Biochar Mitigates the Negative Effects of Microplastics on Sugarcane Growth by Altering Soil Nutrients and Microbial Community Structure and Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Soil, Biochar, and Microplastics
2.2. Experimental Setup and Design
2.3. Sample Collection and Analysis
2.3.1. Plant and Soil Collection
2.3.2. Measurement of Soil Physicochemical Properties
2.3.3. Soil DNA Extraction and Microbial Community Analysis
2.4. Data Analysis
3. Results
3.1. Biomass of Sugarcane
3.2. Physical and Chemical Properties of Soil
3.3. Soil Microorganisms
3.3.1. Diversity of Soil Microbial Communities
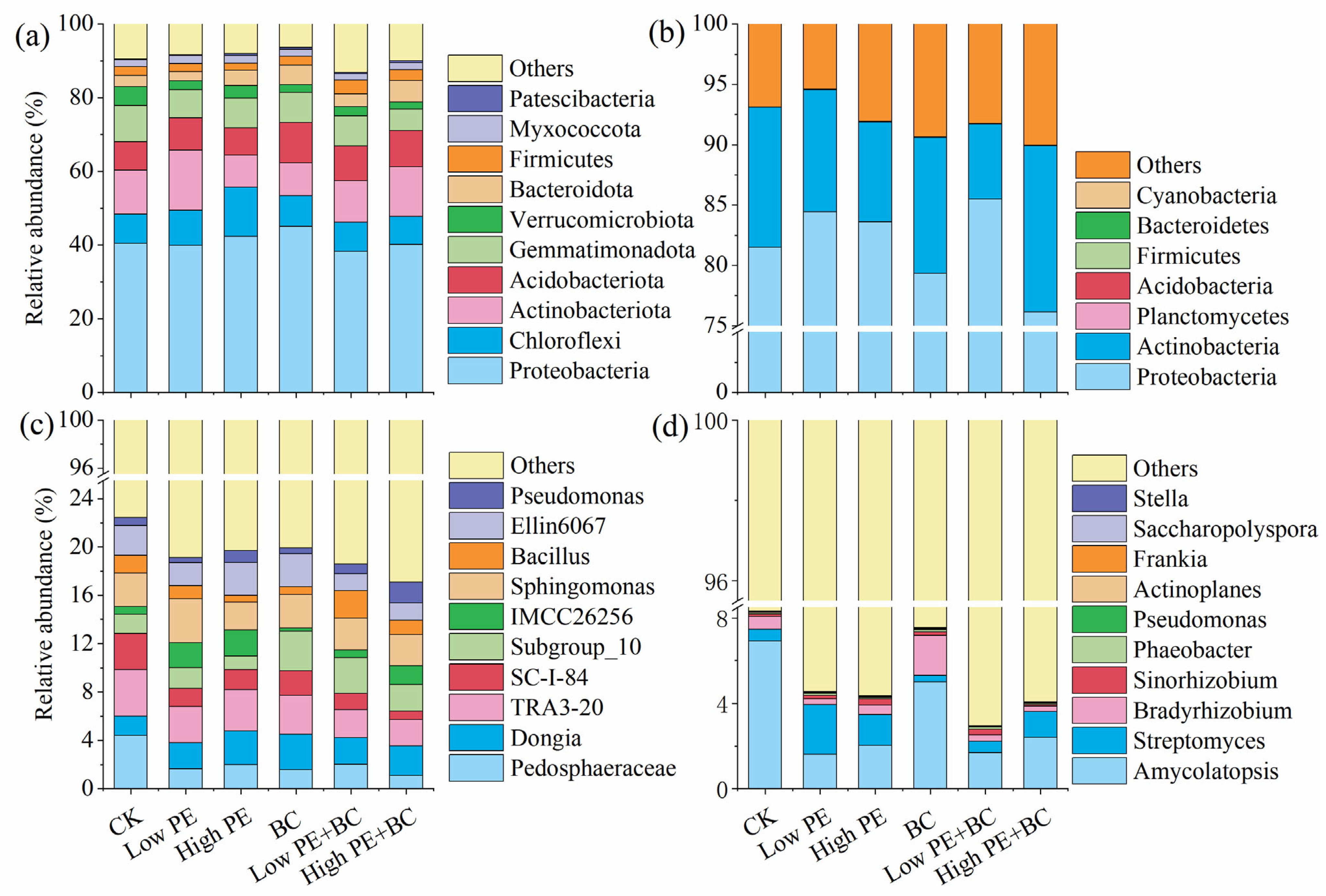
3.3.2. Soil Microbial Community Composition
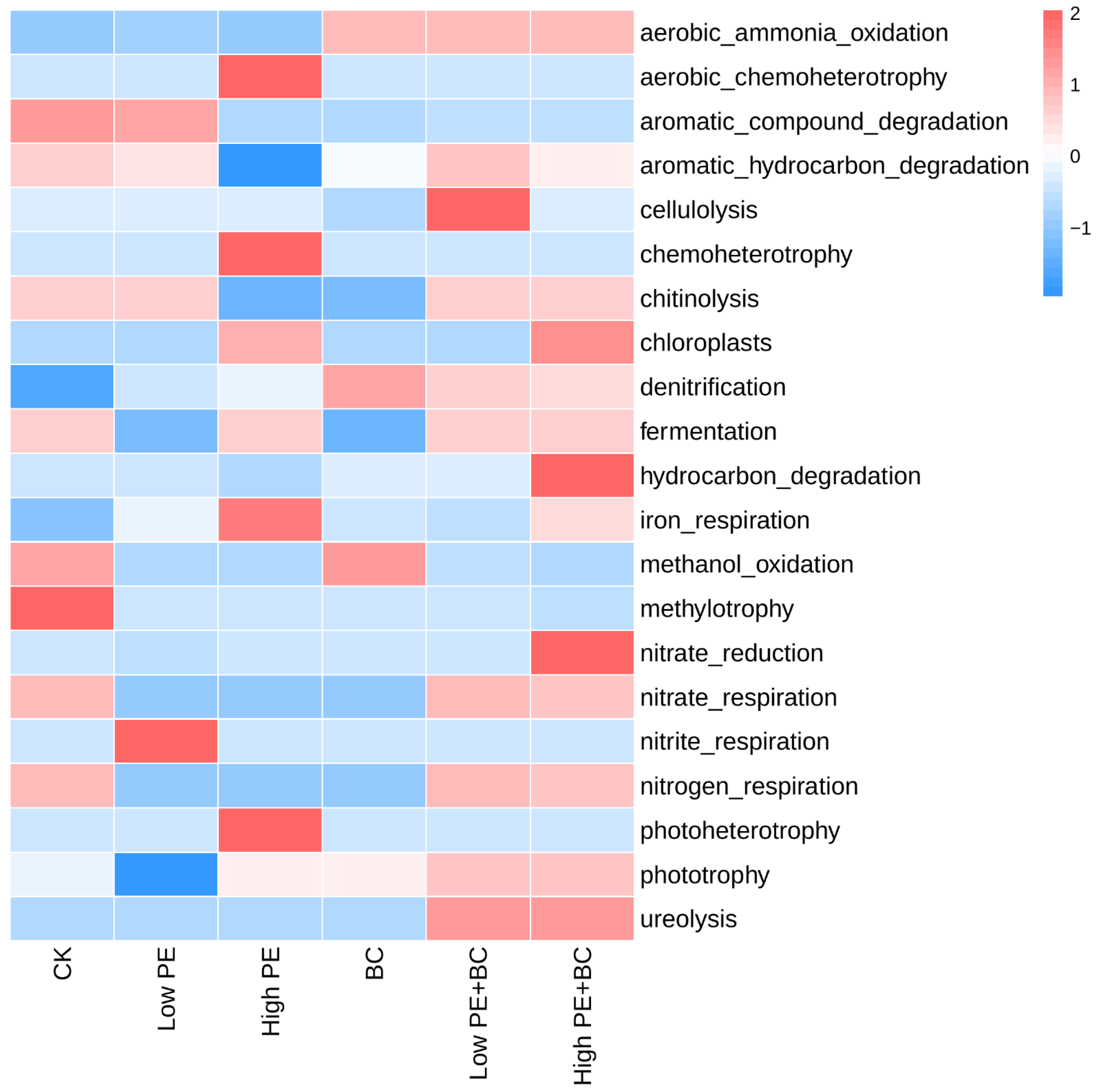
3.3.3. Soil Microbial Function
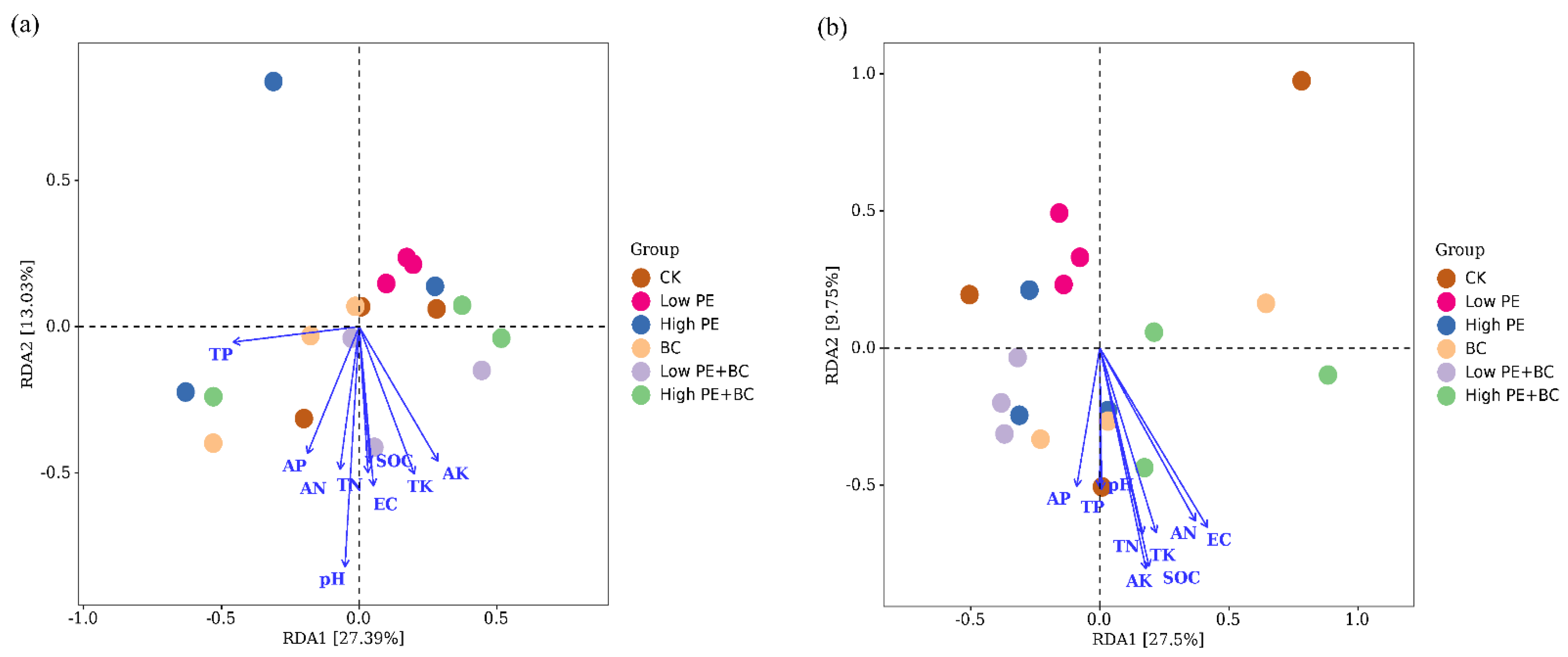
3.4. Correlations between Sugarcane Biomass, Soil Microbes, and Soil Physicochemical Properties
4. Discussion
4.1. Effects of Microplastics and Biochar on Sugarcane Biomass and Soil Physical and Chemical Properties
4.2. Effects of Microplastics and Biochar on Bacterial Community Diversity and Composition
4.3. Effects of Microplastics and Biochar on Microbial Community Structure and Function
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Mishra, S.; Pandey, R.; Yu, Z.G.; Kumar, M.; Khoo, K.S.; Thakur, T.K.; Show, P.L. Microplastics in terrestrial ecosystems: Unignorable impacts on soil characterises, nutrient storage and its cycling. TrAC Trends Anal. Chem. 2023, 158, 116869. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, S.; Xu, W.; Liang, C.; Li, J.; Zhang, H.; Li, Y.; Liu, X.; Jones, D.L.; Chadwick, D.R.; et al. Effects of plastic residues and microplastics on soil ecosystems: A global meta-analysis. J. Hazard. Mater. 2022, 435, 129065. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; An, Y.-J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-R.; Yang, L.-T. Sugarcane Agriculture and Sugar Industry in China. Sugar Tech 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, W.; Chen, D.; Cai, A.; Ao, J.; Huang, Z. Optimizing soil and fertilizer phosphorus management according to the yield response and phosphorus use efficiency of sugarcane in southern China. J. Soil Sci. Plant Nutr. 2020, 20, 1655–1664. [Google Scholar] [CrossRef]
- Jacques, O.; Prosser, R.S. A probabilistic risk assessment of microplastics in soil ecosystems. Sci. Total Environ. 2021, 757, 143987. [Google Scholar] [CrossRef]
- Yao, Y.; Lili, W.; Shufen, P.; Li, G.; Hongmei, L.; Weiming, X.; Lingxuan, G.; Jianning, Z.; Guilong, Z.; Dianlin, Y. Can microplastic mediate soil properties, plant growth and carbon/nitrogen turnover in the terrestrial ecosystem? Ecosyst. Health Sustain. 2022, 8, 2133638. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Gao, B.; Yao, H.; Li, Y.; Zhu, Y. Microplastic addition alters the microbial community structure and stimulates soil carbon dioxide emissions in vegetable-growing soil. Environ. Toxicol. Chem. 2021, 40, 352–365. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Zhao, Y.; Zhou, H.; Xiong, X.; Wu, C. Effects of microplastic biofilms on nutrient cycling in simulated freshwater systems. Sci. Total Environ. 2020, 719, 137276. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Thavamani, P.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Agronomic and remedial benefits and risks of applying biochar to soil: Current knowledge and future research directions. Environ. Int. 2016, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ran, T.; Li, J.; Liao, H.; Zhao, Y.; Yang, G.; Long, J. Effects of biochar amendment on bacterial communities and their function predictions in a microplastic-contaminated Capsicum annuum L. soil. Environ. Technol. Innov. 2023, 31, 103174. [Google Scholar] [CrossRef]
- Khalid, A.R.; Shah, T.; Asad, M.; Ali, A.; Samee, E.; Adnan, F.; Bhatti, M.F.; Marhan, S.; Kammann, C.I.; Haider, G. Biochar alleviated the toxic effects of PVC microplastic in a soil-plant system by upregulating soil enzyme activities and microbial abundance. Environ. Pollut. 2023, 332, 121810. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, W.; Lu, Y.; Li, S.; Shen, D.; Ling, Q.; Chen, D.; Ao, J. Combined Chemical Fertilizers with Molasses Increase Soil Stable Organic Phosphorus Mineralization in Sugarcane Seedling Stage. Sugar Tech 2022, 25, 552–561. [Google Scholar] [CrossRef]
- Page, A.L. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Sakurai, M.; Wasaki, J.; Tomizawa, Y.; Shinano, T.; Osaki, M. Analysis of bacterial communities on alkaline phosphatase genes in soil supplied with organic matter. Soil Sci. Plant Nutr. 2008, 54, 62–71. [Google Scholar] [CrossRef]
- Hammer, E.C.; Forstreuter, M.; Rillig, M.C.; Kohler, J. Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl. Soil Ecol. 2015, 96, 114–121. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of microplastics in soil ecosystems: Above and below ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Liu, W.; Meng, L.; Wu, J.; Zeb, A.; Cheng, L.; Lian, Y.; Sun, H. Effects of microplastics derived from polymer-coated fertilizer on maize growth, rhizosphere, and soil properties. J. Clean. Prod. 2021, 318, 128571. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Z.; Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Effect of polyvinyl chloride microplastics on bacterial community and nutrient status in two agricultural soils. Bull. Environ. Contam. Toxicol. 2021, 107, 602–609. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K.-H. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef]
- Song, D.; Xi, X.; Zheng, Q.; Liang, G.; Zhou, W.; Wang, X. Soil nutrient and microbial activity responses to two years after maize straw biochar application in a calcareous soil. Ecotoxicol. Environ. Saf. 2019, 180, 348–356. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Mujakić, I.; Piwosz, K.; Koblížek, M. Phylum Gemmatimonadota and its role in the Environment. Microorganisms 2022, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Zhu, Y.-G.; Chu, H. Biodiversity of key-stone phylotypes determines crop production in a 4-decade fertilization experiment. ISME J. 2021, 15, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hu, Y.; Cai, G.; Yao, H.; Ye, J.; Sun, Q.; Veresoglou, S.D.; Li, Y.; Zhu, Z.; Guggenberger, G.; et al. Organic phosphorus availability shapes the diversity of phoD-harboring bacteria in agricultural soil. Soil Biol. Biochem. 2021, 161, 108364. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, N.; Condron, L.M.; Dunfield, K.E.; Chen, Z.; Wang, J.; Chen, L. Impact of long-term phosphorus fertilizer inputs on bacterial phoD gene community in a maize field, Northeast China. Sci. Total Environ. 2019, 669, 1011–1018. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Q.; Hui, X.; Ran, J.; Ma, Q.; Wang, X.; Wang, Z. Long-term high-P fertilizer input decreased the total bacterial diversity but not phoD-harboring bacteria in wheat rhizosphere soil with available-P deficiency. Soil Biol. Biochem. 2020, 149, 107918. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Pinto-Tomás, A.A.; Cheng, K.; Huang, Y. Habitat Adaptation drives speciation of a Streptomyces species with distinct habitats and disparate geographic origins. mBio 2022, 13, e0278121. [Google Scholar] [CrossRef]
- Luo, G.; Ling, N.; Nannipieri, P.; Chen, H.; Raza, W.; Wang, M.; Guo, S.; Shen, Q. Long-term fertilisation regimes affect the composition of the alkaline phosphomonoesterase encoding microbial community of a vertisol and its derivative soil fractions. Biol. Fert. Soils 2017, 53, 375–388. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Sang, M.K.; Igalavithana, A.D.; Zhang, M.; Hou, D.; Oleszczuk, P.; Sung, J.; Ok, Y.S. Biochar alters chemical and microbial properties of microplastic-contaminated soil. Environ. Res. 2022, 209, 112807. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, G.; Ren, S.; Li, L.; Li, C.; Li, Y.; Yu, X.; Yin, Y.; Liu, T.; Liu, X. Responses of soil microbial community structure, potential ecological functions, and soil physicochemical properties to different cultivation patterns in cucumber. Geoderma 2023, 429, 116237. [Google Scholar] [CrossRef]
- Ali, A.; Ghani, M.I.; Elrys, A.S.; Ding, H.; Iqbal, M.; Cheng, Z.; Cai, Z. Different cropping systems regulate the metabolic capabilities and potential ecological functions altered by soil microbiome structure in the plastic shed mono-cropped cucumber rhizosphere. Agric. Ecosyst. Environ. 2021, 318, 107486. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D.; et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef] [PubMed]


| pH (H2O) | EC (ms/cm) | SOC (g/kg) | TN (g/kg) | TP (g/kg) | TK (g/kg) | AN (mg/kg) | AP (mg/kg) | AK (mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|
| CK | 3.96 (0.04) bc | 0.82 (0.01) b | 12.71 (0.69) b | 1.46 (0.03) b | 0.54 (0.01) b | 14.34 (0.03) c | 196.02 (4.06) b | 15.55 (2.79) cd | 84.67 (3.06) b |
| Low PE | 3.89 (0.04) cd | 0.81 (0.02) b | 13.32 (0.73) b | 1.45 (0.02) b | 0.53 (0.02) b | 14.31 (0.20) c | 194.73 (7.07) b | 14.08 (1.41) cd | 86.33 (3.51) b |
| High PE | 3.86 (0.05) d | 0.83 (0.02) b | 13.37 (0.53) b | 1.44 (0.03) b | 0.53 (0.03) b | 14.21 (0.12) c | 193.05 (5.41) b | 12.84 (0.33) d | 86.01 (4.00) b |
| BC | 4.08 (0.04) a | 1.06 (0.07) a | 19.14 (1.16) a | 1.66 (0.02) a | 0.60 (0.04) a | 15.05 (0.32) b | 215.69 (5.81) a | 24.44 (1.46) a | 95.33 (4.51) a |
| Low PE + BC | 4.06 (0.04) a | 1.07 (0.04) a | 19.83 (0.49) a | 1.66 (0.02) a | 0.56 (0.02) ab | 15.45 (0.10) a | 213.92 (4.17) a | 19.31 (1.66) b | 97.67 (7.09) a |
| High PE + BC | 4.00 (0.06) ab | 1.11 (0.01) a | 19.65 (0.37) a | 1.66 (0.05) a | 0.55 (0.04) ab | 15.41 (0.15) a | 211.63 (1.6) a | 16.38 (1.99) bc | 98.67 (4.51) a |
| Treatment | Observed OTUs | Chao1 | Shannon | |
|---|---|---|---|---|
| 16S rRNA gene-based bacteria | CK | 1539 (67) ab | 1541 (66) ab | 9.34 (0.07) b |
| Low PE | 1462 (73) bc | 1463 (37) bc | 9.21 (0.05) c | |
| High PE | 1424 (48) c | 1428 (48) c | 9.14 (0.03) c | |
| BC | 1590 (31) a | 1626 (56) a | 9.63 (0.08) a | |
| Low PE + BC | 1554 (43) ab | 1557 (42) ab | 9.56 (0.01) a | |
| High PE + BC | 1538 (66) ab | 1542 (67) ab | 9.55 (0.03) a | |
| phoD-harboring bacteria | CK | 934 (68) c | 1282 (36) c | 3.59 (0.18) c |
| Low PE | 1207 (69) b | 1504 (82) b | 4.58 (0.20) ab | |
| High PE | 1361 (47) a | 1749 (75) a | 4.84 (0.24) a | |
| BC | 1197 (39) b | 1557 (73) b | 4.63 (0.09) ab | |
| Low PE + BC | 1179 (44) b | 1465 (38) b | 4.51 (0.11) b | |
| High PE + BC | 1311 (39) a | 1722 (63) a | 4.75 (0.08) ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Zhou, W.; Chen, D.; Tian, J.; Ao, J. Biochar Mitigates the Negative Effects of Microplastics on Sugarcane Growth by Altering Soil Nutrients and Microbial Community Structure and Function. Plants 2024, 13, 83. https://doi.org/10.3390/plants13010083
Wu Q, Zhou W, Chen D, Tian J, Ao J. Biochar Mitigates the Negative Effects of Microplastics on Sugarcane Growth by Altering Soil Nutrients and Microbial Community Structure and Function. Plants. 2024; 13(1):83. https://doi.org/10.3390/plants13010083
Chicago/Turabian StyleWu, Qihua, Wenling Zhou, Diwen Chen, Jiang Tian, and Junhua Ao. 2024. "Biochar Mitigates the Negative Effects of Microplastics on Sugarcane Growth by Altering Soil Nutrients and Microbial Community Structure and Function" Plants 13, no. 1: 83. https://doi.org/10.3390/plants13010083
APA StyleWu, Q., Zhou, W., Chen, D., Tian, J., & Ao, J. (2024). Biochar Mitigates the Negative Effects of Microplastics on Sugarcane Growth by Altering Soil Nutrients and Microbial Community Structure and Function. Plants, 13(1), 83. https://doi.org/10.3390/plants13010083







