Comprehensive Genomic Analysis of SnRK in Rosaceae and Expression Analysis of RoSnRK2 in Response to Abiotic Stress in Rubus occidentalis
Abstract
1. Introduction
2. Results
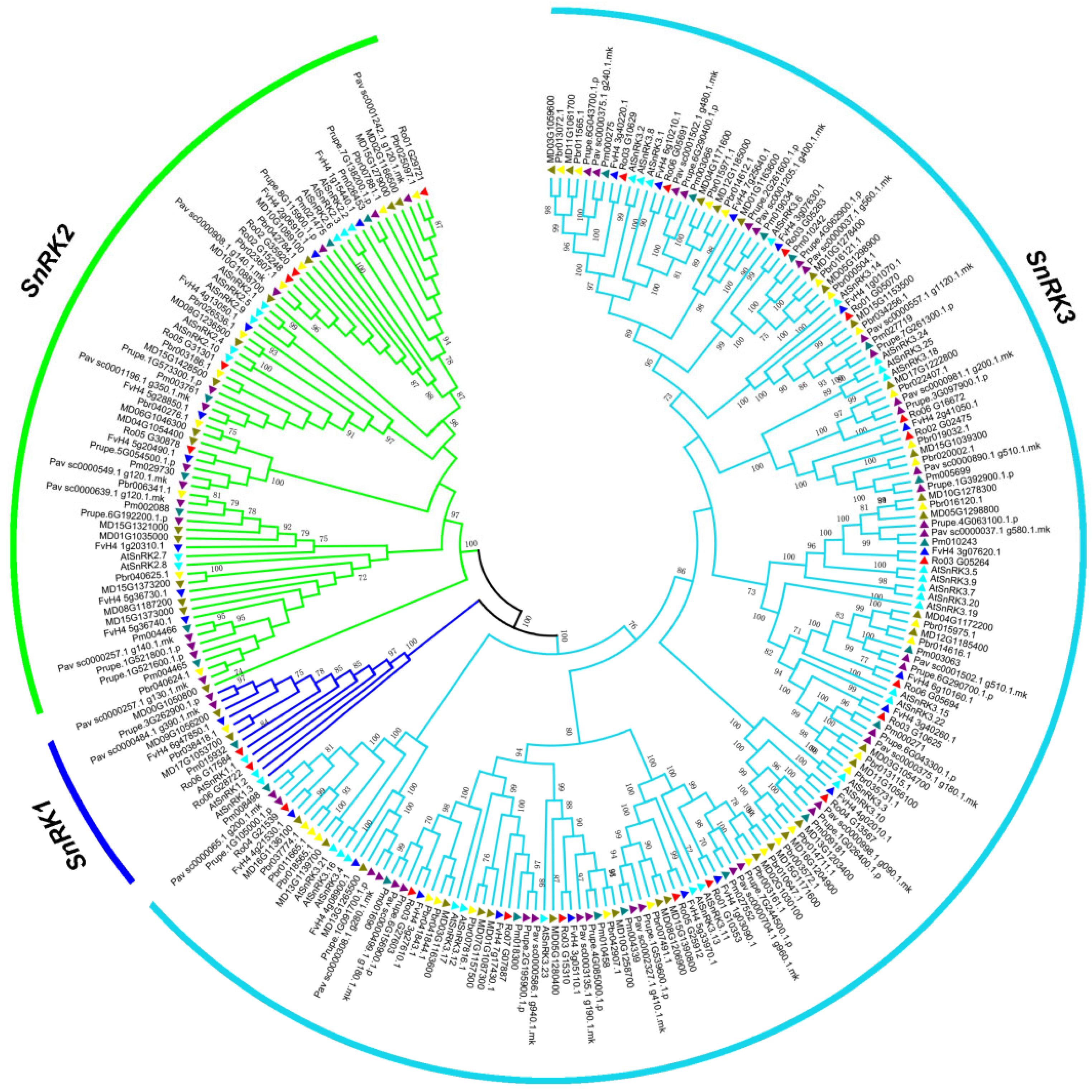
2.1. Identification, Phylogenetic Tree and Classification of SnRK Genes in 7 Rosaceae Species
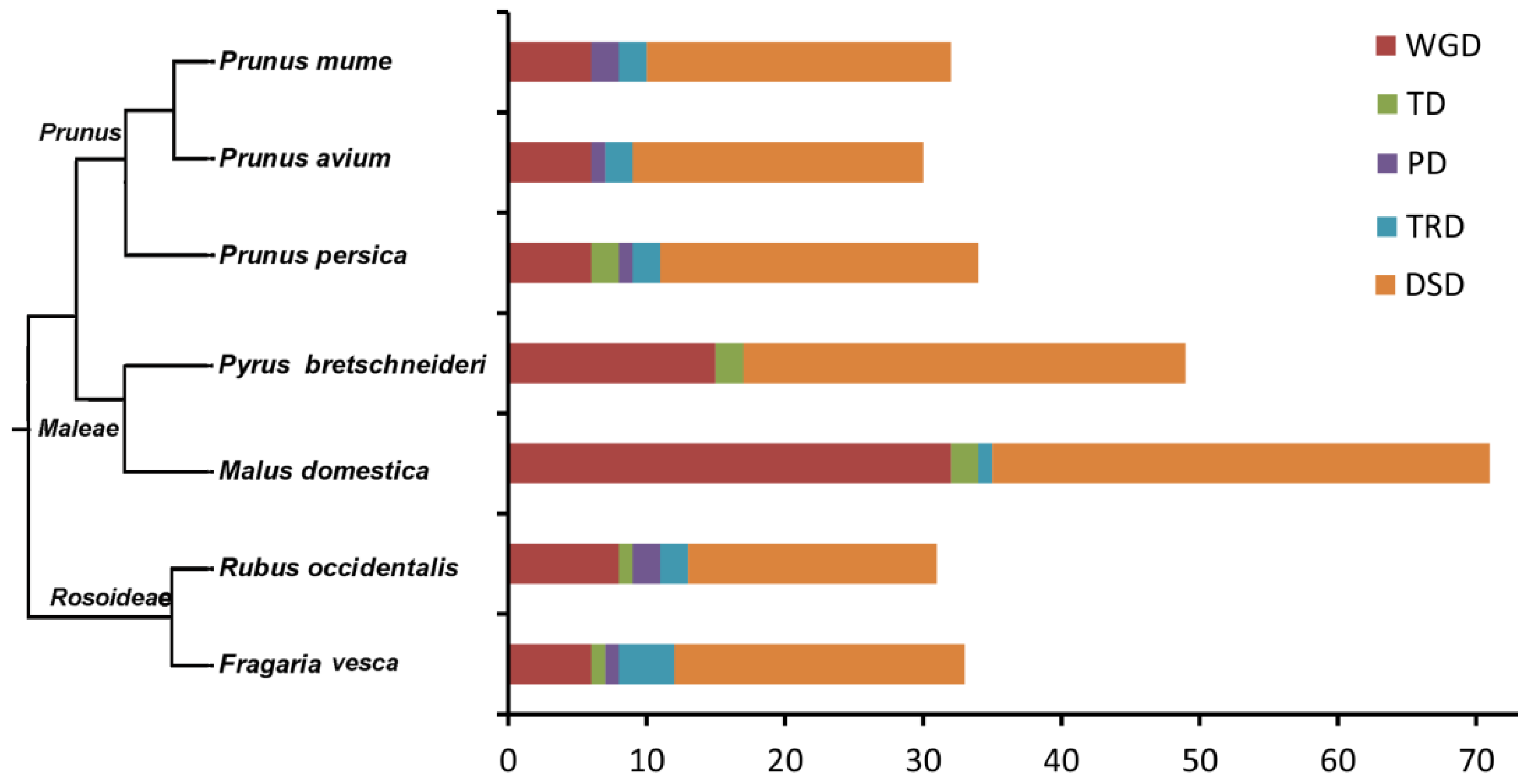
2.2. Chromosome Location and Synteny Analysis of SnRK Gene Family
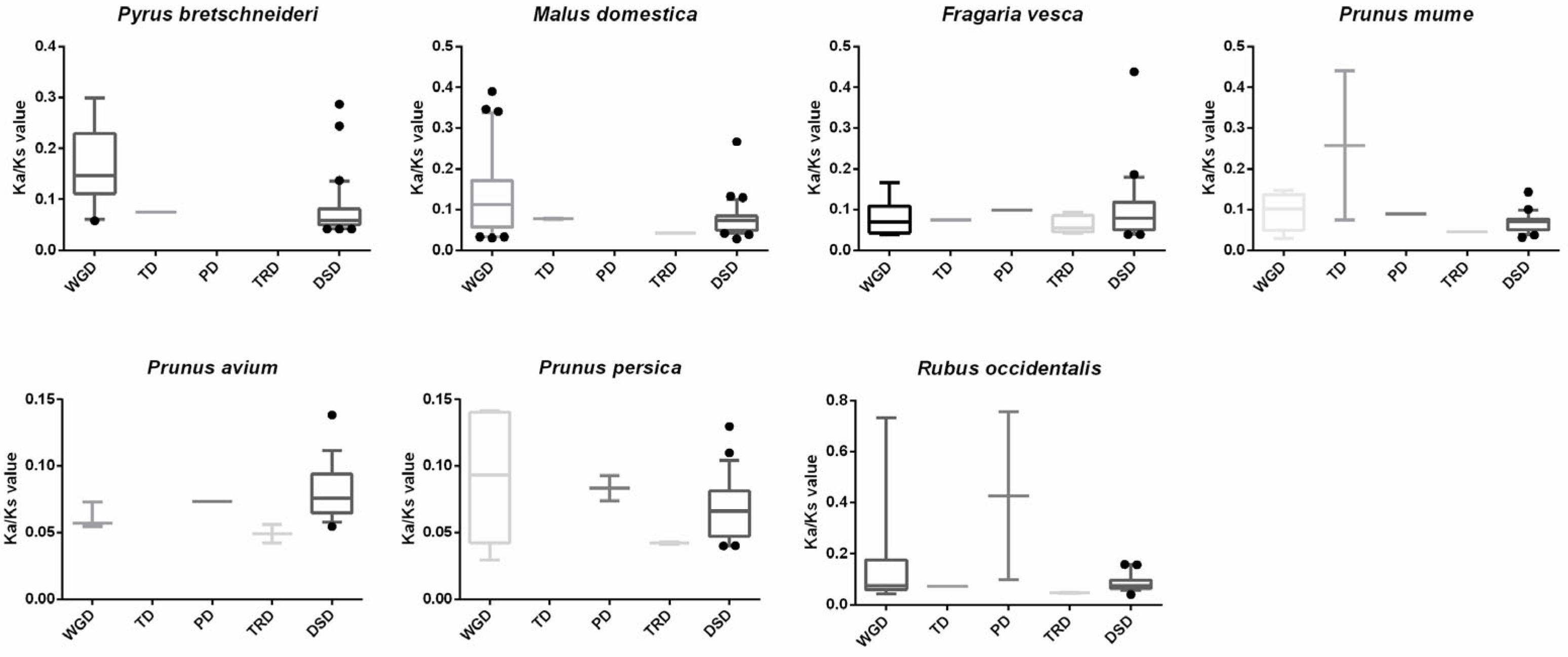
2.3. SnRK Genes Evolved under Strong Purifying Selection
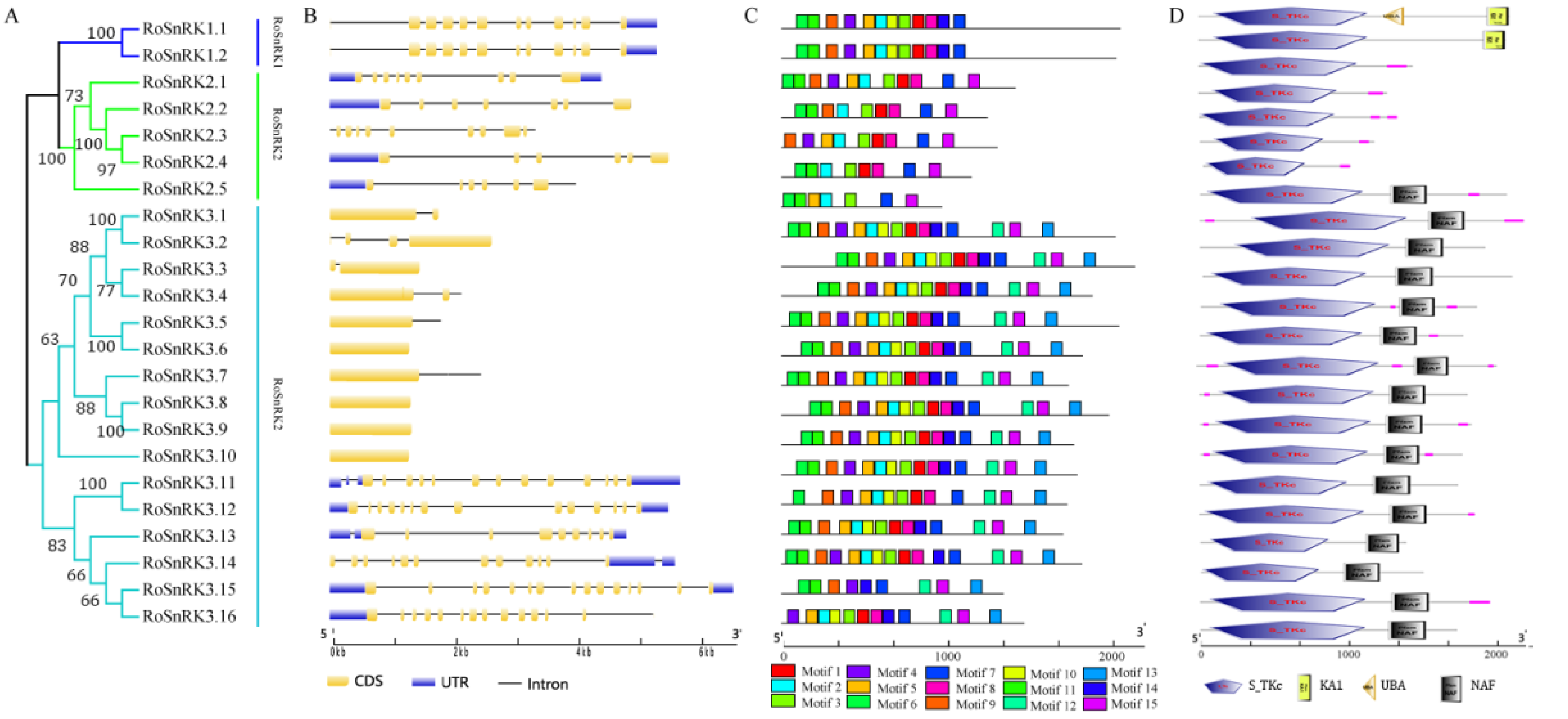
2.4. The Analysis of Gene Structure and Protein Characterization
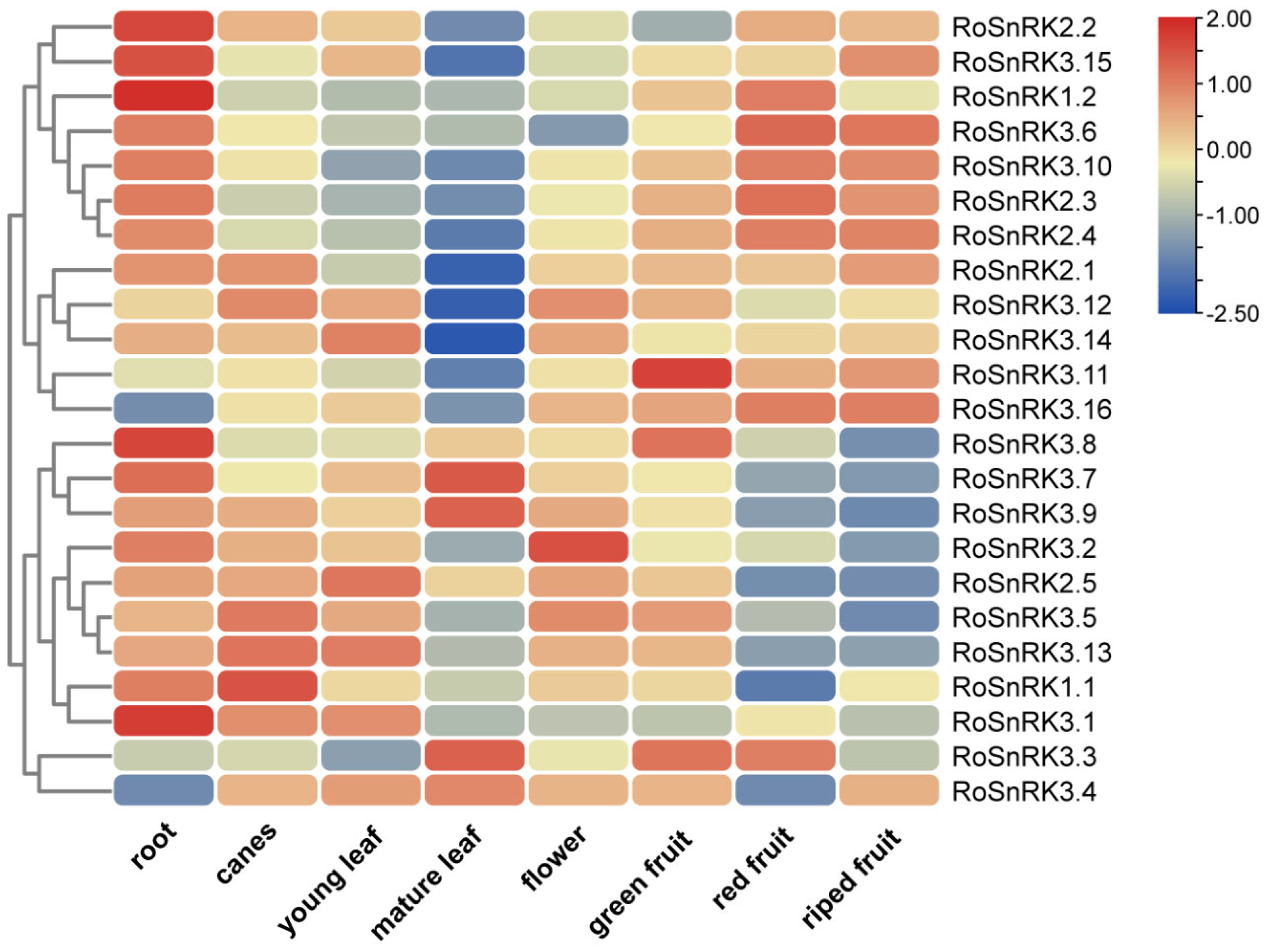
2.5. Expression Analysis of RoSnRK Genes in Different Tissues of Black Raspberry
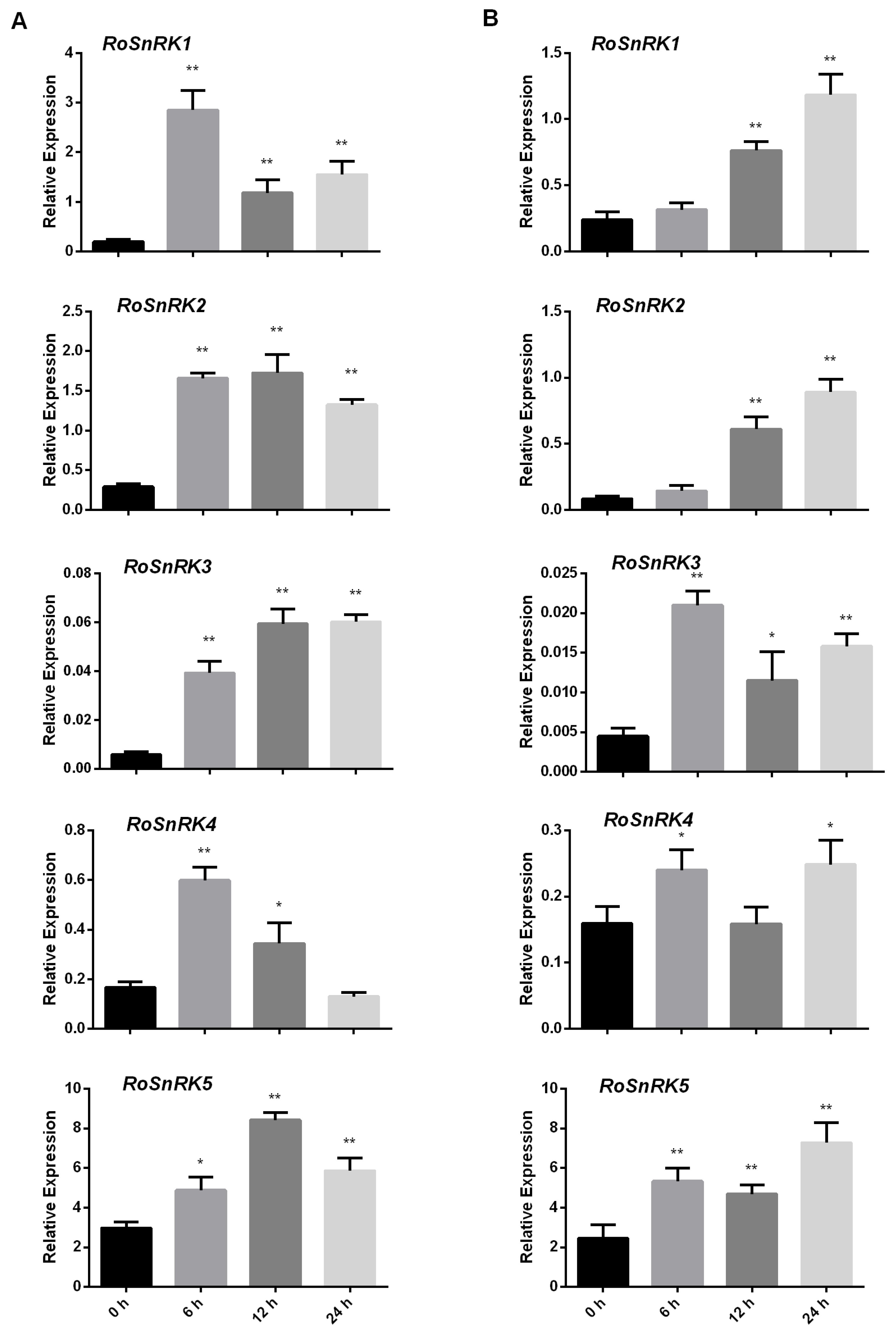
2.6. Expression Analysis of RoSnRK2 Genes under Different Abiotic Stresses
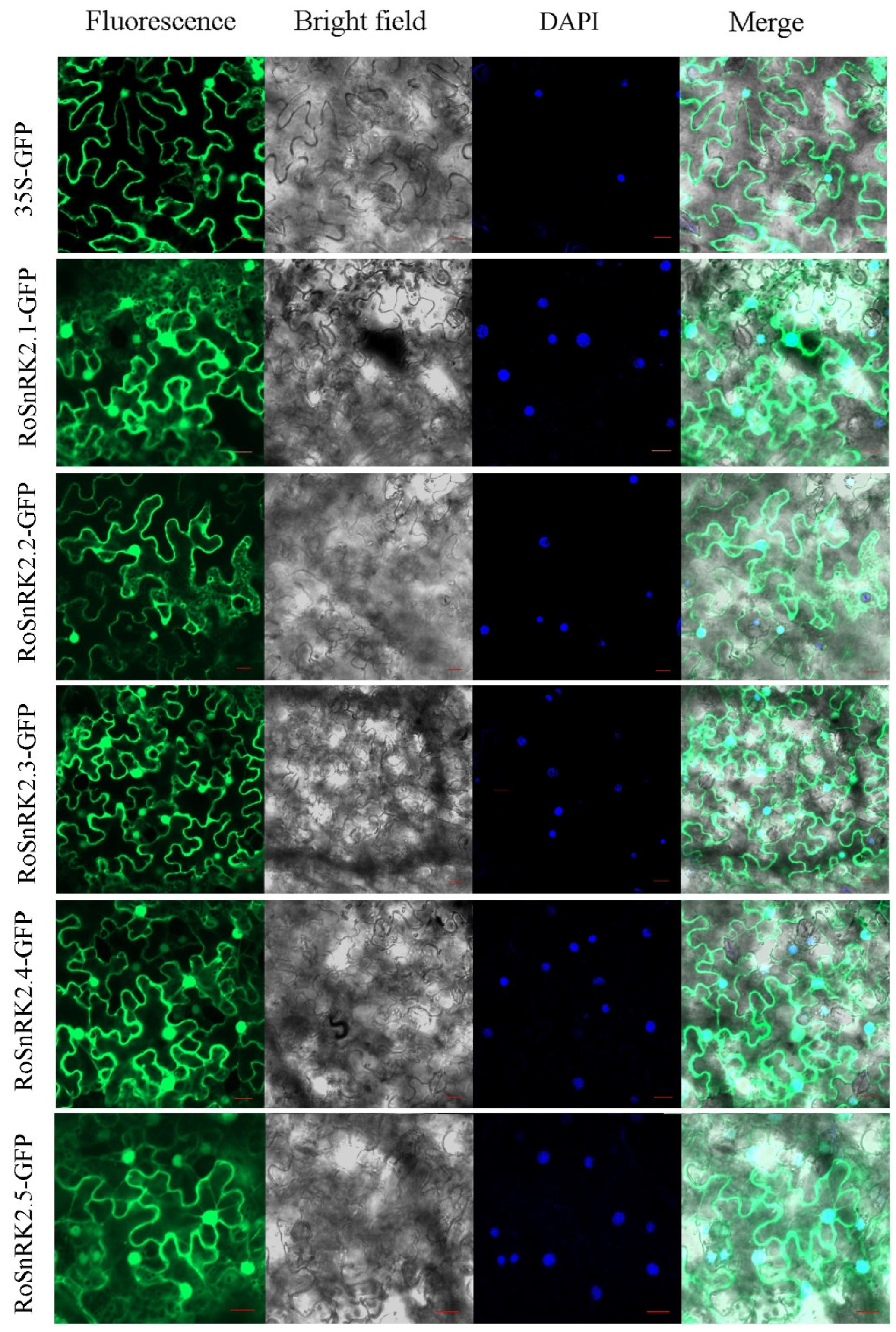
2.7. Subcellular Localization of RoSnRK2 Proteins
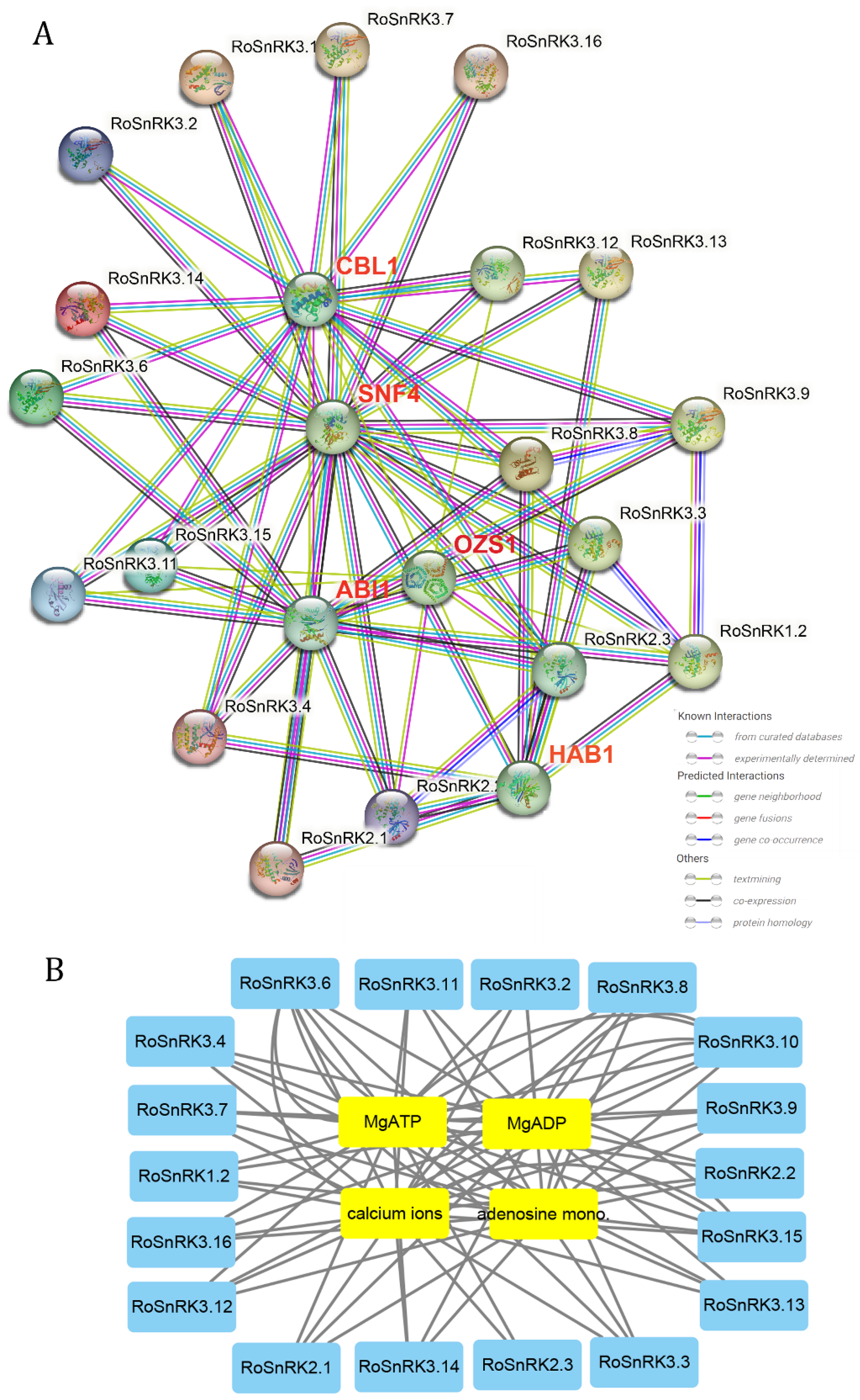
2.8. Protein–Protein and Protein–Chemical Interaction Analysis
3. Discussion
4. Materials and Methods
4.1. Identification of SnRK Genes in 7 Rosaceae Species
4.2. Phylogenetic Tree and Gene Structure Analysis
4.3. Cis-Element Predictions of RoSnRKs
4.4. Location of SnRK Genes on Chromosomes and Synteny Analysis
4.5. Calculating Ka, Ks, and Ka/Ks of Duplicated Gene Pairs
4.6. Expression Analysis of RoSnRKs in Different Tissues
4.7. Plant Materials Treatment and qRT-PCR Analysis
4.8. Subcellular Localization
4.9. Protein–Protein and Protein–Chemical Interaction Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bohnert, H.J.; Gong, Q.; Li, P.; Ma, S. Unraveling abiotic stress tolerance mechanisms—Getting genomics going. Curr. Opin. Plant Biol. 2006, 9, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wu, J.; Jiang, M.; Wang, Y. Plant mitogen-activated protein kinase cascades in environmental stresses. Int. J. Mol. Sci. 2021, 22, 1543. [Google Scholar] [CrossRef]
- Yip Delormel, T.; Boudsocq, M. Properties and functions of calcium-dependent protein kinases and their relatives in Ara-bidopsis thaliana. New Phytol. 2019, 224, 585–604. [Google Scholar] [CrossRef]
- Upadhyay, S.K. Chapter 1—An Overview of Receptor-Like Kinases in Plants. In Plant Receptor-Like Kinases; Upadhyay, S.K., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 1–23. [Google Scholar]
- Karlik, E. Chapter 5—Roles of Plant Receptor-Like Kinases in Response to Abiotic Stress. In Plant Receptor-Like Kinases; Upadhyay, S.K., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 87–119. [Google Scholar]
- Mishra, S.; Sharma, R.; Chaudhary, R.; Kumar, U.; Sharma, P. SNF1-Related Protein Kinase in Plants: Roles in Stress Response and Signaling. In Plant Receptor-Like Kinases; Elsevier: Cham, Switzerland, 2023; pp. 195–209. [Google Scholar]
- Halford, N.G.; Hey, S.J. Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem. J. 2009, 419, 247–259. [Google Scholar] [CrossRef]
- Celenza, J.L.; Carlson, M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science 1986, 233, 1175–1180. [Google Scholar] [CrossRef]
- Hrabak, E.M.; Chan, C.W.M.; Gribskov, M.; Harper, J.F.; Choi, J.H.; Halford, N.; Kudla, J.; Luan, S.; Nimmo, H.G.; Sussman, M.R.; et al. The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 2003, 132, 666–680. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Carling, D.; Carlson, M. The AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 1998, 67, 821–855. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.-Y.; Seo, P.J.; Woo, J.C.; Park, C.-M. AKIN10 delays flowering by inactivating IDD8 transcription factor through protein phosphorylation in Arabidopsis. BMC Plant Biol. 2015, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shewry, P.R.; Jones, H.; Barcelo, P.; Lazzeri, P.A.; Halford, N.G. Expression of antisense SnRK1 protein kinase sequence causes abnormal pollen development and male sterility in transgenic barley. Plant J. 2001, 28, 431–441. [Google Scholar] [CrossRef]
- Purcell, P.C.; Smith, A.M.; Halford, N.G. Antisense expression of a sucrose non-fermenting-1-related protein kinase sequence in potato results in decreased expression of sucrose synthase in tubers and loss of sucrose-inducibility of sucrose synthase transcripts in leaves. Plant J. 1998, 14, 195–202. [Google Scholar] [CrossRef]
- Coello, P.; Hey, S.J.; Halford, N.G. The sucrose non-fermenting-1-related (SnRK) family of protein kinases: Potential for ma-nipulation to improve stress tolerance and increase yield. J. Exp. Bot. 2011, 62, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Née, G.; Kramer, K.; Nakabayashi, K.; Yuan, B.; Xiang, Y.; Miatton, E.; Finkemeier, I.; Soppe, W.J. Delay of germination1 requires PP2C phosphatases of the ABA signalling pathway to control seed dormancy. Nat. Commun. 2017, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qi, K.; Gao, X.; Guo, L.; Cao, P.; Li, Q.; Qiao, X.; Gu, C.; Zhang, S. Genome-wide identification and comparative analysis of the PYL gene family in eight Rosaceae species and expression analysis of seeds germination in pear. BMC Genom. 2022, 23, 233. [Google Scholar] [CrossRef] [PubMed]
- Soma, F.; Takahashi, F.; Suzuki, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Plant Raf-like kinases regulate the mRNA population upstream of ABA-unresponsive SnRK2 kinases under drought stress. Nat. Commun. 2020, 11, 1373. [Google Scholar] [CrossRef]
- Kawa, D.; Meyer, A.J.; Dekker, H.L.; Abd-El-Haliem, A.M.; Gevaert, K.; Van De Slijke, E.; Maszkowska, J.; Bucholc, M.; Dobrowolska, G.; De Jaeger, G. SnRK2 protein kinases and mRNA decapping machinery control root development and response to salt. Plant Physiol. 2020, 182, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Maszkowska, J.; Dębski, J.; Kulik, A.; Kistowski, M.; Bucholc, M.; Lichocka, M.; Klimecka, M.; Sztatelman, O.; Szymańska, K.P.; Dadlez, M. Phosphoproteomic analysis reveals that dehydrins ERD10 and ERD14 are phosphorylated by SNF1-related protein kinase 2.10 in response to osmotic stress. Plant Cell Environ. 2019, 42, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jia, H.; Liu, G.; Yang, S.; Zhang, S.; Yang, Y.; Yang, P.; Cui, H. Cloning and characterization of SnRK2 subfamily II genes from Nicotiana tabacum. Mol. Biol. Rep. 2014, 41, 5701–5709. [Google Scholar] [CrossRef] [PubMed]
- Mustilli, A.-C.; Merlot, S.; Vavasseur, A.; Fenzi, F.; Giraudat, J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 2002, 14, 3089–3099. [Google Scholar] [CrossRef]
- Huai, J.; Wang, M.; He, J.; Zheng, J.; Dong, Z.; Lv, H.; Zhao, J.; Wang, G. Cloning and characterization of the SnRK2 gene family from Zea mays. Plant Cell Rep. 2008, 27, 1861–1868. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yamamoto, S.; Minami, H.; Kagaya, Y.; Hattori, T. Differential activation of the rice sucrose nonfermenting1–related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 2004, 16, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zhang, H.; Tian, S.; Chang, X.; Jing, R. TaSnRK2. 4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J. Exp. Bot. 2010, 61, 683–696. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, X.; Wang, L.; Zhu, L.; Zhou, T.; Deng, J.; Zhang, X. Molecular cloning and functional characterization of a novel cotton CBL-interacting protein kinase gene (GhCIPK6) reveals its involvement in multiple abiotic stress tolerance in transgenic plants. Biochem. Biophys. Res. Commun. 2013, 435, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Lee, J.-S.; Han, H.; Ah Choi, S.; Joo Go, S.; Sun Yoon, I. Isolation and characterization of a novel rice Ca2+-regulated protein kinase gene involved in responses to diverse signals including cold, light, cytokinins, sugars and salts. Plant Mol. Biol. 2003, 52, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.-J.; Wang, C.; Li, K.; Luan, S. The CBL–CIPK calcium signaling network: Unified paradigm from 20 years of discoveries. Trends Plant Sci. 2020, 25, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ishitani, M.; Halfter, U.; Kim, C.-S.; Zhu, J.-K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 2000, 97, 3730–3734. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Halfter, U.; Ishitani, M.; Zhu, J.-K. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 2001, 13, 1383–1400. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.J.; Sun, M.H.; Kang, H.; Lu, J.; You, C.X.; Hao, Y.J. A CIPK protein kinase targets sucrose transporter MdSUT2. 2 at Ser254 for phosphorylation to enhance salt tolerance. Plant Cell Environ. 2019, 42, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Starr, T.K.; Jameson, S.C.; Hogquist, K.A. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003, 21, 139–176. [Google Scholar] [CrossRef] [PubMed]
- VanBuren, R.; Wai, C.M.; Colle, M.; Wang, J.; Sullivan, S.; Bushakra, J.M.; Liachko, I.; Vining, K.J.; Dossett, M.; Finn, C.E. A near complete, chromosome-scale assembly of the black raspberry (Rubus occidentalis) genome. Gigascience 2018, 7, giy094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Wan, S.-Q.; Wang, W.-D.; Chen, J.-F.; Huang, L.-L.; Duan, M.-S.; Yu, Y.-B. Genome-wide identification and characteri-zation of the CsSnRK2 family in Camellia sinensis. Plant Physiol. Bioch. 2018, 132, 287–296. [Google Scholar] [CrossRef]
- Wang, C.; Abbas, F.; Zhou, Y.; Ke, Y.; Li, X.; Yue, Y.; Yu, Y.; Yu, R.; Fan, Y. Genome-wide identification and expression pattern of SnRK gene family under several hormone treatments and its role in floral scent emission in Hedychium coronarium. PeerJ 2021, 9, e10883. [Google Scholar] [CrossRef]
- Santiago, J.; Rodrigues, A.; Saez, A.; Rubio, S.; Antoni, R.; Dupeux, F.; Park, S.Y.; Marquez, J.A.; Cutler, S.R.; Rodriguez, P.L. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J. 2009, 60, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Sun, X.; Guo, Z.; Joldersma, D.; Guo, L.; Qiao, X.; Qi, K.; Gu, C.; Zhang, S. Genome-wide Identification and Evolution of the PP2C Gene Family in Eight Rosaceae Species and Expression Analysis Under Stress in Pyrus bretschneideri. Front. Genet. 2021, 12, 770014. [Google Scholar] [CrossRef] [PubMed]
- Ramon, M.; Dang, T.V.T.; Broeckx, T.; Hulsmans, S.; Crepin, N.; Sheen, J.; Rolland, F. Default activation and nuclear translocation of the plant cellular energy sensor SnRK1 regulate metabolic stress responses and development. Plant Cell 2019, 31, 1614–1632. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, J.; Qiao, X.; Jin, C.; Duan, W.; Sun, X.; Wu, J. Genome-wide survey of sucrose non-fermenting 1-related protein kinase 2 in Rosaceae and expression analysis of PbrSnRK2 in response to ABA stress. BMC Genom. 2020, 21, 781. [Google Scholar] [CrossRef]
- Jeffares, D.C.; Penkett, C.J.; Bähler, J. Rapidly regulated genes are intron poor. Trends Genet. 2008, 24, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.C.; Purugganan, M.D. The early stages of duplicate gene evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 15682–15687. [Google Scholar] [CrossRef]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genom. Biol. 2019, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Wang, X.Y.; Paterson, A.H. Genome and gene duplications and gene expression divergence: A view from plants. Ann. N. Y. Acad. Sci. 2012, 1256, 1–14. [Google Scholar] [CrossRef]
- Li, J.M.; Qin, M.F.; Qiao, X.; Cheng, Y.S.; Li, X.L.; Zhang, H.P.; Wu, J. A New Insight into the Evolution and Functional Divergence of SWEET Transporters in Chinese White Pear (Pyrus bretschneideri). Plant Cell Physiol. 2017, 58, 839–850. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, F.; Galvan-Ampudia, C.S.; Julkowska, M.M.; Caarls, L.; Van Der Does, D.; Laurière, C.; Munnik, T.; Haring, M.A.; Testerink, C. The Snf1-related protein kinases SnRK2.4 and SnRK2.10 are involved in maintenance of root system architecture during salt stress. Plant J. 2012, 72, 436–449. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Zhang, Z.; Liu, X.; Hsu, C.-C.; Du, Y.; Sang, T.; Zhu, C.; Wang, Y.; Satheesh, V. A RAF-SnRK2 kinase cascade mediates early osmotic stress signaling in higher plants. Nat. Commun. 2020, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jia, H.; Wang, X.; Shi, C.; Wang, X.; Ma, P.; Wang, J.; Ren, M.; Li, J. Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6 in guard cells. Mol. Plant 2020, 13, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Inter-active Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Tang, H.B.; Wang, X.Y.; Paterson, A.H. PGDD: A database of gene and genome duplication in plants. Nucleic Acids Res. 2013, 41, D1152–D1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Tang, H.B.; DeBarry, J.D.; Tan, X.; Li, J.P.; Wang, X.Y.; Lee, T.H.; Jin, H.Z.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A Toolkit Incorporating Gamma-Series Methods and Sliding Window Strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, C.; Yang, H.; Lyu, L.; Li, W.; Wu, W. Selection and Validation of Candidate Reference Genes for Gene Expression Analysis by RT-qPCR in Rubus. Int. J. Mol. Sci. 2021, 22, 10533. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Sharma, A.; Dixit, S.; Singh, K.; Upadhyay, S.K. OSCA Genes in Bread Wheat: Molecular Characterization, Expression Profiling, and Interaction Analyses Indicated Their Diverse Roles during Development and Stress Response. Int. J. Mol. Sci. 2022, 23, 14867. [Google Scholar] [CrossRef] [PubMed]
- Mendu, V.; Singh, K.; Upadhyay, S.K. Insight into the roles of proline-rich extensin-like receptor protein kinases of bread wheat (Triticum aestivum L.). Life 2022, 12, 941. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Guan, S.L.; Zhu, N.; Li, Q.; Chong, X.; Wang, T.; Xuan, J. Comprehensive Genomic Analysis of SnRK in Rosaceae and Expression Analysis of RoSnRK2 in Response to Abiotic Stress in Rubus occidentalis. Plants 2023, 12, 1784. https://doi.org/10.3390/plants12091784
Wang G, Guan SL, Zhu N, Li Q, Chong X, Wang T, Xuan J. Comprehensive Genomic Analysis of SnRK in Rosaceae and Expression Analysis of RoSnRK2 in Response to Abiotic Stress in Rubus occidentalis. Plants. 2023; 12(9):1784. https://doi.org/10.3390/plants12091784
Chicago/Turabian StyleWang, Guoming, Sophia Lee Guan, Nan Zhu, Qionghou Li, Xinran Chong, Tao Wang, and Jiping Xuan. 2023. "Comprehensive Genomic Analysis of SnRK in Rosaceae and Expression Analysis of RoSnRK2 in Response to Abiotic Stress in Rubus occidentalis" Plants 12, no. 9: 1784. https://doi.org/10.3390/plants12091784
APA StyleWang, G., Guan, S. L., Zhu, N., Li, Q., Chong, X., Wang, T., & Xuan, J. (2023). Comprehensive Genomic Analysis of SnRK in Rosaceae and Expression Analysis of RoSnRK2 in Response to Abiotic Stress in Rubus occidentalis. Plants, 12(9), 1784. https://doi.org/10.3390/plants12091784






