Advances in Biotechnological Production and Metabolic Regulation of Astragalus membranaceus
Abstract
1. Introduction
1.1. Astragalus Tissue Culture
1.1.1. Establishment of Regeneration Plant System of Astragalus
1.1.2. Research Progress on the Culture of Adventitious Roots of Astragalus
1.1.3. Research Progress of the Hairy Roots Culture of Astragalus
1.1.4. Research Progress on the Culture of Astragalus Suspension Cells
1.1.5. Culture of Astragalus Protoplasts
1.2. Astragalus Metabolism Control Research
1.2.1. Astragalus Saponins
1.2.2. Astragalus Flavone
1.2.3. Astragalus Polysaccharide
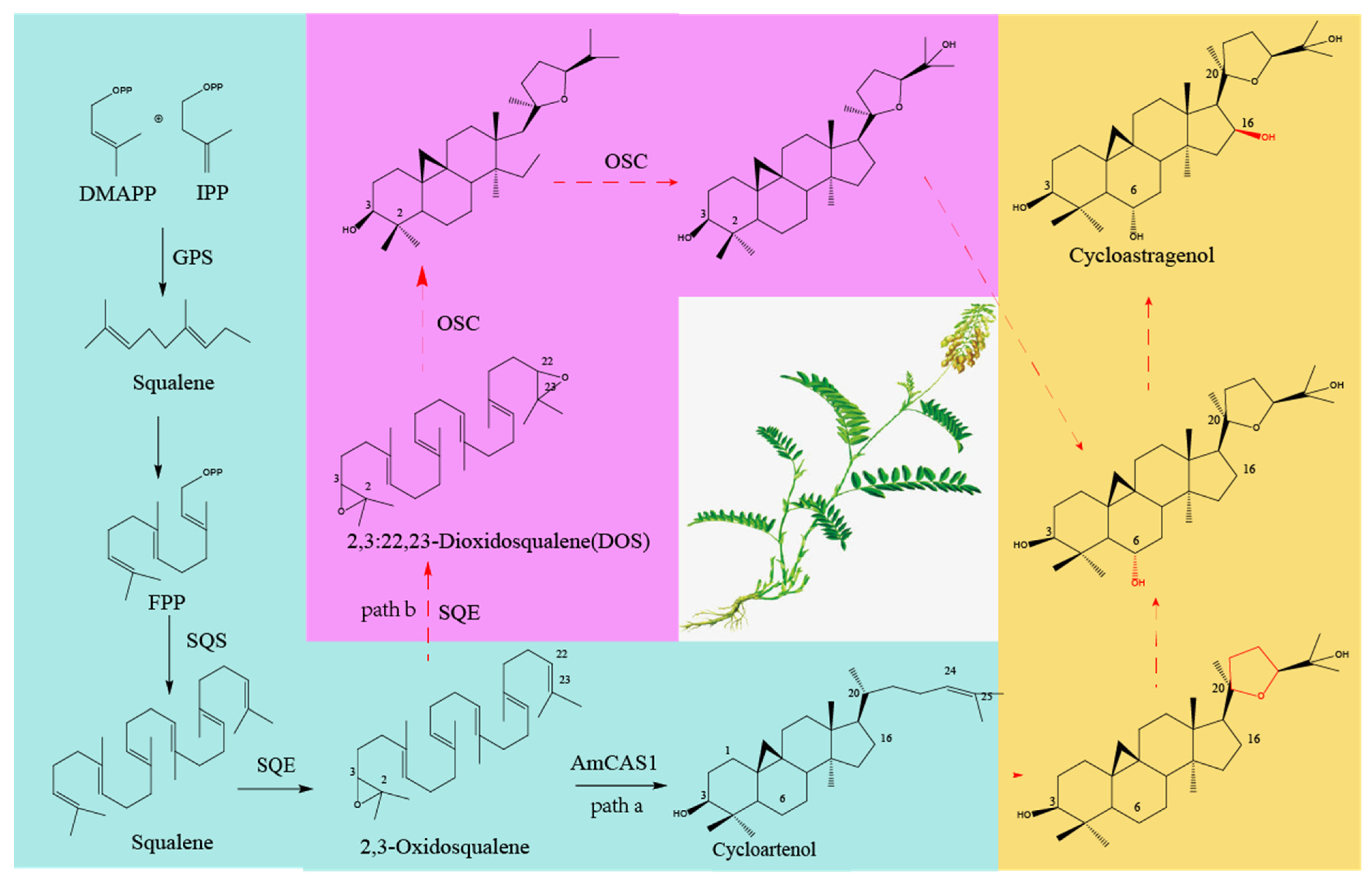
1.3. Research Progress on Synthetic Biological Pathways of Astragalosides
1.3.1. Research Progress on the Biosynthesis Pathway of Astragalosides
1.3.2. Astragalus Saponin Metabolism Engineering Research
1.3.3. Biotransformation of Astragalosides
Microbial Transformation
Enzymolysis
2. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Wang, Y.; Wang, R.; Chi, X.; Zhang, X. Research and utilization of astragalus germplasm resources. Anhui Agric. Sci. Bull. 2020, 26, 34–57. [Google Scholar]
- Ivancheva, S.; Nikolova, M.; Tsvetkova, R. Pharmacological activities and biologically active compounds of bulgarian medicinal plants. Phytochem. Adv. Res. 2006, 37661, 87–103. [Google Scholar]
- Yang, Q.Y.; Chen, K.J.; Lu, S.; Sun, H.R. Research progress on mechanism of action of radix astragalus in the treatment of heart failure. Chin. J. Integr. Med. 2012, 18, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Lysiuk, R.; Darmohray, R. Pharmacology and ethnomedicine of the genus astragalus. Int. J. Pharmacol. Phytochem. Ethnomed. 2016, 3, 46–53. [Google Scholar] [CrossRef]
- Zhao, L.; Lv, X.; Yi, L.; Xia, Q.; Li, X. Research progress of saponins in Astragalus membranaceus. J. Food Saf. Qual. 2021, 12, 4937–4946. [Google Scholar]
- Liu, Y.T.; Lv, W.L. Research progress in Astragalus membranaceus and its active components on immune responses in liver fibrosis. Chin. J. Integr. Med. 2020, 26, 794–800. [Google Scholar] [CrossRef]
- Xu, N.; Wu, X.J. Research advance of pharmacological effects of astragalosides on nervous system diseases. Zhongguo Zhong Yao Za Zhi 2021, 46, 4674–4682. [Google Scholar] [PubMed]
- Zhang, X.P.; Li, Y.D.; Luo, L.L.; Liu, Y.Q.; Li, Y.; Guo, C.; Li, Z.D.; Xie, X.R.; Song, H.X.; Yang, L.P.; et al. Astragalus saponins and liposome constitute an efficacious adjuvant formulation for cancer vaccines. Cancer Biother. Radiopharm. 2018, 33, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Lekmine, S.; Bendjedid, S.; Benslama, O.; Martin-Garcia, A.I.; Boussekine, S.; Kadi, K.; Akkal, S.; Nieto, G.; Sami, R.; Al-Mushhin, A.; et al. Ultrasound-assisted extraction, lc-ms/ms analysis, anticholinesterase, and antioxidant activities of valuablen metabolites from astragalus armatus willd.: In silico molecular docking and in vitro enzymatic studies. Antioxidants 2022, 11, 2000. [Google Scholar] [CrossRef]
- Durazzo, A.; Nazhand, A.; Lucarini, M.; Silva, A.M.; Souto, S.B.; Guerra, F.; Severino, P.; Zaccardelli, M.; Souto, E.B.; Santini, A. Astragalus (Astragalus membranaceus bunge): Botanical, geographical, and historical aspects to pharmaceutical components and beneficial role. Rend. Lincei Sci. Fis. Nat. 2021, 32, 625–642. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, Y.; E, X. Research progress on differences between Astragalus membranaceus and Astragalus membranaceus. J. Chin. Med. Mater. 2020, 43, 1261–1265. [Google Scholar]
- Wu, S.Q.; Lian, M.L.; Gao, R.; Park, S.Y.; Piao, X.C. Bioreactor application on adventitious root culture of Astragalus membranaceus. Vitr. Cell Dev. Biol. Plant 2011, 47, 719–724. [Google Scholar] [CrossRef]
- Zhang, H.; Yasmin, F.; Song, B.H. Neglected treasures in the wild-legume wild relatives in food security and human health. Curr. Opin. Plant Biol. 2019, 49, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Catarino, S.; Duarte, M.C.; Costa, E.; Carrero, P.G.; Romeiras, M.M. Conservation and sustainable use of the medicinal leguminosae plants from angola. PeerJ 2019, 7, e6736. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xiao, P. Application of bioengineering technology to protection of endangered medicinal plant resources. Chin. Tradit. Herb. Drugs 1994, 39, 961–964. [Google Scholar]
- Zhang, H.C.; Liu, J.M.; Chen, H.M.; Gao, C.C.; Lu, H.Y.; Zhou, H.; Li, Y.; Gao, S.L. Up-regulation of licochalcone a biosynthesis and secretion by tween 80 in hairy root cultures of glycyrrhiza uralensis fisch. Mol. Biotechnol. 2011, 47, 50–56. [Google Scholar] [CrossRef]
- Turgut-Kara, N.; Ari, U. In vitro plant regeneration from embryogenic cell suspension culture of astragalus chrysochlorus (leguminoseae). Afr. J. Biotechnol. 2008, 7, 1250–1255. [Google Scholar]
- Feng, Y.C.; Zhao, Y.; Ha, Y.; Li, J.J.; Su, Z.Y.; Quan, X.L.; Wu, S.Q.; Wu, W.L. Drought stress-induced methyl jasmonate accumulation promotes calycosin-7-o-beta-d-glucoside production in Astragalus membranaceus adventitious roots. Plant Cell Tissue Organ. Cult. 2021, 147, 561–568. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H. Study on tissue culture and radiation mutation of Astragalus membranaceus bge. Var. Mongholicus (bge.) Hsiao. Agric. Sci. Technol.-Hunan 2009, 10, 37–40. [Google Scholar]
- Ma, L.; Wang, X.; Gao, W. Callus initiation of Astragalus membranaceus var.mongholicus. Chin. J. Pharm. Biotechnol. 2008, 15, 355–358. [Google Scholar]
- Jiao, J. Optimization of Astragalus membranaceus Hairy Root Cultures and Elicitation of Its Main Active Constituent Biosynthesis. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2016. [Google Scholar]
- Du, M.; Wu, X.J.; Ding, J.; Hu, Z.B.; White, K.N.; Branford-White, C.J. Astragaloside iv and polysaccharide production by hairy roots of Astragalus membranaceus in bioreactors. Biotechnol. Lett. 2003, 25, 1853–1856. [Google Scholar] [CrossRef]
- Halder, M.; Roychowdhury, D.; Jha, S. A critical review on biotechnological interventions for production and yield enhancement of secondary metabolites in hairy root cultures. In Hairy Roots: An Effective Tool of Plant Biotechnology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 21–44. [Google Scholar]
- Bu, Y. Effects of Culture Conditions on the Contents of Several Secondary Components in Astragalus Cells. Master’s Thesis, Dalian Polytechnic University, Dalian, China, 2008. [Google Scholar]
- Liu, Q.; Zhong, Z.; Cong, L.; Zhang, L. Lsolation of protoplast from the Astragalus membranaceus. Guihaia 2008, 3, 411–413. [Google Scholar]
- Patel, H.; Krishnamurthy, R. Elicitors in plant tissue culture. J. Pharmacogn. Phytochem. 2013, 2, 60–65. [Google Scholar]
- Naik, P.M.; Al-Khayri, J.M. Abiotic and biotic elicitors-role in secondary metabolites production through in vitro culture of medicinal plants. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; InTech: Rijeka, Croatia, 2016; pp. 247–277. [Google Scholar]
- Zhang, K.; Liu, Z.; Yan, S.; Ren, W.; Liu, X.; Ma, W. Research progress on the secondary metabolism in astragalus. World Sci. Technol.—Mod. Tradit. Chin. Med. Mater. Med. 2016, 18, 875–882. [Google Scholar]
- Al-Oubaidi, H.K.M.; Kasid, N.M. Increasing phenolic and flavonoids compounds of cicer arietinum l. From embryo explant using titanium dioxide nanoparticle in vitro. World J. Pharm. Res 2015, 4, 1791–1799. [Google Scholar]
- Cakir, O.; Ari, S. Defensive and secondary metabolism in astragalus chrysochlorus cell cultures, in response to yeast extract stressor. J. Env. Biol. 2009, 30, 51–55. [Google Scholar]
- Wei, H.; Cheng, L.; Wu, P.; Han, M.; Yang, L. Short-term water changes response of saponin biosynthesis process in astragalus membranceus. Zhongguo Zhong Yao Za Zhi= China J. Chin. Mater. Med. 2019, 44, 441–447. [Google Scholar]
- Tuan, P.A.; Chung, E.; Thwe, A.A.; Li, X.; Kim, Y.B.; Mariadhas, V.A.; Al-Dhabi, N.A.; Lee, J.H.; Park, S.U. Transcriptional profiling and molecular characterization of astragalosides, calycosin, and calycosin-7-o-beta-d-glucoside biosynthesis in the hairy roots of Astragalus membranaceus in response to methyl jasmonate. J. Agric. Food Chem. 2015, 63, 6231–6240. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.Y.; Wang, Y.G.; Zhang, Y.F.; Zhou, T.S.; Fang, C.M.; Nan, P.; Wang, X.Q.; Li, X.B.; Wei, Y.L.; Chen, J.K. Phenylalanine ammonia lyase functions as a switch directly controlling the accumulation of calycosin and calycosin-7-o-beta-d-glucoside in Astragalus membranaceus var. Mongholicus plants. J. Exp. Bot. 2008, 59, 3027–3037. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.Y.; Niu, L.L.; Wang, X.Q.; Guo, N.; Zang, Y.P.; Fu, Y.J. Enhanced production of two bioactive isoflavone aglycones in Astragalus membranaceus hairy root cultures by combining deglycosylation and elicitation of immobilized edible aspergillus niger. J. Agric. Food Chem. 2017, 65, 9078–9086. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Wang, Y.; Abozeid, A.; Tian, D.M.; Zhang, X.N.; Tang, Z.H. The different resistance of two astragalus plants to uv-b stress is tightly associated with the organ-specific isoflavone metabolism. Photochem. Photobiol. 2018, 94, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Gai, Q.Y.; Wang, W.; Luo, M.; Gu, C.B.; Fu, Y.J.; Ma, W. Ultraviolet radiation-elicited enhancement of isoflavonoid accumulation, biosynthetic gene expression, and antioxidant activity in Astragalus membranaceus hairy root cultures. J. Agric. Food Chem. 2015, 63, 8216–8224. [Google Scholar] [CrossRef]
- Jin, H.Y.; Yu, Y.; Quan, X.L.; Wu, S.Q. Promising strategy for improving calycosin-7-o-beta-d-glucoside production in Astragalus membranaceus adventitious root cultures. Ind. Crops Prod. 2019, 141, 111792. [Google Scholar] [CrossRef]
- Hwang, S. High-yield production of astragalosides from transgenic hairy root cultures of Astragalus membranaceus. KSBB J. 2006, 21, 123–128. [Google Scholar]
- Gai, Q.Y.; Jiao, J.; Luo, M.; Wang, W.; Yao, L.P.; Fu, Y.J. Deacetylation biocatalysis and elicitation by immobilized penicillium canescens in Astragalus membranaceus hairy root cultures: Towards the enhanced and sustainable production of astragaloside iv. Plant Biotechnol. J. 2017, 15, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sun, H.; Zhang, C.; Cao, Q.; Qin, X. Effects of hexanal on growth and accumulation of secondary metabolites in Astragalus membranaceus var. Mongholicus adventitious roots. Plant Physiol. J. 2015, 51, 476–480. [Google Scholar]
- Li, X.H.; Kim, J.K.; Park, S.U. Heterologous expression of three transcription factors differently regulated astragalosides metabolic biosynthesis in Astragalus membranaceus hairy roots. Plants 2022, 11, 1897. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Thwe, A.A.; Li, X.H.; Kim, Y.J.; Kim, J.K.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Triterpene and flavonoid biosynthesis and metabolic profiling of hairy roots, adventitious roots, and seedling roots of Astragalus membranaceus. J. Agric. Food Chem. 2015, 63, 8862–8869. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.R.; Xu, S.H.; Li, J.L.; Hu, Y.L.; Lin, Z.P. Expression profile of a pal gene from Astragalus membranaceus var. Mongholicus and its crucial role in flux into flavonoid biosynthesis. Plant Cell Rep. 2006, 25, 705–710. [Google Scholar] [CrossRef]
- Bao, R.; Liu, J.; Yuan, Y.; Yu, Y.; Quan, X.; Wu, S. Cloning and expression analysis of chalcone reductase gene from Astragalus membranaceus. Nor. Horticul. 2019, 141–146. [Google Scholar]
- Zheng, H.; Yuan, Y.; Jin, H.; Yu, Y.; Quan, X.; Wu, S. Cloning and expression analysis of chs gene in Astragalus membranaceus. J. Agric. Sci. Yanbian Univ. 2019, 41, 9–14. [Google Scholar]
- Liu, X. Responses of Artemisia Annua to Polyploidy Induction and the Regualtion of Agnps on Salvia Miltiorrhiza Hairy Roots. Ph.D Thesis, Northeast Forestry University, Xianyang, China, 2013. [Google Scholar]
- Wang, W. Study on the Nutrition Absorption Characteristics and Second Ary Metabolish Regulation of Astragalus membranaceus (fisch.) bge. Ph.D Thesis, Northwest A&F University, Xianyang, China, 2008. [Google Scholar]
- Xiao, Y.; Wang, R.; Zhao, S. Research progress on biosynthesis of saponins of astragali radix. Acta Univ. Tradit. Med. Sin. Pharmacol. Shanghai 2021, 35, 80–88. [Google Scholar]
- Chen, J.; Wu, X.T.; Xu, Y.Q.; Zhong, Y.; Li, Y.X.; Chen, J.K.; Li, X.; Nan, P. Global transcriptome analysis profiles metabolic pathways in traditional herb Astragalus membranaceus bge. Var. Mongolicus (bge.) Hsiao. BMC Genom. 2015, 16, S15. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, M.; Xu, L.; Yi, Y.; Wang, L.; Wang, H.; Wang, Z.; Xing, J.; Li, P.; Zhang, X.; et al. Identification of oxidosqualene cyclases associated with saponin biosynthesis from Astragalus membranaceus reveals a conserved motif important for catalytic function. J. Adv. Res. 2023, 43, 247–257. [Google Scholar] [CrossRef]
- Yao, M.; Liu, J.; Jin, S.; Jiao, J.; Gai, Q.; Wei, Z.; Fu, Y.; Zhao, J. A novel biotransformation of astragalosides to astragaloside iv with the deacetylation of fungal endophyte penicillium canescens. Process Biochem. 2014, 49, 807–812. [Google Scholar] [CrossRef]
- Duan, Y.; Du, W.; Song, Z.; Chen, R.; Xie, K.; Liu, J.; Chen, D.; Dai, J. Functional characterization of a cycloartenol synthase and four glycosyltransferases in the biosynthesis of cycloastragenol-type astragalosides from Astragalus membranaceus. Acta Pharm. Sin. B 2023, 13, 271–283.58. [Google Scholar] [CrossRef]
- Meng, X.T.; Yue, S.J.; Yang, Z.R.; Liu, J.; Feng, W.W.; Su, J.; Zhang, Y. Investigation on Biotransformation Characteristics of Astragalosides in Human Intestinal Microbiota. Food Drug 2018, 20, 161–167. (In Chinese) [Google Scholar]
- Zhang, J.; Wang, S. Expression of Astragalus membranaceus phenylalanine ammonia-lyase gene in pichia pastoris. J. Zhejiang Univ. Agric. Life Sci. 2014, 40, 1–8. [Google Scholar]
- Chen, C.; Fu, Y.; Zu, Y.; Wang, W.; Mu, F.; Luo, M.; Li, C.; Gu, C.; Zhao, C. Biotransformation of saponins to astragaloside iv from radix astragali by immobilized aspergillus niger. Biocatal. Agric. Biotechnol. 2013, 2, 196–203. [Google Scholar] [CrossRef]
- Ye, L.; Liu, X.H.; Zhou, W.; Feng, M.Q.; Shi, X.L.; Li, J.Y.; Chen, D.F.; Zhou, P. Microbial transformation of astragalosides to astragaloside iv by absidia corymbifera as2. Process Biochem. 2011, 46, 1724–1730. [Google Scholar] [CrossRef]
- Bedir, E.; Kula, C.; Oner, O.; Altas, M.; Tag, O.; Ongen, G. Microbial transformation of astragalus sapogenins using cunninghamella blakesleeana nrrl 1369 and glomerella fusarioides atcc 9552. J. Mol. Catal. B-Enzym. 2015, 115, 29–34. [Google Scholar] [CrossRef]
- Li, Q.; Wu, T.; Zhao, L.; Pei, J.; Wang, Z.; Xiao, W. Highly efficient biotransformation of astragaloside iv to cycloastragenol by sugar-stimulated beta-glucosidase and beta-xylosidase from dictyoglomus thermophilum. J. Microbiol. Biotechnol. 2019, 29, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, X.; Ye, L.; Feng, M.; Zhou, P.; Shi, X. The biotransformation of astragalosides by a novel acetyl esterase from absidia corymbifera as2. Process Biochem. 2014, 49, 1464–1471. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, H.; Liang, H.; Sun, X.; Shen, X.; Wang, J.; Wang, W.; Yuan, Q.; Ri, H.; Kim, T. Enzymatic bioconversion of cycloastragenol-6-o-β-d-glucoside into cycloastragenol by a novel recombinant β-glucosidase from phycicoccus sp. Soil748. Process Biochem. 2020, 90, 81–88. [Google Scholar] [CrossRef]

| Culture Material | Culture Medium | Cultivation Conditions | Result |
|---|---|---|---|
| Callus | MS + 0.5 mg/L 6-BA + 2 mg/L2.4-D + 0.1 mg/LVC+3% sucrose + 0.7% agar | The culture temperature was 25 °C, 24 h dark culture, pH value was 5.8 | The induction rate can reach 100% [16] |
| Suspension cell | MS + 0.1 mg/L (NAA) + 1.0 mg/L (6- BA) + 1.5% (w/v) sucrose and 0.8% (w/v) agar After three weeks, MS + 0.5 mg/L (IAA) + 1.5% (w/v) sucrose and 0.8% (w/v) agar | Seed germination, callus induction and subculture were carried out in a growth chamber illuminated with fluorescent light (ca. 1400 mol m−2 s−1) over a 16/8 day and night at 25 ± 2 °C | The seedlings developed fragile callus within 2 weeks [17] |
| Adventitious root | MS +7 mg/L(IBA)+ 30 g/L sucrose + 7 g/L agar | In dark, at 25 ± 2 °C, for 6 weeks of culture | Adventitious roots were successfully induced [18] |
| Culture Material | Activity Component | Influence Factor | Metabolic Regulation | Result |
|---|---|---|---|---|
| Suspension cells | PAL Activity and Total Phenol | Yeast extract (10 g/L) was added for 36 h on the 13th day of culture | PAL activity induced by yeast extract was positively correlated with total phenol accumulation | Increased PAL activity and total phenol content [30] |
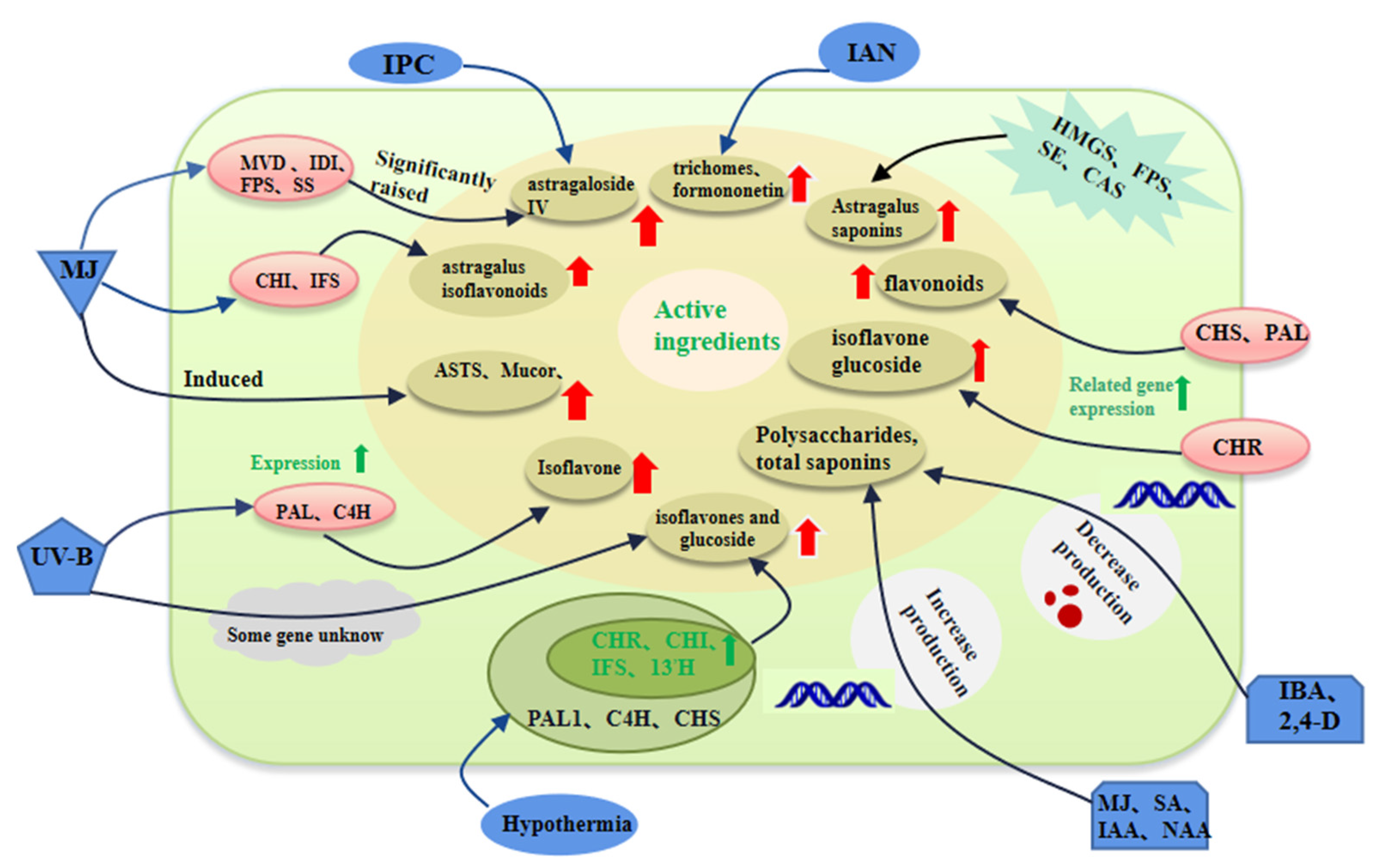
| Astragalus | Astragalus saponin | --- | HMGR, FPS, SE, and CAS are the main regulatory genes | Regulated the synthesis of Astragalus saponin [31] |
| Hairy roots | Saponins and Isoflavone | Regulation of Methyl Jasmonate (MJ), Acetylsalicylic Acid (ASA) and Salicylic Acid (SA) | MVD, IDI, FPS, SS, CHI, IFS | It is revealed that MVD, IDI, FPS and SS are key enzyme genes that MJ induces and which regulate the saponin biosynthesis pathway CHI and IFS are the key factors of the isoflavone biosynthesis pathway [21] |
| Astragalus | CycloAstragalus phenol and Astragalus phenol | Endophytic fungi | --- | Endophytic fungi were found to transform sapogenins (Cycloenosterol and Astragalus cresol) [23] |
| Hairy roots of Astragalus | ASTS, MAO rhzomorphand CG | 100 μM methyl jasmonate (MeJA) treatment | 2127 genes were up-regulated by MeJA and 1247 genes were down-regulated by MeJA | The accumulation of ASTS, MAO rhizomorph and CG in hairy roots treated with MeJA increased significantly [32] |
| Astragalus | Genistein -7-O-β-D- glucoside (CGs) | Low temperature stress, light dependence | CHS, CHR, CHI, IFS, and I3’H PAL1, C4H | Temperature fluctuations up-regulated the transcription of CHS, CHR, CHI, IFS, and I3’H, but had different effects on the transcription of PAL1 and C4H of phenylpropanoid pathway in leaves [33] |
| Hairy roots | Hairy cephalosporins (CA) and formononetin (FO) | AMHRCs were co-cultured with immobilized aspergillus niger (IAN) for 54 h | --- | The CA and FO biosynthetic pathway gene expression was significantly up-regulated, thereby increasing the production of CA and FO [34] |
| Root | Isoflavone | After 10 days of UV-B treatment (λ = 313 nm, 804 j/m) | --- | UV-B radiation significantly induced isoflavone synthesis [35] |
| Hairy roots | Isoflavone | Ultraviolet light (UV-A, UV-B and UV-C) irradiation | PAL, C4H | 86.4 kJ/m (2) UV-B upregulated the transcription and expression of all genes involved in the isoflavone biosynthesis pathway of AMHRCs [36] |
| Culture Material | Active Ingredient | Influence Factor | Increase Multiples |
|---|---|---|---|
| Astragalus adventitious roots | Calycosin isoflavone glycoside | Hydrogen peroxide, the L-phenylalanine | The culture treated with hydrogen peroxide and L-phenylalanine was 8.6 times higher than that treated with hydrogen peroxide alone [37] |
| Astragalus adventitious roots | Calycosin isoflavone glycoside | Drought stress, methyl jasmonate, and L-phenylalanine | The three combinations induced the highest CG content, 3.12 times higher than that of the field plants [18] |
| Astragalus hair root | Astragalus saponin I, Astragalus saponin and Astragalus methyside | Methyl jasmonate | It reached 2.98, 2.85, 2.30, and 1.57 times in the control group, respectively [32] |
| Astragalus hair root | Astragalus methylside | Chitosan | It was 2.1 times higher than that in the control group [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, B.; Xuan, L.; Zhang, Y.; Zhang, G.; Meng, J.; Mu, W.; Liu, J.; Paek, K.-Y.; Park, S.-Y.; Wang, J.; et al. Advances in Biotechnological Production and Metabolic Regulation of Astragalus membranaceus. Plants 2023, 12, 1858. https://doi.org/10.3390/plants12091858
Ji B, Xuan L, Zhang Y, Zhang G, Meng J, Mu W, Liu J, Paek K-Y, Park S-Y, Wang J, et al. Advances in Biotechnological Production and Metabolic Regulation of Astragalus membranaceus. Plants. 2023; 12(9):1858. https://doi.org/10.3390/plants12091858
Chicago/Turabian StyleJi, Baoyu, Liangshuang Xuan, Yunxiang Zhang, Guoqi Zhang, Jie Meng, Wenrong Mu, Jingjing Liu, Kee-Yoeup Paek, So-Young Park, Juan Wang, and et al. 2023. "Advances in Biotechnological Production and Metabolic Regulation of Astragalus membranaceus" Plants 12, no. 9: 1858. https://doi.org/10.3390/plants12091858
APA StyleJi, B., Xuan, L., Zhang, Y., Zhang, G., Meng, J., Mu, W., Liu, J., Paek, K.-Y., Park, S.-Y., Wang, J., & Gao, W. (2023). Advances in Biotechnological Production and Metabolic Regulation of Astragalus membranaceus. Plants, 12(9), 1858. https://doi.org/10.3390/plants12091858






