Flowers and Inflorescences of Selected Medicinal Plants as a Source of Triterpenoids and Phytosterols
Abstract
1. Introduction
2. Results
2.1. Identification of Steroids and Triterpenoids in Obtained Extracts
2.2. The Content of Steroids and Triterpenoids in Berberis vulgaris Inflorescences and Leaves
2.3. The Content of Steroids and Triterpenoids in Crategus laevigata Inflorescences and Leaves
2.4. The Content of Steroids and Triterpenoids in Pulsatilla vulgaris Flowers and Leaves
2.5. The Content of Steroids and Triterpenoids in Rosa rugosa Flowers and Leaves
2.6. The Content of Steroids and Triterpenoids in Inflorescences of Wild S. nigra and Ornamental S. nigra f. Porphyrophylla
2.7. The Content of Steroids and Triterpenoids in Vinca minor Flowers and Leaves
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction
4.3. Fractionation of Diethyl Ether Extracts
4.4. Derivatization of Triterpenoid Acids
4.5. Identification and Quantification of Steroids by Gas Chromatography-Mass Spectrometry
4.6. Statistical Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chrétien, L.T.S.; Khalil, A.; Gershenzon, J.; Lucas-Barbosa, D.; Marcel Dicke, M.; Giron, D. Plant metabolism and defence strategies in the flowering stage: Time-dependent responses of leaves and flowers under attack. Plant Cell Environ. 2022, 45, 2841–2855. [Google Scholar] [CrossRef]
- Skrajda-Brdak, M.; Dąbrowski, G.; Konopka, I. Edible flowers, a source of valuable phytonutrients and their pro-healthy effects—A review. Trends Food Sci. Technol. 2020, 103, 179–199. [Google Scholar] [CrossRef]
- Kumari, P.; Ujala; Bhargava, B. Phytochemicals from edible flowers: Opening a new arena for healthy life style. J. Funct. Foods 2021, 78, 104375. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers: Emerging components in the diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Matyjaszczyk, E.; Śmiechowska, M. Edible flowers. Benefits and risks pertaining to their consumption. Trends Food Sci. Technol. 2019, 91, 670–674. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Anti-hyperglycemic and anticholinergic effects of natural antioxidant contents in edible flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Rezende, F.A.G.G.; Mourac, M.A.F.; Dominguetec, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2020, 129, 108868. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, E.R.T. Edible flowers: More than a gastronomic trend. Ornam. Hortic. 2021, 27, 436–437. [Google Scholar] [CrossRef]
- Janarny, G.; Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S. Nutraceutical potential of dietary phytochemicals in edible flowers—A review. J. Food Biochem. 2021, 45, e13642. [Google Scholar] [CrossRef]
- Faccio, G. Plant complexity and cosmetic innovation. iScience 2020, 23, 101358. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Ziemlewska, A.; Bujak, T.; Nizioł-Łukaszewska, Z.; Hordyjewicz-Baran, Z. Cosmetic and dermatological properties of selected Ayurvedic plant extracts. Molecules 2021, 26, 614. [Google Scholar] [CrossRef] [PubMed]
- Bujak, T.; Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Lal, K.; Wasilewski, T.; Hordyjewicz-Baran, Z. Flower extracts as multifunctional dyes in the cosmetics industry. Molecules 2022, 27, 922. [Google Scholar] [CrossRef] [PubMed]
- Giustra, M.; Cerri, F.; Anadol, Y.; Salvioni, L.; Antonelli Abella, T.; Prosperi, D.; Galli, P.; Colombo, M. Eco-luxury: Making sustainable drugs and cosmetics with Prosopis cineraria natural extracts. Front. Sustain. 2022, 3, 1047218. [Google Scholar] [CrossRef]
- dos Santos Nascimento, L.B.; Gori, A.; Raffaelli, A.; Ferrini, F.; Brunetti, C. Phenolic compounds from leaves and flowers of Hibiscus roseus: Potential skin cosmetic applications of an under-investigated species. Plants 2021, 10, 522. [Google Scholar] [CrossRef]
- Zymone, K.; Raudone, L.; Žvikas, V.; Jakštas, V.; Janulis, V. Phytoprofiling of Sorbus L. inflorescences: A valuable and promising resource for phenolics. Plants 2022, 11, 3421. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, M.C.; Menichini, F.; Xiao, J.; Tundis, R. Edible flowers: A rich source of phytochemicals with antioxidant and hypoglycemic properties. J. Agric. Food Chem. 2016, 64, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Kandylis, P. Phytochemicals and antioxidant properties of edible flowers. Appl. Sci. 2022, 12, 9937. [Google Scholar] [CrossRef]
- Rivas-García, L.; Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Forbes-Hernández, T.Y.; Varela-López, A.; Juan Llopis, J.; Sánchez-González, C.; Quiles, J.L. Edible flowers as a health promoter: An evidence-based review. Trends Food Sci. Technol. 2021, 117, 46–59. [Google Scholar] [CrossRef]
- Fang, S.; Belwal, T.; Li, L.; Limwachiranon, J.; Liu, X.; Luo, Z. Phytosterols and their derivatives: Potential health-promoting uses against lipid metabolism and associated diseases, mechanism and safety issues. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1243–1267. [Google Scholar] [CrossRef]
- Suryamani; Sindhu, R.; Singh, I. Phytosterols: Physiological functions and therapeutic applications. In Bioactive Food Components Activity in Mechanistic Approach; Cazarin, C.B.B., Pastore, G.M., Bicas, J.L., Morostica, M.R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 223–238. [Google Scholar]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The bioavailability and biological activities of phytosterols as modulators of cholesterol metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Adelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar]
- Paduch, R.; Kandefer-Szerszeń, M. Antitumor and antiviral activity of pentacyclic triterpenes. Mini-Rev. Org. Chem. 2014, 11, 262–268. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef]
- González-Coloma, A.; López-Balboa, C.; Santana, O.; Reina, M.; Fraga, B.M. Triterpene-based plant defenses. Phytochem. Rev. 2011, 10, 245–260. [Google Scholar] [CrossRef]
- Yang, X.; Ding, W.; Yue, Y.; Xu, C.; Wang, X.; Wang, L. Cloning and expression analysis of three critical triterpenoid pathway genes in Osmanthus fragrans. Electron. J. Biotechol. 2018, 36, 1–8. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Yasukawa, K.; Kasahara, Y.; Kimura, Y.; Koike, K.; Nikaido, T.; Takido, M. Constituents of compositae plants 2. Triterpene diols, triols, and their 3-O-fatty acid esters from edible Chrysanthemum flower extract and their anti-inflammatory effects. J. Agric. Food Chem. 2001, 49, 3187–3197. [Google Scholar] [CrossRef] [PubMed]
- Vilkickyte, G.; Raudone, L. Optimization, validation and application of HPLC-PDA methods for quantification of triterpenoids in Vaccinium vitis-idaea L. Molecules 2021, 26, 1645. [Google Scholar] [CrossRef] [PubMed]
- Shanaida, M. Determination of triterpenoids in some Lamiaceae species. Res. J. Pharm. Technol. 2018, 11, 3113–3118. [Google Scholar] [CrossRef]
- Han, J.; Chen, X.; Liu, W.; Cui, H.; Yuan, T. Triterpenoid saponin and lignan glycosides from the traditional medicine Elaeagnus angustifolia flowers and their cytotoxic activities. Molecules 2020, 25, 462. [Google Scholar] [CrossRef]
- Lin, L.M.; Zhang, X.G.; Zhu, J.J.; Gao, H.M.; Wang, Z.M.; Wang, W.H. Two new triterpenoid saponins from the flowers and buds of Lonicera japonica. J. Asian Nat. Prod. Res. 2008, 10, 925–929. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Huttunen, S. Triterpenoid content of berries and leaves of bilberry Vaccinium myrtillus from Finland and Poland. J. Agric. Food Chem. 2012, 60, 11839–11849. [Google Scholar] [CrossRef]
- Gadouche, L.; Alsoufi, A.S.M.; Pacholska, D.; Skotarek, A.; Pączkowski, C.; Szakiel, A. Triterpenoid and steroid content of lipophilic extracts of selected medicinal plants of the Mediterranean region. Molecules 2023, 28, 697. [Google Scholar] [CrossRef]
- Mokhber-Dezfuli, N.; Saeidnia, S.; Gohari, A.R.; Kurepaz-Mahmoodabadi, M. Phytochemistry and pharmacology of Berberis species. Pharmacogn. Rev. 2014, 8, 8–15. [Google Scholar]
- Bober, Z.; Stepień, A.; Aebisher, D.; Ożóg, Ł.; Bartusik-Aebisher, D. Fundamentals of the use of Berberis as a medicinal plants. Eur. J. Clin. Exp. Med. 2018, 16, 41–46. [Google Scholar] [CrossRef]
- Khokhlova, K.; Zdoryk, O.; Vyshnevska, L. Chromatographic characterization on flavonoids and triterpenes of leaves and flowers of 15 Crataegus species. Nat. Prod. Res. 2020, 34, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Gleńsk, M.; Włodarczyk, M. Determination of oleanolic and ursolic acids in Sambuci flos using HPLC with a new reversed-phase column packed with naphthalene bounded silica. Nat. Prod. Com. 2017, 12, 1839–1841. [Google Scholar] [CrossRef]
- Martin, R.; Fausten, G.; Bischoff, F. Screening of different species on their content of the triterpenes ursolic and oleanolic acid. J. Med. Spice Plant 2009, 14, 37–43. [Google Scholar]
- Łaska, G.; Sieniawska, E.; Maciejewska-Turska, M.; Świątek, Ł.; Pasco, D.S.; Balachandran, P. Pulsatilla vulgaris inhibits cancer proliferation in signaling pathways of 12 reporter genes. Int. J. Mol. Sci. 2023, 24, 1139. [Google Scholar] [CrossRef]
- Dashbaldan, S.; Rogowska, A.; Pączkowski, C.; Szakiel, A. Distribution of triterpenoids and steroids in developing rugosa rose (Rosa rugosa Thunb.) accessory fruit. Molecules 2021, 26, 5158. [Google Scholar] [CrossRef]
- Cuong, D.M.; Sathasivam, R.; Park, C.H.; Yeo, H.J.; Park, Y.E.; Kim, J.K.; Park, S.U. Analysis of triterpenoids, carotenoids, and phenylpropanoids in the flowers, leaves, roots, and stems of white bitter melon (Cucurbitaceae, Momordica charantia). Trop. J. Pharm. Res. 2021, 20, 155–160. [Google Scholar] [CrossRef]
- Nezi, P.; Cicaloni, V.; Tinti, L.; Salvini, L.; Iannone, M.; Vitalini, S.; Garzoli, S. Metabolomic and proteomic profile of dried hop inflorescences (Humulus lupulus L. cv. Chinook and cv. Cascade) by SPME-GC-MS and UPLC-MS-MS. Separations 2022, 9, 204. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Koivuniemi, H.; Huttunen, S. Comparison of the triterpenoid content of berries and leaves of lingonberry Vaccinium vitis-idaea from Finland and Poland. J. Agric. Food Chem. 2012, 60, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Babotă, M.; Mocan, A.; Vlase, L.; Crişan, O.; Ielciu, I.; Gheldiu, A.M.; Vodnar, D.C.; Crişan, G.; Păltinean, R. Phytochemical analysis, antioxidant and antimicrobial activities of Helichrysum arenarium (L.) Moench. and Antennaria dioica (L.) Gaertn. Flowers. Molecules 2018, 23, 409. [Google Scholar] [CrossRef] [PubMed]
- Stuper-Szablewska, K.; Szablewski, T.; Przybylska-Balcerek, A.; Szwajkowska-Michałek, L.; Krzyżaniak, M.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z. Antimicrobial activities evaluation and phytochemical screening of some selected plant materials used in traditional medicine. Molecules 2023, 28, 244. [Google Scholar] [CrossRef]
- Wrońska, N.; Szlaur, M.; Zawadzka, K.; Lisowska, K. The synergistic effect of triterpenoids and flavonoids—New approaches for treating bacterial infections? Molecules 2022, 27, 847. [Google Scholar] [CrossRef] [PubMed]
- Tonga, J.L.; Kamdem, M.H.K.; Pagna, J.I.M.; Fonkui, T.Y.; Tata, C.M.; Fotsing, M.C.D.; Nkengfack, E.A.; Mmutlane, E.M.; Ndinteh, D.T. Antibacterial activity of flavonoids and triterpenoids isolated from the stem bark and sap of Staudtia kamerunensis Warb. (Myristicaceae). Arab. J. Chem. 2022, 15, 104150. [Google Scholar] [CrossRef]
- Tolufashe, G.F.; Lawal, M.M.; Govender, K.K.; Shode, F.O.; Singh, T. Exploring the bioactivity of pentacyclic triterpenoids as potential antimycobacterial nutraceutics: Insights through comparative biomolecular modeling. J. Mol. Graph. Model. 2021, 105, 1007900. [Google Scholar] [CrossRef]
- Tursun, X.; Zhao, Y.; Talat, Z.; Xin, X.; Tursun, A.; Abdulla, R.; AkbarAisa, H. Anti-inflammatory effect of Rosa rugosa flowers extract in lyposaccharose-stimulated RAW264.7 macrophages. Biomed. Ther. 2016, 24, 184–190. [Google Scholar]
- Petrut Marţis, G.S.; Mureșan, V.; Vlaic Marc, R.M.; Mureșan, C.C.; Pop, C.R.; Buzgău, G.; Mureșan, A.E.; Ungur, R.A.; Muste, S. The physicochemical and antioxidant properties of Sambucus nigra L. and Sambucus nigra Haschberg during growth phases: From buds to ripening. Antioxidants 2021, 10, 1093. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Szakiel, A.; Głowacka, A.; Rozpara, E.; Marszałek, K.; Skąpska, S. Triterpenoids of three apple cultivars—Biosynthesis, antioxidative and anti-inflammatory properties, and fate during processing. Molecules 2023, 28, 2584. [Google Scholar] [CrossRef]
- Han, N.; Bakovic, M. Biologically active triterpenoids and their cardioprotective and anti-inflammatory effects. J. Bioanal. Biomed. 2015, 1, S12. [Google Scholar]
- Rababa’h, A.M.; Al Yacoub, O.N.; El-Elimat, T.; Rabab’ah, M.; Altarabsheh, S.; Deo, S.; Al-Azayzih, A.; Zayed, A.; Alazzam, S.; Alzoubi, K.H. The effect of hawthorn flower and leaf extract (Crataegus spp.) on cardiac hemostasis and oxidative parameters in Sprague Dawley rats. Heliyon 2020, 6, e04617. [Google Scholar] [CrossRef]
- Vrancheva, R.; Ivanov, I.; Dincheva, I.; Badjakov, I.; Pavlov, A. Triterpenoids and other non-polar compounds in leaves of wild and cultivated Vaccinium species. Plants 2021, 10, 94. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The content of selected minerals, bioactive compounds, and the antioxidant properties of the flowers and fruit of selected cultivars and wildly growing plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef]
- Rogowska, A.; Pączkowski, C.; Szakiel, A. Modulation of steroid and triterpenoid metabolism in Calendula officinalis plants and hairy root cultures exposed to cadmium stress. Int. J. Mol. Sci. 2022, 23, 5640. [Google Scholar] [CrossRef]
- Rogowska, A.; Stpiczyńska, M.; Pączkowski, C.; Szakiel, A. The influence of exogenous jasmonic acid on the biosynthesis of steroids and triterpenoids in Calendula officinalis plants and hairy root culture. Int. J. Mol. Sci. 2022, 23, 12173. [Google Scholar] [CrossRef]
- Zhao, M.; Fan, J.; Liu, Q.; Luo, H.; Tang, Q.; Li, C.; Zhao, J.; Zhang, X. Phytochemical profiles of edible flowers of medicinal plants of Dendrobium officinale and Dendrobium devonianum. Food Sci. Nutr. 2021, 9, 6575–6586. [Google Scholar] [CrossRef]
- Benvenuti, S.; Mazzoncini, M. The biodiversity of edible flowers: Discovering new tastes and new health benefits. Front. Plant Sci. 2021, 11, 569499. [Google Scholar] [CrossRef] [PubMed]
- Schmitzer, V.; Choo, W.S.; Urbanek Krajnc, A. Editorial: Phytochemical in ornamental plants: Synthesis, nutraceutical properties and applied focus—Women in Plant Science series. Front. Plant Sci. 2022, 13, 1016968. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, R.; Periago, M.J.; Luna-Recio, C.; Javier, G.A.F.; Navarro-González, I. Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018, 252, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Szakiel, A.; Pączkowski, C.; Marszałek, K.; Skąpska, S.; Kowalska, H.; Jędrzejczak, R. Extraction of triterpenic acid and phytosterols from apple pomace with supercritical carbon dioxide: Impact of process parameters, modelling of kinetics, and scaling-up study. Molecules 2018, 23, 2790. [Google Scholar] [CrossRef] [PubMed]
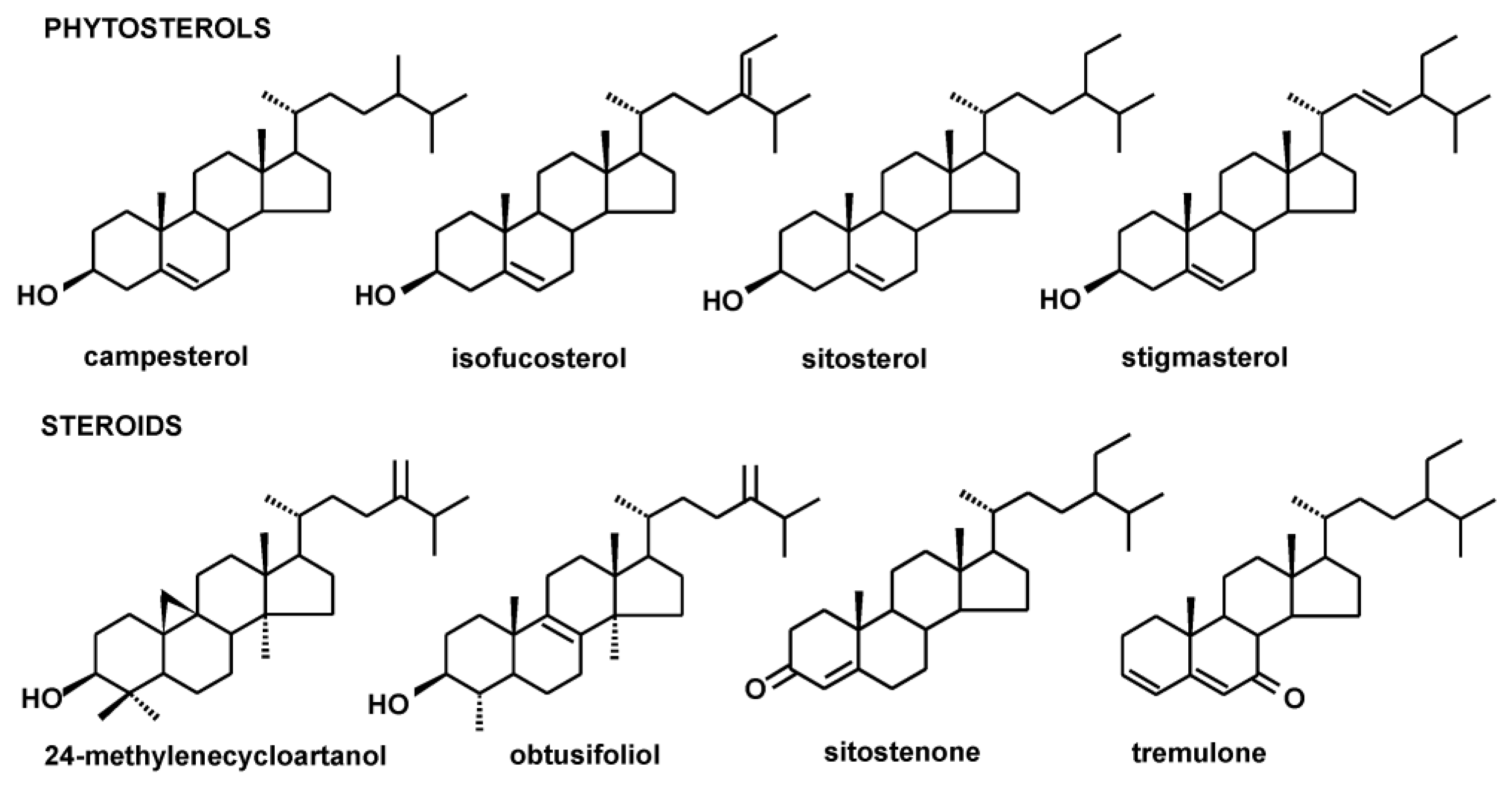

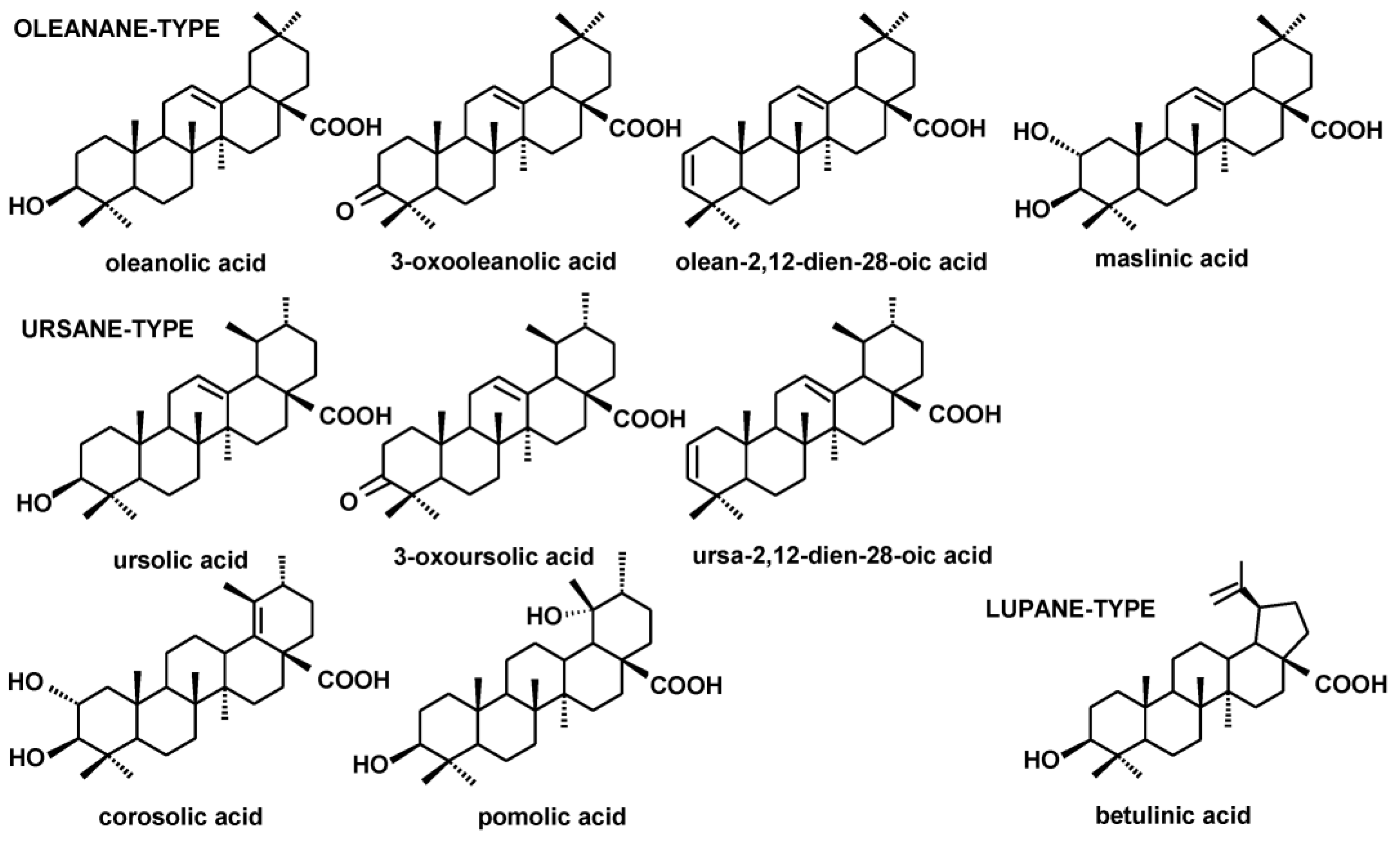

| Compound/Category | Inflorescences | Leaves |
|---|---|---|
 |  | |
| µg/g d.w. | ||
| Steroids: | ||
| Campesterol | 31.50 ± 2.49 a, A | 46.65 ± 4.26 b, A |
| Stigmasterol | 11.71 ± 0.96 a, A | 11.77 ± 0.57 a, A |
| Sitosterol | 55.45 ± 4.76 a, A | 285.56 ± 19.38 b, A |
| 24-methylenecycloartanol | 18.51 ± 1.37 a, A | 26.77 ± 1.88 b, A |
| Tremulone | 38.56 ± 2.84 a, A | 177.05 ± 15.18 b, A |
| Sum of steroids | 155.73 | 547.82 |
| Triterpenoid acids: | ||
| Oleanolic acid | 45.82 ± 3.48 a, A | 38.92 ± 3.22 a, A |
| Ursolic acid | 76.82 ± 5.69 a, A | 79.68 ± 6.65 a, A |
| Olean-2,12-dien-28-oic acid | 3.28 ± 0.40 A | n.d. |
| Ursa-2,12-dien-28-oic acid | 10.94 ± 0.88 A | n.d. |
| 3-oxo-oleanolic acid | 1.89 ± 0.02 A | n.d. |
| 3-oxo-ursolic acid | 3.62 ± 0.24 A | n.d. |
| Sum of triterpenoid acids | 142.37 | 118.60 |
| Total | 298.10 | 666.42 |
| Compound/Category | Flowers | Leaves |
|---|---|---|
 |  | |
| µg/g d.w. | ||
| Steroids: | ||
| Sitosterol | 62.80 ± 5.64 a, A | 118.92 ± 10.06 b, B |
| Tremulone | 61.75 ± 5.97 a, B | 55.64 ± 5.02 b, B |
| Sum of steroids | 124.55 | 174.56 |
| neutral triterpenoids: | ||
| α-amyrine/lupeol | 1346.22 ± 108.94 a, A | 34.87 ± 3.50 b, A |
| α-amyrenone | n.d. | 32.60 ± 3.09 a, A |
| β-amyrine | 94.81 ± 10.20 a, A | 25.98 ± 2.62 b, A |
| Erythrodiol | n.d. | 34.51 ± 3.35 a, A |
| Germanicol | n.d. | 56.98 ± 5.26 a, A |
| Uvaol | n.d. | 37.14 ± 3.58 a, A |
| Oleanolic aldehyde | 17.62 ± 1.68 A | n.d. |
| Ursolic aldehyde | 186.80 ± 20.45 A | n.d. |
| Sum of neutral triterpenoids | 1645.45 | 222.08 |
| Triterpenoid acids: | ||
| Oleanolic acid | 357.57 ± 30.15 a, B | 1277.52 ± 118.84 b, B |
| Betulinic acid | 21.09 ± 2.11 a, A | 86.66 ± 8.50 b, A |
| Ursolic acid | 769.14 ± 68.64 a, B | 4506.87 ± 401.11 b, B |
| Olean-2,12-dien-28-oic acid | 13.96 ± 1.42 a, B | 27.80 ± 3.12 b, A |
| Ursa-2,12-dien-28-oic acid | 47.84 ± 4.06 a, B | 102.55 ± 11.70 b, A |
| 3-oxo-oleanolic acid | 23.56 ± 2.22 a, B | n.d. |
| 3-oxo-ursolic acid | 27.94 ± 2.86 a, B | n.d. |
| Sum of triterpenoid acids | 1261.10 | 6001.40 |
| Total | 3031.10 | 6398.04 |
| Compound/Category | Flowers | Leaves |
|---|---|---|
 |  | |
| µg/g d.w. | ||
| Steroids: | ||
| Campesterol | 160.40 ± 14.86 a, B | 25.72 ± 2.60 b, B |
| Isofucosterol | 57.39 ± 5.40 a, A | n.d. |
| Stigmasterol | 171.44 ± 15.26 a, B | 22.06 ± 2.28 b, B |
| Sitosterol | 645.31 ± 65.05 a, B | 101.19 ± 12.55 b, B |
| Sitostenone | 73.68 ± 7.18 a, A | 27.99 ± 2.31 b, A |
| Tremulone | 28.71 ± 3.05 a, C | 63.22 ± 6.08 b, A |
| Sum of steroids | 1136.93 | 240.18 |
| Triterpenoid acids | ||
| Oleanolic acid | 20.58 ± 1.94 a, C | 22.32 ± 2.46 a, C |
| Ursolic acid | 5.18 ± 0.46 a, C | 6.26 ± 0.58 a, C |
| Sum of triterpenoid acids | 25.76 | 28.58 |
| Total | 1162.69 | 268.76 |
| Compound/Category | Flowers | Leaves |
|---|---|---|
 |  | |
| µg/g d.w. | ||
| Steroids: | ||
| Campesterol | 16.62 ± 1.58 a, C | 55.74 ± 6.01 b, A |
| Isofucosterol | 33.78 ± 4.02 a, B | 60.12 ± 5.94 b, A |
| Stigmasterol | 8.12 ± 0.98 a, C | 39.93 ± 4.05 b, C |
| Sitosterol | 329.30 ± 20.71 a, C | 410.41 ± 29.95 b, C |
| 24-methylenecycloartanol | 21.44 ± 2.66 a, A | 55.26 ± 5.72 b, B |
| Obtusifoliol | 18.17 ± 1.85 a, A | 28.79 ± 3.11 b, A |
| Sitostenone | 6.39 ± 0.62 a, B | 18.62 ± 2.04 b, B |
| Tremulone | 22.61 ± 2.30 a, C | 25.50 ± 2.63 a, C |
| Sum of steroids | 456.43 | 694.37 |
| Neutral triterpenoids: | ||
| α-amyrine/lupeol | 284.78 ± 30.04 a, B | 158.92 ± 16.88 b, B |
| α-amyrenone | 32.54 ± 3.30 a, A | 22.15 ± 2.31 b, B |
| β-amyrine | 92.04 ± 8.98 a, A | 66.32 ± 6.74 b, B |
| Erythrodiol | 48.12 ± 5.04 a, A | n.d. |
| Uvaol | 142.58 ± 15.66 a, A | n.d. |
| Oleanolic aldehyde | 12.88 ± 1.31 a, B | 28.34 ± 3.02 b, A |
| Ursolic aldehyde | 45.13 ± 4.48 a, B | 45.86 ± 4.23 a, A |
| Sum of neutral triterpenoids | 530.07 | 321.59 |
| Triterpenoid acids: | ||
| Betulinic acid | 35.75 ± 3.60 a, B | 10.82 ± 1.07 b, B |
| Corosolic acid | 138.99 ± 14.55 a, A | 147.06 ± 15.83 a, A |
| Maslinic acid | 36.04 ± 3.46 a, A | 14.23 ± 1.57 b, A |
| Pomolic acid | 48.33 ± 5.01 a, A | 102.93 ± 10.55 b, A |
| Oleanolic acid | 250.41 ± 26.13 a, D | 152.34 ± 14.08 b, D |
| Ursolic acid | 490.82 ± 50.04 a, D | 362.20 ± 35.16 b, D |
| Olean-2,12-dien-28-oic acid | n.d. | 20.62 ± 2.44 a, A |
| Ursa-2,12-dien-28-oic acid | n.d. | 30.77 ± 3.05 a, B |
| 3-oxo-oleanolic acid | n.d. | 22.71 ± 2.33 a, A |
| 3-oxo-ursolic acid | n.d. | 61.74 ± 6.28 a, A |
| Sum of triterpenoid acids | 1000.34 | 925.42 |
| Total | 1986.84 | 1941.38 |
| Compound/Category | Wild | Ornamental |
|---|---|---|
 |  | |
| µg/g d.w. | ||
| Steroids: | ||
| Campesterol | 90.58 ± 8.69 a, D | 71.60 ± 6.23 b, E |
| Isofucosterol | 6.84 ± 0.58 a, C | 8.21 ± 0.79 a, C |
| Sitosterol | 431.16 ± 51.73 a, D | 363.84 ± 22.67 b, C |
| Sitostanol | 7.23 ± 0.58 a, A | 6.11 ± 0.56 a, A |
| 24-methylenecycloartanol | 72.23 ± 6.57 a, B | 51.50 ± 3.92 b, C |
| Tremulone | 94.24 ± 10.36 a, D | 60.07 ± 5.89 b, B |
| Sum of steroids | 702.28 | 561.33 |
| Neutral triterpenoids: | ||
| α-amyrine/lupeol | 99.24 ± 5.76 a, C | 77.14 ± 5.25 b, D |
| α-amyrenone | 42.04 ± 3.49 a, B | 21.46 ± 1.95 b, C |
| β-amyrine | 42.42 ± 3.08 a, B | 28.41 ± 2.61 b, C |
| Taraxasterol | 3.27 ± 0.21 a, A | 3.52 ± 0.29 a, A |
| Erythrodiol | 4.58 ± 0.48 a, B | 3.31 ± 0.31 a, B |
| Germanicol | 15.61 ± 1.23 a, A | 7.88 ± 0.66 b, B |
| Uvaol | 20.93 ± 1.75 a, B | 17.30 ± 1.29 a, B |
| Oleanolic aldehyde | 1.50 ± 0.13 a, C | 12.95 ± 0.89 b, B |
| Ursolic aldehyde | 144.87 ± 9.28 a, A | 93.89 ± 5.45 b, C |
| Sum of neutral triterpenoids | 374.46 | 265.86 |
| Triterpenoid acids: | ||
| Oleanolic acid | 3686.88 ± 365.00 a, E | 2137.63 ± 198.79 b, F |
| Ursolic acid | 7684.76 ± 423.20 a, E | 5840.09 ± 443.85 b, F |
| Olean-2,12-dien-28-oic acid | 343.54 ± 28.17 a, C | 157.40 ± 15.32 b, D |
| Ursa-2,12-dien-28-oic acid | 989.00 ± 72.20 a, C | 457.37 ± 36.13 b, D |
| 3-oxo-oleanolic acid | 46.62 ± 3.21 a, C | 25.42 ± 2.41 b, B |
| 3-oxo-ursolic acid | 84.65 ± 8.63 a, C | 67.85 ± 4.82 b, D |
| Oleanolic acid methyl ester | 25.41 ± 1.91 a, A | 6.53 ± 0.38 b, B |
| Ursolic acid methyl ester | 3.42 ± 0.28 a, A | 5.77 ± 0.42 b, B |
| Sum of triterpenoid acids | 12,864.28 | 8698.06 |
| Total | 13,941.02 | 9525.25 |
| Compound/Category | Flowers | Leaves |
|---|---|---|
 |  | |
| µg/g d.w. | ||
| Steroids: | ||
| Campesterol | 89.53 ± 9.75 a, D | 151.19 ± 14.80 b, C |
| Stigmasterol | 40.42 ± 4.14 a, D | 61.08 ± 6.22 b, D |
| Sitosterol | 280.13 ± 26.05 a, C | 362.63 ± 3.75 b, C |
| 24-methylenecycloartanol | 321.16 ± 30.98 a, D | n.d. |
| Sitostenone | 107.97 ± 9.86 a, C | 95.03 ± 9.55 a, C |
| Tremulone | n.d. | 93.47 ± 9.20 a, D |
| Sum of steroids | 839.21 | 763.40 |
| Neutral triterpenoids: | ||
| α-amyrine/lupeol | 2999.49 ± 280.15 a, E | 400.75 ± 38.01 b, C |
| α-amyrenone | 99.22 ± 8.86 a, D | n.d. |
| β-amyrine | 55.65 ± 5.20 a, D | 214.47 ± 20.13 b, C |
| Betulin | n.d. | 258.25 ± 24.90 a, A |
| Lupeol acetate | 2263.36 ± 203.72 a, A | n.d. |
| Oleanolic aldehyde | n.d. | 50.37 ± 4.95 a, B |
| Ursolic aldehyde | n.d. | 281.06 ± 25.88 a, B |
| Sum of neutral triterpenoids | 5417.72 | 1204.90 |
| Triterpenoid acids: | ||
| Oleanolic acid | 219.76 ± 20.04 a, D | 26.78 ± 2.76 b, C |
| Oleanolic acid acetate | 106.51 ± 9.35 a, A | n.d. |
| Ursolic acid | 654.55 ± 63.01 a, B | 83.05 ± 8.40 b, A |
| Sum of triterpenoid acids | 980.82 | 109.83 |
| Total | 7237.75 | 2028.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edorh Tossa, P.; Belorgey, M.; Dashbaldan, S.; Pączkowski, C.; Szakiel, A. Flowers and Inflorescences of Selected Medicinal Plants as a Source of Triterpenoids and Phytosterols. Plants 2023, 12, 1838. https://doi.org/10.3390/plants12091838
Edorh Tossa P, Belorgey M, Dashbaldan S, Pączkowski C, Szakiel A. Flowers and Inflorescences of Selected Medicinal Plants as a Source of Triterpenoids and Phytosterols. Plants. 2023; 12(9):1838. https://doi.org/10.3390/plants12091838
Chicago/Turabian StyleEdorh Tossa, Pauline, Morgan Belorgey, Soyol Dashbaldan, Cezary Pączkowski, and Anna Szakiel. 2023. "Flowers and Inflorescences of Selected Medicinal Plants as a Source of Triterpenoids and Phytosterols" Plants 12, no. 9: 1838. https://doi.org/10.3390/plants12091838
APA StyleEdorh Tossa, P., Belorgey, M., Dashbaldan, S., Pączkowski, C., & Szakiel, A. (2023). Flowers and Inflorescences of Selected Medicinal Plants as a Source of Triterpenoids and Phytosterols. Plants, 12(9), 1838. https://doi.org/10.3390/plants12091838










