New Sesquiterpene Glycosides from the Flowers of Aster koraiensis and Their Inhibition Activities on EGF- and TPA-Induced Cell Transformation
Abstract
1. Introduction
2. Results and Discussion
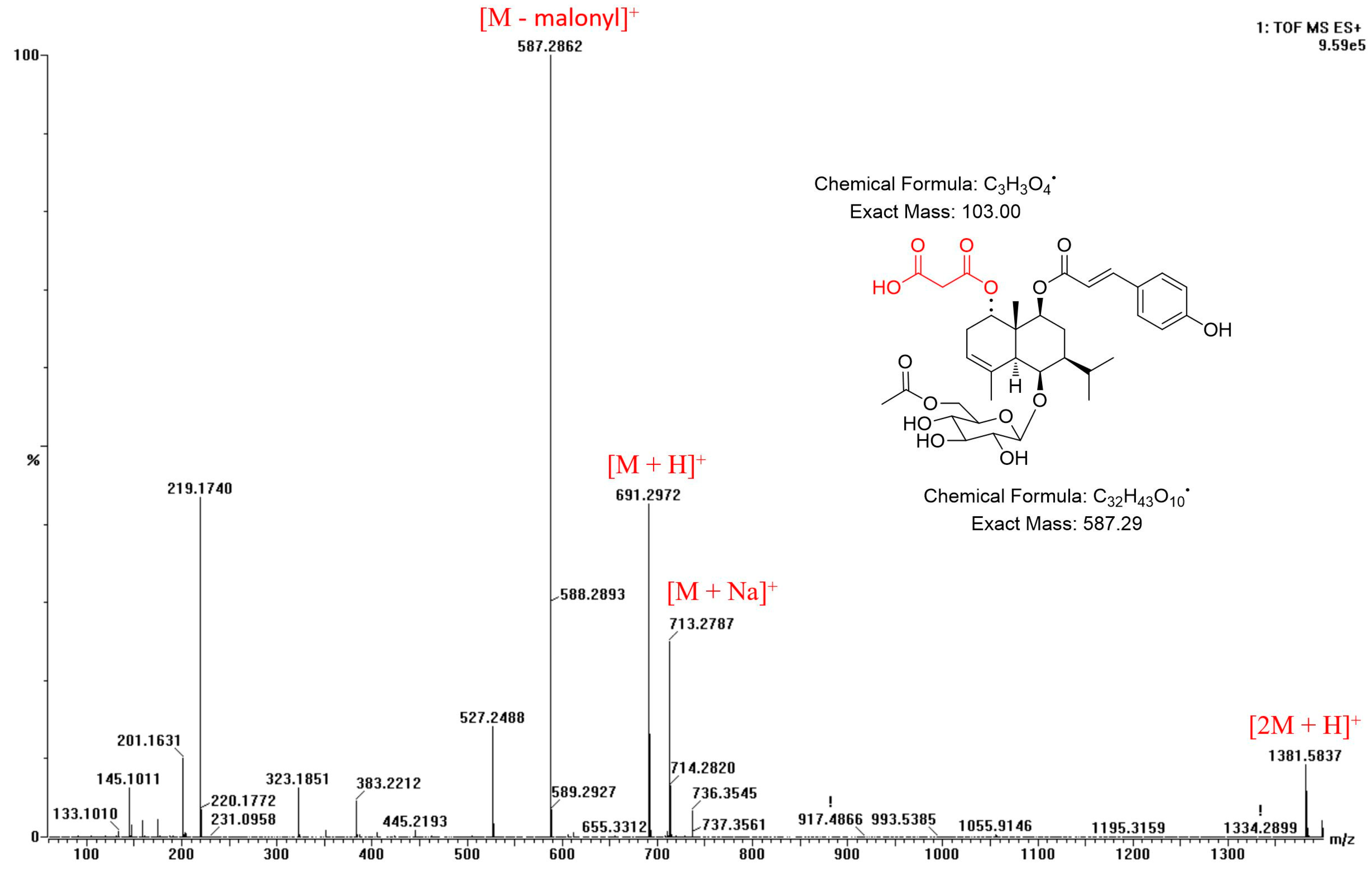
2.1. Structural Elucidation of Compounds
2.2. Effect of Isolated Compounds on EGF- and TPA-Induced Cell Transformation
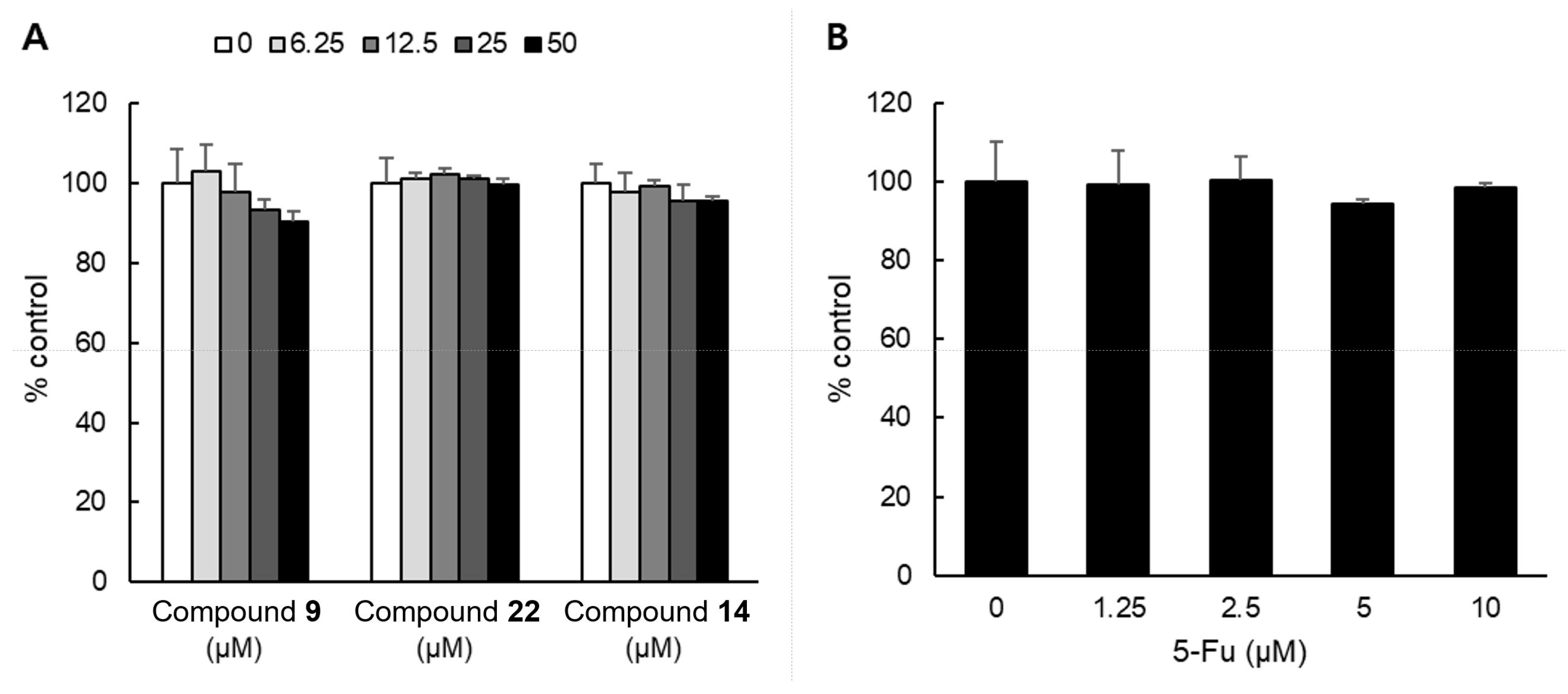
2.3. Cytotoxicity of Compounds on NHDF Cell
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Askoseoside A (Compound 1)
3.3.2. Askoseoside B (Compound 2)
3.3.3. Askoseoside C (Compound 3)
3.3.4. Askoseoside D (Compound 4)
3.4. Acid Hydrolysis and Sugar Identification
3.5. Computational Methods
3.6. EGF- or TPA-Induced Cell Transformation (Soft Agar) Assay
3.7. WST-8 Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahn, D. Illustrated Book of Korean Medicinal Herbs; Kyo-hak Publishing Co.: Seoul, Republic of Korea, 1998; p. 107. [Google Scholar]
- Lee, J.; Lee, Y.M.; Lee, B.W.; Kim, J.-H.; Kim, J.S. Chemical constituents from the aerial parts of Aster koraiensis with protein glycation and aldose reductase inhibitory activities. J. Nat. Prod. 2012, 75, 267–270. [Google Scholar] [CrossRef]
- Sohn, E.; Kim, J.; Kim, C.-S.; Kim, Y.S.; Jang, D.S.; Kim, J.S. Extract of the aerial parts of Aster koraiensis reduced development of diabetic nephropathy via anti-apoptosis of podocytes in streptozotocin-induced diabetic rats. Biochem. Biophys. Res. Commun. 2010, 391, 733–738. [Google Scholar] [CrossRef]
- Kim, J.; Hyun, S.-W.; Lee, I.S.; Jo, K.; Kim, Y.S.; Kim, J.S.; Kim, C.-S. Aster koraiensis extract lowers postprandial glucose in normoglycemic and high-fat-diet-induced obese mice. Food Sci. Biotechnol. 2019, 28, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Sim, Y.-B.; Kim, S.-M.; Kang, Y.-J.; Lee, J.-K.; Lim, S.-S.; Kim, J.-K.; Suh, H.-W. Antinociceptive profiles and mechanisms of orally administered Aster Koraiensis extract in the mouse. J. Med. Plants Res. 2011, 5, 6267–6272. [Google Scholar]
- Choe, S.Y.; Seo, Y.; Bang, C.Y.; Woo, S.H.; Kang, M. Protective effects of Gymnaster koraiensis extract on high fat diet-induced fatty liver in mice. Adv. Tradit. Med. 2021, 21, 361–369. [Google Scholar] [CrossRef]
- Hong, S.-C.; Ha, J.-H.; Lee, J.K.; Jung, S.H.; Kim, J.-C. In vivo anti-inflammation potential of Aster koraiensis extract for dry eye syndrome by the protection of ocular surface. Nutrients 2020, 12, 3245. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Seo, Y.H.; Lee, I.-H.; Choi, H.Y.; Kwon, H.C.; Choi, J.-H.; Lee, J.; Jang, D.S. New Eudesmane-Type Sesquiterpene Glycosides from the Leaves of Aster koraiensis. Plants 2020, 9, 1811. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-J.; Min, B.-S.; Park, J.-Y.; Kim, Y.-H.; Lee, H.-K.; Bae, K.-H. Gymnasterkoreaynes A− F, Cytotoxic Polyacetylenes from Gymnaster koraiensis. J. Nat. Prod. 2002, 65, 897–901. [Google Scholar] [CrossRef]
- Kwon, J.; Ko, K.; Zhang, L.; Zhao, D.; Yang, H.O.; Kwon, H.C. An autophagy inducing triterpene saponin derived from Aster koraiensis. Molecules 2019, 24, 4489. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, J.-Y.; Kwon, H.C.; Jeon, S.; Lee, S.J.; Jung, H.; Kim, S.; Jang, D.S.; Lee, C.J. Astersaponin I from Aster koraiensis is a natural viral fusion blocker that inhibits the infection of SARS-CoV-2 variants and syncytium formation. Antivir. Res. 2022, 208, 105428. [Google Scholar] [CrossRef]
- Akihisa, T.; Franzblau, S.G.; Ukiya, M.; Okuda, H.; Zhang, F.; Yasukawa, K.; Suzuki, T.; Kimura, Y. Antitubercular activity of triterpenoids from Asteraceae flowers. Biol. Pharm. Bull. 2005, 28, 158–160. [Google Scholar] [CrossRef]
- Akihisa, T.; Yasukawa, K.; Oinuma, H.; Kasahara, Y.; Yamanouchi, S.; Takido, M.; Kumaki, K.; Tamura, T. Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. Phytochemistry 1996, 43, 1255–1260. [Google Scholar] [CrossRef]
- Lopes, D.C.D.X.P.; de Oliveira, T.B.; Viçosa, A.L.; Valverde, S.S.; Júnior, E.R. Anti-inflammatory activity of the compositae family and its therapeutic potential. Planta Med. 2021, 87, 71–100. [Google Scholar] [CrossRef]
- Piątkowska, E.; Biel, W.; Witkowicz, R.; Kępińska-Pacelik, J. Chemical Composition and Antioxidant Activity of Asteraceae Family Plants. Appl. Sci. 2022, 12, 12293. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The plants of the Asteraceae family as agents in the protection of human health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Faridchehr, A. Constituents and biological activities of selected genera of the Iranian Asteraceae family. J. Herb. Med. 2021, 25, 100405. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Yasukawa, K.; Kasahara, Y.; Kimura, Y.; Koike, K.; Nikaido, T.; Takido, M. Constituents of compositae plants. 2. Triterpene diols, triols, and their 3-o-fatty acid esters from edible chrysanthemum flower extract and their anti-inflammatory effects. J. Agric. Food Chem. 2001, 49, 3187–3197. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.-H.; Shin, S.-L.; Lee, C.-H. Antioxidant effects of ethanol extracts from flower species of compositae plant. J. Korean Soc. Food Sci. Nutr. 2010, 39, 159–164. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.-J.; Ding, J. Natural products in cancer therapy: Past, present and future. Nat. Prod. Bioprospecting 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Hwang, S.-Y.; Chae, J.-I.; Kwak, A.-W.; Lee, M.-H.; Shim, J.-H. Alternative options for skin cancer therapy via regulation of AKT and related signaling pathways. Int. J. Mol. Sci. 2020, 21, 6869. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Lee, H.S.; Ryu, H.W.; Lee, M.-H.; Lee, J.Y.; Li, Y.; Dong, Z.; Lee, H.-K.; Oh, S.-R.; Cho, Y.-Y. Targeting of magnolin on ERKs inhibits Ras/ERKs/RSK2-signaling-mediated neoplastic cell transformation. Carcinogenesis 2014, 35, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-Y.; Lee, M.-H.; Lee, C.-J.; Yao, K.; Lee, H.S.; Bode, A.M.; Dong, Z. RSK2 as a key regulator in human skin cancer. Carcinogenesis 2012, 33, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhao, R.; Yoon, G.; Shim, J.-H.; Choi, B.Y.; Yin, F.; Xu, B.; Laster, K.V.; Liu, K.; Dong, Z. 3-deoxysappanchalcone inhibits skin cancer proliferation by regulating t-lymphokine-activated killer cell-originated protein kinase in vitro and in vivo. Front. Cell Dev. Biol. 2021, 9, 638174. [Google Scholar] [CrossRef]
- Oi, N.; Yamamoto, H.; Langfald, A.; Bai, R.; Lee, M.-H.; Bode, A.M.; Dong, Z. LTA4H regulates cell cycle and skin carcinogenesis. Carcinogenesis 2017, 38, 728–737. [Google Scholar] [CrossRef]
- Cheng, Z.-H.; Wu, T.; Bligh, S.A.; Bashall, A.; Yu, B.-Y. Cis-eudesmane sesquiterpene glycosides from liriope m uscari and ophiopogon j aponicus. J. Nat. Prod. 2004, 67, 1761–1763. [Google Scholar] [CrossRef]
- Xu, X.; Xie, H.; Hao, J.; Jiang, Y.; Wei, X. Eudesmane sesquiterpene glucosides from lychee seed and their cytotoxic activity. Food chem. 2010, 123, 1123–1126. [Google Scholar] [CrossRef]
- Kitajima, J.; Kimizuka, K.; TANAK, Y. Three new sesquiterpenoid glucosides of Ficus pumila fruit. Chem. Pharm. Bull. 2000, 48, 77–80. [Google Scholar] [CrossRef]
- An, J.-P.; Ha, T.K.Q.; Kim, H.W.; Ryu, B.; Kim, J.; Park, J.; Lee, C.H.; Oh, W.K. Eudesmane glycosides from Ambrosia artemisiifolia (Common Ragweed) as potential neuroprotective agents. J. Nat. Prod. 2019, 82, 1128–1138. [Google Scholar] [CrossRef]
- Nhoek, P.; Ahn, J.; Chae, H.-S.; Pel, P.; Kim, Y.-M.; Lee, S.E.; Lee, J.H.; Kim, J.; Choi, Y.H.; Lee, K. Isolation of polyacetylenes with proprotein convertase/kexin type 9 downregulating activity and two new sesquiterpenes from the aerial parts of Aster koraiensis. Tetrahedron Lett. 2020, 61, 151957. [Google Scholar] [CrossRef]
- Teles, Y.C.; Horta, C.C.R.; Agra, M.D.F.; Siheri, W.; Boyd, M.; Igoli, J.O.; Gray, A.I.; De Souza, M.D.F.V. New sulphated flavonoids from Wissadula periplocifolia (L.) C. Presl (Malvaceae). Molecules 2015, 20, 20161–20172. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Ambe, Y.; Nakamura, M.; Yamakawa, T.; Noguchi, H.; Kodama, T. Quercetin 3-O-β-d-Glucopyranoside and Isorhamnetin 3-O-β-d-Glucopyranoside Formation from Quercetin by Cell Cultures of Ipomoea batatas and Crocus sativum. Agric. Biol. Chem. 1991, 55, 613–614. [Google Scholar] [CrossRef]
- Lim, S.S.; Jung, Y.J.; Hyun, S.K.; Lee, Y.S.; Choi, J.S. Rat lens aldose reductase inhibitory constituents of Nelumbo nucifera stamens. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 825–830. [Google Scholar]
- Sawabe, A.; Nesumi, C.; Morita, M.; Matsumoto, S.; Matsubara, Y.; Komemushi, S. Glycosides in African dietary leaves, Hibiscus sabdariffa. J. Oleo Sci. 2005, 54, 185–191. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Y.; Foo, L.Y.; McNabb, W.; Molan, A. Phenolic glycosides of forage legume Onobrychis viciifolia. Phytochemistry 2000, 55, 67–75. [Google Scholar] [CrossRef]
- Han, X.H.; Hong, S.S.; Hwang, J.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Monoamine oxidase inhibitory components from Cayratia japonica. Arch. Pharmacal Res. 2007, 30, 13–17. [Google Scholar] [CrossRef]
- Lee, M.H.; Son, Y.K.; Han, Y.N. Tissue factor inhibitory flavonoids from the fruits of Chaenomeles sinensis. Arch. Pharmacal Res. 2002, 25, 842–850. [Google Scholar] [CrossRef]
- Park, J.; Min, B.; Jung, H.; Kim, Y.; Lee, H.; Bae, K. Polyacetylene glycosides from Gymnaster koraiensis. Chem. Pharm. Bull. 2002, 50, 685–687. [Google Scholar] [CrossRef]
- Dat, N.T.; Cai, X.F.; Shen, Q.; Im, S.L.; Lee, E.J.; Park, Y.K.; Bae, K.; Kim, Y.H. Gymnasterkoreayne G, a new inhibitory polyacetylene against NFAT transcription factor from Gymnaster koraiensis. Chem. Pharm. Bull. 2005, 53, 1194–1196. [Google Scholar] [CrossRef]
- Cardoso, C.L.; Bolzani, V.d.S.; Silva, D.H.S.; Ishii, H.; Berova, N.; Nakanishi, K. The Absolute Configuration of 1-(3’, 4’-Dihydroxycinnamoyl) cyclopentane-2, 3-diol from the Amazonian Tree Chimarrhis t urbinata. J. Nat. Prod. 2006, 69, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-K.; Kim, K.-H.; Ryu, S.-Y.; Choi, S.-U.; Lee, K.-R. Phytochemical constituents from the flowers of Gymnaster koraiensis and their cytotoxic activities in vitro. Bull. Korean Chem. Soc. 2010, 31, 227–229. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, Y.; Lv, H.; Zhang, H.; Liang, T.; Zhou, G.; Huang, L.; Tian, Y.; Liang, W. Apigenin in cancer therapy: From mechanism of action to nano-therapeutic agent. Food Chem. Toxicol. 2022, 168, 113385. [Google Scholar] [CrossRef] [PubMed]
- Jho, E.H.; Kang, K.; Oidovsambuu, S.; Lee, E.H.; Jung, S.H.; Shin, I.-S.; Nho, C.W. Gymnaster koraiensis and its major components, 3, 5-di-O-caffeoylquinic acid and gymnasterkoreayne B, reduce oxidative damage induced by tert-butyl hydroperoxide or acetaminophen in HepG2 cells. BMB Rep. 2013, 46, 513. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef]
- Seo, Y.H.; Jeon, J.-H.; Jeong, M.; Ryu, S.M.; Jeon, W.K.; Jang, D.S.; Shim, S.H.; Lee, D.; Choi, J.-H.; Lee, J. Chemical constituents of Apios americana tubers and their inhibitory activities on nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Nat. Prod. 2018, 81, 1598–1603. [Google Scholar] [CrossRef]
- Ryu, S.M.; Nam, H.-h.; Kim, J.S.; Song, J.-h.; Seo, Y.H.; Kim, H.S.; Lee, A.Y.; Kim, W.J.; Lee, D.; Moon, B.C. Chemical Constituents of the Egg Cases of Tenodera angustipennis (Mantidis ootheca) with Intracellular Reactive Oxygen Species Scavenging Activity. Biomolecules 2021, 11, 556. [Google Scholar] [CrossRef]


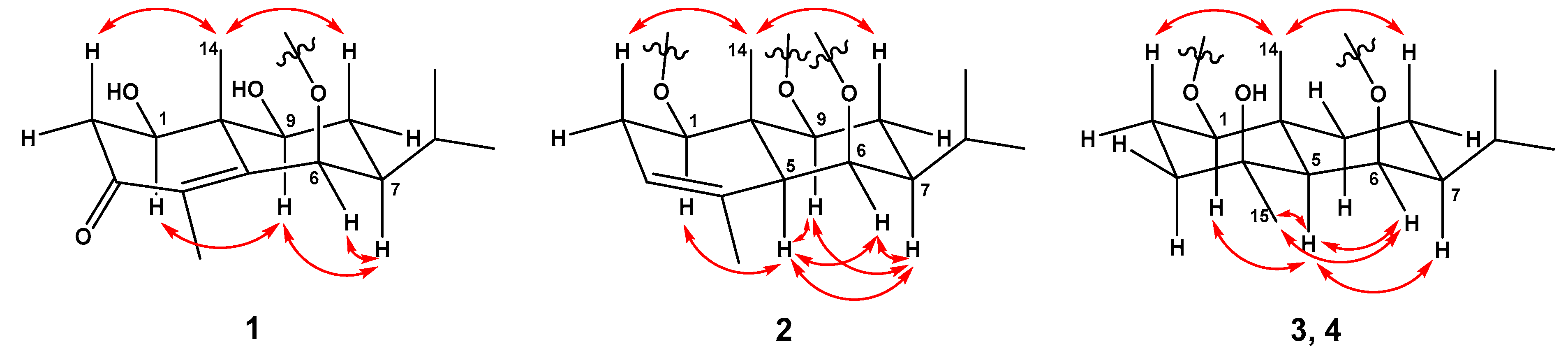


| 1 a,e | 2 a,e | 2 b,f | ||||
|---|---|---|---|---|---|---|
| Position | δC, Type | δH Multi (J in Hz) | δC, Type | δH Multi (J in Hz) | δC, Type | δH Multi (J in Hz) |
| 1 | 77.1, CH | 3.99, dd (12.9, 4.7) | 80.5, CH | 4.89, dd (10.0, 5.9) | 76.9, CH | 4.68, dd (9.7, 6.2) |
| 2 | 42.8, CH2 | 2.45, dd (15.8, 4.7), 2.66, dd (16.4, 12.9) | 29.7, CH2 | 2.090, m c, 2.25, m | 28.5, CH2 | 1.89, m, 2.07, m |
| 3 | 200.8, C | 120.4, CH | 5.26, br d (2.3) | 118.9, CH | 5.20, br s | |
| 4 | 134.1, C | 135.4, C | 133.9, C | |||
| 5 | 159.0, C | 52.2, CH | 2.093, s | 49.9, CH | 2.09, s | |
| 6 | 78.23, CH | 4.89, br d (1.8) | 75.0, CH | 4.47, s | 72.1, CH | 4.36, s |
| 7 | 48.4, CH | 1.04, m c | 51.4, CH | 1.17, m | 49.1, CH | 1.16, m |
| 8 | 29.5, CH2 | 1.78, m, 1.86, m c | 28.9, CH2 | 1.91, m | 27.3, CH2 | 1.70, m, 184, m |
| 9 | 81.7, CH | 3.65, dd (11.7, 4.7) | 80.3, CH | 4.97, dd (10.6, 5.9) | 77.7, CH | 4.83, dd (11.4, 4.8) |
| 10 | 46.7, C | 42.2, C | 40.2, C | |||
| 11 | 28.8, CH | 2.03, m | 29.2, CH | 1.94, m | 27.2, CH | 1.90, m |
| 12 | 21.47, CH3 | 0.99, d (6.5) | 21.5, CH3 | 1.02, d (6.5) | 20.9, CH3 | 0.95, d (6.5) |
| 13 | 21.51, CH3 | 1.03, d (6.5) | 21.8, CH3 | 0.92, d (6.5) | 21.4, CH3 | 0.84, d (6.5) |
| 14 | 12.3, CH3 | 1.36, s | 11.6, CH3 | 1.36, s | 10.7, CH3 | 1.22, s |
| 15 | 11.8, CH3 | 1.85, s | 21.6, CH3 | 1.83, s | 20.4, CH3 | 1.78, s |
| 1′ | 106.7, CH | 4.37, d (7.6) | 103.7, CH | 4.40, d (7.6) | 101.7, CH | 4.27, d (7.3) |
| 2′ | 75.9, CH | 3.16, (8.2) | 76.1, CH | 3.15, td (7.0, 2.3) | 74.3, CH | 2.93, m |
| 3′ | 78.17, CH | 3.33, m c | 78.3, CH | 3.308, m c | 76.8, CH | 3.11, t (8.6) |
| 4′ | 71.7, CH | 3.28, t (8.8) | 71.5, CH | 3.310, m c | 70.1, CH | 3.07, t (9.2) |
| 5′ | 77.9, CH | 3.12, m | 74.9, CH | 3.36, m | 73.4, CH | 3.26, ddd (9.2, 6.8, 1.9) |
| 6′ | 62.8, CH2 | 3.60, m | 64.7, CH2 | 4.14, dd (11.7, 5.3), 4.41, dd (11.7, 1.8) | 63.6, CH2 | 3.99, dd (11.6, 6.8), 4.28, m c |
| 7′ | 172.7, C | 170.2, C | ||||
| 8′ | 20.8, CH3 | 2.05, s | 20.6, CH3 | 1.99, s | ||
| 1″ | 127.3, C | 124.9, C | ||||
| 2″ | 131.3, CH | 7.47, d (8.8) | 130.1, CH | 7.51, d (8.8) | ||
| 3″ | 116.9, CH | 6.80, d (8.8) | 115.8, CH | 6.78, d (8.8) | ||
| 4″ | 161.3, C | 160.0, C | ||||
| 5″ | 116.9, CH | 6.80, d (8.8) | 115.8, CH | 6.78, d (8.8) | ||
| 6″ | 131.3, CH | 7.47, d (8.8) | 130.1, CH | 7.51, d (8.8) | ||
| 7″ | 146.6, CH | 7.61, d (15.8) | 144.1, CH | 7.48, d (15.8) | ||
| 8″ | 116.2, CH | 6.32, d (15.8) | 114.9, CH | 6.29, d (15.8) | ||
| 9″ | 168.7, C | 165.7, C | ||||
| 1′′′ | 168.8, C | |||||
| 2′′′ | 45.7, CH2 | 2.54, m c, 2.73, m | ||||
| 3′′′ | nt d | |||||
| 3 b | 4 b | |||
|---|---|---|---|---|
| Position | δC, Type | δH Multi (J in Hz) | δC, Type | δH Multi (J in Hz) |
| 1 | 81.8, CH | 3.11, m | 85.1, CH | 3.17, m a |
| 2 | 27.8, CH2 | 1.45, m, 1.91, m | 22.8, CH2 | 1.67, m, 1.75, m a |
| 3 | 41.0, CH2 | 1.42, m, 1.67, m | 40.5, CH2 | 1.38, m, 1.72, m a |
| 4 | 73.5, C | 73.4, C | ||
| 5 | 55.2, CH | 0.99, br s | 55.5, CH | 1.05, s |
| 6 | 76.9, CH | 4.65, s | 76.8, CH | 4.65, s |
| 7 | 54.2, CH | 0.85, m | 54.1, CH | 0.87, m |
| 8 | 21.7, CH2 | 1.62, m, 1.72, m | 21.7, CH2 | 1.62, m, 1.74, m a |
| 9 | 41.6, CH2 | 1.01, m, 1.88, m | 42.3, CH2 | 1.98, dt (13.4, 2.9), 1.03, m |
| 10 | 41.1, C | 40.8, C | ||
| 11 | 27.5, CH | 2.03, m | 27.5, CH | 2.03, m |
| 12 | 21.6, CH3 | 0.90, d (6.5) | 21.5, CH3 | 0.91, d (6.7) |
| 13 | 22.5, CH3 | 1.01, d (6.5) | 22.4, CH3 | 1.02, d (6.7) |
| 14 | 14.9, CH3 | 1.28, s | 15.9, CH3 | 1.32, s |
| 15 | 29.8, CH3 | 1.35, s | 29.7, CH3 | 1.37, s |
| 1′ | 105.3, CH | 4.48, d (7.6) | 105.3, CH | 4.48, d (7.6) |
| 2′ | 76.2, CH | 3.17, t (8.2) | 76.1, CH | 3.16, m a |
| 3′ | 78.1, CH | 3.32, m a | 78.1, CH | 3.32, m a |
| 4′ | 71.5, CH | 3.33, m a | 71.5, CH | 3.33, m a |
| 5′ | 78.4, CH | 3.34, m a | 78.4, CH | 3.34, m a |
| 6′ | 62.5, CH2 | 3.70, dd (11.7, 4.7), 3.90, d (11.7) | 62.5, CH2 | 3.70, dd (12.4, 5.7), 3.90, d (12.4) |
| 1″ | 98.0, CH | 4.82, s | ||
| 2″ | 73.3, CH | 3.74, m a | ||
| 3″ | 72.7, CH | 3.64, dd (9.5, 2.9) | ||
| 4″ | 74.0, CH | 3.38, t (9.5) | ||
| 5″ | 70.3, CH | 3.73, m a | ||
| 6″ | 18.1, CH3 | 1.24, d (5.7) | ||
| Compounds | Inhibitory Activity % (EGF) a | Inhibitory Activity % (TPA) b |
|---|---|---|
| 1 | 22.5 ± 9.5 | 51.4 ± 5.6 |
| 2 | 60.4 ± 2.0 | - c |
| 3 | 44.2 ± 4.4 | 52.5 ± 4.5 |
| 4 | 57.8 ± 1.5 | 67.1 ± 6.6 |
| 5 | 36.8 ± 7.8 | 52.2 ± 5.1 |
| 6 | 25.4 ± 3.6 | 58.6 ± 6.3 |
| 7 | 37.9 ± 2.7 | 45.7 ± 6.6 |
| 8 | 36.1 ± 9.9 | 45.8 ± 7.2 |
| 9 | 88.6 ± 2.8 | 80.2 ± 2.3 |
| 10 | 56.9 ± 1.2 | 40.7 ± 6.9 |
| 11 | 51.5 ± 2.4 | 52.0 ± 6.3 |
| 12 | 52.6 ± 5.5 | 36.4 ± 2.8 |
| 13 | 64.1 ± 8.3 | 66.2 ± 4.2 |
| 14 | 79.2 ± 4.2 | 70.7 ± 1.1 |
| 15 | 50.0 ± 5.5 | 58.1 ± 4.7 |
| 16 | 27.7 ± 7.2 | 72.1 ± 1.8 |
| 17 | 59.3 ± 3.8 | 73.7 ± 3.9 |
| 18 | 65.5 ± 5.6 | 66.4 ± 1.6 |
| 22 | 60.0 ± 0.6 | 72.1 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.-H.; Kim, J.-Y.; Ryu, S.-M.; Hwang, S.-Y.; Lee, M.-H.; Kim, N.; Son, H.; Lee, A.-Y.; Kim, H.-S.; Moon, B.-C.; et al. New Sesquiterpene Glycosides from the Flowers of Aster koraiensis and Their Inhibition Activities on EGF- and TPA-Induced Cell Transformation. Plants 2023, 12, 1726. https://doi.org/10.3390/plants12081726
Seo Y-H, Kim J-Y, Ryu S-M, Hwang S-Y, Lee M-H, Kim N, Son H, Lee A-Y, Kim H-S, Moon B-C, et al. New Sesquiterpene Glycosides from the Flowers of Aster koraiensis and Their Inhibition Activities on EGF- and TPA-Induced Cell Transformation. Plants. 2023; 12(8):1726. https://doi.org/10.3390/plants12081726
Chicago/Turabian StyleSeo, Young-Hye, Ji-Young Kim, Seung-Mok Ryu, Sun-Young Hwang, Mee-Hyun Lee, Nahyun Kim, Hojun Son, A-Yeong Lee, Hyo-Seon Kim, Byeong-Cheol Moon, and et al. 2023. "New Sesquiterpene Glycosides from the Flowers of Aster koraiensis and Their Inhibition Activities on EGF- and TPA-Induced Cell Transformation" Plants 12, no. 8: 1726. https://doi.org/10.3390/plants12081726
APA StyleSeo, Y.-H., Kim, J.-Y., Ryu, S.-M., Hwang, S.-Y., Lee, M.-H., Kim, N., Son, H., Lee, A.-Y., Kim, H.-S., Moon, B.-C., Jang, D.-S., & Lee, J. (2023). New Sesquiterpene Glycosides from the Flowers of Aster koraiensis and Their Inhibition Activities on EGF- and TPA-Induced Cell Transformation. Plants, 12(8), 1726. https://doi.org/10.3390/plants12081726







