ScATG8 Gene Cloned from Desert Moss Syntrichia caninervis Exhibits Multiple Stress Tolerance
Abstract
:1. Introduction
2. Results
2.1. Cloning and Phylogenetic Analysis of ScATG8
2.2. Expression Pattern of the ScATG8 Gene under Different Abiotic Stresses
2.3. Subcellular Localization of ScATG8
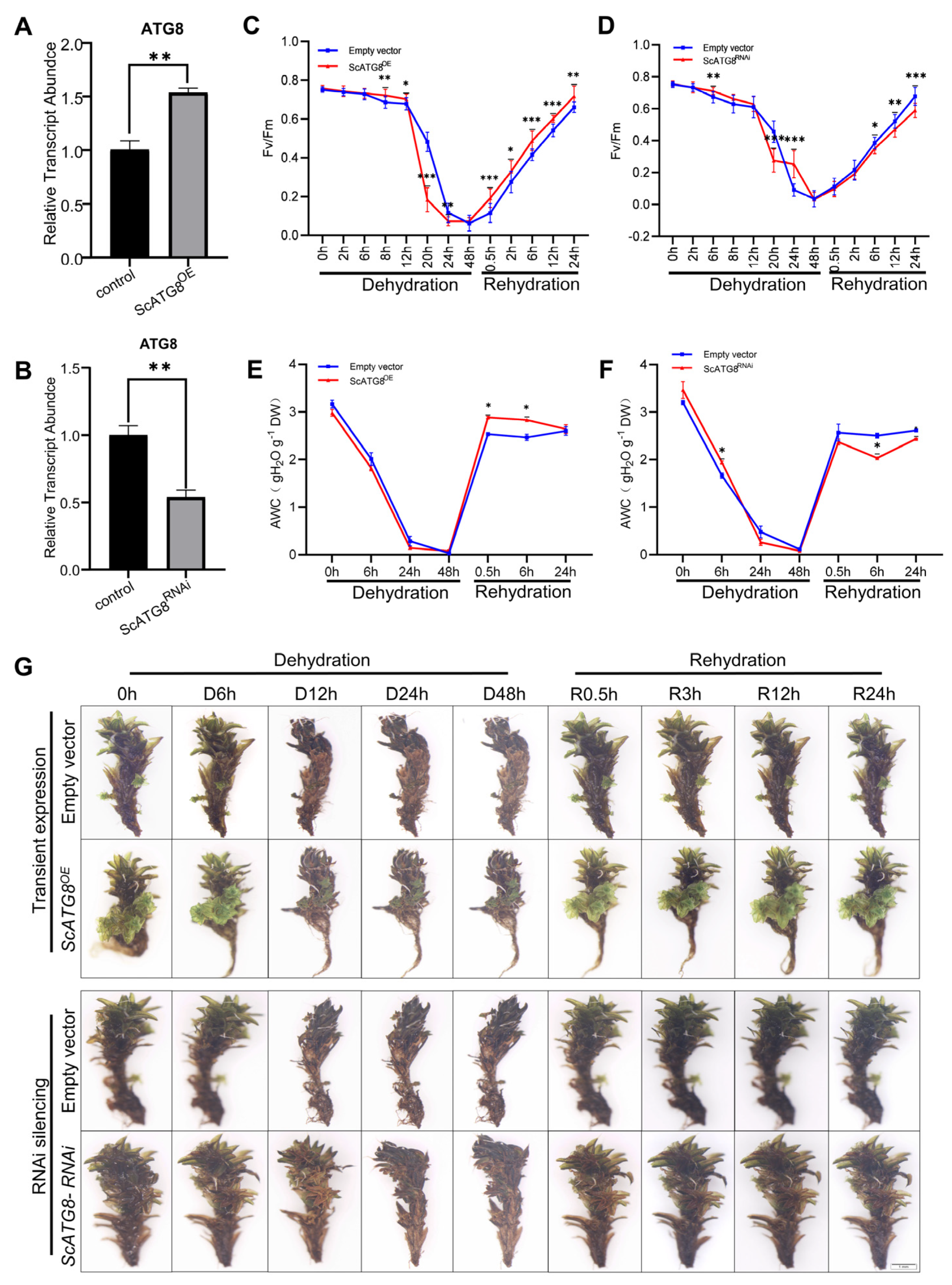
2.4. Function of ScATG8 during Dehydration–Rehydration
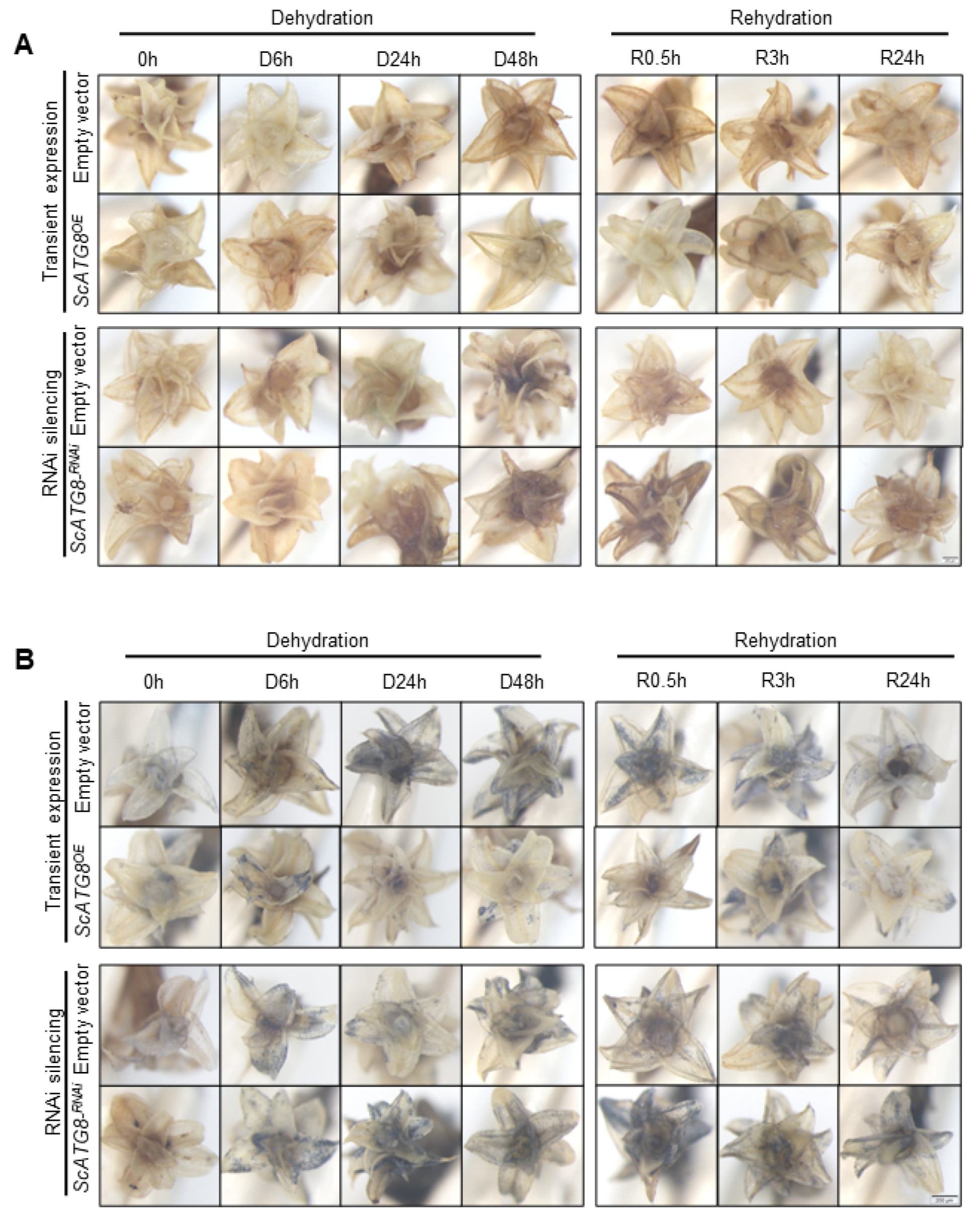
2.5. Overexpression of ScATG8 Promotes ROS Scavenging in S. caninervis under Dehydration–Rehydration
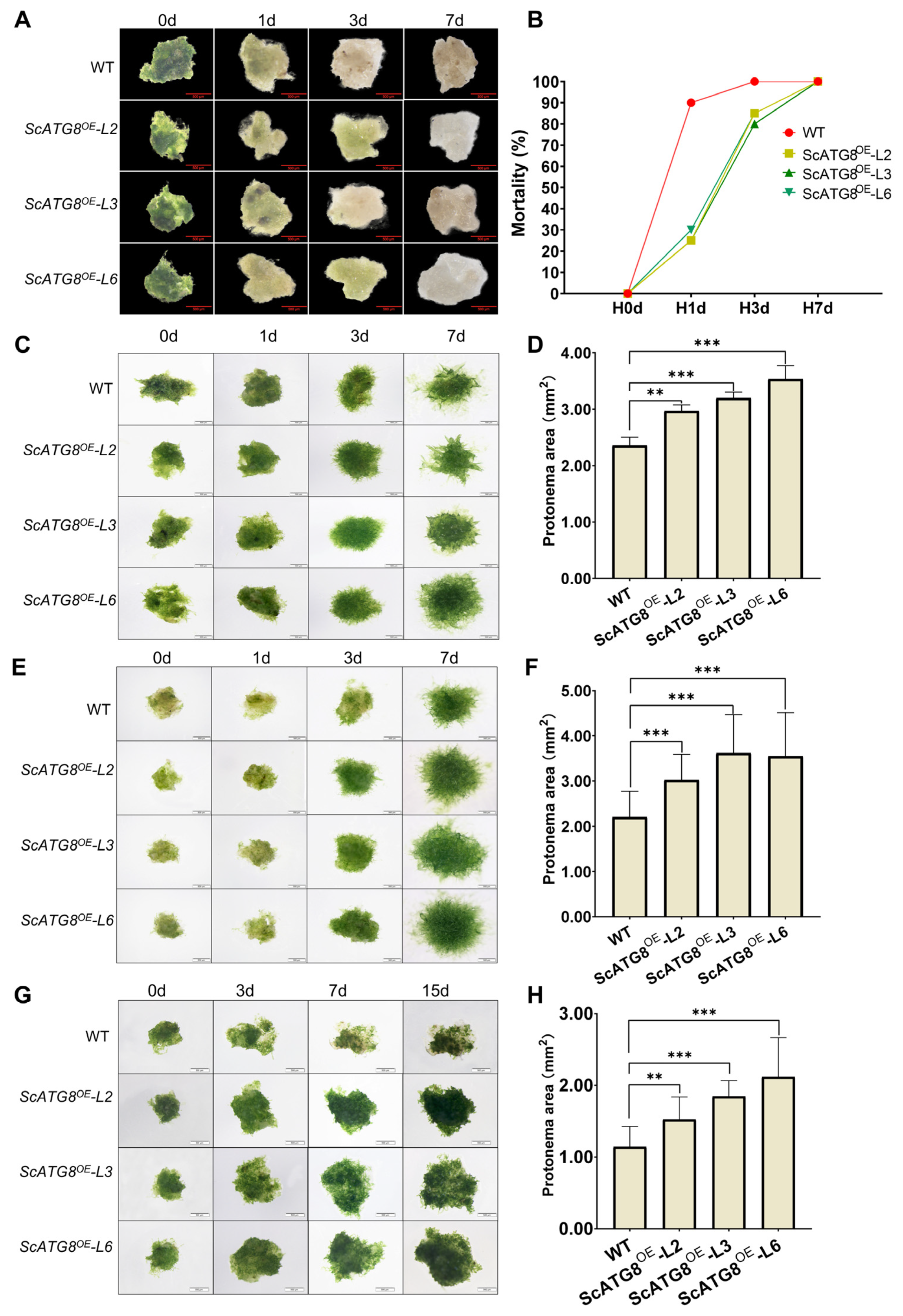
2.6. ScATG8 Overexpression Promotes Cold, Heat, and Salt Tolerance in Transgenic Physcomitrium Patens
3. Discussion
4. Materials and Methods
4.1. Sequence and Phylogenetic Analysis of the ScATG8 Gene
4.2. Plant Growth
4.3. Stress Treatment for S. caninervis and Transgenic Physcomitrium Patens
4.4. RNA Extraction and RT-qPCR Analysis
4.5. Plasmid Construction
4.6. S. caninervis Transformation
4.7. Generation of Transgenic P. patens
4.8. Confocal Microscopy of Leaves in N. benthamiana and S. caninervis
4.9. Measurement of Fv/Fm (Maximum Quantum Yield of PS II)
4.10. Measurement of Absolute Water Content (AWC)
4.11. NBT and DAB Staining
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nguyen, T.N.; Lazarou, M. A unifying model for the role of the ATG8 system in autophagy. J. Cell Sci. 2022, 135, 6. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, K.; Hanaoka, H.; Sato, S.; Kato, T.; Tabata, S.; Noda, T.; Ohsumi, Y. Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell 2004, 16, 2967–2983. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Yang, M.K.; Guo, X.; Huang, W.; Chen, L. Multiple Functions of ATG8 Family Proteins in Plant Autophagy. Front. Cell Dev. Biol. 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Kuzuoglu-Ozturk, D.; Yalcinkaya, O.C.; Akpinar, B.A.; Mitou, G.; Korkmaz, G.; Gozuacik, D.; Budak, H. Autophagy-related gene, TdAtg8, in wild emmer wheat plays a role in drought and osmotic stress response. Planta 2012, 236, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Zhang, W.; Sun, H.; Wei, X.J.; Yue, J.Y.; Wang, H.Z. Identification of autophagy-related genes ATG4 and ATG8 from wheat (Triticum aestivum L.) and profiling of their expression patterns responding to biotic and abiotic stresses. Plant Cell Rep. 2014, 33, 1697–1710. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.Y.; Wang, W.W.; Dou, X.T.; Wang, Y.J.; Jiao, J.L.; Wang, H.Z. Overexpression of the autophagy-related gene TaATG8 enhances wheat seedling tolerance to salt stress by increasing autophagic activity. Crop Pasture Sci. 2022, 73, 1325–1333. [Google Scholar] [CrossRef]
- Sun, X.; Pan, B.S.; Xu, W.Y.; Chen, Q.M.; Wang, Y.; Ban, Q.Y.; Xing, C.H.; Zhang, S.L. Genome-wide identification and expression analysis of the pear autophagy-related gene PbrATG8 and functional verification of PbrATG8c in Pyrus bretschneideri Rehd. Planta 2021, 253, 14. [Google Scholar] [CrossRef]
- Zhai, Y.F.; Wang, H.; Liang, M.M.; Lu, M.H. Both silencing- and over-expression of pepper CaATG8c gene compromise plant tolerance to heat and salt stress. Environ. Exp. Bot. 2017, 141, 10–18. [Google Scholar] [CrossRef]
- Haxim, Y.; Ismayil, A.; Jia, Q.; Wang, Y.; Zheng, X.Y.; Chen, T.Y.; Qian, L.C.; Liu, N.; Wang, Y.J.; Han, S.J.; et al. Autophagy functions as an antiviral mechanism against geminiviruses in plants. eLife 2017, 6, 17. [Google Scholar] [CrossRef]
- Dagdas, Y.F.; Belhaj, K.; Maqbool, A.; Chaparro-Garcia, A.; Pandey, P.; Petre, B.; Tabassum, N.; Cruz-Mireles, N.; Hughes, R.K.; Sklenar, J.; et al. An effector of the Irish potato famine pathogen antagonizes a host autophagy cargo receptor. eLife 2016, 5, 23. [Google Scholar] [CrossRef]
- Che, R.M.; Liu, C.H.; Wang, Q.; Tu, W.Y.; Wang, P.; Li, C.; Gong, X.Q.; Mao, K.; Feng, H.; Huang, L.L.; et al. The Valsa Mali effector Vm1G-1794 protects the aggregated MdEF-Tu from autophagic degradation to promote infection in apple. Autophagy 2023, 19, 1745–1763. [Google Scholar] [CrossRef]
- Bartels, D.; Schneider, K.; Terstappen, G.; Piatkowski, D.; Salamini, F. Molecular cloning of abscisic acid-modulated genes which are induced during desiccation of the resurrection plant Craterostigma plantagineum. Planta 1990, 181, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Farrant, J.M.; Cooper, K.; Nell, H. Desiccation Tolerance; Cabi Publishing-C a B Int: Wallingford, UK, 2012; pp. 238–265. [Google Scholar]
- Oliver, M.J.; Cushman, J.C.; Koster, K.L. Dehydration Tolerance in Plants. In Plant Stress Tolerance: Methods and Protocols; Sunkar, R., Ed.; Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2010; Volume 639, pp. 3–24. [Google Scholar]
- Liu, Z.; Cheng, D. Plant Drought-resistant Physiology Research Progress and Breeding. Chin. Agric. Sci. Bull. 2011, 27, 249–252. [Google Scholar]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhang, D.; Li, X.; Yang, H.; Wood, A.J. De novo assembly and characterization of the transcriptome in the desiccation-tolerant moss Syntrichia caninervis. BMC Res. Notes 2014, 7, 490. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhang, D.Y.; Li, X.S.; Guan, K.Y.; Yang, H.L. Novel DREB A-5 subgroup transcription factors from desert moss (Syntrichia caninervis) confers multiple abiotic stress tolerance to yeast. J. Plant Physiol. 2016, 194, 45–53. [Google Scholar] [CrossRef]
- Silva, A.T.; Gao, B.; Fisher, K.M.; Mishler, B.D.; Ekwealor, J.T.B.; Stark, L.R.; Li, X.S.; Zhang, D.Y.; Bowker, M.A.; Brinda, J.C.; et al. To dry perchance to live: Insights from the genome of the desiccation-tolerant biocrust moss Syntrichia caninervis. Plant J. 2021, 105, 1339–1356. [Google Scholar] [CrossRef]
- Rensing, S.A.; Goffinet, B.; Meyberg, R.; Wu, S.Z.; Bezanilla, M. The Moss Physcomitrium (Physcomitrella) patens: A Model Organism for Non-Seed Plants. Plant Cell 2020, 32, 1361–1376. [Google Scholar] [CrossRef]
- Koster, K.L.; Balsamo, R.A.; Espinoza, C.; Oliver, M.J. Desiccation sensitivity and tolerance in the moss Physcomitrella patens: Assessing limits and damage. Plant Growth Regul. 2010, 62, 293–302. [Google Scholar] [CrossRef]
- Oliver, M.J.; Velten, J.; Mishler, B.D. Desiccation tolerance in bryophytes: A reflection of the primitive strategy for plant survival in dehydrating habitats? Integr. Comp. Biol. 2005, 45, 788–799. [Google Scholar] [CrossRef]
- Zhao, H.; Zha, X.; He, Y.; Liu, X. Physcomitrella patens, a Potential Model System in Plant Molecular Biology (Chinese with abstract in English). Chin. Bull. Bot. 2004, 21, 129–138. [Google Scholar]
- Yang, H.; Zhang, D.; Li, H.; Dong, L.; Lan, H. Ectopic overexpression of the aldehyde dehydrogenase ALDH21 from Syntrichia caninervis in tobacco confers salt and drought stress tolerance. Plant Physiol. Biochem. 2015, 95, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Yang, R.R.; Liang, Y.Q.; Gao, B.; Li, S.M.; Bai, W.W.; Oliver, M.J.; Zhang, D.Y. The ScAPD1-like gene from the desert moss Syntrichia caninervis enhances resistance to Verticillium dahliae via phenylpropanoid gene regulation. Plant J. 2023, 113, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kellner, R.; De la Concepcion, J.C.; Maqbool, A.; Kamoun, S.; Dagdas, Y.F. ATG8 Expansion: A Driver of Selective Autophacy Diversification? Trends Plant Sci. 2017, 22, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Njaci, I.; Moghaddam, L.; Long, H.; Dickman, M.B.; Zhang, X.; Mundree, S. Trehalose Accumulation Triggers Autophagy during Plant Desiccation. PLoS Genet 2015, 11, e1005705. [Google Scholar] [CrossRef]
- Mukae, K.; Sakil, M.A.; Kotake, T.; Inoue-Aono, Y.; Moriyasu, Y. Autophagy accelerates cell death after desiccation and hydration stress in Physcomitrium. Environ. Exp. Bot. 2023, 213, 12. [Google Scholar] [CrossRef]
- Zhen, X.X.; Xu, F.; Zhang, W.Z.; Li, N.; Li, X. Overexpression of rice gene OsATG8b confers tolerance to nitrogen starvation and increases yield and nitrogen use efficiency (NUE) in Arabidopsis. PLoS ONE 2019, 14, 18. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhang, Y.G.; Yang, H.L.; Liang, Y.Q.; Li, X.S.; Oliver, M.J.; Zhang, D.Y. Functional Aspects of Early Light-Induced Protein (ELIP) Genes from the Desiccation-Tolerant Moss Syntrichia caninervis. Int. J. Mol. Sci. 2020, 21, 1411. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; Benina, M.; Obata, T.; Tohge, T.; Sujeeth, N.; Minkov, I.; Hille, J.; Temanni, M.R.; Marriott, A.S.; Bergström, E.; et al. Molecular mechanisms of desiccation tolerance in the resurrection glacial relic Haberlea rhodopensis. Cell. Mol. Life Sci. 2013, 70, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Q.; Li, X.S.; Zhang, J.; Zhuo, L.; Liu, X.J.; Yang, R.R.; Zhang, D.Y. Dehydration rates impact physiological, biochemical and molecular responses in desert moss Bryum argenteum. Environ. Exp. Bot. 2021, 183, 10. [Google Scholar] [CrossRef]
- Coelho, M.C.M.; Gabriel, R.; Ah-Peng, C. Characterizing and Quantifying Water Content in 14 Species of Bryophytes Present in Azorean Native Vegetation. Diversity 2023, 15, 295. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, T.; Haxim, Y.; Liu, X.; Yang, Q.; Hawar, A.; Waheed, A.; Li, X.; Zhang, D. ScATG8 Gene Cloned from Desert Moss Syntrichia caninervis Exhibits Multiple Stress Tolerance. Plants 2024, 13, 59. https://doi.org/10.3390/plants13010059
Cao T, Haxim Y, Liu X, Yang Q, Hawar A, Waheed A, Li X, Zhang D. ScATG8 Gene Cloned from Desert Moss Syntrichia caninervis Exhibits Multiple Stress Tolerance. Plants. 2024; 13(1):59. https://doi.org/10.3390/plants13010059
Chicago/Turabian StyleCao, Ting, Yakupjan Haxim, Xiujin Liu, Qilin Yang, Amangul Hawar, Abdul Waheed, Xiaoshuang Li, and Daoyuan Zhang. 2024. "ScATG8 Gene Cloned from Desert Moss Syntrichia caninervis Exhibits Multiple Stress Tolerance" Plants 13, no. 1: 59. https://doi.org/10.3390/plants13010059
APA StyleCao, T., Haxim, Y., Liu, X., Yang, Q., Hawar, A., Waheed, A., Li, X., & Zhang, D. (2024). ScATG8 Gene Cloned from Desert Moss Syntrichia caninervis Exhibits Multiple Stress Tolerance. Plants, 13(1), 59. https://doi.org/10.3390/plants13010059








