Testing the Tropical Niche Conservatism Hypothesis: Climatic Niche Evolution of Escallonia Mutis ex L. F. (Escalloniaceae)
Abstract
1. Introduction
2. Results
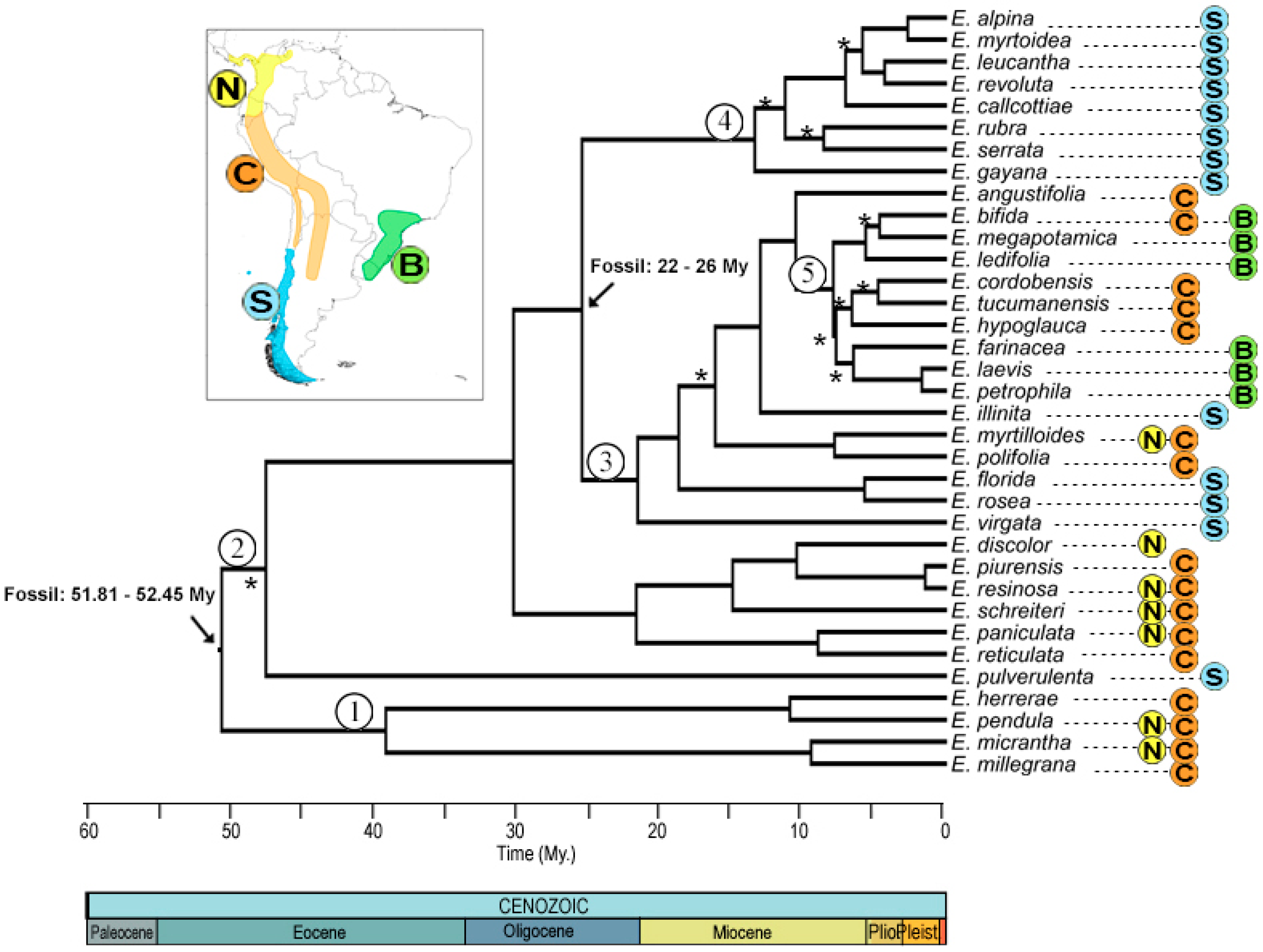
2.1. Phylogenetic Reconstruction
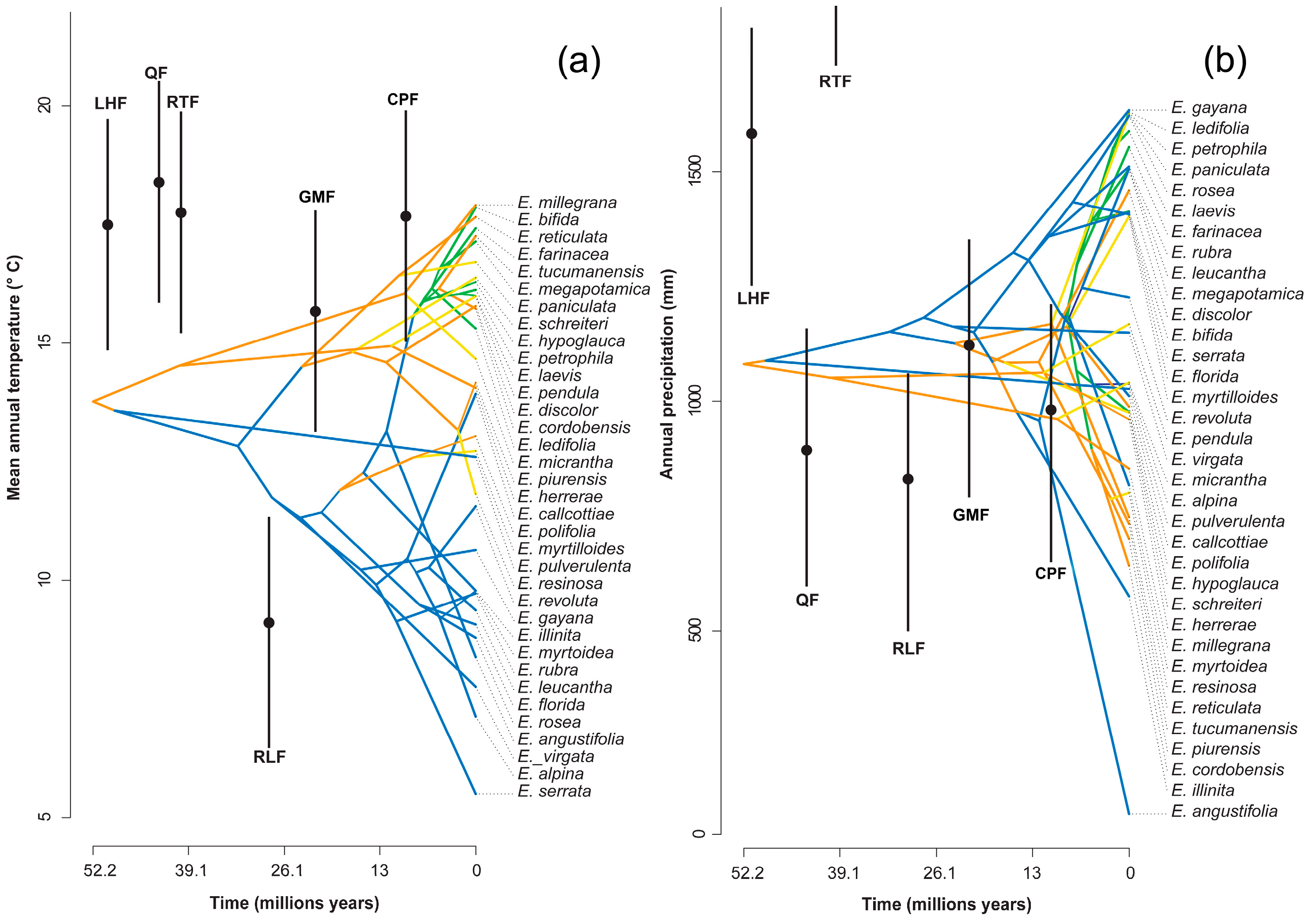
2.2. Ancestral Climate Reconstruction
2.3. Evolution Models and Niche Conservatism
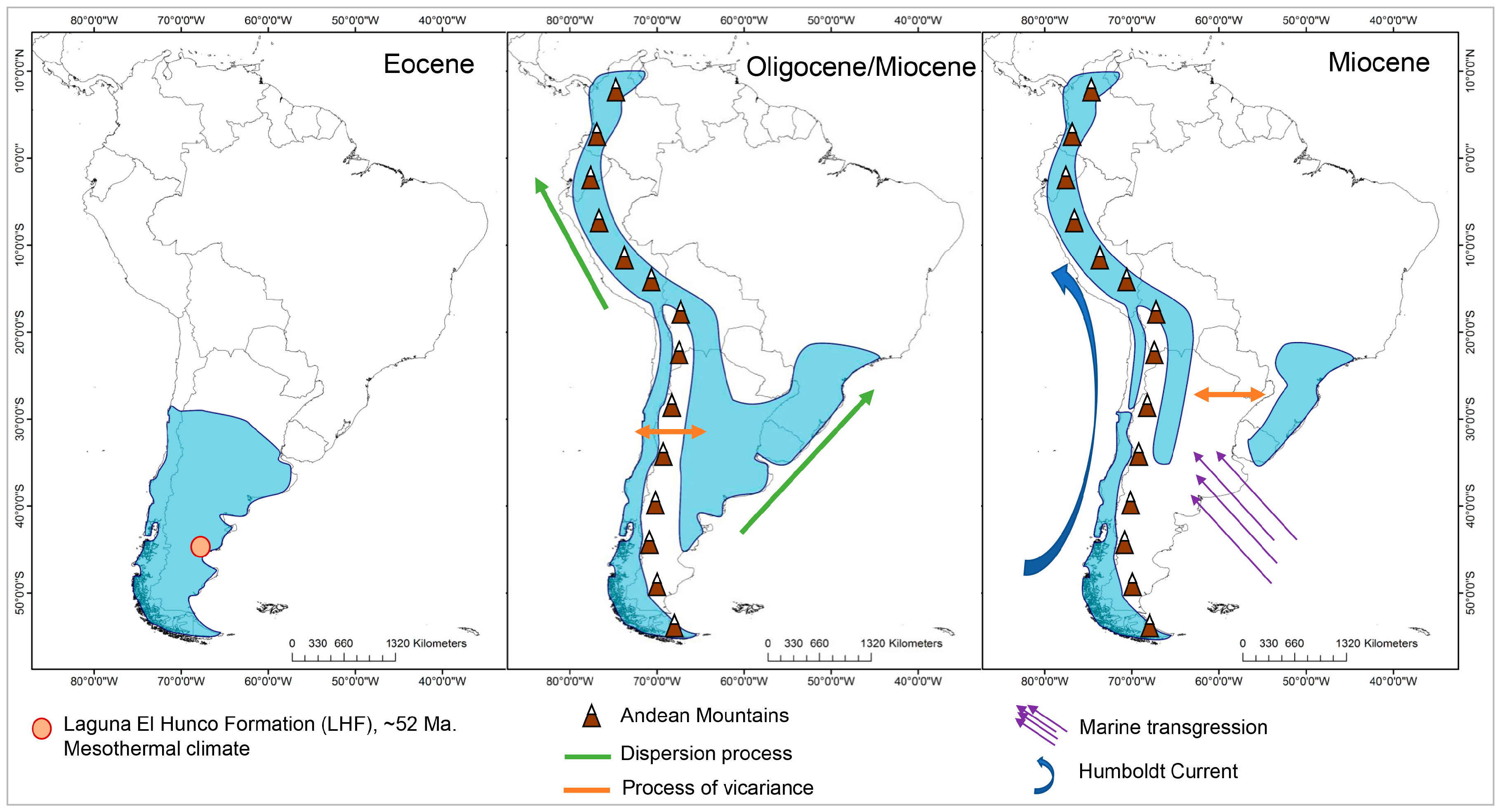
2.4. Ancestral Area Reconstruction
3. Discussion
3.1. Phylogenetic Reconstruction
3.2. Ancestral Climate Reconstruction
3.3. Evolution Models and Niche Conservatism
4. Materials and Methods
4.1. Niche Modeling
4.2. Phylogenetic Reconstruction
4.3. Evolution Models and Niche Conservatism
4.4. Ancestral Area Reconstruction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, D.H. Energy supply and patterns of species richness on local and regional scales. In Species Diversity in Ecological Communities: Historical and Geographical Perspectives, 1st ed.; Ricklefs, R.E., Schluter, D., Eds.; University of Chicago Press: Chicago, IL, USA, 1993; Volume 1, pp. 66–74. [Google Scholar]
- Hawkins, B.A.; Field, R.; Cornell, H.V.; Currie, D.J.; Guégan, J.F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; O’Brien, E.M.; et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology 2003, 84, 3105–3117. [Google Scholar] [CrossRef]
- Field, R.; Hawkins, B.A.; Cornell, H.V.; Currie, D.J.; Diniz-Filho, J.A.; Guégan, J.F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; et al. Spatial species-richness gradients across scales: A meta-analysis. J. Biogeogr. 2009, 36, 132–147. [Google Scholar] [CrossRef]
- Clarke, A.; Gaston, K.J. Climate, energy and diversity. Proc. R. Soc. B 2006, 273, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Ricklefs, R.E. Taxon richness and climate in angiosperms: Is there a globally consistent relationship that precludes region effects? Am. Nat. 2004, 163, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.J.; Donoghue, M.J. Historical biogeography, ecology and species richness. Trends Ecol. Evol. 2004, 19, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Crane, P.R.; Lidgard, S. Angiosperm diversification and paleolatitudinal gradients in Cretaceous floristic diversity. Science 1989, 246, 675–678. [Google Scholar] [CrossRef]
- Francis, A.P.; Currie, D.J. A globally consistent richness-climate relationship for angiosperms. Am. Nat. 2003, 161, 523–536. [Google Scholar] [CrossRef]
- Behrensmeyer, A.K.; Damuth, J.D.; Dimichelle, W.A.; Potts, R.; Sues, H.; Wing, S. Terrestrial ecosystems through time. In Evolutionary Paleoecology of Terrestrial Plants and Animals, 1st ed.; University of Chicago Press: Chicago, IL, USA, 1992. [Google Scholar]
- Donoghue, M.J. A phylogenetic perspective on the distribution of plant diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11549–11555. [Google Scholar] [CrossRef]
- Kerkhoff, A.J.; Moriarty, P.E.; Weiser, M.D. The latitudinal species richness gradient in New World woody angiosperms is consistent with the tropical conservatism hypothesis. Proc. Natl. Acad. Sci. USA 2014, 111, 8125–8130. [Google Scholar] [CrossRef]
- Ricklefs, R.E. Historical and ecological dimensions of global patterns in plant diversity. Biol. Skr. 2005, 55, 583–603. [Google Scholar]
- Hinojosa, L.F.; Villagrán, C. Historia de los bosques del sur de Sudamérica, I: Antecedentes paleobotánicos, geológicos y climáticos del Terciario del cono sur de América. Rev. Chil. Hist. Nat. 1997, 70, 225–240. [Google Scholar]
- Villagrán, C.; Hinojosa, L.F. Historia de los bosques del sur de Sudamérica, II: Análisis fitogeográfico. Rev. Chil. Hist. Nat. 1997, 70, 1–267. [Google Scholar]
- Villagrán, C.; Armesto, J.J. Fitogeografía histórica de la Cordillera de la Costa de Chile. In Historia, Biodiversidad y Ecología de los Bosques Costeros de Chile, 1st ed.; Editorial Universitaria: Santiago, Chile, 2005; pp. 99–116. [Google Scholar]
- Richardson, J.E.; Pennington, R.T.; Pennington, T.D.; Hollingsworth, P.M. Rapid diversification of a species-rich genus of neotropical rain forest trees. Science 2001, 293, 2242–2245. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, R.A.; Gil, P.R.; Hoffman, M.; Pilgrm, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J.; da Fonseca, G.A.B. Hotspots Revisited: Earths’s Biologically Richest and Most Endangered Terrestrial Ecoregions, 1st ed.; Conservation International, University of Chicago Press: Chicago, IL, USA, 2005; 392p. [Google Scholar]
- Kier, G.; Kreft, H.; Lee, T.M.; Jetz, W.; Ibisch, P.L.; Nowicki, C.; Mutke, J.; Barthlott, W. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. USA 2009, 106, 9322–9327. [Google Scholar] [CrossRef] [PubMed]
- Luebert, F.; Hilger, H.H.; Weigend, M. Diversification in the Andes: Age and origins of South American Heliotropium lineages (Heliotropiaceae, Boraginales). Mol. Phylogenet. Evol. 2011, 61, 90–102. [Google Scholar] [CrossRef]
- Kozak, K.H.; Wiens, J.J. Climatic zonation drives latitudinal variation in speciation mechanisms. Proc. R. Soc. B 2007, 274, 2995–3003. [Google Scholar] [CrossRef] [PubMed]
- Young, K.R.; Ulloa, C.U.; Luteyn, J.L.; Knapp, S. Plant evolution and endemism in Andean South America: An introduction. Bot. Rev. 2002, 68, 4–21. [Google Scholar] [CrossRef]
- Zapata, F. A multilocus phylogenetic analysis of Escallonia (Escalloniaceae): Diversification in montane South America. Am. J. Bot. 2013, 100, 526–545. [Google Scholar] [CrossRef]
- Sede, S.M.; Dürnhöfer, S.I.; Morello, S.; Zapata, F. Phylogenetics of Escallonia (Escalloniaceae) based on plastid DNA sequence data. Bot. J. Linn. 2013, 173, 442–451. [Google Scholar] [CrossRef]
- Gansser, A. Facts and theories on the Andes Twenty-sixth William Smith Lecture. J. Geol. Soc. Lond. 1973, 129, 93–131. [Google Scholar] [CrossRef]
- Peyre, G.; Montesinos, D.; Giraldo, D.; de Mera, A.G.; Ruthsatz, B.; Luebert, F.; Ontivero, M.; García, N.; Álvarez, M.; Meneses, R.I.; et al. VegAndes: The vegetation database for the Latin American highlands. Veg. Classif. Surv. 2022, 3, 287–296. [Google Scholar] [CrossRef]
- Weigend, M. Observations on the biogeography of the Amotape-Huancabamba zone in northern Peru. Bot. Rev. 2002, 68, 38–54. [Google Scholar] [CrossRef]
- Brako, L.; Zarucchi, J.L. Catalogue of the flowering plants and Gymnosperms of Peru. Catálogo de las Angiospermas y Gimnospermas del Perú. Monogr. Syst. Bot. 1993, 45, 1–1286. [Google Scholar]
- Lundberg, J. Phylogenetic Studies in the Euasterids II: With Particular Reference to Asterales and Escalloniaceae. Ph.D. Thesis, Faculty of Science and Technology, Uppsala University, Uppsala, Sweden, 2001. [Google Scholar]
- Tank, D.C.; Donoghue, M.J. Phylogeny and phylogenetic nomenclature of the Campanulidae based on an expanded sample of genes and taxa. Syst. Bot. 2010, 35, 425–441. [Google Scholar] [CrossRef]
- Nix, H.A. An environmental analysis of Australian rainforests. In The Rainforest Legacy: Australian National Rainforests Study, 3rd ed.; Australian Heritage Commission: Canberra, Australia, 1991; Volume 2, pp. 1–26. [Google Scholar]
- Pagel, M. Inferring the historical patterns of biological evolution. Nature 1999, 401, 877. [Google Scholar] [CrossRef] [PubMed]
- Matzke, N.J. Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Syst. Biol. 2014, 63, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Kvasov, D.D.; Verbitsky, M.Y. Causes of Antarctic glaciation in the Cenozoic. Quat. Res. 1981, 15, 1–17. [Google Scholar] [CrossRef]
- Gutiérrez, N.M.; Le Roux, J.P.; Vásquez, A.; Carreño, C.; Pedroza, V.; Araos, J.; Oyarzún, J.L.; Pino, J.P.; Rivera, H.A.; Hinojosa, L.F. Tectonic events reflected by palaeocurrents, zircon geochronology, and palaeobotany in the Sierra Baguales of Chilean Patagonia. Tectonophysics 2017, 695, 76–99. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Molina, A.; Farias, M. Andean uplift, ocean cooling and Atacama hyperaridity: A climate modeling perspective. Earth Planet. Sci. Lett. 2010, 292, 39–50. [Google Scholar] [CrossRef]
- Alpers, C.N.; Brimhall, G.H. Middle Miocene climatic change in the Atacama Desert, northern Chile: Evidence from supergene mineralization at La Escondida. Geol. Soc. Am. Bull. 1988, 100, 1640–1656. [Google Scholar] [CrossRef]
- Hernández, R.M.; Jordan, T.E.; Farjat, A.D.; Echavarría, L.; Idleman, B.D.; Reynolds, J.H. Age, distribution, tectonics, and eustatic controls of the Paranense and Caribbean marine transgressions in southern Bolivia and Argentina. J. S. Am. Earth Sci. 2005, 19, 495–512. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, C.; Cárdenas, A. Global warming and neotropical rainforests: A historical perspective. Annu. Rev. Earth Planet. Sci. 2013, 41, 741–766. [Google Scholar] [CrossRef]
- Wilf, P.; Cúneo, N.R.; Johnson, K.R.; Hicks, J.F.; Wing, S.L.; Obradovich, J.D. High plant diversity in Eocene South America: Evidence from Patagonia. Science 2003, 300, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, L.F. Cambios climáticos y vegetacionales inferidos a partir de paleofloras cenozoicas del sur de Sudamérica. Rev. Geol. Chile 2005, 32, 95–115. [Google Scholar] [CrossRef]
- Hansen, T.F. Stabilizing selection and the comparative analysis of adaptation. Evolution 1997, 51, 1341–1351. [Google Scholar] [CrossRef]
- Cooper, N.; Jetz, W.; Freckleton, R.P. Phylogenetic comparative approaches for studying niche conservatism. J. Evol. Biol. 2010, 23, 2529–2539. [Google Scholar] [CrossRef]
- Butler, M.A.; King, A.A. Phylogenetic comparative analysis: A modeling approach for adaptive evolution. Am. Nat. 2004, 164, 683–695. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Evans, M.E.; Smith, S.A.; Flynn, R.S.; Donoghue, M.J. Climate, niche evolution, and diversification of the “bird-cage” Evening Primroses (Oenothera, Sections Anogra and Kleinia). Am. Nat. 2009, 173, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Heibl, C. PHYLOCLIM: Integrating Phylogenetics and Climatic Niche Modelling. R Package Version 0.9-4. 2011. Available online: https://cran.r-project.org/web/packages/phyloclim/phyloclim.pdf (accessed on 20 May 2016).
- Hall, T. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, A.; San Martín, J. Presencia del Género Escallonia (Magnoliopsida, Escalloniaceae) en el Terciario de Chile Central. Bol. Mus. Nac. Hist. Nat. 1999, 48, 29–36. [Google Scholar] [CrossRef]
- Flynn, J.J.; Charrier, R.; Croft, D.A.; Gans, P.B.; Herriott, T.M.; Wertheim, J.A.; Wyss, A.R. Chronologic implications of new Miocene mammals from the Cura-Mallín and Trapa Trapa formations, Laguna del Laja area, south central Chile. J. S. Am. Earth Sci. 2008, 26, 412–423. [Google Scholar] [CrossRef]
- Gandolfo, M.A.; Wilf, P. Lista de Plantas de la Laguna del Hunco. 2013. Available online: http://bhort.bh.cornell.edu/histology/taxonesLH.html (accessed on 20 May 2016).
- Rambaut, A.; Drummond, A.J. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar]
- Guerstein, G.R.; Guler, M.V.; Brinkhuis, H.; Warnaar, J. Mid-Cenozoic palaeoclimatic and palaeoceanographic trends inthe southwest Atlantic basins: A dinoflagellate view. In The Paleontology of Gran Barranca: Evolution and Environmental Change through the Middle Cenozoic of Patagonia, 1st ed.; Madden, R.H., Carlini, A.A., Vucetich, M.G., Kay, R.F., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 398–409. [Google Scholar]
- Gutiérrez, N.M.; Hinojosa, L.F.; Le Roux, J.P.; Pedroza, V. Evidence for an Early-Middle Miocene age of the Navidad Formation (central Chile): Paleontological, paleoclimatic and tectonic implications. Andean Geol. 2013, 40, 66–78. [Google Scholar]
- Hinojosa, F.; Pérez, F.; Rougier, D.; Villagrán, C.; Armesto, J.J. Legados históricos de la vegetación de bosque en Chile. In Ciencias Ecológicas 1983–2013. Treinta años de Investigaciones Chilenas, 1st ed.; Montecinos, V., Orlando, J., Eds.; Editorial Universitaria: Santiago, Chile, 2015; pp. 123–138. [Google Scholar]
- Pino, J.P. Cambios en la Composición y Diversidad Florística Asociados al Clima Durante el Mioceno en el Sur de Sudamérica: El caso de la Formación Navidad (Chile Central). Master’s Thesis, Faculty of Science, Universidad de Chile, Santiago, Chile, 2016. [Google Scholar]
- Harmon, L.J.; Losos, J.B.; Davies, J.T.; Gillespie, R.G.; Gittleman, J.L.; Jennings, W.B.; Kozak, K.H.; McPeek, M.A.; Moreno-Roark, F.; Near, T.J.; et al. Early bursts of body size and shape evolution are rare in comparative data. Evolution 2010, 64, 2385–2396. [Google Scholar] [CrossRef]
- Ree, R.H.; Smith, S.A.; Baker, A. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst. Biol. 2008, 57, 4–14. [Google Scholar] [CrossRef]
- Yu, Y.; Harris, A.J.; He, X. S-DIVA (Statistical Dispersal-Vicariance Analysis): A tool for inferring biogeographic histories. Mol. Phylogenet. Evol. 2010, 56, 848–850. [Google Scholar] [CrossRef]
- Landis, M.J.; Matzke, N.J.; Moore, B.R.; Huelsenbeck, J.P. Bayesian analysis of biogeography when the number of areas is large. Syst. Biol. 2013, 62, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Matzke, N.J. Probabilistic historical biogeography: New models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Front. Biogeogr. 2013, 5, 242–248. [Google Scholar] [CrossRef]
- Hinojosa, L.F.; Gaxiola, A.; Pérez, M.F.; Carvajal, F.; Campano, M.F.; Quattrocchio, M. Non-congruent fossil and phylogenetic evidence on the evolution of climatic niche in the Gondwana genus Nothofagus. J. Biogeogr. 2016, 43, 555–567. [Google Scholar] [CrossRef]


| Bioclimatic Variable | Southern Andes | Central Andes | Northern Andes | Brazil | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean | Min. | Max. | Mean | Min. | Max. | Mean | Min. | Max. | Mean | |
| Mean annual temperature (Bio 1) | 5.6 | 14.4 | 9.6 | 8.4 | 18.7 | 15.2 | 11.1 | 16.5 | 14.5 | 15.3 | 17.3 | 16.5 |
| Temperature seasonality (Bio 4) | 243.0 | 410.6 | 359.4 | 51.0 | 472.3 | 208.7 | 43.4 | 224.2 | 91.4 | 228.9 | 323.1 | 279.0 |
| Mean temperature of wettest quarter (Bio 4) | 3.3 | 12.8 | 6.1 | 7.0 | 22.2 | 16.9 | 11.3 | 18.6 | 15.1 | 17.7 | 20.0 | 18.7 |
| Mean temperature of warmest quarter (Bio 10) | 9.5 | 17.6 | 14.3 | 11.8 | 22.6 | 17.6 | 11.6 | 18.9 | 15.4 | 18.5 | 21.2 | 19.9 |
| Mean temperature of coldest quarter (Bio 11) | 1.4 | 11.5 | 5.1 | 4.9 | 15.9 | 12.3 | 10.1 | 15.8 | 13.1 | 11.8 | 13.4 | 12.8 |
| Mean annual precipitation (Bio 12) | 591.8 | 1852.3 | 1284.6 | 78.8 | 1372.9 | 807.9 | 871.2 | 1674.3 | 1185.1 | 1484.5 | 1809.1 | 1584.9 |
| Precipitation of the wettest quarter (Bio 16) | 345.6 | 925.7 | 628.7 | 51.5 | 516.8 | 375.5 | 436.9 | 642.4 | 526.6 | 479.7 | 750.1 | 549.1 |
| Precipitation of the warmest quarter (Bio 18) | 29 | 357.9 | 126 | 21.9 | 437.7 | 309.7 | 211.5 | 464.1 | 346.4 | 449.1 | 714.1 | 529 |
| Precipitation of the coldest quarter (Bio 19) | 328.6 | 866.8 | 580.2 | 26.8 | 440.2 | 94 | 49.6 | 369.7 | 219.6 | 219.7 | 354.1 | 283.8 |
| Altitude (m) | 239.4 | 1585.8 | 890.5 | 855 | 2811.2 | 2059.8 | 2132.9 | 3162.5 | 2531.2 | 678.6 | 1284.9 | 921.3 |
| Variable | BM | OU | WN | Lambda | p-Value (Different from 1) | p-Value (Different from 0) |
|---|---|---|---|---|---|---|
| Bio 1 | 0.68 | 0.32 | <0.01 | 1 | 1 | <0.01 |
| Bio 4 | 0.73 | 0.27 | <0.01 | 1 | 1 | <0.01 |
| Bio 8 | 0.77 | 0.23 | <0.01 | 1 | 1 | <0.01 |
| Bio 10 | 0.15 | 0.84 | <0.01 | 0.95 | 1 | <0.01 |
| Bio 11 | 0.71 | 0.29 | <0.01 | 0.97 | 1 | <0.01 |
| Bio 12 | 0.04 | 0.65 | 0.31 | 0 | <0.01 | 1 |
| Bio 16 | 0.08 | 0.58 | 0.33 | 0 | <0.01 | 1 |
| Bio 18 | 0.56 | 0.44 | <0.01 | 1 | 1 | <0.01 |
| Bio 19 | 0.72 | 0.28 | <0.01 | 1 | 1 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibán, M.J.; Hinojosa, L.F. Testing the Tropical Niche Conservatism Hypothesis: Climatic Niche Evolution of Escallonia Mutis ex L. F. (Escalloniaceae). Plants 2024, 13, 133. https://doi.org/10.3390/plants13010133
Dibán MJ, Hinojosa LF. Testing the Tropical Niche Conservatism Hypothesis: Climatic Niche Evolution of Escallonia Mutis ex L. F. (Escalloniaceae). Plants. 2024; 13(1):133. https://doi.org/10.3390/plants13010133
Chicago/Turabian StyleDibán, María José, and Luis Felipe Hinojosa. 2024. "Testing the Tropical Niche Conservatism Hypothesis: Climatic Niche Evolution of Escallonia Mutis ex L. F. (Escalloniaceae)" Plants 13, no. 1: 133. https://doi.org/10.3390/plants13010133
APA StyleDibán, M. J., & Hinojosa, L. F. (2024). Testing the Tropical Niche Conservatism Hypothesis: Climatic Niche Evolution of Escallonia Mutis ex L. F. (Escalloniaceae). Plants, 13(1), 133. https://doi.org/10.3390/plants13010133





