8O4 | 5.2 | -H | 179.0350 | 135.0450 |
| Chlorogenic acid | C16H18O9 | 5.3 | -H | 353.0878 | 191.0562 |
| Syringic acid | C9H10O5 | 6.5 | -H | 197.0455 | 182.0221 |
| Epigallocatechin gallate | C22H18O11 | 6.7 | -H | 457.0776 | 169.0142 |
| Ferulic acid | C10H10O4 | 8.8 | -H | 193.0506 | 134.0377 |
| Rutin | C27H30O16 | 11.8 | -H | 609.1461 | 300.0272 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaru, O.T.; Nitulescu, G.M.; Codreanu, A.M.; Calmuc, V.-A.; Venables, L.; van de Venter, M.; Gird, C.E.; Duta-Bratu, C.-G.; Nitulescu, G. Inhibitory Effects on Staphylococcus aureus Sortase A by Aesculus sp. Extracts and Their Toxicity Evaluation. Plants 2024, 13, 1405. https://doi.org/10.3390/plants13101405
Olaru OT, Nitulescu GM, Codreanu AM, Calmuc V-A, Venables L, van de Venter M, Gird CE, Duta-Bratu C-G, Nitulescu G. Inhibitory Effects on Staphylococcus aureus Sortase A by Aesculus sp. Extracts and Their Toxicity Evaluation. Plants. 2024; 13(10):1405. https://doi.org/10.3390/plants13101405
Chicago/Turabian StyleOlaru, Octavian Tudorel, George Mihai Nitulescu, Andreea Miruna Codreanu, Valentina-Andreea Calmuc, Luanne Venables, Maryna van de Venter, Cerasela Elena Gird, Cosmina-Gabriela Duta-Bratu, and Georgiana Nitulescu. 2024. "Inhibitory Effects on Staphylococcus aureus Sortase A by Aesculus sp. Extracts and Their Toxicity Evaluation" Plants 13, no. 10: 1405. https://doi.org/10.3390/plants13101405
APA StyleOlaru, O. T., Nitulescu, G. M., Codreanu, A. M., Calmuc, V.-A., Venables, L., van de Venter, M., Gird, C. E., Duta-Bratu, C.-G., & Nitulescu, G. (2024). Inhibitory Effects on Staphylococcus aureus Sortase A by Aesculus sp. Extracts and Their Toxicity Evaluation. Plants, 13(10), 1405. https://doi.org/10.3390/plants13101405









