Enhanced Cytotoxicity and Antimelanoma Activity of Novel Semisynthetic Derivatives of Betulinic Acid with Indole Conjugation
Abstract
:1. Introduction
2. Results
2.1. Compounds Used in the Study
2.2. Cell Viability Assay
2.2.1. B164A5
2.2.2. HaCaT
2.3. Evaluation of the Cytotoxic Potential by Lactate Dehydrogenase (LDH) Assay
2.4. Cytotoxic Effects by Means of the Neutral Red (NR) Assay
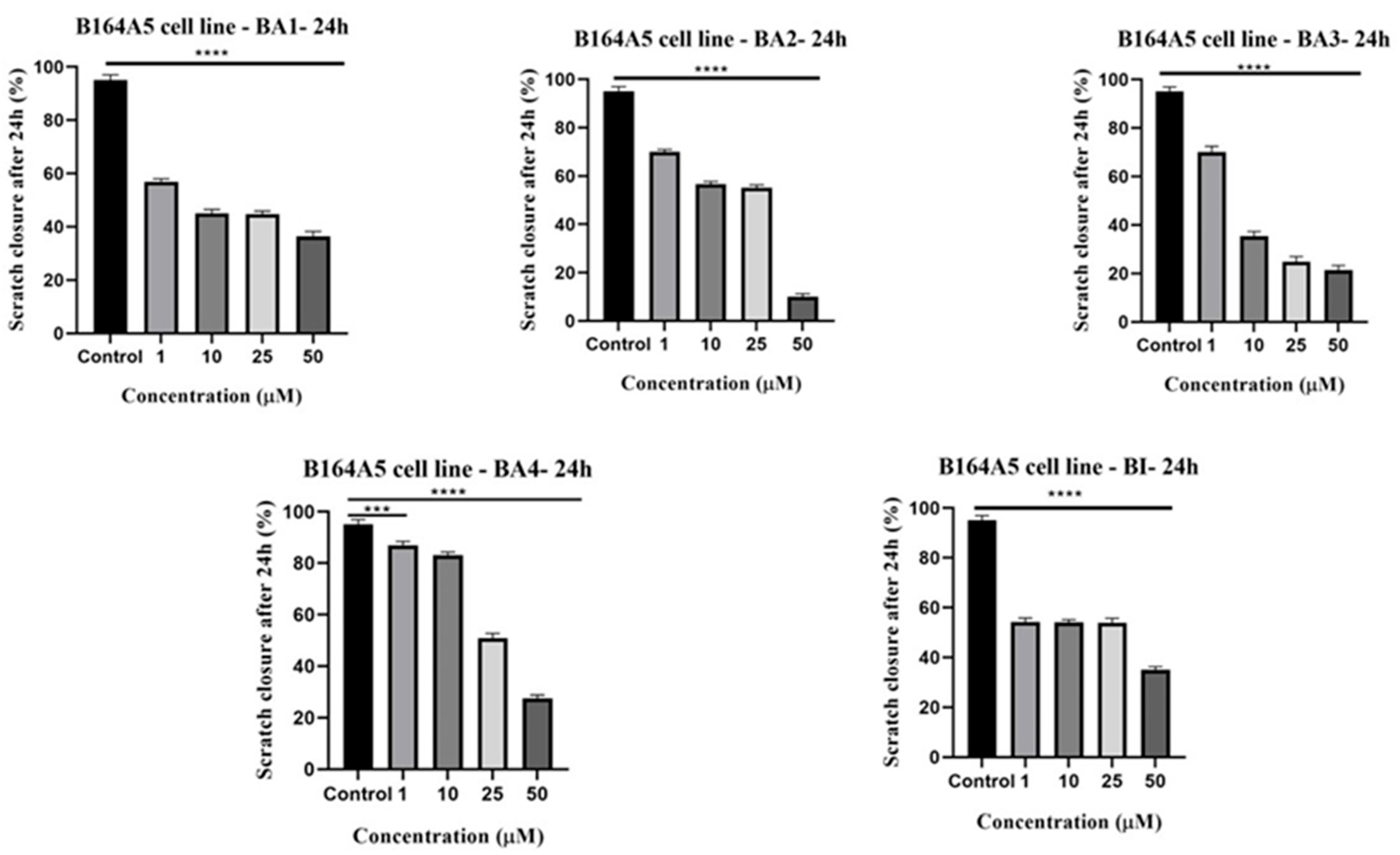
2.5. Anti-Migratory Activity Evaluation Employing the Scratch Assay Method
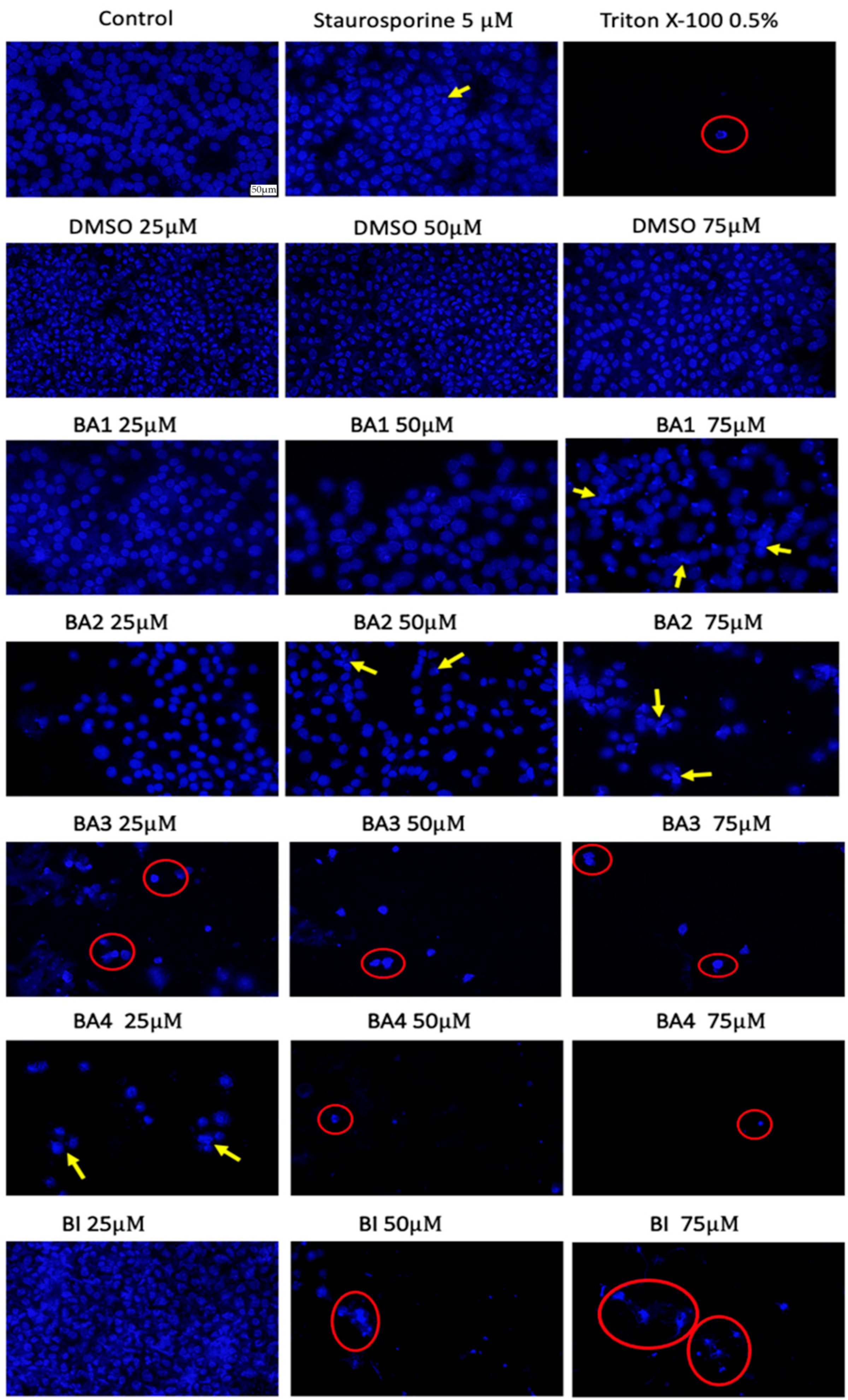
2.6. Nuclear Staining Using Hoechst 33342
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cellular Viability
4.3. Evaluation of the Cytotoxic Potential by Lactate Dehydrogenase (LDH) Assay
4.4. Neutral Red (NR) Assay
4.5. Anti-Migratory Potential—Scratch Assay Method
4.6. Hoechst Staining
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siddiqui, A.J.; Jahan, S.; Singh, R.; Saxena, J.; Ashraf, S.A.; Khan, A.; Choudhary, R.K.; Balakrishnan, S.; Badraoui, R.; Bardakci, F.; et al. Review Article Plants in Anticancer Drug Discovery: From Molecular Mechanism to Chemoprevention. BioMed Res. Int. 2022, 2022, 5425485. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J.; Snader, K.M. The Influence of Natural Products upon Drug Discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar]
- Butler, M.S. The Role of Natural Product Chemistry in Drug Discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Bhusnure, O.G.; Shinde, M.C.; Vijayendra, S.S.M.; Gholve, S.B.; Giram, P.S.; Birajdar, M.J. Phytopharmaceuticals: An Emerging Platform for Innovation and Development of New Drugs from Botanicals. J. Drug Deliv. Ther. 2019, 9, 1046–1057. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Rates, S.M.K. Plants as Source of Drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Izzo, A.A.; Hoon-Kim, S.; Radhakrishnan, R.; Williamson, E.M.; Fürst, R.; Zündorf, I.; Bhusnure, O.G.; Shinde, M.C.; Vijayendra, S.S.M.; Gholve, S.B.; et al. A Critical Approach to Evaluating Clinical Efficacy, Adverse Events and Drug Interactions of Herbal Remedies. Phytother. Res. 2020, 149, 413–425. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Ignatenko, V.A.; Han, Y.; Tochtrop, G.P. Molecular Library Synthesis Using Complex Substrates: Expanding the Framework of Triterpenoids. J. Org. Chem. 2013, 78, 410–418. [Google Scholar] [CrossRef]
- Blundell, R.; Azzopardi, J.; Briffa, J.; Rasul, A.; Vargas-de la Cruz, C.; Shah, M.A. Analysis of Pentaterpenoids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 457–475. [Google Scholar] [CrossRef]
- Haque, A.; Brazeau, D.; Amin, A.R. Perspectives on Natural Compounds in Chemoprevention and Treatment of Cancer: An Update with New Promising Compounds. Eur. J. Cancer 2021, 149, 165–183. [Google Scholar] [CrossRef]
- Hoenke, S.; Heise, N.V.; Kahnt, M.; Deigner, H.P.; Csuk, R. Betulinic Acid Derived Amides Are Highly Cytotoxic, Apoptotic and Selective. Eur. J. Med. Chem. 2020, 207, 112815. [Google Scholar] [CrossRef] [PubMed]
- Lombrea, A.; Scurtu, A.D.; Avram, S.; Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Dehelean, C.A.; Soica, C.; Danciu, C. Anticancer Potential of Betulonic Acid Derivatives. Int. J. Mol. Sci. 2021, 22, 3676. [Google Scholar] [CrossRef] [PubMed]
- Pavel, I.Z.; Pârvu, A.E.; Dehelean, C.A.; Vlase, L.; Csuk, R.; Muntean, D.M. Assessment of the Antioxidant Effect of a Maslinic Acid Derivative in an Experimental Model of Acute Inflammation. Farmacia 2017, 65, 390–395. [Google Scholar]
- Saxena, B.B.; Zhu, L.; Hao, M.; Kisilis, E.; Katdare, M.; Oktem, O.; Bomshteyn, A.; Rathnam, P. Boc-Lysinated-Betulonic Acid: A Potent, Anti-Prostate Cancer Agent. Bioorg. Med. Chem. 2006, 14, 6349–6358. [Google Scholar] [CrossRef]
- Kozubek, M.; Hoenke, S.; Deigner, H.P.; Csuk, R. Betulinic Acid and Glycyrrhetinic Acid Derived Piperazinyl Spacered Rhodamine B Conjugates Are Highly Cytotoxic and Necrotic. Results Chem. 2022, 4, 10–17. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Thu, H.N.T.; Tu, A.L.T.; Thanh, T.N.; Thi, C.B.; Babkov, D.A.; Kazakova, O.B. Structural Modifications of 2,3-Indolobetulinic Acid: Design and Synthesis of Highly Potent α-Glucosidase Inhibitors. Bioorg. Chem. 2019, 88, 102957. [Google Scholar] [CrossRef]
- Isah, M.B.; Ibrahim, M.A.; Mohammed, A.; Aliyu, A.B.; Masola, B.; Coetzer, T.H.T. A Systematic Review of Pentacyclic Triterpenes and Their Derivatives as Chemotherapeutic Agents against Tropical Parasitic Diseases. Parasitology 2016, 143, 1219–1231. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Jonnalagadda, S.C.; Suman, P.; Morgan, D.C.; Seay, J.N. Recent Developments on the Synthesis and Applications of Betulin and Betulinic Acid Derivatives as Therapeutic Agents, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 53. [Google Scholar] [CrossRef]
- Pavlova, N.I.; Savinova, O.V.; Nikolaeva, S.N.; Boreko, E.I.; Flekhter, O.B. Antiviral Activity of Betulin, Betulinic and Betulonic Acids against Some Enveloped and Non-Enveloped Viruses. Fitoterapia 2003, 74, 489–492. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniyatullina, G.V.; Mustafin, A.G.; Babkov, D.A.; Sokolova, E.V.; Spasov, A.A. Evaluation of Cytotoxicity and A-glucosidase Inhibitory Activity of Amide and Polyamino-derivatives of Lupane Triterpenoids. Molecules 2020, 25, 4833. [Google Scholar] [CrossRef]
- Tsepaeva, O.V.; Nemtarev, A.V.; Salikhova, T.I.; Abdullin, T.I.; Grigor’eva, L.R.; Khozyainova, S.A.; Mironov, V.F. Synthesis, Anticancer, and Antibacterial Activity of Betulinic and Betulonic Acid C-28-Triphenylphosphonium Conjugates with Variable Alkyl Linker Length. Anticancer Agents Med. Chem. 2019, 20, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Plewka, K.; Kandefer-Szerszeń, M. Betulin, Betulinic Acid and Butein Are Inhibitors of Acetaldehyde-Induced Activation of Liver Stellate Cells. Pharmacol. Rep. 2011, 63, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Regueiro-Ren, A.; Liu, Z.; Chen, Y.; Sin, N.; Sit, S.-Y.; Swidorski, J.J.; Chen, J.; Venables, B.L.; Zhu, J.; Nowicka-Sans, B. Discovery of BMS-955176, a Second Generation HIV-1 Maturation Inhibitor with Broad Spectrum Antiviral Activity. ACS Med. Chem. Lett. 2016, 7, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, I.V.; Tolstikova, T.G.; Zhukova, N.A.; Petrenko, N.I.; Schults, E.E.; Uzenkova, N.V.; Grek, O.R.; Pozdnyakova, S.V.; Tolstikov, G.A. Betulonic Acid and Derivatives, a New Group of Agents Reducing Side Effects of Cytostatics. Dokl. Biol. Sci. 2004, 399, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.L.C.; Freire, C.S.R.; Silvestre, A.J.D.; Silva, A.M.S. Recent Developments in the Functionalization of Betulinic Acid and Its Natural Analogues: A Route to New Bioactive Compounds. Molecules 2019, 24, 355. [Google Scholar] [CrossRef] [PubMed]
- Lugiņina, J.; Linden, M.; Bazulis, M.; Kumpiņš, V.; Mishnev, A.; Popov, S.A.; Golubeva, T.S.; Waldvogel, S.R.; Shults, E.E.; Turks, M. Electrosynthesis of Stable Betulin-Derived Nitrile Oxides and Their Application in Synthesis of Cytostatic Lupane-Type Triterpenoid-Isoxazole Conjugates. Eur. J. Org. Chem. 2021, 2021, 2557–2577. [Google Scholar] [CrossRef]
- Mierina, I.; Vilskersts, R.; Turks, M. Delivery Systems for Birch-Bark Triterpenoids and Their Derivatives in Anticancer Research. Curr. Med. Chem. 2020, 27, 1308–1336. [Google Scholar] [CrossRef] [PubMed]
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M. Discovery of Betulinic Acid as a Selective Inhibitor of Human Melanoma That Functions by Induction of Apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Coricovac, D.; Dehelean, C.A.; Pinzaru, I.; Mioc, A.; Aburel, O.-M.; Macasoi, I.; Draghici, G.A.; Petean, C.; Soica, C.; Boruga, M. Assessment of Betulinic Acid Cytotoxicity and Mitochondrial Metabolism Impairment in a Human Melanoma Cell Line. Int. J. Mol. Sci. 2021, 22, 4870. [Google Scholar] [CrossRef]
- Takada, Y.; Aggarwal, B.B. Betulinic Acid Suppresses Carcinogen-Induced NF-κB Activation through Inhibition of IκBα Kinase and P65 Phosphorylation: Abrogation of Cyclooxygenase-2 and Matrix Metalloprotease-9. J. Immunol. 2003, 171, 3278–3286. [Google Scholar] [CrossRef]
- Melzig, M.F.; Bormann, H. Betulinic Acid Inhibits Aminopeptidase N Activity. Planta Med. 1998, 64, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Shim, J.S.; Kim, J.H.; Cho, H.Y.; Yum, Y.N.; Kim, S.H.; Yu, J. Betulinic Acid Inhibits Growth Factor-induced in Vitro Angiogenesis via the Modulation of Mitochondrial Function in Endothelial Cells. Jpn. J. Cancer Res. 2002, 93, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liang, N.; Yang, L.I.U.; Cheng, M.-S. Recent Progress on Betulinic Acid and Its Derivatives as Antitumor Agents: A Mini Review. Chin. J. Nat. Med. 2021, 19, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Nistor, G.; Mioc, A.; Mioc, M.; Balan-Porcarasu, M.; Ghiulai, R.; Racoviceanu, R.; Avram, Ș.; Prodea, A.; Semenescu, A.; Milan, A.; et al. Novel Semisynthetic Betulinic Acid−Triazole Hybrids with In Vitro Antiproliferative Potential. Processes 2022, 11, 101. [Google Scholar] [CrossRef]
- Nistor, G.; Mioc, M.; Mioc, A.; Balan-Porcarasu, M.; Racoviceanu, R.; Prodea, A.; Milan, A.; Ghiulai, R.; Semenescu, A.; Dehelean, C.; et al. The C30-Modulation of Betulinic Acid Using 1,2,4-Triazole: A Promising Strategy for Increasing Its Antimelanoma Cytotoxic Potential. Molecules 2022, 27, 7807. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.H.L.; Pezzuto, J.M.; Pisha, E. Synthesis of Betulinic Acid Derivatives with Activity against Human Melanoma. Bioorg. Med. Chem. Lett. 1998, 8, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Rani, N.; Aggarwal, P.; Sanna, V.K.; Singh, A.T.; Jaggi, M.; Joshi, N.; Sharma, P.K.; Irchhaiya, R.; Burman, A.C. Synthesis and Cytotoxic Activity of Heterocyclic Ring-Substituted Betulinic Acid Derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 5058–5062. [Google Scholar] [CrossRef]
- Yang, S.-J.; Liu, M.-C.; Xiang, H.-M.; Zhao, Q.; Xue, W.; Yang, S. Synthesis and in Vitro Antitumor Evaluation of Betulin Acid Ester Derivatives as Novel Apoptosis Inducers. Eur. J. Med. Chem. 2015, 102, 249–255. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Tilaoui, M.; Ait Mouse, H.; Zyad, A. Update and New Insights on Future Cancer Drug Candidates from Plant-Based Alkaloids. Front. Pharmacol. 2021, 12, 719694. [Google Scholar] [CrossRef]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological Trends in Skin Cancer What Does the Future Hold. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Strashilov, S.; Yordanov, A. Aetiology and Pathogenesis of Cutaneous Melanoma: Current Concepts and Advances. Int. J. Mol. Sci. 2021, 22, 6395. [Google Scholar] [CrossRef] [PubMed]
- Becquart, O.; Oriano, B.; Dalle, S.; Mortier, L.; Leccia, M.T.; Dutriaux, C.; Dalac, S.; Montaudié, H.; De Quatrebarbes, J.; Brunet-Possenti, F. Tolerance and Effectiveness of Targeted Therapies in Aged Patients with Metastatic Melanoma. Cancers 2021, 13, 3042. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, S.; Przybyło, M.; Stępień, E. The Clinical Trial Landscape for Melanoma Therapies. J. Clin. Med. 2019, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Nistor, M.; Rugina, D.; Diaconeasa, Z.; Socaciu, C.; Socaciu, M.A. Pentacyclic Triterpenoid Phytochemicals with Anticancer Activity: Updated Studies on Mechanisms and Targeted Delivery. Int. J. Mol. Sci. 2023, 24, 12923. [Google Scholar] [CrossRef] [PubMed]
- Lombrea, A.; Semenescu, A.D.; Magyari-Pavel, I.Z.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Muntean, D.; Dehelean, C.A.; Dinu, S.; Danciu, C. Comparison of In Vitro Antimelanoma and Antimicrobial Activity of 2,3-Indolo-Betulinic Acid and Its Glycine Conjugates. Plants 2023, 12, 1253. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Fukunaga-Kalabis, M.; Herlyn, M. Crosstalk in Skin: Melanocytes, Keratinocytes, Stem Cells, and Melanoma. J. Cell Commun. Signal. 2016, 10, 191–196. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, Z.; Rao, Y. Current Advances in the Biotechnological Synthesis of Betulinic Acid: New Findings and Practical Applications. Syst. Microbiol. Biomanuf. 2023, 3, 179–192. [Google Scholar] [CrossRef]
- Jiang, W.; Li, X.; Dong, S.; Zhou, W. Betulinic Acid in the Treatment of Tumour Diseases: Application and Research Progress. Biomed. Pharmacother. 2021, 142, 111990. [Google Scholar] [CrossRef]
- Grymel, M.; Zawojak, M.; Adamek, J. Triphenylphosphonium Analogues of Betulin and Betulinic Acid with Biological Activity: A Comprehensive Review. J. Nat. Prod. 2019, 82, 1719–1730. [Google Scholar] [CrossRef]
- Drag-Zalesinska, M.; Kulbacka, J.; Saczko, J.; Wysocka, T.; Zabel, M.; Surowiak, P.; Drag, M. Esters of Betulin and Betulinic Acid with Amino Acids Have Improved Water Solubility and Are Selectively Cytotoxic toward Cancer Cells. Bioorg. Med. Chem. Lett. 2009, 19, 4814–4817. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and Its Derivatives as Novel Compounds with Different Pharmacological Effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef] [PubMed]
- Govdi, A.I.; Sokolova, N.V.; Sorokina, I.V.; Baev, D.S.; Tolstikova, T.G.; Mamatyuk, V.I.; Fadeev, D.S.; Vasilevsky, S.F.; Nenajdenko, V.G. Synthesis of New Betulinic Acid–Peptide Conjugates and in Vivo and in Silico Studies of the Influence of Peptide Moieties on the Triterpenoid Core Activity. Med. Chem. Commun. 2015, 6, 230–238. [Google Scholar] [CrossRef]
- Popov, S.A.; Semenova, M.D.; Baev, D.S.; Sorokina, I.V.; Zhukova, N.A.; Frolova, T.S.; Tolstikova, T.G.; Shults, E.E.; Turks, M. Lupane-Type Conjugates with Aminoacids, 1, 3, 4-Oxadiazole and 1, 2, 5-Oxadiazole-2-Oxide Derivatives: Synthesis, Anti-Inflammatory Activity and in Silico Evaluation of Target Affinity. Steroids 2019, 150, 108443. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.A.; Semenova, M.D.; Baev, D.S.; Frolova, T.S.; Shults, E.E.; Wang, C.; Turks, M. Synthesis of Cytotoxic Urs-12-Ene-and 28-Norurs-12-Ene-Type Conjugates with Amino-and Mercapto-1, 3, 4-Oxadiazoles and Mercapto-1, 2, 4-Triazoles. Steroids 2020, 153, 108524. [Google Scholar] [CrossRef]
- Popov, S.A.; Wang, C.; Qi, Z.; Shults, E.E.; Turks, M. Synthesis of Water-Soluble Ester-Linked Ursolic Acid–Gallic Acid Hybrids with Various Hydrolytic Stabilities. Synth. Commun. 2021, 51, 2466–2477. [Google Scholar] [CrossRef]
- Semenova, M.D.; Popov, S.A.; Golubeva, T.S.; Baev, D.S.; Shults, E.E.; Turks, M. Synthesis and Cytotoxicity of Sulfanyl, Sulfinyl and Sulfonyl Group Containing Ursane Conjugates with 1, 3, 4-Oxadiazoles and 1, 2, 4-Triazoles. ChemistrySelect 2021, 6, 6472–6477. [Google Scholar] [CrossRef]
- Khlebnicova, T.S.; Piven, Y.A.; Baranovsky, A.V.; Lakhvich, F.A.; Shishkina, S.V.; Zicāne, D.; Tetere, Z.; Rāviņa, I.; Kumpiņš, V.; Rijkure, I. Synthesis of Novel Lupane Triterpenoid-Indazolone Hybrids with Oxime Ester Linkage. Steroids 2017, 117, 77–89. [Google Scholar] [CrossRef]
- Luo, M.L.; Zhao, Q.; He, X.H.; Xie, X.; Zhu, H.P.; You, F.M.; Peng, C.; Zhan, G.; Huang, W. Research Progress of Indole-Fused Derivatives as Allosteric Modulators: Opportunities for Drug Development. Biomed. Pharmacother. 2023, 162, 114574. [Google Scholar] [CrossRef]
- Luo, M.L.; Huang, W.; Zhu, H.P.; Peng, C.; Zhao, Q.; Han, B. Advances in Indole-Containing Alkaloids as Potential Anticancer Agents by Regulating Autophagy. Biomed. Pharmacother. 2022, 149, 112827. [Google Scholar] [CrossRef]
- Han, B.; He, X.H.; Liu, Y.Q.; He, G.; Peng, C.; Li, J.L. Asymmetric Organocatalysis: An Enabling Technology for Medicinal Chemistry. Chem. Soc. Rev. 2021, 50, 1522–1586. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Zhao, Q.; Han, B.; Zhu, H.P.; Peng, C.; Zhan, G.; Huang, W. Indole-Based Small Molecules as Potential Therapeutic Agents for the Treatment of Fibrosis. Front. Pharmacol. 2022, 13, 845892. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.L.; Horswill, J.G.; Anavi-Goffer, S.; Reggio, P.H.; Bolognini, D.; Abood, M.E.; McAllister, S.; Strange, P.G.; Stephens, G.J.; Pertwee, R.G.; et al. CB1 Receptor Allosteric Modulators Display Both Agonist and Signaling Pathway Specificity. Mol. Pharmacol. 2013, 83, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Yan, W.; Chapman, K.; Ramesh, K.; Ferrell, A.J.; Yin, J.; Wang, X.; Xu, Q.; Rosenbaum, D.M. Structure of an Allosteric Modulator Bound to the CB1 Cannabinoid Receptor. Nat. Chem. Biol. 2019, 15, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Farcas, C.G.; Moaca, E.A.; Dragoi, R.; Vaduva, D.B.; Marcovici, I.; Mihali, C.V.; Loghin, F. Preliminary Results of Betulinic Acid-Loaded Magnetoliposomes—A Potential Approach to Increase Therapeutic Efficacy in Melanoma. Rev. Chim. 2019, 70, 3372–3377. [Google Scholar] [CrossRef]
- Soica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupko, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic Acid in Complex with a Gamma-Cyclodextrin Derivative Decreases Proliferation and in Vivo Tumor Development of Non-Metastatic and Metastatic B164A5 Cells. Int. J. Mol. Sci. 2014, 15, 8235–8255. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.F.; Petrova, A.V.; Apryshko, G.N.; Kukovinets, O.S.; Kazakova, O.B. Synthesis and Cytotoxicity of Indole Derivatives of Betulin, Erythrodiol, and Uvaol. Russ. J. Bioorg. Chem. 2018, 44, 322–329. [Google Scholar] [CrossRef]
- Jeong, H.J.; Chai, H.B.; Park, S.Y.; Kim, D.S.H.L. Preparation of Amino Acid Conjugates of Betulinic Acid with Activity against Human Melanoma. Bioorg. Med. Chem. Lett. 1999, 9, 1201–1204. [Google Scholar] [CrossRef]
- Wróblewska-Łuczka, P.; Cabaj, J.; Bąk, W.; Bargieł, J.; Grabarska, A.; Góralczyk, A.; Łuszczki, J.J. Additive Interactions between Betulinic Acid and Two Taxanes in In Vitro Tests against Four Human Malignant Melanoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 9641. [Google Scholar] [CrossRef]
- Decker, T.; Lohmann-Matthes, M.-L. A Quick and Simple Method for the Quantitation of Lactate Dehydrogenase Release in Measurements of Cellular Cytotoxicity and Tumor Necrosis Factor (TNF) Activity. J. Immunol. Methods 1988, 115, 61–69. [Google Scholar] [CrossRef]
- Ates, G.; Vanhaecke, T.; Rogiers, V.; Rodrigues, R.M. Assaying Cellular Viability Using the Neutral Red Uptake Assay. In Cell Viability Assays: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017; pp. 19–26. [Google Scholar]
- Ghiƫu, A.; Pavel, I.Z.; Avram, S.; Kis, B.; Minda, D.; Dehelean, C.A.; Buda, V.; Folescu, R.; Danciu, C. An In Vitro-In Vivo Evaluation of the Antiproliferative and Antiangiogenic Effect of Flavone Apigenin against SK-MEL-24 Human Melanoma Cell Line. Anal. Cell. Pathol. 2021, 2021, 5552664. [Google Scholar] [CrossRef] [PubMed]
- Ghițu, A.; Schwiebs, A.; Radeke, H.H.; Avram, S.; Zupko, I.; Bor, A.; Pavel, I.Z.; Dehelean, C.A.; Oprean, C.; Bojin, F. A Comprehensive Assessment of Apigenin as an Antiproliferative, Proapoptotic, Antiangiogenic and Immunomodulatory Phytocompound. Nutrients 2019, 11, 858. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Muntean, D.; Alexa, E.; Farcas, C.; Oprean, C.; Zupko, I.; Bor, A.; Minda, D.; Proks, M.; Buda, V. Phytochemical Characterization and Evaluation of the Antimicrobial, Antiproliferative and pro-Apoptotic Potential of Ephedra Alata Decne. Hydroalcoholic Extract against the MCF-7 Breast Cancer Cell Line. Molecules 2018, 24, 13. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Cioanca, O.; Hancianu, M.; Racoviceanu, R.; Muntean, D.; Zupko, I.; Oprean, C.; Tatu, C.; Paunescu, V.; Proks, M. Botanical Therapeutics (Part II): Antimicrobial and In Vitro Anticancer Activity against Mcf7 Human Breast Cancer Cells of Chamomile, Parsley and Celery Alcoholic Extracts. Anti-Cancer Agents Med. Chem. 2021, 21, 187–200. [Google Scholar] [CrossRef]
- Felice, F.; Zambito, Y.; Belardinelli, E.; Fabiano, A.; Santoni, T.; Di Stefano, R. Effect of Different Chitosan Derivatives on in Vitro Scratch Wound Assay: A Comparative Study. Int. J. Biol. Macromol. 2015, 76, 236–241. [Google Scholar] [CrossRef]

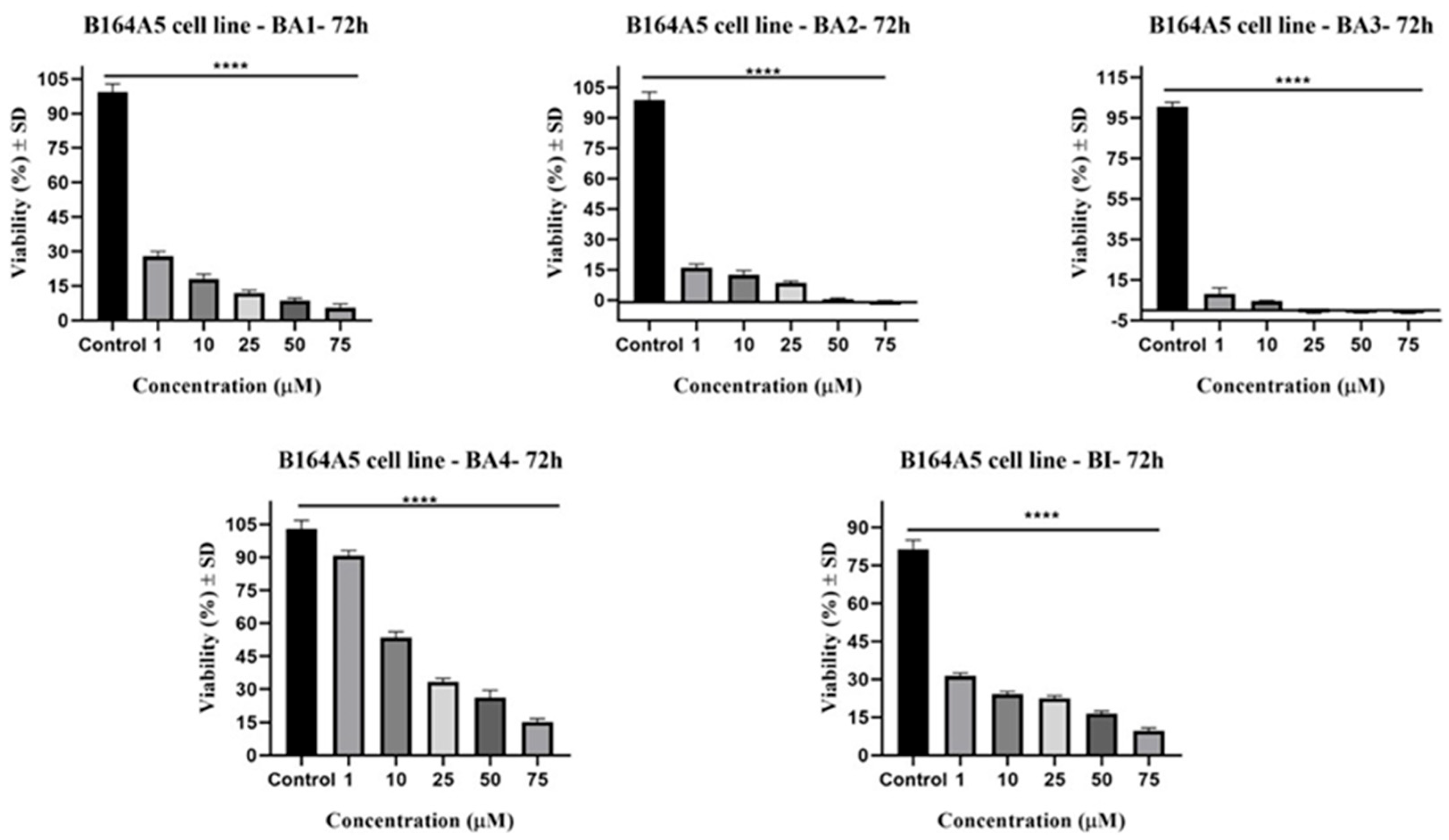
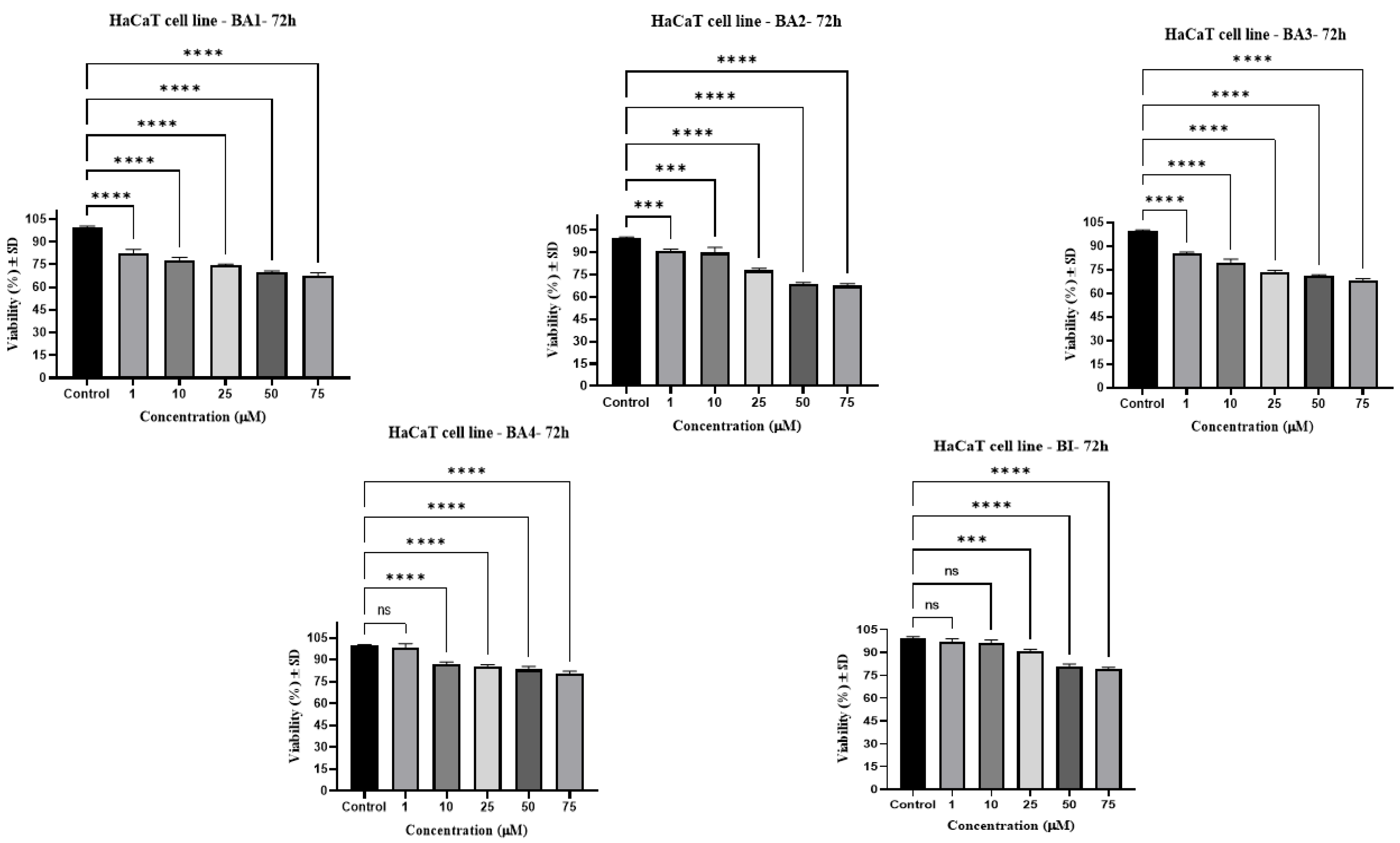
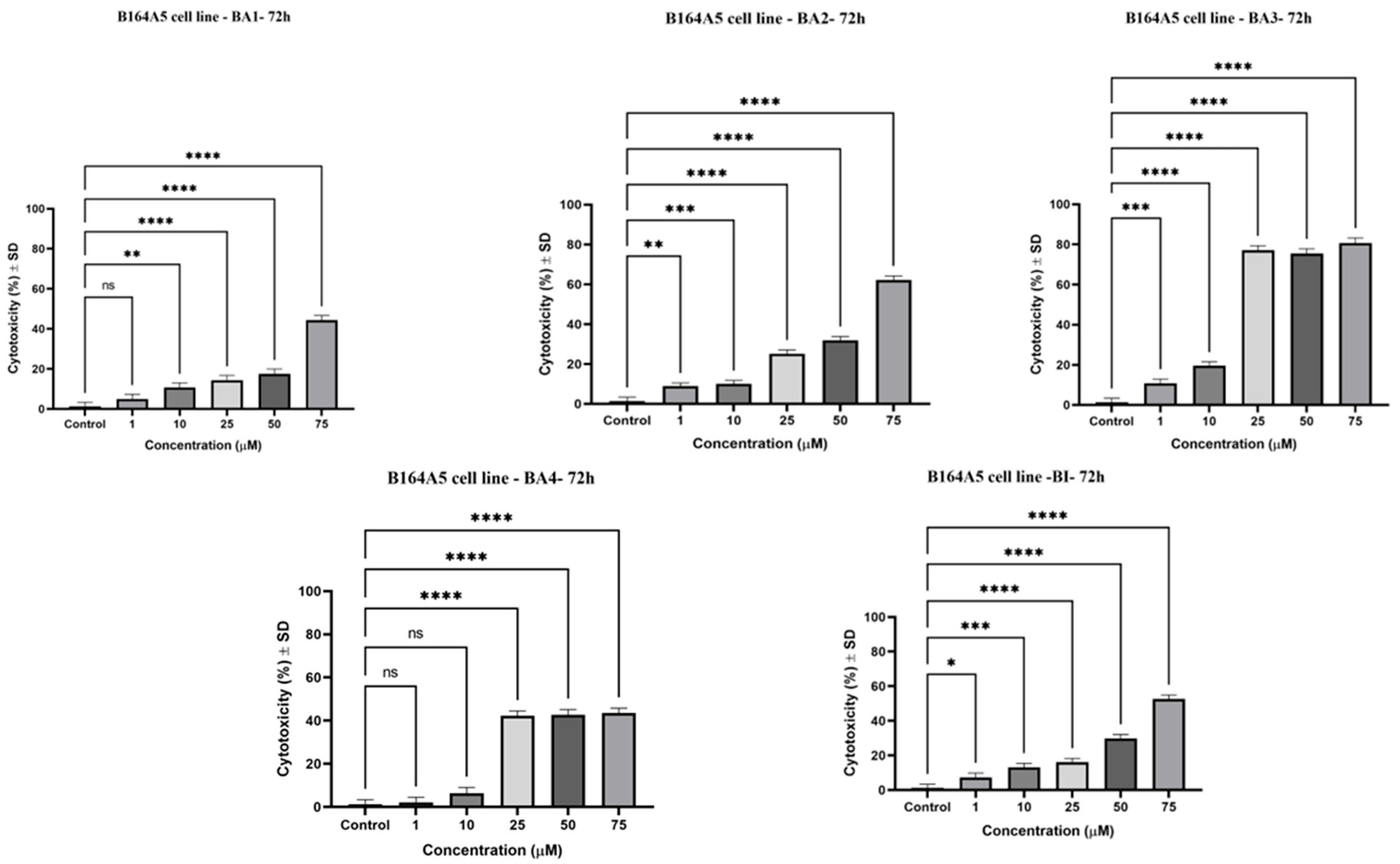
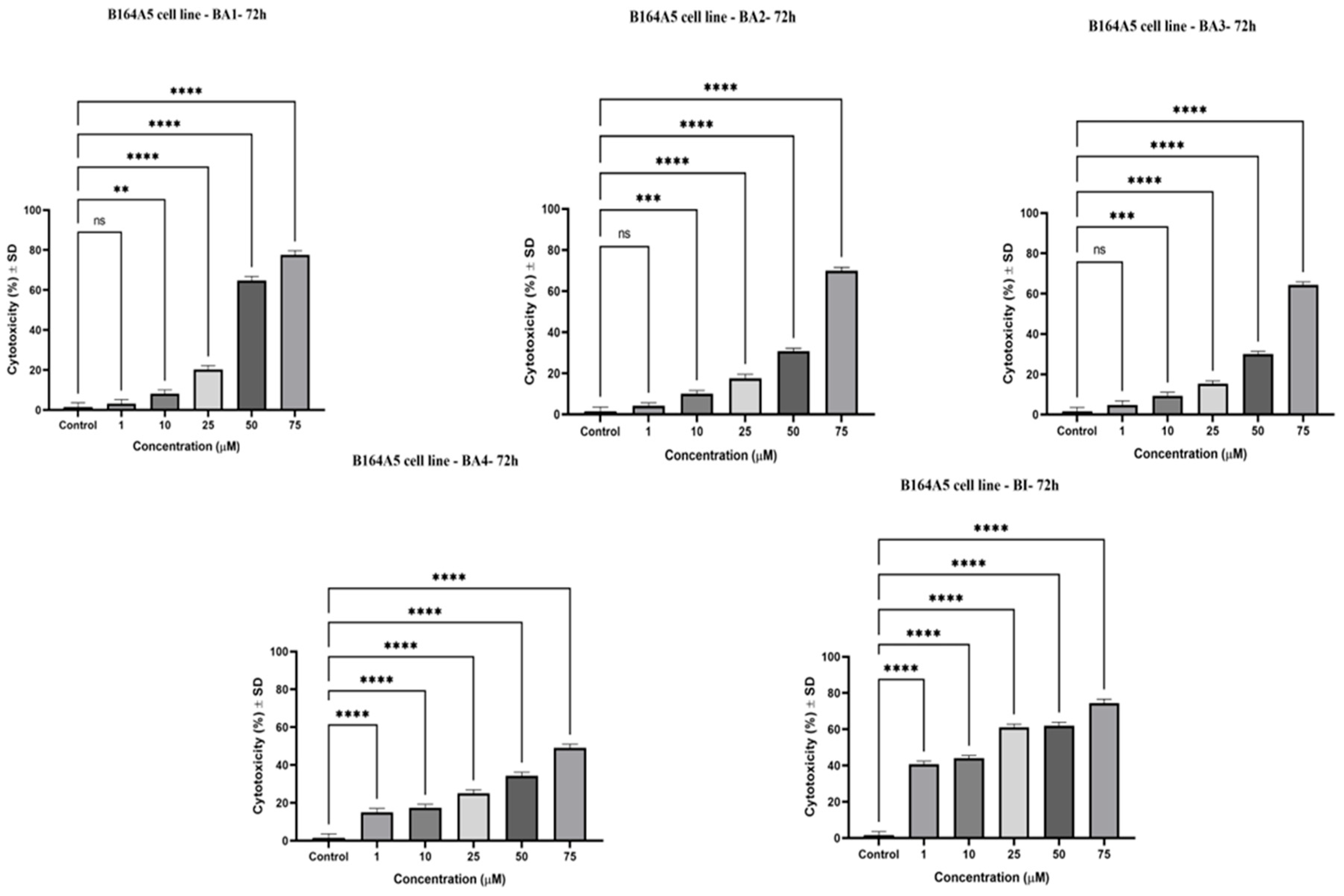
| Compound | IC50 (μM) |
|---|---|
| BI | 21.14 ± 0.08 |
| BA1 | 10.34 ± 0.06 |
| BA2 | 9.15 ± 0.05 |
| BA3 | 8.11 ± 0.13 |
| BA4 | 17.62 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombrea, A.; Watz, C.G.; Bora, L.; Dehelean, C.A.; Diaconeasa, Z.; Dinu, S.; Turks, M.; Lugiņina, J.; Peipiņš, U.; Danciu, C. Enhanced Cytotoxicity and Antimelanoma Activity of Novel Semisynthetic Derivatives of Betulinic Acid with Indole Conjugation. Plants 2024, 13, 36. https://doi.org/10.3390/plants13010036
Lombrea A, Watz CG, Bora L, Dehelean CA, Diaconeasa Z, Dinu S, Turks M, Lugiņina J, Peipiņš U, Danciu C. Enhanced Cytotoxicity and Antimelanoma Activity of Novel Semisynthetic Derivatives of Betulinic Acid with Indole Conjugation. Plants. 2024; 13(1):36. https://doi.org/10.3390/plants13010036
Chicago/Turabian StyleLombrea, Adelina, Claudia Geanina Watz, Larisa Bora, Cristina Adriana Dehelean, Zorita Diaconeasa, Stefania Dinu, Māris Turks, Jevgeņija Lugiņina, Uldis Peipiņš, and Corina Danciu. 2024. "Enhanced Cytotoxicity and Antimelanoma Activity of Novel Semisynthetic Derivatives of Betulinic Acid with Indole Conjugation" Plants 13, no. 1: 36. https://doi.org/10.3390/plants13010036
APA StyleLombrea, A., Watz, C. G., Bora, L., Dehelean, C. A., Diaconeasa, Z., Dinu, S., Turks, M., Lugiņina, J., Peipiņš, U., & Danciu, C. (2024). Enhanced Cytotoxicity and Antimelanoma Activity of Novel Semisynthetic Derivatives of Betulinic Acid with Indole Conjugation. Plants, 13(1), 36. https://doi.org/10.3390/plants13010036











