Abstract
The rhizosphere is a rich source of actinomycetes which can produce several potential biologically active secondary metabolites. The principal goal for this research is to extract, purify, and characterize the bioactive secondary metabolites produced by three different strains of actinomycetes isolated from the rhizosphere of rosemary, black locust, and olive. The plant growth-promoting effect (PGPE) of the studied strains of actinomycetes on Ocimum basilicum L. (basil) and the disease-control effect on necrotic stem lesions of “black leg” caused by Fusarium tabacinum on basil were evaluated in silico. The cell-free culture filtrates from the studied actinomycetes isolates were evaluated in vitro for their antimicrobial activity against some common phytopathogens. The secondary metabolites obtained from the cell-free culture filtrates have been chemically characterized using high-resolution electrospray ionization of liquid-chromatography/mass-spectrometric detection (ESI-(HR)Orbitrap-MS). Results of the in silico trial showed that all studied isolates demonstrated PGPE on basil seedlings, improved some eco-physiological characteristics, and reduced the disease incidence of F. tabacinum. The extracted metabolites from the studied actinomycetes demonstrated antimicrobial activity in a Petri-plates assay. The chemical analysis revealed the presence of 20 different components. This research emphasizes how valuable the examined isolates are for producing bioactive compounds, indicating their putative antimicrobial activity and their potential employment as fungal biocontrol agents. In particular, the obtained results revealed the possibility of green synthesis of some important secondary metabolites, such as N-Acetyl-l-histidinol, Rhizocticin A, and Eponemycin, from actinomycetes. The bioactive metabolites may be successively used to develop novel bio-formulations for both crop protection and/or PGPE.
1. Introduction
Bioactive substances are abundantly produced by soil microorganisms []. Due to bacteria’s capacity to produce a variety of useful products, such as antibiotics, fungicides, herbicides, hydrolytic enzymes, antitumor, antivirals, and immune suppressants, there is increased interest in employing them for medical and agricultural applications [,,]. Recently, pathogen resistance demands the discovery of novel antimicrobial substances that are effective against serious phytopathogens; thus, there is significant interest in screening new microbes with antimicrobial activity from various environments in order to explore novel potential medications against infections that are resistant to drugs. Among these new isolates are actinomycetes, unicellular filamentous Gram-positive bacteria, which are found throughout nature in a wide range of environments. Actinomycetes are prominent and significant producers of numerous biological by-products such as antibiotics and plant growth-promoting substances []. Actinomycetes are very similar to fungi due to their ability to form a mycelium (hyphae), but their hyphae are much smaller than fungal ones [,].
Actinobacteria is regarded as one of the major groups of actinomycetes, and it includes the genus Streptomyces, which produces several known antibiotics []. According to Berdy [], the majority of the discovered bioactive substances are produced by the genus Streptomyces. Girão et al. [] reported the presence of several bioactive compounds, which account for around 45% of all known microbial bioactive metabolites, and they have been isolated from actinobacteria from terrestrial sources. Girão et al. [] investigated the ability of several actinobacteria isolated from Laminaria ochroleucae to control Candida albicans and Staphylococcus aureus. As a result, new actinomycetes strains can be isolated for the purpose of discovering novel bioactive substances for use in agriculture, especially for the suppression of harmful phytopathogens.
Ocimum basilicum L. (basil) is a culinary herb of the Lamiaceae family. It is native of tropical zones of central Africa and southeast Asia, and it can be also found in Mediterranean region []. Basil can be infected by several phytopathogens, such as Fusarium sp. (wilt disease), Pythium sp. (damping off), Botrytis sp. (gray mold), Colletotrichum sp. (black spot), and Peronospora sp. (downy mildew) []. In particular, F. tabacinum W. Gams (Beyma) causes necrotic stem lesions (“black leg disease”) on basil [].
The primary goals of the present research were: (i) the extraction and the purification of the extracts produced from three studied actinomycetes isolates; (ii) the chemical characterization of the metabolites of the three isolates using HR-LC-ESI-Orbitrap-MS; (iii) the in vitro evaluation of their antimicrobial activity against some phytopathogenic strains; and (iv) the in silico evaluation of the promoting effect of the three actinomycetes strains on O. basilicum, as well as an evaluation of the control of “black leg disease” on basil caused by F. tabacinum.
2. Results
2.1. Morphological and Molecular Identification
The morphological examination, using an optical microscope, of the three pure cultures showed the typical mycelia-like structure of actinomycetes, which is characterized by branching and filamentous growth. In particular, the colony of isolate Act1 produced well-developed vegetative radial-hyphae ranged between 1.5 and 2.0 µm Ø, consisting of long, semi-straight filaments bearing colonies arranged vertically in whorls with irregular shapes (Figure 1A,B). The isolate Act2 showed a dense radial-hyphae ranged between 1.5 and 2.0 µm Ø and consisted of long, semi-straight filaments bearing colonies arranged vertically in whorls with irregular shapes (Figure 1C,D). The isolate Act3 showed a radial-hyphae and consisted of long, straight filaments bearing colonies with regular coccoid-shape (Figure 1E,F). The extracted DNA from the studied isolates and amplified by PCR produced amplicons with a molecular weight of about 350, as expected. The negative control did not show any amplification. The amplified DNA were sequenced at BMR Genomics (Padova, Italy), as previously reported by Elshafie and Camele []. BLAST software was used for comparing the obtained sequences with those available in GenBank. The analysis demonstrated strong similarity percentages with Streptomyces sp., Streptomyces atratus, and Arthrobacter humicola, which are present in GenBank. One sequence for each studied isolate was deposited in the NCBI GenBank with the following accession numbers ON241810, ON241816, and ON241806 for Streptomyces sp. (Act1), Streptomyces atratus (Act2), and Arthrobacter humicola (Act3), respectively.
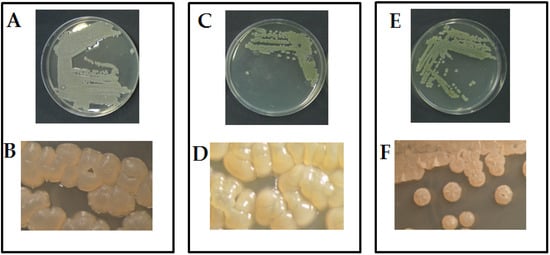
Figure 1.
Microscopic morphological features of the three studied actinomycetes isolates. (A,C,E) are cultures of Streptomyces sp. (Act1), Streptomyces atratus (Act2), and Arthrobacter humicola (Act3) grown in PY-CA media, respectively. (B,D,F) are the colonies of Streptomyces sp. (Act1), Streptomyces atratus (Act2), and Arthrobacter humicola (Act3), respectively, examined under stereo microscope (1000×).
2.2. Growth-Promoting and Disease-Control Effects
2.2.1. Eco-Physiological Characteristics
Bacterization of all plants with the actinomycete isolates resulted in stimulation of basil seedling growth and showed higher values of eco-physiological characteristics compared to the negative control (non-bacterized plants) (Table 1). In particular, regarding the healthy seedlings, only plants inoculated with Act1 showed the highest significant values of NL and TDwS (Table 1). Whereas, plants inoculated with Act1 and Act2 showed higher significant values regarding NT, SL and TFwS (Table 1).

Table 1.
Eco-physiological characteristics of basil after actinomycetes inoculation for healthy and infected plants.
On the other hand, Table 1 reports also the eco-physiological characteristics of bacterized basil infected with F. tabacinum. In particular, infected seedlings inoculated with Act2 demonstrated higher significant values of SL, whereas infected seedlings inoculated with Act1 showed higher significant values of NT and SL (Table 1). Generally, all inoculated seedlings after infection showed moderate values regarding all measured eco-physiological parameters which considered as promising (Table 1).
2.2.2. Disease-Control Effect
The plants bacterized with Streptomyces sp. and A. humicola exhibited no foliar or radical symptoms due to F. tabacinum infection. In fact, the disease indexes of Streptomyces sp. and A. humicola were 0.3 and 0.2%, respectively, whereas the control effects were 99.2 and 99.5%, respectively (Table 2). On the other hand, S. atratus-bacterized seedlings had a moderate disease index higher than 7% and a control effect higher than 79% (Figure 2 and Figure 3).

Table 2.
Determination of disease index and control effect on basil after different applications.
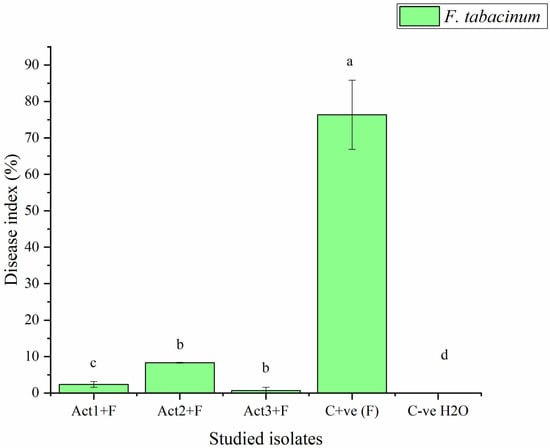
Figure 2.
Disease index of basil infected with F. tabacinum. Where: Act1+F; Act2+F; Act3+F are inoculated plants with Streptomyces sp., S. atratus and A. humicola, respectively. Bars with different letters between different treatments are significantly different at p < 0.05 according to Tukey’s B multiple comparison post hoc test using SPSS software. Values are mean of 3 replicates ±SDs.
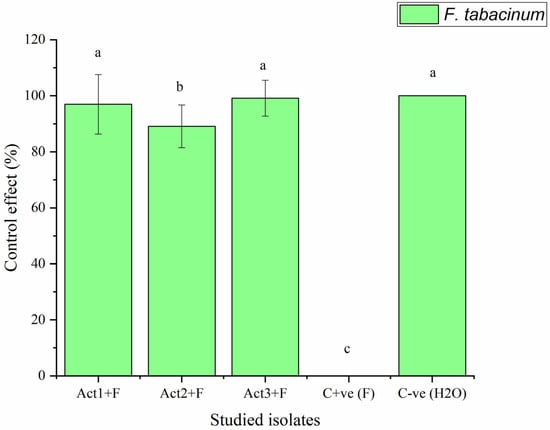
Figure 3.
Control effect of basil after infection with pathogen. Bars with different letters between different treatments are significantly different at p < 0.05 according to Tukey’s B multiple comparison post hoc test using SPSS software. Values are mean of 3 replicates ±SDs.
The results also showed that the C+ve (plants only inoculated with F. tabacinum) developed leaf chlorosis at 20 DPI, and it later turned necrotic. In addition, high percentages of leaf wilt and root necrosis were also observed at 35 DPI. In particular, a significant high percentage of symptomatic leaves was observed in the case of seedlings infected with F. tabacinum, where the disease index was higher than 36% compared to C-ve (plants not inoculated with F. tabacinum or actinomycetes isolates) and plants bacterized with actinomycetes isolates (Figure 2, Table 2). F. tabacinum was always re-isolated from the infected-plants.
2.3. Antimicrobial Assay of Metabolites
2.3.1. Antifungal Activity
Crude metabolite extracts obtained from actinomycete cultural media were evaluated for their antifungal activity against some food and phytopathogenic fungi following the incorporation method (Figure 4). A. humicola showed the highest antifungal effect against all tested fungi. Streptomyces sp. showed a moderate antifungal effect, which was higher than S. atratus, particularly against Monilinia fructigena, Rhizoctonia solani, Fusarium oxysporum, Penicillium expansum, and P. digitatum. On the other hand, S. atratus showed a promising effect against Sclerotinia sclerotiorum and P. commune.
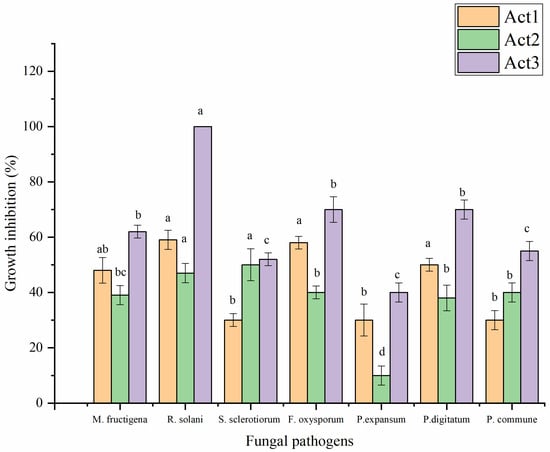
Figure 4.
Antifungal activity of extracted metabolites. Bars with different letters between different phytopathogenic fungi for each treatment are significantly different at p < 0.05 according to Tukey’s B multiple comparison post hoc test using SPSS software. Values are mean of 3 replicates ±SDs.
2.3.2. Antibacterial Activity
Regarding antibacterial activity, the crude metabolite extracts were evaluated against some food and plant pathogenic bacteria following the disc diffusion method (Figure 5). The highest significant antibacterial activity of S. atratus was observed against P. aeruginosa and X. campestris, showing the bacterial growth inhibition (BGI) of 88.9 and 76.7%, respectively. A. humicola showed the highest activity against P. fluorescens, X. campestris, P. syringae, and P. aeruginosa, showing BGI of 58.9, 57.8, 55.6 and 51.1%, respectively. Streptomyces sp. showed moderate activity against P. aeruginosa, P. syringae, and X. campestris, with BGI of 36.7, 27.8 and 24.0%, respectively. In addition, the lowest activity was observed in the case of S. atratus against B. cereus and E. coli, with BGI of 21.1 and 20.0%, respectively. On the other hand, Streptomyces sp. appeared to not be active against E. coli, B. cereus, and B. megaterium. In case of A. humicola, no activity was observed against E. coli and B. megaterium.
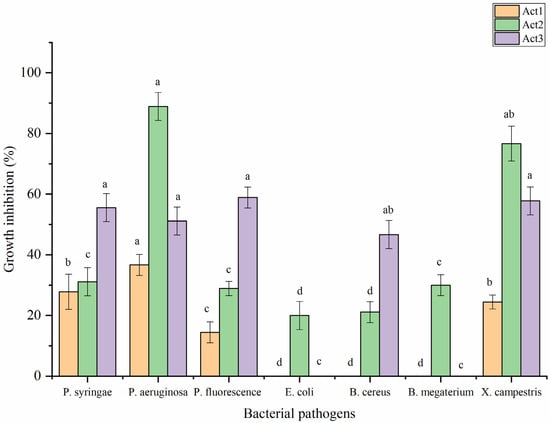
Figure 5.
Antibacterial activity of extracted metabolites. Bars with different letters between different phytopathogenic bacteria for each treatment are significantly different at p < 0.05 according to Tukey’s B multiple comparison post hoc test using SPSS software. Values are mean of 3 replicates ±SDs.
2.4. ESI/(HR)orbitrap/MS Metabolic Profiles
The chemical analysis of 3 studied actinomycetes isolates revealed the presence of 20 components, as illustrated in Table 3, (Figures S1–S3). Particularly, compound 1: N-Acetyl-L-histidinol, identified both in Streptomyces sp. (Act1) and S. atratus (Act2) extracts, which eluted at a retention time of 0.87 min, showed a peak [M + H]+ ion at m/z 184.1076, corresponding to a protonated molecular ion. From the spectrum, a produced ion at m/z 166, corresponding to a loss of oxydrile group, was also evident. We noted that the compound generating the protonated ion at 184.1076 exhibited a prominent peak in all of the extracts, and this compound was previously isolated from Streptomyces coelicolor [].

Table 3.
Chromatographic analysis of extracted secondary metabolites from studied actinomycetes isolates.
Compound 2: N,N′-Diacetyl-2-deoxystreptamine was identified only in A. humicola (Act3) extract, and it has molecular ion [M + H]+ at m/z 247.1283. 2-Deoxystreptamine antibiotics constitute a large group of aminoglycoside antibiotics to which over 20 members have been assigned to date, including the paromomycins, the kanamycins, the gentamicins, the nebramycins, ribostamycin, the lividomycins, validomycin, and ambutyrosin [].
Compound 3: Indolactam V displayed a sodiated ion of 324.1659, which is consistent with Indolacatm V; it was only identified in Streptomyces sp. (Act1) extract, and it had a retention time of 1.18 min. This compound was previously isolated from Streptomyces blastmyceticus and showed antagonistic activity toward protein kinase C [].
Compound 4: Hexahydro-2H-pyrido [1,2-a]pyrazin-3(4H)-one showed a protonated molecular ion [M + H]+ (m/z 155.117), detected only in S. atratus (Act2) extract. This compound was previously found in a Streptomyces extract, as reported by Nithya et al. [].
Compounds 5,6: Compound 5 corresponds to valyldetoxinine, with a molecular ion [M + H]+ at m/z 275.159, whereas compound 6 corresponds to paromomycin, with a potassium molecular ion [M+K]+ at m/z 654.265. These two compounds were found only in A. humicola (Act3) extract. Valyldetoxinine is a member of detoxin complex, a group of depsipeptide metabolites produced by Streptomyces caesputosus var. detoxus, which is present in the soil []. The detoxins exhibit antimicrobial activity against some microorganisms. Paromomycin is a naturally occurring aminoglycoside antibiotic, produced by Streptomyces rimosus sp. paromomycinus, that affects both prokaryotes and eukaryotes through the chelation with A-site of ribosome [,]. Valyldetoxinine and paromomycin had not been previously reported in A. humicola.
Compound 7: N-[3-[5-(2-methylpropyl)-3,6-dioxo-2-piperazinyl]propyl]-N′-nitro-,(2S-cis)-Guanidine was present in both extracts of Streptomyces sp. (Act1) and S. atratus (Act2), and it was eluted at 7.28 min, with a hydrate molecular ion [M + H2O]+ at m/z 332.1812 []. This compound was a cyclic dipeptide (CDP), widely biosynthesized during the cyclo-dipeptide synthesis cycle within prokaryotic and/or eukaryotic cells []. This compound was also found in Streptomyces strains [].
Compound 8: n-Hexyl-β-d-glucoside was detected in Streptomyces sp. (Act1) and S. atratus (Act2) extracts, with an [M + H]+ ion at m/z 265.1654; it is a substrate of β-glucosidases, which are hydrolytic enzymes normally present in Streptomyces strains that cleave β-glycosidic bonds of carbohydrates [].
Compounds 9–11: Nonactic acid (9) and homononactinic acid (11) were identified only in S. atratus (R3) extract, with an [M + H]+ ion at m/z 203.1274 and m/z 217.1430, corresponding to protonated molecular ions, respectively. These two compounds were isolated from Streptomyces and assayed against a panel of cancer cell lines, as reported by Lu et al. [].
Compound 10: (11aS)-1,2,3,11a-Tetrahydro-8-hydroxy-7-methoxy-5H-pyrrolo [2,1-c][1,4]benzodiazepin-5-one, also known as antibiotic DC81, was present in Streptomyces sp. (Act1) and S. atratus (Act2), with m/z 247.1073, and it was previously reported as an antitumor and antibiotic compound that originated from some actinomycetes [].
Compounds 12–15: These compounds were identified only in Streptomyces sp. (Act1) extract. Compound 12 gave an [M + H]+ ion at m/z 307.1646, attributed to phthoxazolins B, C, or D. These compounds were isolated from the culture broth of Streptomyces sp., as reported by Shiomi et al. [], but the limitation of LC-MS is that metabolites are structural isomers which cannot be distinguished. Compound 13 was identified as Chandrananimycin D, based on an accurate mass at m/z 301.0811, and it was attributed to a protonated molecular ion [M + H]+: The phenoxazinone chandrananimycin D was first characterized and isolated from Streptomyces griseus; its antiproliferative activity has also been reported []. Compound 14 gave an [M + H]+ ion at m/z 299.0659, which was attributed to a protonated molecular ion of carboxyexfoliazone. This compound was first isolated from a wild-type Streptomyces strain, as reported by Abdelfattah et al. []. Compound 15 eluted at 10.52 min, and it had a molecular ion [M + H]+ at m/z 283.0705, corresponding to a protonated molecular ion of phencomycin, which was previously reported by Chatterjee et al. [].
Compounds 16–18: These compounds were detected only in A. humicola (Act3) extract, giving an [M + H]+ ion at m/z 288.2889 and an [M + H]+ ion at 346.2220, respectively. Particularly, compound 16 was attributed to a protonated molecular ion of 2-amino-3-hydroxyhexadecanoic acid, and has already been reported in the Arthrobacter genus []. Compound 18 was attributed to a protonated molecular ion of Maoxianamides A or B. These two compounds were isolated from Streptomyces maoxianensis, as reported by Li et al. []. Compound 17: 1,1-Dimethylethyl 2-[2-(ethoxycarbonyl)-1-cyclopenten-1-yl]diazenecarboxylate, found only in S. atratus (Act2) extract, gave an [M + H]+ ion at m/z 269.14923.
Compound 19: This compound was attributed to a protonated molecular ion Rhizocticin A, and it was identified both in Streptomyces sp. (Act1) and S. atratus (Act2) extracts, and it gave an [M + H]+ ion at m/z 352.30490. This compound is a natural phosphonate antibiotic produced by the bacterial strain Bacillus subtilis [], and it is very similar to plumbemycin, isolated from Streptomyces plumbeus, by the amino acid (Z)-2-amino-5-phosphono-3-pentenoic acid, which is present in both antibiotics [].
Compound 20: This compound was attributed to a protonated molecular ion of Eponemycin, and it was identified in all three studied isolates. It gave an [M + H]+ ion at m/z 399.24997. This compound was reported to be produced by Streptomyces hygroscopicus and showed potent growth inhibition of various tumor cells []. Recently, Fitri et al. [] reported that this compound is a potential candidate for a new antimalarial drug due to its efficacy against Plasmodium berghei.
3. Discussion
The obtained results are promising for biocontrol of F. tabacinum; the treatments with actinomycetes isolates showed a high reduction of disease symptoms on O. basilicum seedlings, demonstrating the control effect exerted by Streptomyces sp. and Arthrobacter humicola against F. tabacinum. Furthermore, the treatment with the three studied isolates of actinomycetes may induce also the resistance effect against F. tabacinum. In addition, the obtained results of the current study are in agreement with the results reported by Elshafie and Camele [], where the same studied strains showed the capacity to stimulate the development of Solanum lycopersicum and reduce the disease incidence of S. sclerotiorum. The results agree with several studies which reported that many actinomycetes from the soil can inhibit some harmful phytopathogenic fungi [,,].
The PGPE of the studied isolates also showed significant influences on the eco-physiological characteristics of basil plants, which may be due to synthesis of phytohormones such as gibberellic acid, Indole 3-acetic acid, and zeatine (Z) produced by symbiotic and saprophytic actinomycetes [].
Chaudhary et al. [] investigated the antagonistic behavior of some actinomycetes isolates obtained from various niche environments in India, and observed the bioactivity of some of the studied isolates against Bacillus cereus, Shigella dysenteriae, and Methicillin-resistant Staphylococcus. The same authors also noted that none of the studied isolates were able to stop mycelial growth inside cells; however, all were able to stop the extracellular growth of the filaments of the studied bacteria [].
Regarding the secondary metabolites produced by various Streptomyces species, Odumosu et al. [] reported the presence of some bioactive substances with antibiotic properties; thus, they are a potential source of novel antibiotics.
Javoreková et al. [] investigated the addition of Streptomyces sampsonii and S. flavovariabilis to vermicompost, and the results revealed the strongest antagonistic action against a number of phytopathogenic fungi, including Rhizoctonia solani, Alternaria tenuissima, Aspergillus niger, and Penicillium expansum.
Our analysis showed the presence of several components, including phenoxazinones and a detoxin complex, to which the bioactivities could be attributed. In particular, chandrananimycin D was reported in the literature [] for its antimicrobial activity. Valyldetoxinine, a member of detoxin complex, was distinguished by its remarkable detoxifying effect against the antibiotic blasticidin S, both in animal and plant cells []. Paromomycin, an antibiotic depsipeptide, can be used as a biocontrol agent to suppress soilborne diseases; the compound can also be used as plant protection agent, in particular against Pectobacterium carotovorum, the agent responsible for bacterial soft-rot-producing pectolytic enzymes that hydrolyze pectin between individual plant cells, and against Phytophthora capsici, the agent responsible for blight and fruit rot of peppers and other important commercial crops [].
Jizba et al. [] reported stimulatory effects of nonactic and homonanctinic acids on Cucumis sativus growth, and the substances were also considered later as pesticidal metabolites by Jizba and Skibova []. Li et al. [] reported a moderate antifungal activity of maoxianamide A and B against S. sclerotiorum, the fungal phytopathogen responsible for white mold, which is a ubiquitous and highly destructive disease. In addition, rhizocticin A was reported for its effect on the inhibition of Rhizoctonia solani, the fungal pathogen that causes brown patch [].
4. Materials and Methods
4.1. Actinomycetes Isolation
The three studied actinomycetes used in this study have been isolated from the rhizosphere of black locust (Robinia pseudoacacia L.) (Act1), rosemary (Rosmarinus officinalis L.) (Act2), and olive (Olea europaea L.) (Act3) at Potenza city (Basilicata region, Sothern Italy), following the membrane filter technique []. Briefly, soil samples were collected from the studied area and dried under laminar flow for one week. The soil samples were further held in a water bath at 50 °C for 30 min to destroy vegetative microorganisms. Starch casein media (SCM) was prepared for isolating actinomycetes as follows: [g/L]: starch (10); K2HPO4 (2); KNO3 (2); casein (0.3); MgSO4.7H2O (0.05); CaCO3 (0.02); FeSO4.7H2O (0.01); agar (15). The pH was adjusted to 7.0. The prepared media was autoclaved at 121 °C for 15 min and then was poured in plates at 45 °C and allowed to settle. The isolation on agar plates was carried out by placing filter (mixed cellulose ester, pore size = 0.2 mm, Advantec) on the center of the Petri dishes, and 0,5 g of soil was sprinkled on the membrane filters pre-moistened with 100 µL of sterile distilled water (SDW). All plates were incubated at 28 °C for 4 days, and then the membrane filters were removed. The plates were further incubated until the actinomycete colonies became visible, after which a sub-culturing was performed. The isolates were sub-cultured and conserved in peptone yeast calcium agar (PY-CA), which contained [g/L]: peptone (5), yeast extract (3), and calcium chloride (0.7). This method is preferred due to its selectivity of actinomycetes and due to the inability of other microbes to produce mycelia that can penetrate the membrane filter.
4.2. Morphological and Molecular Identification
The morphological identification of the three actinomycetes isolates was carried out previously based on their microscopic features using a light microscope (Axioskop—ZEISS, Oberkochen, Germany). Molecular identification based on PCR techniques was carried out as follows: Briefly, PCR primers Y1 (5′-TGG CTC AGG ACG AAC GCT GGC GGC-3′) and Y2 (5′-CCT ACT GCT GCC TCC CGT AGG AGT-3′) [] were used to amplify a 348-bp fragment from the 16S rRNA gene. The PCR reaction was performed under the following conditions: 1 cycle of 94 °C for 5 min, followed by 34 cycles of 94 °C for 30 s; 57 °C for 30 s; and 72 °C for 1 min, with a final extension step (1 cycle) of 72 °C for 5 min. Preparation of the samples for PCR was performed as described by Ward et al. [], and the eventual obtained amplicons were observed by electrophoresis in a 1.5% agarose gel.
4.3. Growth-Promoting and Disease-Control Effects
A greenhouse trial was undertaken out to evaluate the PGPE of the three isolates for basil, as well as to evaluate their disease-control effect (DC) against the necrotic stem lesions (“black leg”) caused by F. tabacinum (Figure S4). Basil seeds were surface sterilized with ethanol (70%), rinsed three times with sterile distilled water, and then were sowed in polystyrene seed trays. The greenhouse’s temperature and relative humidity were maintained at 24 ± 2 °C and 60–70%, respectively, during the entire experiment.
Regarding actinomycetes treatment, a suspension 106 CFU.mL-1 of each isolate obtained from 5-days-fresh PY-Ca culture and inoculated into minimal mineral (MM) media was prepared as follows [g/L]: dipotassium phosphate (10.5), potassium dihydrogen phosphate (4.5), ammonium sulfate (1.5), trisodium citrate dihydrate (0.5), magnesium sulfate (0.2), and dextrose (5.0), and the pH value was adjusted to 7.0. The broth culture was incubated in a rotary incubator at 28 °C and 180 rpm for 8 days. The broth culture (100 mL/pot) was poured into the basil-rhizosphere at 15 days post-seed germination (DPSg).
For artificial fungal-infection, a conidial-suspension (108 spore/mL) of F. tabacinum was inoculated in a potato dextrose broth (PDB) flask and incubated under stirring (180 rpm) for 7 days at 22 °C. In total, 50 mL of broth was inoculated into the basil-rhizosphere 10 days after actinomycetes treatment. Twenty seedlings were used for each experiment: (i) untreated healthy; (ii) treated only with actinomycetes; and (iii) treated only with fungi.
At the end of the trial, plant growth was examined for the eco-physiological characteristics 40 DPSg following the method explained by Elshafie et al. []. The stem length (SL), leaf number (NL), twigs number (NT), total shoot fresh-weight (TFwS) and total shoot dry-weight (TDwS) were measured. The disease incidence was monitored daily for 15 days post-infection (DPI) using the following scale: 0 = less than 5% symptomatic leaf; 1 = 6 to 20% of symptomatic leaf; 2 = 21 to 50% of symptomatic leaf; 3 = 51 to 80 % of symptomatic leaf; and 4 ≥ 80% of symptomatic leaf []. Using Formula 1, the infection proportion (IP%) was calculated, and Formulas 2 and 3 were used for evaluating the disease index (DI%) and control effect (CE%), respectively [].
where: NSL = number of symptomatic leaves; TLN = total leaf number; TL = total number of leaves; Hi.S = highest scale; DI-P = disease index of pathogen treatment; DI-B = disease index of actinomycetes-treated.
4.4. Extraction of Metabolites
The secondary metabolites of the three studied strains were obtained following the method of Lavermicocca et al. [], with minor changes, as follows: an Erlenmeyer flask was filled with 140 mL of MM broth and seeded with 2.0 mL of each actinomycete suspension at 107 CFU/mL. It was incubated in a rotary incubator at 28 °C and 180 rpm for 8 days. The broth culture of each isolate was centrifuged at 20,000× g/10 min, the precipitate was discarded, and the upper-phase was filtered using Millipore 0.22 μM. The purified filtrate was extracted in equal volume of suitable organic solvent (ethyl-acetate) by shaking for 5 min and separated using a separator funnel. The combined organic fractions were concentrated using an evaporator (Heidolf 2000, Schwabach, Germany) at 180 rpm/80 °C for 20 min. The dried extracts were resuspended in 1 mL of sterile distilled water [].
4.5. Microbicidal Test of Metabolites
4.5.1. Antifungal Assay
The in vitro antifungal activity of the extracted metabolites from the three studied isolates was evaluated against the following phytopathogenic fungi: M. fructigena, R. solani, F. oxysporum, S. sclerotiorum, P. expansum, P. digitatum, and P. commune, using the incorporation method [,]. In total, 10 μL of each extract, in concentrations of 100 and 50%, was deposited on a potato dextrose agar (PDA) [] pre-inoculated with each tested fungal disc. To assess the antifungal activity, we determined the diameter of the grown mycelium in millimeters. The inhibition percentage of fungal growth (FGI%) was calculated following Formula (4) []. Cycloheximide was used as positive control (C+ve) at 100 µg/mL.
where: FGI (%) is the mycelium inhibition percentage: D.MGt is the mean diameter of the fungal mycelium in the treated Petri dish (mm); and D.MGc is the mean diameter of the mycelium control Petri dish (mm).
4.5.2. Antibacterial Assay
The in vitro antibacterial activity of the extracts was evaluated against the following bacteria: Pseudomonas syringae, P. fluorescence, P. aeruginosa, E. coli, Bacillus cereus, B. megaterium, and Xanthomonas campestris, using the disc diffusion method [,,]. King’B (KB) nutrient media was used for reculturing the studied bacterial strains []. The bacterial suspensions were prepared in SDW and adjusted to 106 CFU/mL. In total, 4 mL of each bacterial suspension diluted in soft agar (0.7%) at 9:1 v/v were poured into a KB Petri dish (90 mm). A total of 15 μL of each extract, at concentrations of 100 and 50%, was deposited on filter discs (6 mm-OXOID) previously placed on plates and left for 30 min under laminar flow. Finally, the bactericidal effect was evaluated by measuring the diameter of the inhibition zone (D.Iz) in millimeters compared to tetracycline (1600 µg/mL), used as C+ve. The bacterial growth inhibition percentage (BGI%) was calculated following Formula (5). All tested treatments were carried out in triplicate ± standard deviations (SDs).
where: BGI (%) represents the bacterial inhibition percentage; D.Iz is the mean diameter of inhibition zone in the treated Petri dish (mm); and D.Cc is the mean diameter of the bacteria grown in control Petri dish (mm).
BGI (%) = D.Iz/D.Cc × 100
4.6. ESI/(HR)orbitrap/MS Metabolic Profiles
The qualitative analysis of three studied actinomycetes extracts was performed by high performance liquid chromatography-mass spectrometry (HPLC-MS) using an LTQ-XL Ion Trap mass spectrometer (Thermo Fischer Scientific Spa, Rodano, Italy) equipped with an Ultimate 3000 HPLC (Agilent Technology, Cernusco sul Naviglio, Italy). Chromatographic separation was obtained using a Kinetex Polar C18 column (100 × 3.0 mm, 100 Å, 2.6 µm) (Phenomenex, Torrance, CA, USA). The injection volume was 0.5 mL/min and a mobile phase consisting of a combination of A (0.1% formic acid in water, v/v) and B (0.1% formic acid in acetonitrile MeCN); a linear gradient, which ranged between 5 and 60% B in 25 min, from 60 to 95% B in 10 min, and held at 95% B for 5 min, was used. The mass spectrometer was set in positive ion mode. ESI source conditions were the following: capillary voltage −48 V; tube lens voltage −176.47; capillary temperature 280 °C; sheath 15 and auxiliary gas flow (N2) 5; sweep gas 0; and spray voltage 5. MS spectra were obtained at 30,000 resolutions by full-range acquisition with a scan range between 150 and 1500 m/z.
4.7. Statistical Analysis
For the statistical analysis, the collected results were analyzed via a one-way ANOVA using Statistical Package for the Social Sciences (SPSS) version 13.0, 2004 (Chicago, IL, USA). The Tukey’s B post hoc multiple comparison test was applied to determine the significance level with a probability of p ≤ 0.05.
5. Conclusions
This research revealed the biological activity of actinomycetes, especially Streptomyces and Arthrobacter. This study also highlighted the value of the new studied strains in terms of their capacity to produce significant bioactive by-products that can be employed as biocontrol agents against several harmful fungi, including Fusarium species. In conclusion, some metabolites, detected in the extracts of the studied isolates, were recognized to be effective against phytopathogenic fungi and bacteria. This may represent the potential for their use in future sustainable strategies to control outbreaks in a vast range of crops. This research demonstrated the possibility of green synthesis of some important secondary metabolites, such as N-Acetyl-L-histidinol, rhizocticin A, and Eponemycin from actinomycete. In particular, N-Acetyl-L-histidinol (detected in the current study from Streptomyces sp. and S. atratus), is considered an important derivative of the primary metabolite L-Histidinol, in agreement with Ballio et al. [], who reported that N-acetyl-L-histidinol was produced in cultures of Streptomyces coelicolor. Regarding rhizocticin A (detected in the current study from Streptomyces sp. and S. atratus), it is considered a natural phosphonate antibiotic and hydrophilic phosphono-oligopeptides extracted from different biocontrol agents such as Bacillus subtilis []. Eponemycin (detected in the current study from the three studied isolates) is considered an important antibiotic with specific in vivo antitumor effects against B16 melanoma []. These potential bioactive compounds may be used to develop new commercial formulations, either as plant-growth promoters or for crop protection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12091869/s1, Figure S1: LC-MS chromatogram of the metabolites extracted from Streptomyces spp. (Act1); Figure S2: LC-MS chromatogram of the metabolites extracted from S. atratus (Act2); Figure S3: LC-MS chromatogram of the metabolites extracted from A. humicola (Act3); Figure S4: Methodological procedures of in silico experiment in greenhouse.
Author Contributions
Conceptualization, H.S.E. and I.C.; Data curation, V.D.F.; Formal analysis, C.F.; Funding acquisition, I.C.; Investigation, L.D.M., L.C. and I.C.; Methodology, H.S.E., L.D.M., C.F. and L.C.; Supervision, V.D.F. and I.C.; Writing—original draft, H.S.E.; Writing—review and editing, H.S.E., L.D.M., V.D.F. and I.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions; neither the European Union nor the European Commission can be considered responsible for them.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chaudhary, H.S.; Yadav, J.; Shrivastava, A.R.; Singh, S.; Singh, A.K.; Gopalan, N. Antibacterial activity of actinomycetes isolated from different soil samples of Sheopur (A city of central India). J. Adv. Pharm. Technol. Res. 2013, 4, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Vikineswary, S.; Nadaraj, P.; Wong, W.H.; Balabaskaran, S. Actinomycetes from a tropical mangrove ecosystem—Antifungal activity of selected strains. Asia Pac. J. Mol. Biol. Biotechnol. 1997, 5, 81–86. [Google Scholar]
- Jeffrey, L.S.H.; Norzaimawati, A.N.; Rosnah, H. Prescreening of bioactivities from actinomycetes isolated from forest peat soil in Sarawak. J. Trop. Agric. Food Sci. 2011, 39, 245–254. [Google Scholar]
- Manivasagan, P.; Kang, K.-H.; Sivakumar, K.; Li-Chan, E.C.; Oh, H.M.; Kim, S.K. Marine actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014, 38, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Pepper, I.L.; Gentry, T.J. Chapter 4—Earth Environments. In Environmental Microbiology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 59–88. [Google Scholar]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williams and Wilkins: Baltimore, MD, USA, 1994. [Google Scholar]
- Berdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- Girão, M.; Ribeiro, I.; Ribeiro, T.; Azevedo, I.C.; Pereira, F.; Urbatzka, R.; Leão, P.N.; Carvalho, M.F. Actinobacteria Isolated from Laminaria ochroleuca: A Source of New Bioactive Compounds. Front. Microbiol. 2019, 10, 683. [Google Scholar] [CrossRef]
- Phippen, W.B.; Simon, J.E. Anthocyanin inheritance and instability in purple basil (Ocimum basilicum L.). J. Heredity 2000, 91, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, A.; Minuto, A.; Minuto, G.; Gullino, M.L. First Report of Downy Mildew on Basil (Ocimum basilicum) in Italy. Plant Dis. 2004, 88, 312. [Google Scholar] [CrossRef] [PubMed]
- Matta, A. Fusarium tabacinum (Beyma) W. Gams patogeno in natura su basilico e pomodoro. Riv. Di Patol. Veg. 1978, 4, 119–125. [Google Scholar]
- Elshafie, H.S.; Camele, I. Rhizospheric actinomycetes revealed antifungal and plant-growth-promoting activities under controlled environment. Plants 2022, 11, 1872. [Google Scholar] [CrossRef] [PubMed]
- Ballio, A.; Russi, S.; Vlasić, D. Isolation and identification of N-acetyl-L-histidinol in cultures of mutants of Streptomyces coelicolor requiring histidine. Ann. Ist. Super. Sanita 1966, 2, 523–530. [Google Scholar] [PubMed]
- Seligman, S.J. Frequency of resistance to kanamycin, tobramycin, netilmicin, and amikacin in gentamicin-resistant gram-negative bacteria. Antimicrob. Agents Chemother. 1978, 13, 70–73. [Google Scholar] [CrossRef]
- He, F.; Takahiro, M.; Iori, M.; Hitomi, N.; Miroslava, A.; Shotaro, H.; Takayoshi, A.; Ikuro, A. Molecular basis for the P450-catalyzed C-N bond formation in indolactam biosynthesis. Nature Chem. Biol. 2019, 15, 1206–1213. [Google Scholar] [CrossRef]
- Nithya, K.; Muthukumar, C.; Biswas, B.; Alharbi, N.S.; Kadaikunn, S.; Khaled, J.M.; Dhanasekaran, D. Desert actinobacteria as a source of bioactive compounds production with a special emphasis on Pyridine-2,5-diacetamide a new pyridine alkaloid produced by Streptomyces sp. DA3-7. Microbiol. Res. 2018, 207, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Han, S.Y.; Joullie, M.M. Total synthesis of (+)-valyldetoxinine and (−)-detoxin D1. Tetrahedron 1993, 49, 785–802. [Google Scholar] [CrossRef]
- El-Housseiny, G.S.; Ibrahim, A.A.; Yassien, M.A.; Aboshanab, K.M. Production and statistical optimization Paromomycin by Streptomyces rimosus NRRL 2455 in solid state fermentation. BMC Microbiol. 2021, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.M.; Kogan, H.V.; Elikan, A.B.; Snow, J.W. Paromomycin Reduces Vairimorpha (Nosema) ceranae Infection in Honey Bees but Perturbs Microbiome Levels and Midgut Cell Function. Microorganisms 2022, 10, 1107. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, T.; Akutagawa, T.; Funatani, H.; Uchida, T.; Hotta, Y.; Niwa, M.; Takaya, Y. Cyclic dipeptides exhibit potency for scavenging radicals. Bioorg. Med. Chem. 2012, 20, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.; Berube, C.; Voyer, N.; Grenier, D. Anti-biofilm and anti-adherence properties of novel cyclic dipeptides against oral pathogens. Bioorg. Med. Chem. 2019, 27, 2323–2331. [Google Scholar] [CrossRef]
- Tatsuta, K.; Tsuchiya, T.; Umezawa, S.; Naganawa, H.; Umezawa, H. Revised structure for arglecin. J. Antibiot. 1972, 25, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Ryan, B.; Henehan, G.T.M. b-Glucosidase from Streptomyces griseus: Nanoparticle immobilization and application to alkyl glucoside synthesis. Protein Exp. Purif. 2017, 132, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Hu, J.; Xie, X.; Zhou, R.; Li, F.; Huang, R.; He, J. Secondary Metabolites with Cytotoxic Activities from Streptomyces sp. BM-8 Isolated from the Feces of Equusquagga. Molecules 2021, 26, 7556. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, Y.K.; Wang, J.J.; Hsu, C.C.; Tsai, F.Y.; Sung, P.J.; Lin, H.C.; Chang, L.S.; Hu, W.P. DC-81-enediyne induces apoptosis of human melanoma A375 cells: Involvement of the ROS, p38 MAPK, and AP-1 signaling pathways. Cell Biol. Toxicol. 2013, 29, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K.; Arai, N.; Shinose, M.; Takahashi, Y.; Yoshida, H.; Iwabuchi, J.; Tanaka, Y.; Omura, S. New antibiotics phthoxazolins B, C and D produced by Streptomyces sp. KO-7888. J. Antibiot. 1995, 48, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.B.; Nett, M.; Dahse, H.M.; Hertweck, C.P. Structural merger of a phenoxazinone with an epoxyquinone antibiotic. J. Nat. Prod. 2010, 73, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, M.S. A new bioactive aminophenoxazinone alkaloid from a marine-derived actinomycete. Nat. Prod. Res. 2013, 27, 2126–2131. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Vijayakumar, E.K.; Franco, C.M.; Maurya, R.; Blumbach, J.; Ganguli, B.N. Phencomycin, a new antibiotic from a Streptomyces species HIL Y-9031725. J. Antibiot. 1995, 8, 1353–1354. [Google Scholar] [CrossRef] [PubMed]
- Hidetsugu, N.; Hitoshi, E.; Koji, K.; Okumura, K. Biological Method of Producing Serine and Serinol Derivatives. U.S. Patent No. 3871958A, 18 March 1975. [Google Scholar]
- Li, J.M.; Yan, K.; Zhang, H.; Qi, H.; Zhang, J.; Xiang, W.S.; Wang, J.D.; Wang, X.J. New aliphatic acid amides from Streptomyces maoxianensis sp. nov. J. Antibiot. 2017, 70, 187–189. [Google Scholar] [CrossRef]
- Gahungu, M.; Arguelles-Arias, A.; Fickers, P.; Zervosen, A.; Joris, B.; Damblon, C.; Luxen, A. Synthesis and biological evaluation of potential threonine synthase inhibitors: Rhizocticin A and Plumbemycin A. Bioorg. Med. Chem. 2013, 21, 4958–4967. [Google Scholar] [CrossRef]
- Fredenhagen, A.; Angst, C.; Peter, H.H. Digestion of rhizocticins to (Z)-L-2-amino-5-phosphono-3-pentenoic acid: Revision of the absolute configuration of plumbemycins A and B. J. Antibiot. 1995, 48, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, K.; Hatori, M.; Nishiyama, Y.; Tomita, K.; Kamei, H.; Konishi, M.; Oki, T. Eponemycin, a new antibiotic active against B16 melanoma. I. Production, isolation, structure and biological activity. J. Antibiot. 1990, 43, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Fitri, L.E.; Cahyono, A.W.; Nugraha, R.Y.B.; Alkarimah, A.; Ramadhani, N.N.; Laksmi, D.A.; Trianty, L.; Noviyanti, R. Analysis of eponemycin (α′β′ epoxyketone) analog compound from Streptomyces hygroscopicus subsp. Hygroscopicus extracts and its antiplasmodial activity during Plasmodium berghei infection. Biomed. Res. 2017, 28, 164–172. [Google Scholar]
- Suwan, N.; Wassamon, B.; Sarunya, N. Antifungal activity of soil actinomycetes to control chilli anthracnose caused by Colletotrichum gloeosporioides. J. Agric. Technol. 2012, 8, 725–737. [Google Scholar]
- Sacramento, D.R.; Coelho, R.R.R.; Wigg, M.D.; Wigg, M.D.; Linhares, L.F.T.; Santos, M.G.M.; Semêdo, L.T.A.; Silva, A.J.R. Antimicrobial and antiviral activities of an actinomycete (Streptomyces sp.) isolated from a Brazilian tropical forest soil. World J. Microbiol. Biotechnol. 2004, 20, 225–229. [Google Scholar] [CrossRef]
- Prapangdee, B.; Kuekulvong, C.; Mongkolsuk, S. Antifungal Potential of Extracellular Metabolites Produced by Streptomyces hygroscopicus against Phytopathogenic Fungi. Int. J. Biol. Sci. 2008, 4, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Vobis, G.; Cassa’n, F.; Luna, V.; Wall, L.G. Production of phytohormones by root-associated saprophytic actinomycetes isolated from the actinorhizal plant Ochetophila trinervis. World J. Microbiol. Biotechnol. 2011, 27, 2195–2202. [Google Scholar] [CrossRef]
- Odumosu, B.T.; Buraimoh, O.M.; Okeke, C.J.; Ogah, J.O.; Michel, F.C., Jr. Antimicrobial activities of the Streptomyces ceolicolor strain AOBKF977550 isolated from a tropical estuary. J. Taibah Univ. Sci. 2017, 11, 836–841. [Google Scholar] [CrossRef]
- Javoreková, S.; Kovácsová, S.; Medo, J.; Maková, J.; Petrová, J.; Hleba, L.; Košťálová, D.; Cinkocki, R. Soil amended with organic fertilizers as a source of actinomycetes with high potential as biocontrol agents. J. Microbiol. Biotechnol. Food Sci. 2021, 8, 1352–1359. [Google Scholar] [CrossRef]
- Zhang, X.M.; Liu, X.; Wang, Z.; Tian, Z.H.; Xie, W.D. Viridobrunnines A B, antimicrobial phenoxazinone alkaloids from a soil associated Streptomyces sp. Heterocycles 2015, 91, 1809–1814. [Google Scholar]
- Ogita, T.; Seto, H.; Ōtake, N.; Yonehara, H. The Structures of Minor Congeners of the Detoxin Complex. Agric. Biol. Chem. 1981, 45, 2605–2611. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, S.W.; Balaraju, K.; Kim, C.J.; Park, K. Chili-Pepper Protection from Phytophthora capsici and Pectobacterium carotovorum SCC1 by Encapsulated Paromomycin Derived from Streptomyces sp. AMG-P1. J. Pure Appl. Microbiol. 2012, 6, 1517–1522. [Google Scholar]
- Jizba, J.; Pfukrylova, V.; Ujhelyova, L.; Varkonda, S. Insecticidal properties of nonactic acid and homononactic acid, the precursors of macrotetrolide antibiotics. Folia Microbiol. 1992, 37, 299–303. [Google Scholar] [CrossRef]
- Jizba, J.; Skibova, I. Regulation of biosynthesis of pesticidal metabolic complexes in Streptomyces griseus. Folia Microbiol. 1994, 39, 119–128. [Google Scholar] [CrossRef]
- Kugler, M.; Loeffler, W.; Rapp, C.; Kern, A.; Jung, G. Rhizocticin A, an antifungal phosphono-oligopeptide of Bacillus subtilis ATCC 6633: Biological properties. Arch. Microbiol. 1990, 153, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Njenga, W.P.; Mwaura, F.B.; Wagacha, J.M.; Gathuru, E.M. Methods of Isolating Actinomycetes from the Soils of Menengai Crater in Kenya. Arch. Clin. Microbiol. 2017, 8, 45. [Google Scholar] [CrossRef]
- Young, J.P.W.; Downer, H.L.; Eardly, B.D. Phylogeny of the phototrophic Rhizobium strain BTAil by polymerase chain reaction-based sequencing of a 16S rRNA gene segment. J. Bacteriol. 1991, 173, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.J.H.; Brown, J.C.S.; Davey, G.P. Detection of dairy Leuconostoc strains using the polymerase chain reaction. Lett. Appl. Microbiol. 1995, 20, 204–208. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.; Bufo, S.A.; Camele, I. An attempt of biocontrol the tomato-wilt disease caused by Verticillium dahliae using Burkholderia gladioli pv. agaricicola and its bioactive secondary metabolites. Int. J. Plant Biol. 2017, 8, 57–60. [Google Scholar] [CrossRef]
- Lee, K.J.; Kamala-Kannan, S.; Sub, H.S.; Seong, C.K.; Lee, G.W. Biological control of Phytophthora blight in red pepper (Capsicum annuum L.) using Bacillus subtilis. World J. Microbiol. Biotechnol. 2007, 24, 1139–1145. [Google Scholar] [CrossRef]
- Lavermicocca, P.; Iacobellis, N.S.; Simmaco, M.; Graniti, A. Biological properties and spectrum of activity of Pseudomonas syringae pv. syringae toxins. Physiol. Mol. Plant Pathol. 1997, 50, 129–140. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Viggiani, L.; Mostafa, M.S.; El-Hashash, M.A.; Bufo, S.A.; Camele, I. Biological activity and chemical identification of ornithine lipid produced by Burkholderia gladioli pv. agaricicola ICMP 11096 using LC-MS and NMR analyses. J. Biol. Res. 2017, 90, 96–103. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Amato, M.; De Feo, V.; Camele, I. Chemical composition and antimicrobial activity of Chia (Salvia hispanica L.) Essential oil. Eur. Food Res. Technol. 2018, 244, 1675–1682. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Gruľová, D.; Baranová, B.; Caputo, L.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Antimicrobial activity and chemical composition of essential oil extracted from Solidago canadensis L. growing wild in Slovakia. Molecules 2019, 24, 1206. [Google Scholar] [CrossRef]
- Aryal, S. Potato Dextrose Agar (PDA)—Principle, Uses, Composition, Procedure and Colony Characteristics. 2018. Available online: https://microbiologyinfo.com/potato-dextrose-agar-pda-principle-uses-composition-procedure-and-colony-characteristics/ (accessed on 5 April 2023).
- Zygadlo, J.A.; Guzman, C.A.; Grosso, N.R. Antifungal properties of the leaf oils of Tagetes minuta L. and Tagetes filifolia Lag. J. Essent. Oil Res. 1994, 6, 617–621. [Google Scholar] [CrossRef]
- Bhunia, A.; Johnson, M.C.; Ray, B. Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J. Appl. Bacteriol. 1988, 65, 261–268. [Google Scholar] [CrossRef]
- Camele, I.; Elshafie, H.S.; Caputo, L.; Sakr, S.H.; De Feo, V. Bacillus mojavensis: Biofilm formation and biochemical investigation of its bioactive metabolites. J. Biol. Res. 2019, 92, 39–45. [Google Scholar] [CrossRef]
- Gru’ová, D.; Caputo, L.; Elshafie, H.S.; Baranová, B.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Thymol chemotype Origanum vulgare L. essential oil as a potential selective bio-based herbicide on monocot plant species. Molecules 2020, 25, 595. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).