Silvicultural Practices for Diversity Conservation and Invasive Species Suppression in Forest Ecosystems of the Bundala National Park, Sri Lanka
Abstract
1. Introduction
2. Results
2.1. Number of Plant Species and Individuals
2.2. Species Diversity
2.3. Interaction among Species Diversity, Plant Density, and Treatments over Time
3. Discussion
4. Materials and Methods
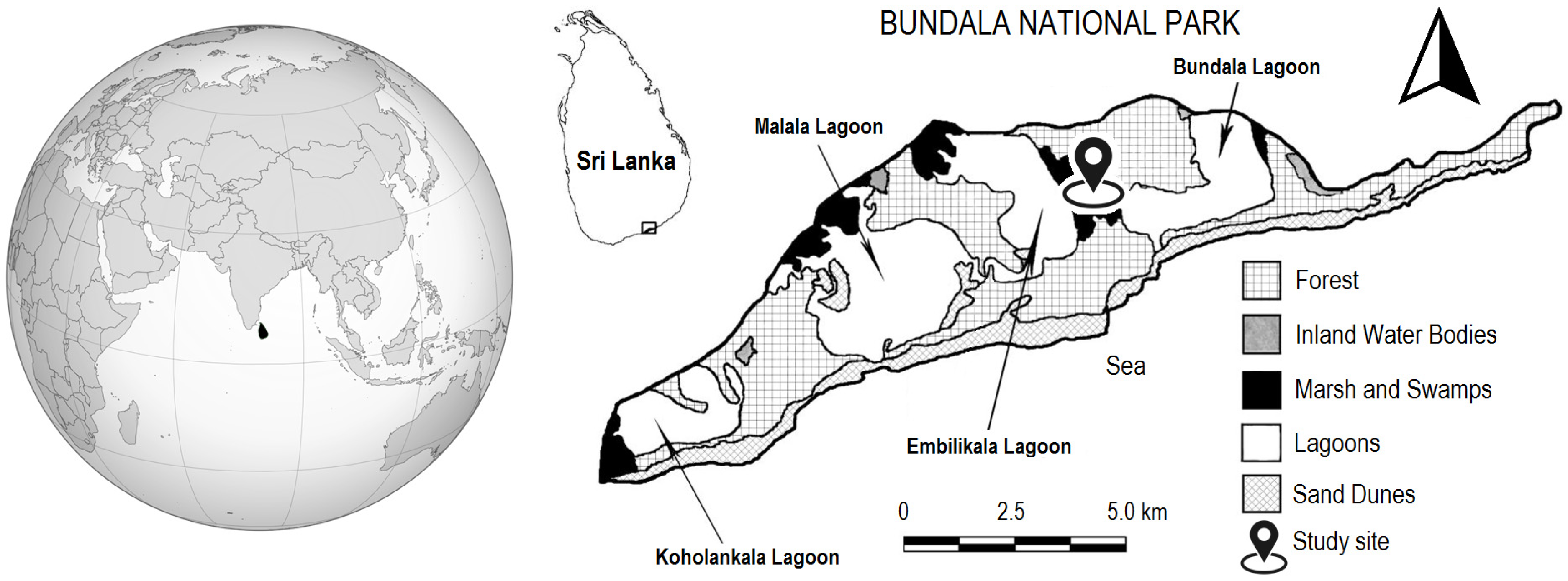
4.1. Study Area
4.2. Data Collection and Treatments
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellis, E.C.; Kaplan, J.O.; Fuller, D.Q.; Verburg, P.H. Used planet: A global history. Proc. Natl. Acad. Sci. USA 2013, 110, 7978–7985. [Google Scholar] [CrossRef] [PubMed]
- Davison, C.W.; Rahbek, C.; Morueta-Holme, N. Land-use change and biodiversity: Challenges for assembling evidence on the greatest threat to nature. Glob. Chang. Biol. 2021, 27, 5414–5429. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.C.; Goldewijk, K.K.; Siebert, S.; Lightman, D.; Ramankutty, N. Anthropogenic transformation of the biomes, 1700 to 2000. Glob. Ecol. Biogeogr. 2010, 19, 589–606. [Google Scholar] [CrossRef]
- Kareiva, P.; Watts, S.; McDonald, R.; Boucher, T. Domesticated nature: Shaping landscapes and ecosystems for human welfare. Science 2007, 316, 1866–1869. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Hadly, E.A.; Bascompte, J.; Berlow, E.L.; Brown, J.H.; Fortelius, M.; Getz, W.M.; Harte, J.; Hastings, A.; Marquet, P.A.; et al. Approaching a state shift in Earth’s biosphere. Nature 2012, 486, 52–58. [Google Scholar] [CrossRef]
- Hilson, G. An overview of land use conflicts in mining communities. Land Use Policy 2002, 19, 65–73. [Google Scholar] [CrossRef]
- Vacek, Z.; Cukor, J.; Vacek, S.; Podrázský, V.; Linda, R.; Kovařík, J. Forest biodiversity and production potential of post-mining landscape: Opting for afforestation or leaving it to spontaneous development? Cent. Eur. For. J. 2018, 64, 116–126. [Google Scholar] [CrossRef]
- Vacek, Z.; Cukor, J.; Vacek, S.; Linda, R.; Prokůpková, A.; Podrázský, V.; Gallo, J.; Vacek, O.; Šimůnek, V.; Drábek, O.; et al. Production potential, biodiversity and soil properties of forest reclamations: Opportunities or risk of introduced coniferous tree species under climate change? Eur. J. For. Res. 2021, 140, 1243–1266. [Google Scholar] [CrossRef]
- Seki, H.A.; Thorn, J.P.; Platts, P.J.; Shirima, D.D.; Marchant, R.A.; Abeid, Y.; Baker, N.; Annandale, M.; Marshall, A.R. Indirect impacts of commercial gold mining on adjacent ecosystems. Biol. Conserv. 2022, 27, 109782. [Google Scholar] [CrossRef]
- WWF. Living Planet Report. In Risk and Resilience in a New Era; WWF International: Gland, Switzerland, 2016; 144p. [Google Scholar]
- IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Díaz, S., Settele, S., Brondízio, E., Ngo, H.T., Guèze, M., Agard, J., Arneth, A., Balvanera, P., Brauman, K., Butcharet, S., et al., Eds.; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- Newbold, T.; Hudson, L.N.; Phillips, H.R.P.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Blandon, A.; Butchart, S.H.M.; Booth, H.L.; Day, J.; et al. A global model of the response of tropical and sub-tropical forest biodiversity to anthropogenic pressures. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141371. [Google Scholar] [CrossRef]
- Hermosilla-Palma, K.; Pliscoff, P.; Folchi, M. Sixty years of land-use and land-cover change dynamics in a global biodiversity hotspot under threat from global change. J. Land Use Sci. 2021, 16, 467–478. [Google Scholar] [CrossRef]
- Radwan, T.M.; Blackburn, G.A.; Whyatt, J.D.; Atkinson, P.M. Global land cover trajectories and transitions. Sci. Rep. 2021, 11, 12814. [Google Scholar] [CrossRef] [PubMed]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Jetz, W.; Wilcove, D.S.; Dobson, A.P. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 2007, 5, e157. [Google Scholar] [CrossRef] [PubMed]
- Pekin, B.K.; Pijanowski, B.C. Global land use intensity and the endangerment status of mammal species. Divers. Distrib. 2012, 18, 909–918. [Google Scholar] [CrossRef]
- Phillips, H.R.P.; Newbold, T.; Purvis, A. Land-use effects on local biodiversity in tropical forests vary between continents. Biodivers. Conserv. 2017, 26, 2251–2270. [Google Scholar] [CrossRef] [PubMed]
- Dirzo, R.; Raven, P.H. Global state of biodiversity and loss. Annu. Rev. Environ. Resour. 2003, 28, 137–167. [Google Scholar] [CrossRef]
- Wright, S.J. Tropical forests in a changing environment. Trends Ecol. Evol. 2005, 20, 553–560. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Wright, S.J.; Muller-Landau, H.C. The future of tropical forest species. Biotropica 2006, 38, 287–301. [Google Scholar] [CrossRef]
- Lewis, S.L.; Edwards, D.P.; Galbraith, D. Increasing human dominance of tropical forests. Science 2015, 349, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Chazdon, R.L.; Peres, C.A.; Dent, D.; Sheil, D.; Lugo, A.L.; Lamb, D.; Stork, N.E.; Miller, S.E. The potential for species conservation in tropical secondary forests. Conserv. Biol. 2009, 23, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.V. The invasibility of tropical forests by exotic plants. J. Trop. Ecol. 2002, 18, 687–705. [Google Scholar] [CrossRef]
- Kerns, B.K.; Tortorelli, C.; Day, M.A.; Nietupski, T.; Barros, A.M.; Kim, J.B.; Krawchuk, M.A. Invasive grasses: A new perfect storm for forested ecosystems? For. Ecol. Manag. 2020, 463, 11798. [Google Scholar] [CrossRef]
- Lapin, K.; Oettel, J.; Steiner, H.; Langmaier, M.; Sustic, D.; Starlinger, F.; Kindermann, G.; Frank, G. Invasive Alien Plant Species in Unmanaged Forest Reserves, Austria. NeoBiota 2019, 48, 71–96. [Google Scholar] [CrossRef]
- Perrings, C.; Naeem, S.; Ahrestani, F.; Bunker, D.E.; Burkill, P.; Canziani, G.; Elmqvist, T.; Ferrati, R.; Fuhrman, J.; Jaksic, F.; et al. Ecosystem services for 2020. Science 2010, 330, 323–324. [Google Scholar] [CrossRef]
- McNeely, J.A. (Ed.) The Great Reshuffling: Human Dimensions of Invasive Alien Species; IUCN: Gland, Switzerland; Cambridge, UK, 2001. [Google Scholar]
- Bambaradeniya, C.N.B. The status and implications of alien invasive species in Sri Lanka. Zoos’ Print J. 2002, 17, 930–935. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P.; Rejmanek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Felton, A.; Boberg, J.; Björkman, C.; Widenfalk, O. Identifying and managing the ecological risks of using introduced tree species in Sweden’s production forestry. For. Ecol. Manag. 2013, 307, 165–177. [Google Scholar] [CrossRef]
- Vacek, Z.; Vacek, S.; Eşen, D.; Yildiz, O.; Král, J.; Gallo, J. Effect of Invasive Rhododendron ponticum L. on Natural Regeneration and Structure of Fagus orientalis Lipsky Forests in the Black Sea Region. Forests 2020, 11, 603. [Google Scholar] [CrossRef]
- Vacek, Z.; Linda, R.; Cukor, J.; Vacek, S.; Šimůnek, V.; Gallo, J.; Vančura, K. Scots pine (Pinus sylvestris L.), the suitable pioneer species for afforestation of reclamation sites? For. Ecol. Manag. 2021, 485, 118951. [Google Scholar] [CrossRef]
- Alizoti, P.; Bastien, J.-C.; Chakraborty, D.; Klisz, M.M.; Kroon, J.; Neophytou, C.; Schueler, S.; Loo, M.; Westergren, M.; Konnert, M.; et al. Non-Native Forest Tree Species in Europe: The Question of Seed Origin in Afforestation. Forests 2022, 13, 273. [Google Scholar] [CrossRef]
- Nandasena, W.D.K.V.; Brabyn, L.; Serrao-Neumann, S. Monitoring invasive pines using remote sensing: A case study from Sri Lanka. Environ. Monit. Assess. 2023, 195, 347. [Google Scholar] [CrossRef]
- Moodley, D.; Foxcroft, L.C.; Novoa, A.; Pyšková, K.; Pergl, J.; Pyšek, P. Invasive alien species add to the uncertain future of protected areas. Neobiota 2020, 57, 1–5. [Google Scholar] [CrossRef]
- Kariyawasam, C.S.; Kumar, L.; Ratnayake, S.S. Invasive plants distribution modeling: A tool for tropical biodiversity conservation with special reference to Sri Lanka. Trop. Conserv. Sci. 2019, 12, 1940082919864269. [Google Scholar] [CrossRef]
- Ekanayake, E.M.B.P.; Xie, Y.; Ibrahim, A.S.; Karunaratne, N.T.P.; Ahmad, S. Effective governance for management of invasive alien plants: Evidence from the perspective of forest and wildlife officers in Sri Lanka. PeerJ 2020, 8, e8343. [Google Scholar] [CrossRef]
- Herath, H.M.R.P.; Wijesundara, C.B. Factors Affecting for Online Purchase Decisions of Sri Lankan Consumer (with Special Reference to Western Province). In Proceedings of the International Conference on Social Sceinces Sri lanka 2008 (ICSSL), Colombo, Sri Lanka, 18–20 July 2008. [Google Scholar]
- Bambaradeniya, C.N.B. Guide to Bundala: A Guide to the Biodiversity of Bundala National Park, a Ramsar Wetland in Sri Lanka; IUCN, International Union for Conservation of Nature: Grann, Switzerland; Colombo, Sri Lanka, 2001; 54p, ISBN 955-8177-12-1. Available online: https://policycommons.net/artifacts/1371762/guide-to-bundala/1985936/ (accessed on 15 February 2023).
- Perera, N. An Overview of Bundala National Park: An exceptional wetland facing multitude of problems. Siyoth 2007, 2, 4–8. [Google Scholar]
- Gunarathne, R.M.U.K.; Perera, G.A.D. Die-out of Manilkara hexandra from Bundala National Park, Sri Lanka: Causes and some possible underlying mechanisms. J. Trop. For. Environ. 2014, 4, 14–27. [Google Scholar] [CrossRef]
- Suraweera, C.; Kumari, B.; Dahanayaka, D.D.G.L. Assessment of invasive alien species at Bundala Ramsar wetland, Sri Lanka, in order to their control and management. In Proceedings of the National Symposium on Invasive Alien Species; Biodiversity Secratariet: Colombo, Sri Lanka, 2017; p. 14. [Google Scholar]
- ISAC. Invasive Species Advisory Committee: Invasive Species Definition Clarification and Guidance White Paper; The National Invasive Species Council (NISC): Beltsville, MA, USA, 2006. Available online: http://www.invasivespeciesinfo.gov/docs/council/isacdef.pdf (accessed on 4 April 2023).
- IUCN. IUCN Sri Lanka and the Ministry of Environment and Natural Resources. In The 2007 Red List of Threatened Fauna and Flora of Sri Lanka; The World Conservation Union (IUCN) and Ministry of Environment and Natural Resources: Colombo, Sri Lanka, 2007; 148p. [Google Scholar]
- Yang, Q.; Ye, W.; Liao, F.; Yin, X. Effects of allelochemicals on seed germination. Chin. J. Ecol. 2005, 24, 1459–1465. [Google Scholar]
- Alford, E.R.; Perry, L.G.; Qin, B.; Vivanco, J.M.; Paschke, M.W. A putative allelopathic agents of Russian knapweed occurs in invaded soils. Soil Biol. Biochem. 2007, 39, 1812–1815. [Google Scholar] [CrossRef]
- Shipunov, A.; Newcombe, G.; Raghavendra, A.K.H.; Andersen, C.L. Hidden diversity of endophytic fungi in an invasive plant. Am. J. Bot. 2008, 95, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Aschehoug, E.T.; Metlen, K.L.; Callaway, R.M.; Newcombe, G. Fungal endophytes directly increase the competitive effects of an invasive forb. Ecology 2012, 93, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, H.D.; Smarakoon, S.P.A.G.V.; Karunaratne, Y.G.P. Case Study on Community Participation in the Management and Conservation of the Bundala National Park. J. Natn. Sci. Found. Sri Lanka 2003, 31, 73–78. [Google Scholar] [CrossRef]
- DWC. Biodiversity Baseline Survey. In Sri Lanka Protected Areas Management and Wildlife Conservation Project (PAM&WCP/CONSULT/02/BDBS); De Alwis, S.M.D.A.U., Dayawansa, P.N., How, R., Padmalal, U.K.G.K., Singhakumara, B.M.P., Weerakoon, D., Wijesinghe, M.R., Infotechs IDEAS in association with GREENTECH Consultants, Bundala National Park Consultancy Services, Green, M.J.B., Eds.; Department of Wildlife Conservation, Ministry of Environment and Natural Resources: Colombo, Sri Lanka, 2008; p. 46. [Google Scholar]
- Mwangi, E.; Swallow, B. Prosopis juliflora Invasion and Rural Livelihoods in the Lake Baringo Area of Kenya. Conserv. Soc. 2008, 6, 130–140. [Google Scholar]
- Weerawardane, N.D. Status of Forest Invasive Species in Sri Lanka; Forest Department: Colombo, Sri Lanka, 2018. [Google Scholar]
- Walter, K. Prosopis, an Alien among the Sacred Trees of South India. Ph.D. Dissertation, University of Helsinki, Helsinki, Finland, 2011. [Google Scholar]
- Royal Botanic Gardens Kew. Opuntia dillenii (Ker Gawl.) Haw. Plants of the World Online. Kew Science. 2022. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:136534-1 (accessed on 4 April 2023).
- Bambaradeniya, C.N.B.; Ekanayake, S.P.; Fernando, R.H.S.S.; Perera, W.P.N.; Somaweera, R. A Biodiversity Status Profile of Bundala National Park: A Ramsar National Wetland of Sri Lanka; Occasional Paper of IUCN Sri Lanka; IUCN Sri Lanka: Colombo, Sri Lanka, 2002. [Google Scholar]
- Galappaththi, M.C.A.; Patabendige, N.M.; Amarasinghe, S.S.; Ranawana, K.B.; Karunaratne, W.A.I.P. Cochineal Scale Dactylopius opuntiae controls Opuntia dillenii in Bundala National Park, Sri Lanka. Ceylon J. Sci. 2021, 50, 297–301. [Google Scholar] [CrossRef]
- Barlow, J.; Gardner, T.A.; Araujo, I.S.; Ávila-Pires, T.C.; Bonaldo, A.B.; Costa, J.E.; Esposito, M.C.; Ferreira, L.V.; Hawes, J.; Hernandez, M.I.M.; et al. Quantifying the biodiversity value of tropical primary, secondary, and plantation forests. Proc. Natl. Acad. Sci. USA 2007, 104, 18555–18560. [Google Scholar] [CrossRef]
- Barlow, J.; Overal, W.L.; Araujo, I.S.; Gardener, T.A.; Peres, C.A. The value of primary, secondary and plantation forests for fruit-feeding butterflies in the Brazilian Amazon. J. Appl. Ecol. 2007, 44, 1001–1012. [Google Scholar] [CrossRef]
- Berry, N.J.; Phillips, O.L.; Lewis, S.L.; Hill, J.K.; Edwards, D.P.; Tawatao, N.B.; Ahmad, N.; Magintan, D.; Khen, C.V.; Maryati, M.; et al. The high value of logged tropical forests: Lessons from northern Borneo. Biodivers. Conserv. 2010, 19, 985–997. [Google Scholar] [CrossRef]
- Struebig, M.J.; Turner, A.; Giles, E.; Lasmana, F.; Tollington, S.; Bernard, H.; Bell, D. Quantifying the biodiversity value of repeatedly logged rainforests: Gradient and comparative approaches from Borneo. Adv. Ecol. Res. 2013, 48, 183–224. [Google Scholar] [CrossRef]
- Floren, A.; Linsenmair, K.E. The importance of primary tropical rain forest for species diversity: An investigation using arboreal ants as an example. Ecosystems 2005, 8, 559–567. [Google Scholar] [CrossRef]
- Bihn, J.H.; Verhaagh, M.; Brändle, M.; Brandl, R. Do secondary forests act as refuges for old growth forest animals? Recovery of ant diversity in the Atlantic forest of Brazil. Biol. Conserv. 2008, 141, 733–743. [Google Scholar] [CrossRef]
- Gibson, L.; Lee, T.M.; Koh, L.P.; Brook, B.; Gardner, T.A.; Barlow, J.; Peres, C.; Bradshaw, C.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. Arid Zone Programme, A Note on the Climate and Vegetation of the Arid Zone of Ceylon; United Nations Educational Scientific and Cultural Organization: Paris, France, 1951; pp. 1–4. [Google Scholar]
- Ranwala, S. Flora of Sri Lanka. In Training Course for National Tourist Guides; Department of Plant Science, University of Colombo: Colombo, Sri Lanka, 2012; pp. 1–11. [Google Scholar]
- DeWalt, S.J.; Maliakal, S.K.; Denslow, J.S. Changes in vegetation structure and composition along a tropical forest chronosequence: Implications for wildlife. For. Ecol. Manag. 2003, 182, 139–151. [Google Scholar] [CrossRef]
- Veddeler, D.; Schulze, C.H.; Steffan-Dewenter, I.; Buchori, D.; Tscharntke, T. The contribution of tropical secondary forest fragments to the conservation of fruit-feeding butterflies: Effects of isolation and age. Biodivers. Conserv. 2005, 14, 3577–3592. [Google Scholar] [CrossRef]
- Martin, P.; Bullock, J.; Newton, A. Carbon pools recover more rapidly than plant biodiversity in secondary tropical forests. Philos. Trans. R. Soc. B 2013, 280, 20132236. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Norden, N.; Angarita, H.A.; Bongers, F.; Martínez-Ramos, M.; Granzow-de la Cerda, I.; Van Breugel, M.; Lebrija-Trejos, E.; Meave, J.A.; Vandermeer, J.; Williamson, G.B.; et al. Successional dynamics in Neotropical forests are as uncertain as they are predictable. Proc. Natl. Acad. Sci. USA 2015, 112, 8013–8018. [Google Scholar] [CrossRef]
- Edirisinghe, E.A.S.R.; Ranaweera, B.; Suraweera, P.A.C.N.B. Evaluation of Uprooting Method to Control Prosopis juliflora in Bundala National Park of Sri Lanka. In Proceedings of the 17th Agricultural Research Symposium, Colombo, Sri Lanka, 28–29 November 2018; Volume 1. No. 5. [Google Scholar]
- Kotagama, S.W.; Bambaradeniya, C.N.B. An Overview of the Wetlands of Sri Lanka and Their Conservation Significance; National Wetland Directory of Sri Lanka: Colombo, Sri Lanka, 2006; pp. 7–16. [Google Scholar]
- Hájek, V.; Vacek, Z.; Vacek, S.; Bílek, L.; Prausová, R.; Linda, R.; Bulušek, D.; Králíček, I. Changes in diversity of protected scree and herb-rich beech forest ecosystems over 55 years. Cent. Eur. For. J. 2020, 66, 202–217. [Google Scholar] [CrossRef]
- Slanař, J.; Vacek, Z.; Vacek, S.; Bulušek, D.; Cukor, J.; Štefančík, I.; Bílek, L.; Král, J. Long-term transformation of submontane spruce-beech forests in the Jizerské hory Mts.: Dynamics of natural regeneration. Cent. Eur. For. J. 2017, 63, 213–225. [Google Scholar] [CrossRef]
- Mulya, H.; Santosa, Y.; Hilwan, I. Comparison of four species diversity indices in mangrove community. Biodivers. J. Biol. Divers. 2021, 22, 3648–3655. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Wright, J.P.; Cadotte, M.W.; Carroll, I.T.; Hector, A.; Srivastava, D.S.; Loreau, M.; Weis, J.J. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl. Acad. Sci. USA 2007, 104, 18123–18128. [Google Scholar] [CrossRef] [PubMed]
- Ramoliya, P.J.; Patel, H.M.; Pandey, A.N. Effect of salinization of soil on growth and macro-and micro-nutrient accumulation in seedlings of Salvadora persica (Salvadoraceae). For. Ecol. Manag. 2004, 202, 181–193. [Google Scholar] [CrossRef]
- Kumar, S.; Rani, C.; Mangal, M. A Critical review on Salvadora persica: An important medicinal plant of arid zone. Int. J. Phytomedicine 2012, 4, 292–303. [Google Scholar]
- Shiferaw, H.; Teketay, D.; Nemomissa, S.; Assefa, F. Some biological characteristics that foster the invasion of Prosopis juliflora (Sw.) DC. at Middle Awash Rift Valley Area, north-eastern Ethiopia. J. Arid. Environ. 2004, 58, 135–154. [Google Scholar] [CrossRef]
- Hussain, M.I.; Shackleton, R.; El-Keblawy, A.; González, L.; Trigo, M.M. Impact of the Invasive Prosopis juliflora on Terrestrial Ecosystems. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2021; p. 52. [Google Scholar]
- Collette, J.C.; Sommerville, K.D.; Lyons, M.B.; Offord, C.A.; Errington, G.; Newby, Z.-J.; von Richter, L.; Emery, N.J. Stepping up to the thermogradient plate: A data framework for predicting seed germination under climate change. Ann. Bot. 2022, 129, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Brundu, G.; Richardson, D.M. Planted forests and invasive alien trees in Europe: A code for managing existing and future plantings to mitigate the risk of negative impacts from invasions. NeoBiota 2016, 30, 5–47. [Google Scholar] [CrossRef]
- Preeti, K.; Avatar, S.R.; Mala, A. Pharmacology and Therapeutic Application of Prosopis juliflora: A Review. J. Plant Sci. 2015, 3, 234–240. [Google Scholar] [CrossRef]
- de Brito Damasceno, G.A.; Souto, A.L.; da Silva, I.B.; Roque, A.A.; Ferrari, M.; Giordani, R.B. Prosopis juliflora: Phytochemical, Toxicological, and Allelochemicals. In Co-Evolution of Secondary Metabolites; Merillon, J.M., Ramawat, K., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, J.; Leng, D.; Hu, S.; Yong, J.W.H.; Chen, X. Invasive plant promotes its arbuscular Mycorrhizal symbioses and competitiveness through its secondary metabolite: Indirect evidence from activated carbon. PLoS ONE 2014, 9, e97163. [Google Scholar] [CrossRef]
- Haregeweyn, N.; Tsunekawa, A.; Tsubo, M.; Meshesha, D.; Melkie, A. Analysis of the invasion rate, impacts and control measures of Prosopis juliflora: A case study of Amibara District, Eastern Ethiopia. Environ. Monit. Assess. 2013, 185, 7527–7542. [Google Scholar] [CrossRef]
- Elfadl, M.A.; Luukkanen, O. Effect of pruning on Prosopis juliflora: Considerations for tropical dryland agroforestry. J. Arid Environ. 2003, 53, 441–455. [Google Scholar] [CrossRef]
- Kahi, C.H.; Ngugi, R.K.; Mureithi, S.M.; Ng’ethe, J.C. The canopy effects of Prosopis juliflora (dc.) and Acacia tortilis (hayne) trees on herbaceous plants species and soil physico-chemical properties in Njemps flats, Kenya. Trop. Subtrop. Agroecosyst. 2009, 10, 441–449. [Google Scholar]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Morey, A.C.; Venette, R.C. A participatory method for prioritizing invasive species: Ranking threats to Minnesota’s terrestrial ecosystems. J. Environ. Manag. 2021, 290, 112556. [Google Scholar] [CrossRef] [PubMed]
- DWC. Bundala National Park Management Plan; Department of Wildlife Conservation, Ministry of Environment and Natural Resources: Colombo, Sri Lanka, 2005. [Google Scholar]
- Perera, N.P. A physiognomic vegetation map of Sri Lanka (Ceylon). J. Biogeogr. 1975, 2, 185–203. [Google Scholar] [CrossRef]
- Ashton, P.S. Toward a regional classification of the humid tropics of Asia. Tropics 1991, 1, 1–12. [Google Scholar] [CrossRef][Green Version]
- Suraweera, C.; Dahanayaka, D.D.G.L. Assessment, Monitoring and Management of Invasive Alien Species at Bundala National Park (A Ramsar Wetland) in Sri Lanka; Wetlands Sri Lanka, Central Environmental Authority: Battaramulla, Sri Lanka, 2017; pp. 80–85. [Google Scholar]
- Fox, T.R.; Haas, C.A.; Smith, D.W.; Loftis, D.L.; Zedaker, S.M.; Jones, R.H.; Hammett, A.L. Alternative silvicultural practices in Appalachian forest ecosystems: Implications for species diversity, ecosystem resilience, and commercial timber production. In Proceedings of the 15th Central Hardwood Forest Conference. USDA Forest Service General Technical Report SRS-101, Southern Research Station, Asheville, NC, USA, 27 February–1 March 2006; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2006; pp. 276–280. [Google Scholar]
- Puettmann, K.J.; Wilson, S.M.G.; Baker, S.C.; Donoso, P.J.; Drössler, L.; Amente, G.; Harvey, B.D.; Knoke, T.; Lu, Y.; Nocentini, S.; et al. Silvicultural alternatives to conventional even-aged forest management—What limits global adoption? For. Ecosyst. 2015, 2, 1–16. [Google Scholar] [CrossRef]
- Puettmann, K.J.; Tappeiner, J.C. Multi-scale assessments highlight silvicultural opportunities to increase species diversity and spatial variability in forests. Forestry 2014, 87, 1–10. [Google Scholar] [CrossRef]
- Andreu, M.; Zobrist, K.; Hinckley, T. Management Practices to Support Increased Biodiversity in Managed Loblolly Pine Plantations; UF/IFAS Extension; The School of Forest Resources and Conservation Department: Seattle, WA, USA, 2014. [Google Scholar]
- Benjamin, L.R. Growth Analysis, Crops. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Brian, T., Murray, B.G., Murphy, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 23–28. [Google Scholar] [CrossRef]
- Margalef, R. Information theory in ecology. Gen. Syst. 1958, 3, 36–71. Available online: http://hdl.handle.net/10261/284346 (accessed on 26 December 2023).
- Menhinick, C.F. A comparison of some species—Individuals diversity indices applied to samples of field insects. Ecology 1964, 45, 859–861. [Google Scholar] [CrossRef]
- Simpson, H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communications. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. Ecological Diversity, 1st ed.; Wiley: New York, NY, USA, 1975; 165p. [Google Scholar]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Siegel, S.; Castellan, N.J. Nonparametric Statistics for the Behavioural Sciences; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]

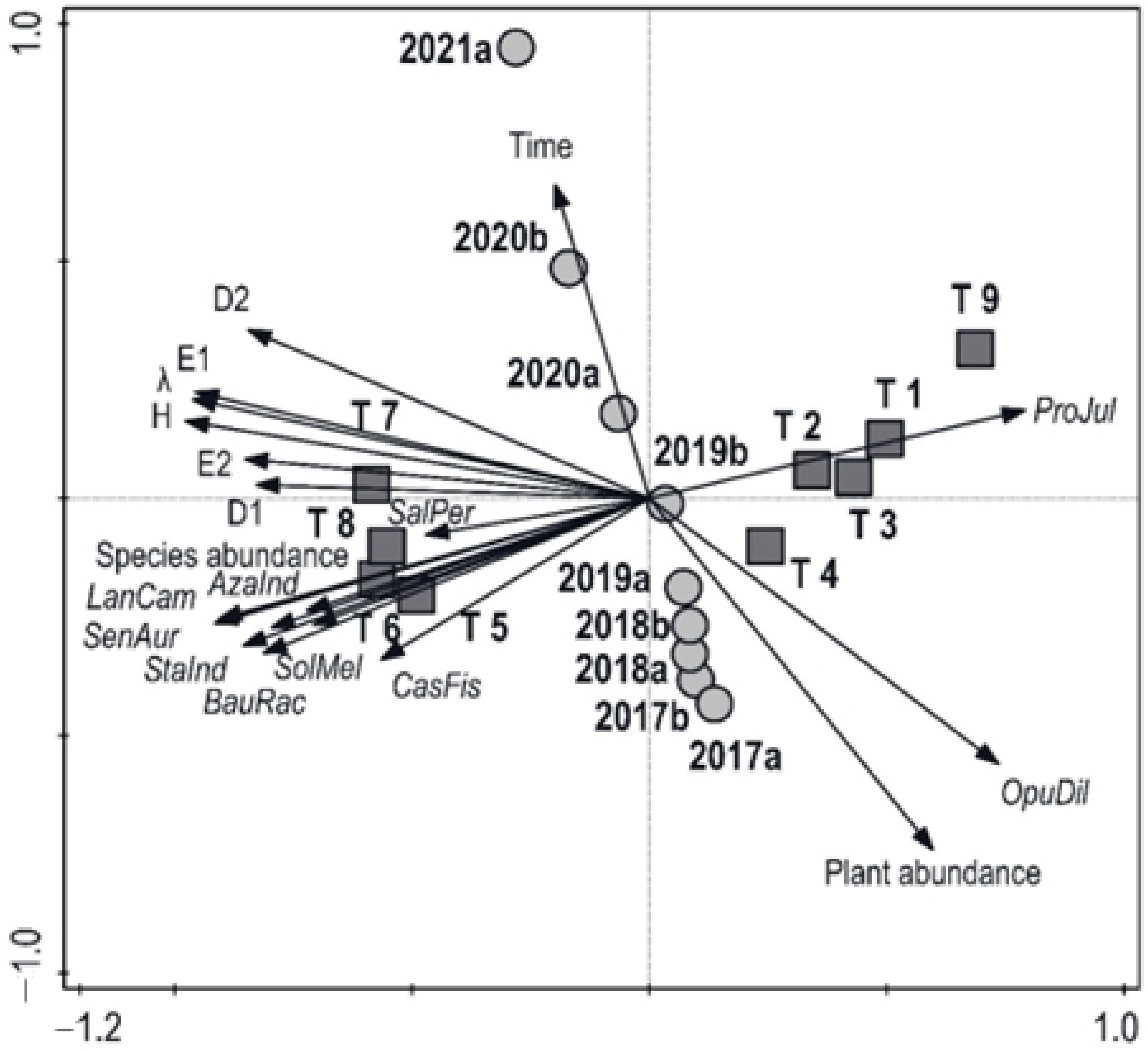
| Treatment | 1. Prosopis juliflora | 2. Opuntia dillenii | 3. Salvadora persica | 4. Lantana camara | 5. Senna auriculata | 6. Limonia acidissima | 7. Bauhinia racemosa | 8. Azadirachta indica | 9. Drypetes sepiaria | 10. Manilkara hexandra | 11. Randia dumetorrum | 12. Derris spp. | 13. Flueggea leucopyrus | 14. Solanum melongena | 15. Stachytarpheta indica | 16. Senna tora | 17. Achyranthes aspera | 18. Pongamia pinnata | 19. Tamarindus indicus | 20. Chloroxylon swietenia | 21. Cassia fistula | 22. Schleichera oleosa | 23. Madhuca longifolia | 24. Syzygium cumini | 25. Vitex altissima | 26. Terminalia arjuna | 27. Phyllanthus emblica | Overall number of Individuals |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 24cd | 324b | 3a | 1a | 1a | 1 | 2a | 1a | 1 | 0 | 1b | 0 | 1a | 1a | 2a | 0a | 0 | 0 | 0 | 1 | 1a | 0 | 0 | 0 | 0 | 0 | 0 | 363b |
| T2 | 26d | 299b | 3a | 2a | 2a | 0 | 2a | 2b | 0 | 0 | 0a | 0 | 1a | 1a | 2a | 0 | 0a | 5bc | 2b | 0 | 2 | 2 | 1 | 3 | 1 | 1 | 1 | 358b |
| T3 | 23c | 306b | 3ab | 2a | 3a | 0 | 2a | 1a | 0 | 0 | 1b | 0 | 2b | 2a | 4a | 2a | 1b | 2a | 0 | 1 | 1a | 0 | 0 | 0 | 0 | 0 | 0 | 355b |
| T4 | 23c | 308b | 3ab | 3a | 2a | 0 | 2a | 2b | 0 | 0 | 1bc | 0 | 3c | 4ab | 6a | 3a | 3c | 4b | 1a | 0 | 2b | 2 | 1 | 3 | 1 | 1 | 1 | 381b |
| T5 | 9b | 26a | 5d | 13b | 17b | 0 | 5b | 3c | 1 | 0 | 1c | 0 | 3c | 40d | 36d | 22d | 10e | 0 | 0 | 0 | 3cd | 0 | 0 | 1 | 0 | 0 | 0 | 196a |
| T6 | 11b | 28a | 4bc | 14b | 19b | 0 | 5b | 3c | 1 | 0 | 1c | 0 | 3c | 33c | 28c | 20d | 6d | 6c | 3c | 0 | 3d | 2 | 1 | 3 | 1 | 1 | 1 | 198a |
| T7 | 4a | 28a | 4cd | 13b | 24bc | 1 | 5b | 2b | 1 | 1 | 2d | 0 | 3c | 7ab | 23bc | 14c | 5d | 5bc | 2ab | 1 | 2bc | 2 | 1 | 3 | 1 | 1 | 1 | 153a |
| T8 | 5a | 22a | 4bc | 18c | 28c | 1 | 5b | 5d | 1 | 0 | 1b | 1 | 2b | 10b | 17b | 8b | 6d | 0 | 0 | 0 | 3d | 0 | 0 | 0 | 0 | 0 | 0 | 135a |
| T9 | 39e | 325b | 3a | 1a | 0a | 0 | 1a | 1a | 0 | 0 | 1b | 0 | 1a | 3a | 3a | 1a | 0 | 0 | 0 | 0 | 1a | 0 | 0 | 0 | 0 | 0 | 0 | 378b |
| Test, p-value | KW, p < 0.001 | KW, p < 0.001 | KW, p < 0.001 | KW, p < 0.001 | KW, p < 0.001 | – | KW, p < 0.001 | KW, p < 0.001 | – | – | KW, p < 0.001 | – | KW, p < 0.001 | KW, p < 0.001 | KW, p < 0.001 | KW, p < 0.001 | KW, p = 0.028 | KW, p < 0.001 | KW, p < 0.001 | – | KW, p < 0.001 | – | – | – | – | – | – | KW, p < 0.001 |
| Treatment | 1. Prosopis juliflora | 2. Opuntia dillenii | 3. Salvadora persica | 4. Lantana camara | 5. Senna auriculata | 6. Limonia acidissima | 7. Bauhinia racemosa | 8. Azadirachta indica | 9. Drypetes sepiaria | 10. Manilkara hexandra | 11. Randia dumetorrum | 12. Derris spp. | 13. Flueggea leucopyrus | 14. Solanum melongena | 15. Stachytarpheta indica | 16. Senna tora | 17. Achyranthes aspera | 18. Pongamia pinnata | 19. Tamarindus indicus | 20. Chloroxylon swietenia | 21. Cassia fistula | 22. Schleichera oleosa | 23. Madhuca longifolia | 24. Syzygium cumini | 25. Vitex altissima | 26. Terminalia arjuna | 27. Phyllanthus emblica | Total Number of Individuals |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 0 | −49,775 | 0 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ↗ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | −1219 |
| T2 | 0 | −59,800 | 0 | 33 | 0 | 0 | 0 | −14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ↗ | −78 | −100 | 0 | −60 | −14 | −33 | −9 | 0 | ↗ | ↗ | −753 |
| T3 | 38 | −180,800 | 0 | 63 | 50 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | −866 |
| T4 | 33 | −89,850 | 0 | 56 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −27 | −129 | −140 | 0 | −60 | 0 | 0 | 0 | 0 | 100 | 100 | −579 |
| T5 | 41 | −900 | 38 | 57 | 40 | ↗ | 39 | 27 | 50 | 0 | 0 | 0 | 0 | 2 | 15 | 33 | −71 | 0 | 0 | 50 | 36 | 0 | 0 | 75 | 0 | 0 | 0 | 13 |
| T6 | 35 | ↙ | 35 | 40 | 35 | 0 | 23 | 42 | 50 | ↗ | 0 | 0 | 0 | 20 | 8 | 23 | −40 | −39 | −9 | ↗ | 20 | 0 | 0 | 0 | 0 | 100 | 100 | 9 |
| T7 | 7 | −1633 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 33 | 0 | 0 | 0 | 10 | 3 | 0 | −50 | −88 | −100 | 0 | −33 | −14 | 0 | 0 | 0 | 100 | 100 | −20 |
| T8 | 10 | −914 | 0 | 11 | 7 | 0 | 0 | 5 | 25 | 0 | 0 | 0 | 0 | 0 | 7 | 22 | −22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −8 |
| T9 | −1 | −19,110 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | −870 |
| Number Species | Species Diversity | Species Heterogeneity | Species Evenness | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | D1 | D2 | Λ | H′ | E1 | E2 | ||||||||
| T1 | 12b | ↗ | 2.046b | ↗ | 0.820bc | ↗ | 0.284a | ↗ | 0.670a | ↗ | 0.282a | ↗ | 0.400a | ↗ |
| T2 | 16c | ↗ | 2.661c | ↗ | 1.015cd | ↗ | 0.369ab | ↗ | 0.979bc | ↗ | 0.351a | ↗ | 0.385a | ↗ |
| T3 | 12b | ↗ | 1.945b | ↗ | 0.751ab | ↗ | 0.313ab | ↗ | 0.786ab | ↗ | 0.319a | ↗ | 0.404a | ↗ |
| T4 | 18d | ↗ | 2.936c | ↗ | 1.054cd | ↗ | 0.392b | ↗ | 1.088c | ↗ | 0.373a | ↗ | 0.371a | ↗ |
| T5 | 15c | ↗ | 2.737c | ↗ | 1.104d | ↗ | 0.844c | ↗ | 2.192d | ↗ | 0.800b | ↘ | 0.751d | ↘ |
| T6 | 21e | ↗ | 3.718d | ↗ | 1.476e | ↗ | 0.873c | ↗ | 2.461de | ↘ | 0.811b | ↘ | 0.728d | ↘ |
| T7 | 23f | ↗ | 4.283e | ↗ | 1.829f | ↗ | 0.867c | ↘ | 2.489e | ↗ | 0.798b | ↘ | 0.677c | ↘ |
| T8 | 15c | → | 2.764c | ↗ | 1.246de | ↗ | 0.852c | ↘ | 2.235de | ↗ | 0.836b | ↘ | 0.761d | ↘ |
| T9 | 9a | → | 1.400a | ↗ | 0.551a | ↗ | 0.288ab | ↗ | 0.635a | ↗ | 0.294a | ↗ | 0.468b | ↗ |
| Test | KW, p < 0.001 | KW, p < 0.001 | KW, p < 0.001 | KW, p < 0.001 | KW, p < 0.001 | KW, p < 0.001 | KW, p < 0.001 | |||||||
| Treatment | Description |
|---|---|
| T1 | Hard pruning of Prosopis juliflora trees, which allows for regeneration of natural vegetation. This only allows keeping one straight branch or main stem of the tree. Most trees do not have straight stems but have more lateral branches. |
| T2 | Hard pruning of Prosopis juliflora trees followed by replanting of the chosen indigenous species. |
| T3 | Thinning of Prosopis juliflora trees, which allows for regeneration of natural vegetation. |
| T4 | Thinning of Prosopis juliflora trees and the replanting of the chosen indigenous species. |
| T5 | Complete uprooting of Prosopis juliflora trees, which allows for regeneration of natural vegetation. |
| T6 | Complete uprooting of Prosopis juliflora trees and replanting of the chosen indigenous species. |
| T7 | Replanting of the chosen indigenous species on a Prosopis juliflora-free site. |
| T8 | Allowing for natural regeneration of vegetation on a Prosopis juliflora-free site. |
| T9 | Control. Examination of the plot that contains Prosopis juliflora without any silvicultural measures. |
| Criterion | Reference | Evaluation | Equation |
|---|---|---|---|
| Species diversity | Margalef (1958) [102] | The number of species determined based on the number of plant species on the plot and the number of plants; minimum D = 0; higher D = higher values. | |
| Menhinick (1964) [103] | |||
| Species heterogeneity | Simpson (1949) [104] | The index combining species richness and evenness; calculated based on the number of individual plants; minimum λ/H′ = 0, higher λ/H′ = higher values. | |
| Shannon (1948) [105] | |||
| Species evenness | Pielou (1975) [106] | The level of evenness in the representation of individual plant species in the plot; range 0–1; minimum E = 0, maximum E = 1. | |
| Hill (1973) [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suraweera, C.; Gallo, J.; Vacek, Z.; Cukor, J.; Vacek, S.; Baláš, M. Silvicultural Practices for Diversity Conservation and Invasive Species Suppression in Forest Ecosystems of the Bundala National Park, Sri Lanka. Plants 2024, 13, 121. https://doi.org/10.3390/plants13010121
Suraweera C, Gallo J, Vacek Z, Cukor J, Vacek S, Baláš M. Silvicultural Practices for Diversity Conservation and Invasive Species Suppression in Forest Ecosystems of the Bundala National Park, Sri Lanka. Plants. 2024; 13(1):121. https://doi.org/10.3390/plants13010121
Chicago/Turabian StyleSuraweera, Channa, Josef Gallo, Zdeněk Vacek, Jan Cukor, Stanislav Vacek, and Martin Baláš. 2024. "Silvicultural Practices for Diversity Conservation and Invasive Species Suppression in Forest Ecosystems of the Bundala National Park, Sri Lanka" Plants 13, no. 1: 121. https://doi.org/10.3390/plants13010121
APA StyleSuraweera, C., Gallo, J., Vacek, Z., Cukor, J., Vacek, S., & Baláš, M. (2024). Silvicultural Practices for Diversity Conservation and Invasive Species Suppression in Forest Ecosystems of the Bundala National Park, Sri Lanka. Plants, 13(1), 121. https://doi.org/10.3390/plants13010121








