Antifungal Potency of Amaranth Leaf Extract: An In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Polyphenol Content
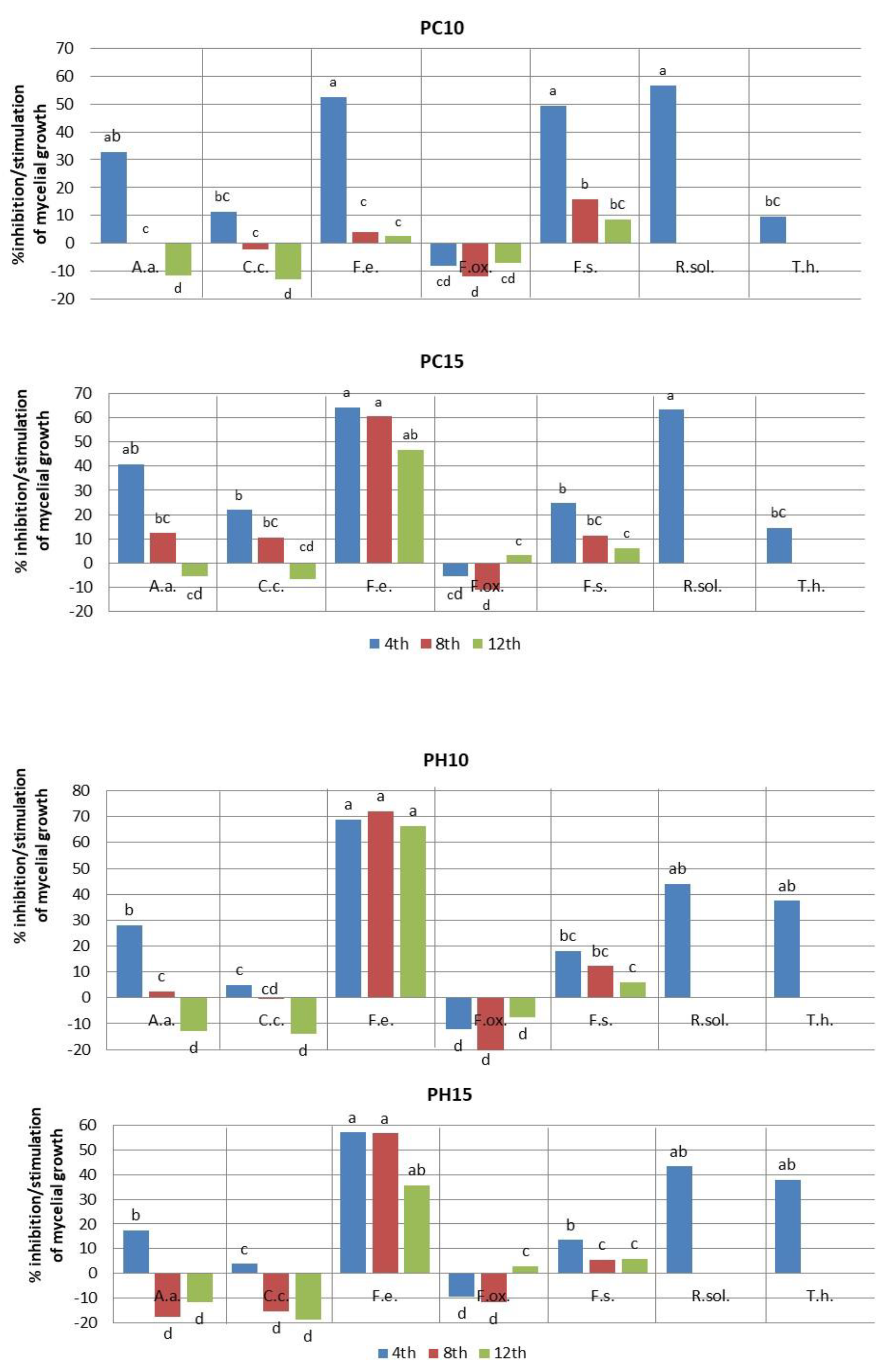
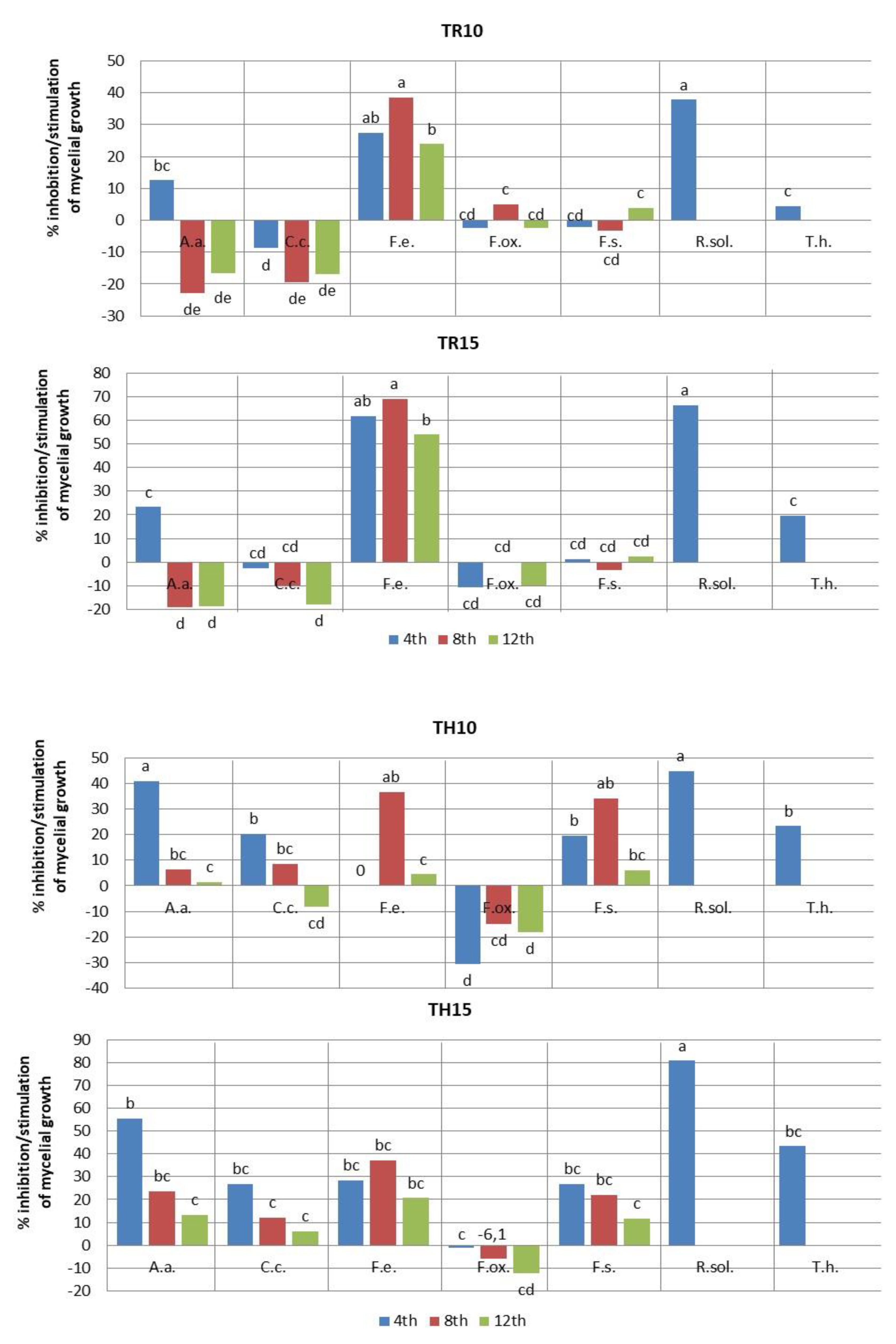
2.2. Fungistatic Activity
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Biochemical Characteristics of Extracts
4.2.1. Extract preparation
4.2.2. Extraction
4.2.3. Total Polyphenol Analysis
4.2.4. Assessment of Extract Antioxidant Activities
4.3. Fungal Cultures
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hestbjerg, H.; Nielse, N.K.F.; Thrane, U.; Elmoholt, S. Production of trichothecenes and other secondary metabolites by Fusarium culmorum and Fusarium equiseti on common laboratory media and a soil organic matter agar an ecological interpretation. J. Agric. Food Chem. 2002, 50, 7593–7599. [Google Scholar] [CrossRef]
- Yli-Mattila, T. Ecology and evolution of toxigenic Fusarium species in cereals in Northern Europe and Asia. J. Plant. Pathol. 2010, 92, 7–18. Available online: https://www.jstor.org/stable/41998764 (accessed on 22 March 2023).
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef]
- Chen, A.; Mao, X.; Sun, Q.; Wei, Z.; Li, J.; You, Y.; Zhao, J.; Jiang, G.; Wu, Y.; Wang, L.; et al. Alternaria Mycotoxins: An Overview of Toxicity, Metabolism, and Analysis in Food. J. Agric. Food Chem. 2021, 69, 7817–7830. [Google Scholar] [CrossRef]
- Meena, M.; Samal, S. Alternaria host-specific (HSTs) toxins: An overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019, 17, 745–758. [Google Scholar] [CrossRef]
- Rotem, J. The Genus Alternaria. In Biology, Epidemiology and Pathogenicity; APS Press: Saint Paul, MN, USA, 1994. [Google Scholar]
- Official Journal of the European Union. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:309:0071:0086:en:PDF (accessed on 22 March 2023).
- Jamiołkowska, A. Preparaty biotechniczne i biologiczne w ochronie papryki słodkiej (Capsicum annuum L.) przed grzybami chorobotwórczymi i indukowaniu reakcji obronnych roślin; Uniwersytet Przyrodniczy w Lublinie: Lublin, Poland, 2013; Volume 379, pp. 1–117. [Google Scholar]
- Jamiołkowska, A. Natural Compounds as Elicitors of Plant Resistance Against Diseases and New Biocontrol Strategies. Agronomy 2020, 10, 173. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Abubakar, E.M. Antibacterial potential of crude leaf extracts of Eucalyptus camaldulensis against some pathogenic bacteria. Afr. J. Plant Sci. 2010, 4, 202–209. [Google Scholar]
- Jamiołkowska, A.; Kowalski, R. Laboratory effect of Silphium perfoliatum L. on the growth of tested fungi. Acta Sci. Pol. Hortorum Cultus 2012, 11, 43–55. [Google Scholar]
- Kursa, W.; Jamiołkowska, A.; Skwaryło-Bednarz, B.; Kowalski, R.; Wyrostek, J.; Patkowska, E.; Kopacki, M. In vitro efficacy of herbal plant extracts on some phytopathogenic fungi. Acta Sci. Pol. Hortorum Cultus 2022, 21, 79–90. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Ahmad, M.; Mehjabeen, J.N.; Ahmad, S.; Qayum, M.; Marwat, I.K. Antimicrobial screening of selected flora of Pakistan. Arch. Biol. Sci. 2011, 63, 691–695. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Shah, M.R.; Qayum, M.; Ercisli, S. Biological screening of selected flora of Pakistan. Biol. Res. 2012, 45, 375–379. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Sałata, A.; Kniaziewicz, M. Tansy (Tanacetum vulgare L.)—A Wild-Growing Aromatic Medicinal Plant with a Variable Essential Oil Composition. Agronomy 2022, 12, 277. [Google Scholar] [CrossRef]
- Kursa, W.; Jamiołkowska, A.; Wyrostek, J.; Kowalski, R. Antifungal Effect of Plant Extracts on the Growth of the Cereal Pathogen Fusarium spp.—An In Vitro Study. Agronomy 2022, 12, 3204. [Google Scholar] [CrossRef]
- Šernaite, L. Plant extracts: Antimicrobial and antifungal activity and appliance in plant protection. Sodininkystė Ir Daržininkystėv 2017, 36, 58–68. Available online: http://www.lsdi.lt/straipsniai/36-3ir (accessed on 22 March 2023).
- Chłopicka, J.; Pasko, P.; Gorinstein, S.; Jedryas, A.; Zagrodzki, P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT Food Sci. Technol. 2012, 46, 548–555. [Google Scholar] [CrossRef]
- Jadhav, V.; Biradar, S.D. Evaluation of Antifungal Activity of Amaranthus spinosus L. (Amaranthaceae). Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 38–43. [Google Scholar] [CrossRef]
- Skwaryło-Bednarz, B.; Nalborczyk, E. Uprawa i wykorzystanie amarantusa. Wieś Jutra 2006, 4, 52–55. [Google Scholar]
- Skwaryło-Bednarz, B.; Jamiołkowska, A.; Kopacki, M.; Patkowska, E.; Golan, K.; Krasowska, P.; Klikocka, H. Assessment of catalase soil activity under amaranth cultivation not exposed to chemical protection methods. Acta Sci. Pol. Hortorum Cultus 2022, 21, 101–110. [Google Scholar] [CrossRef]
- Korzeniowska, K.; Żmudzki, S.; Ambroziak, K.; Wieczorek, P.P. Możliwości zastosowania ekstraktów roślinnych zawierających związki fenolowe w rolnictwie ekologicznym. Przem. Chem. 2017, 1, 100–104. [Google Scholar] [CrossRef]
- Jimoh, M.O.; Afolayan, A.J.; Lewu, F.B. Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages. Sci. Rep. 2019, 9, 12965. [Google Scholar] [CrossRef]
- WorldData.info. Climate Comparison: Poland vs. Turkey. 2023. Available online: https://www.worlddata.info/climate-comparison.php?r1=poland&r2=turkey (accessed on 21 March 2023).
- Hilou, A.; Nacoulma, O.G.; Guiguemde, T.R. In vivo antimalarial activities of extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in mice. J. Ethnopharmacol. 2006, 103, 236–240. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Kammerer, D.; Schieber, A.; Adama, H.; Nacoulma, O.G.; Carle, R. Betacyanins and phenolic compounds from Amaranthus spinosus L. and Boerhavia erecta L. Z. Naturforsch. C. J. Biosci. 2004, 59, 1–8. [Google Scholar] [CrossRef]
- Quiroga, A.V.; Barrio, D.A.; Añón, M.C. Amaranth lectin presents potential antitumor properties. LWT Food Sci. Technol. 2015, 60, 478–485. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, fower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.M.; Grusak, M.A. Minerals, vitamin C, phenolics, favonoids and antioxidant activity of Amaranthus leafy vegetables. J. Food Compos. Anal. 2017, 58, 33–39. [Google Scholar] [CrossRef]
- Ivanescu, B.; Tuchilus, C.; Corciova, A.; Lungu, C.; Mihai, C.T.; Gheldiu, A.M.; Vlase, L. Antioxidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia 2018, 66, 282–288. Available online: https://farmaciajournal.com/wp-content/uploads/2018-02-art-13-Ivanescu_Tuchilus_Vlase_282-288.pdf (accessed on 22 March 2023).
- Kaczorová, D.; Karalija, E.; Dahija, S.; Bešta-Gajević, R.; Parić, A.; Ćavar Zeljković, S. Influence of Extraction Solvent on the Phenolic Profile and Bioactivity of Two Achillea Species. Molecules 2021, 26, 1601. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Polyphenol and flavonoid profiles and radical scavenging activity in leafy vegetable Amaranthus gangeticus. BMC Plant Biol. 2020, 20, 499. [Google Scholar] [CrossRef]
- Bączek, K.; Kosakowska, O.; Przybył, J.; Kuźma, P.; Ejdys, M.; Obiedziński, M.; Węglarz, Z. Intraspecific variability of yarrow (Achillea millefolium L.) in respect of developmental and chemical traits. Herba Pol. 2015, 61, 7–52. [Google Scholar] [CrossRef]
- Dekić, M.S.; Radulović, N.S.; Stojanović, N.M.; Randjelović, P.J.; Stojanović-Radić, Z.Z.; Najman, S.; Stojanović, S. Spasmolytic, antimicrobial and cytotoxic activities of 5-phenylpentyl isothiocyanate, a new glucosinolate autolysis product from horseradish (Armoracia rusticana P. Gaertn., B. Mey. and Scherb., Brassicaceae). Food Chem. 2017, 232, 329–339. [Google Scholar] [CrossRef]
- Akbar, M.; Sherazi, I.N.; Khalil, T.; Iqbal, M.S.; Akhtar, S.; Khan, S.N. Identification of antifungal compounds from slender amaranth. Planta Daninha 2020, 38, e020207096. [Google Scholar] [CrossRef]
- Jamiołkowska, A. Fungi colonizing stems and leaves of hot pepper plants (Capsicum annuum L.) cultivated in field. EJPAU Hortic. 2009, 12, 20093199741. Available online: www.ejpau.media.pl (accessed on 22 March 2023).
- Jamiołkowska, A.; Wagner, A.; Sawicki, K. Fungi colonizing roots of zucchini (Cucurbita pepo L. var. giromontina) plants and pathogenicity of Fusarium spp. to zucchini seedlings. Acta Agrobot. 2011, 64, 73–78. [Google Scholar] [CrossRef]
- Thanoon, A.H.; Jamiołkowska, A.; Buczkowska, H. Biodiversity of fungi colonizing hull-less seed squash (Cucurbita pepo subsp. pepo var. styriaca Greb.) cultivated in an organic farm. Ann. Univ. Maria Curie-Skłodowska. Sect. EEE Hortic. 2015, 25, 37–47. [Google Scholar]
- Germán, B.; Alani Zanon, M.S.; Palazzini, J.M.; Haidukowski, M.; Pascale, M.; Chulze, S. Trichothecenes and zearalenone production by Fusarium equiseti and Fusarium semitectum species isolated from Argentinean soybean. Food Addit. Contam. Part A 2012, 29, 1342–1436. [Google Scholar] [CrossRef]
- Carminate, B.; Martin, G.B.; Barcelos, R.M.; Gontijo, I.; Suzart de Almeida, M.; Belinelo, V.J. Evaluation of Antifungal Activity of Amaranthus viridis L. (Amaranthaceae) on Fusariosis by Piper nigrum L. and on Anthracnose by Musa sp. Agric. J. 2012, 7, 215–219. [Google Scholar] [CrossRef]
- Shirazi, M.; Abid, M.; Sitara, U. Antifungal activity of some medicinal plant extracts against soil-borne phytopathogens. Pak. J. Bot. 2020, 52, 679–685. Available online: http://www.journalijdr.com (accessed on 22 March 2023). [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wyrostek, J.; Kowalski, R. Effect of ultrasound and fragmentation of the raw material on the extraction of phenolic compounds from peppermint leaves and black tea. Przem. Chem. 2022, 101, 928–933. [Google Scholar]
| Plant Extract | Polyphenols (mg GAE/mL) ± SD | Antioxidant Activity, Free Radical Scavenging Ability | |
|---|---|---|---|
| % Inhibition ± SD | mM TE/mL ± SD | ||
| A. cruentus (PC) | 4.31 d ± 0.308 | 30.42 d ± 2.553 | 8.77 d ± 0.805 |
| A. hypochondriacus × A. hybridus L. (PH) | 5.39 c ± 0.259 | 46.51 c ± 0.225 | 13.84 c ± 0.071 |
| A. retroflexus (TR) | 5.81 b ± 0.082 | 56.59 b ± 1.137 | 17.02 b ± 0.359 |
| A. hybridus (TH) | 6.75 a ± 0.162 | 69.34 a ± 1.240 | 21.04 a ± 0.391 |
| Experimental Combination | Concentration (%) | Number of Days ± SD | ||
|---|---|---|---|---|
| 4 | 8 | 12 | ||
| Alternaria alternata | ||||
| PC | 10 | 23.0 ± 0.81 ef | 51.0 ± 0.0 b | 75.7 ± 1.62 ab |
| 15 | 20.3 ± 0.47 f | 44.7 ± 2.05 d | 71.7 ± 1.24 bc | |
| PH | 10 | 24.7 ± 0.47 de | 49.7 ± 0.47 bc | 76.7 ± 0.47 ab |
| 15 | 28.3 ± 0.81 bc | 60.0 ± 0.0a | 76.0 ± 0.47 ab | |
| TR | 10 | 30.0 ± 0.47 b | 62.7 ± 0.47a | 79.3 ± 0.47 ab |
| 15 | 26.3 ± 1.24 cd | 60.7 ± 0.47 a | 80.7 ± 0.47 a | |
| TH | 10 | 20.3 ± 0.47 f | 47.7 ± 1.24 cd | 67.0 ± +1.63 c |
| 15 | 15.3 ± 0.47 g | 39.0 ± 0.81 e | 59.0 ± 2.16 d | |
| CE | 10 | 34.3 ± 1.24 a | 51.0 ± 0.81 b | 68.0 ± 2.16 c |
| 15 | 34.3 ± 1.24 a | 51.0 ± 0.81 b | 68.0 ± 2.16 c | |
| F | 108.8507 | 132.152 | 37.9273 | |
| p | 5.27 × 10−15 | 7.94 × 10−16 | 1.23 × 10−10 | |
| LSD | 2.9 | 3.3 | 5.3 | |
| Colletotrichum coccodes | ||||
| PC | 10 | 23.0 ± 1.24 cd | 51.0 ± 0.0 bc | 76.0 ± 0.47 ab |
| 15 | 20.3 ± 0.00 de | 44.7 ± 0.47 d | 71.7 ± 0.81 ab | |
| PH | 10 | 24.7 ± 0.81 bc | 49.7 ± 0.47cd | 76.7 ± 0.47 ab |
| 15 | 25.0 ± 0.00 bc | 57.7 ± 0.47 a | 80.0 ± 0.00 a | |
| TR | 10 | 28.3 ± 1.24 a | 59.7 ± 0.47 a | 78.7 ± 1.88 a |
| 15 | 26.7 ± 1.24 ab | 55.0 ± 2.44 ab | 79.3 ± 1.69 a | |
| TH | 10 | 20.7 ± 0.47 de | 45.7 ± 2.86 de | 72.7 ± 1.88 abc |
| 15 | 19.0 ± 0.81 e | 44.0 ± 2.44 e | 64.0 ± 7.78 c | |
| CE | 10 | 26.0 ± 0.81 abc | 50.0 ± 0.00 bcd | 67.3 ± 2.05 bc |
| 15 | 26.0 ± 0.81 abc | 50.0 ± 0.00 bcd | 67.3 ± 2.05 bc | |
| F | 29.8333 | 29.8333 | 32.84678 | 10.3675 |
| p | 1.12 × 10−9 | 1.12 × 10−9 | 4.64 × 10−10 | 8.72 × 10−6 |
| LSD | 0.7 | 3.1 | 5.1 | 10.0 |
| Trichoderma harzianum | ||||
| PC | 10 | 81.3 ± 0.47 c | * No measurements | |
| 15 | 77.0 ± 0.81 d | |||
| PH | 10 | 56.3 ± 0.47 g | ||
| 15 | 56.0 ± 0.81 g | |||
| TR | 10 | 86.0 ± 0.81 b | ||
| 15 | 72.3 ± 2.05 e | |||
| TH | 10 | 69.0 ± 0.81 f | ||
| 15 | 51.0 ± 0.81 h | |||
| CE | 10 | 90.0 ± 0.00 a | ||
| 15 | 90.0 ± 0.00 a | |||
| F | 527.6574 | No correlations | ||
| p | 9.09 × 10−22 | |||
| LSD | 3.2 | |||
| Experimental Combination | Concentration (%) | Number of Days ± SD | ||
|---|---|---|---|---|
| 4 | 8 | 12 | ||
| Fusarium equiseti | ||||
| PC | 10 | 13.3 ± 1.24 c | 68.0 ± 2.16 a | 86.7 ± 0.47 a |
| 15 | 10.0 ± 0.00 cd | 28.0 ± 1.63 cde | 47.3 ± 3.68 d | |
| PH | 10 | 8.7 ± 0.94 d | 19.7 ± 0.47 e | 30.0 ± 0.00 e |
| 15 | 12.0 ± 0.81 cd | 30.7 ± 047 c | 57.3 ± 2.05 c | |
| TR | 10 | 20.3 ± 1.24 b | 43.7 ± 2.05 b | 67.7 ± 6.12 b |
| 15 | 10.7 ± 0.47 cd | 22.0 ± 1.63 de | 41.0 ± 0.81 d | |
| TH | 10 | 28.0 ± 2.94 a | 45.0 ± 5.31 b | 85.0 ± 4.08 a |
| 15 | 20.0 ± 0.81 b | 44.7 ± 0.47 b | 70.7 ± 0.94 b | |
| CE | 10 | 28.0 ± 0.81 a | 71.0 ± 2.94 a | 89.0 ± 1.41 a |
| 15 | 28.0 ± 0.81 a | 71.0 ± 2.94 a | 89.0 ± 1.41 a | |
| F | 102.3873 | 140.2651 | 120.3965 | |
| P | 9.56 × 10−15 | 4.43 × 10−16 | 1.97 × 10−15 | |
| LSD | 4.4 | 8.7 | 9.9 | |
| Fusarium oxysporum | ||||
| PC | 10 | 26.7 ± 2.35 b | 60.0 ± 4.08 bc | 75.0 ± 0.00 bc |
| 15 | 26.0 ± 0.00 b | 59.7 ± 0.47 bcd | 67.7 ± 2.05 d | |
| PH | 10 | 27.7 ± 1.69 ab | 67.0 ± 0.00 a | 75.3 ± 0.47 bc |
| 15 | 27.0 ± 2.16 b | 60.0 ± 0.00 bc | 68.0 ± 0.81 d | |
| TR | 10 | 25.3 ± 0.47 b | 51.0 ± 0.00 d | 71.7 ± 0.47 cd |
| 15 | 27.3 ± 0.81 b | 53.7 ± 1.24 cd | 77.0 ± 0.81 b | |
| TH | 10 | 32.2 ± 0.41 a | 61.7 ± 3.68 ab | 82.7 ± 2.05 a |
| 15 | 25.0 ± 0.00 b | 57.0 ± 1.63 bcd | 78.7 ± 1.24 ab | |
| CE | 10 | 24.7 ± 1.24 b | 53.7 ± 1.24 cd | 70.0 ± 1.36 d |
| 15 | 24.7 ± 1.24 b | 53.7 ± 1.24 cd | 70.0 ± 1.36 d | |
| F | 5.942576 | 12.40261 | 28.65224 | |
| P | 0.000453 | 2.14 × 10−6 | 1.61 × 10−9 | |
| LSD | 4.8 | 6.9 | 4.6 | |
| Fusarium solani | ||||
| PC | 10 | 15.0 ± 0.00 e | 52.7 ± 0.47 cd | 77.7 ± 1.24 d |
| 15 | 22.3 ± 1.24 cd | 55.7 ± 1.24 bc | 79.7 ± 0.47 cd | |
| PH | 10 | 24.3 ± 0.47 bc | 55.0 ± 2.44 bc | 80.0 ± 0.00 cd |
| 15 | 25.7 ± 0.47 b | 59.3 ± 0.94 ab | 80.0 ± 0.00 cd | |
| TR | 10 | 30.3 ± 0.47 a | 64.7 ± 0.47 a | 81.7 ± 0.47 bc |
| 15 | 29.3 ± 0.47 a | 64.7 ± 2.05 a | 83.0 ± 1.63 ab | |
| TH | 10 | 24.0 ± 0.81 bc | 41.3 ± 2.44 e | 79.7 ± 0.47 cd |
| 15 | 21.7 ± 0.47 d | 49.0 ± 0.00 d | 75.0 ± 0.00 e | |
| CE | 10 | 29.7 ± 0.47 a | 62.7 ± 1.69 a | 85.0 ± 0.00 a |
| 15 | 29.7 ± 0.47 a | 62.7 ± 1.69 a | 85.0 ± 0.00 a | |
| F | 122.9346 | 22.87798 | 40.0 | |
| P | 1.61 × 10−15 | 1.21 × 10−8 | 7.52 × 10−11 | |
| LSD | 2.2 | 5.6 | 2.5 | |
| Rhizoctonia solani | ||||
| PC | 10 | 39.0 ± 0.81 c | *—No measurements | |
| 15 | 30.3 ± 1.24 c | |||
| PH | 10 | 50.3 ± 0.47 b | ||
| 15 | 51.0 ± 0.47 b | |||
| TR | 10 | 56.0 ± 3.26 b | ||
| 15 | 49.7 ± 6.12 b | |||
| TH | 10 | 30.3 ± 0.47 c | ||
| 15 | 17.3 ± 2.05 d | |||
| CE | 10 | 90.0 ± 0.00 a | ||
| 15 | 90.0 ± 0.00 a | |||
| F | 158.4078 | No correlation | ||
| P | 1.34 × 10−16 | |||
| LSD | 9.5 | |||
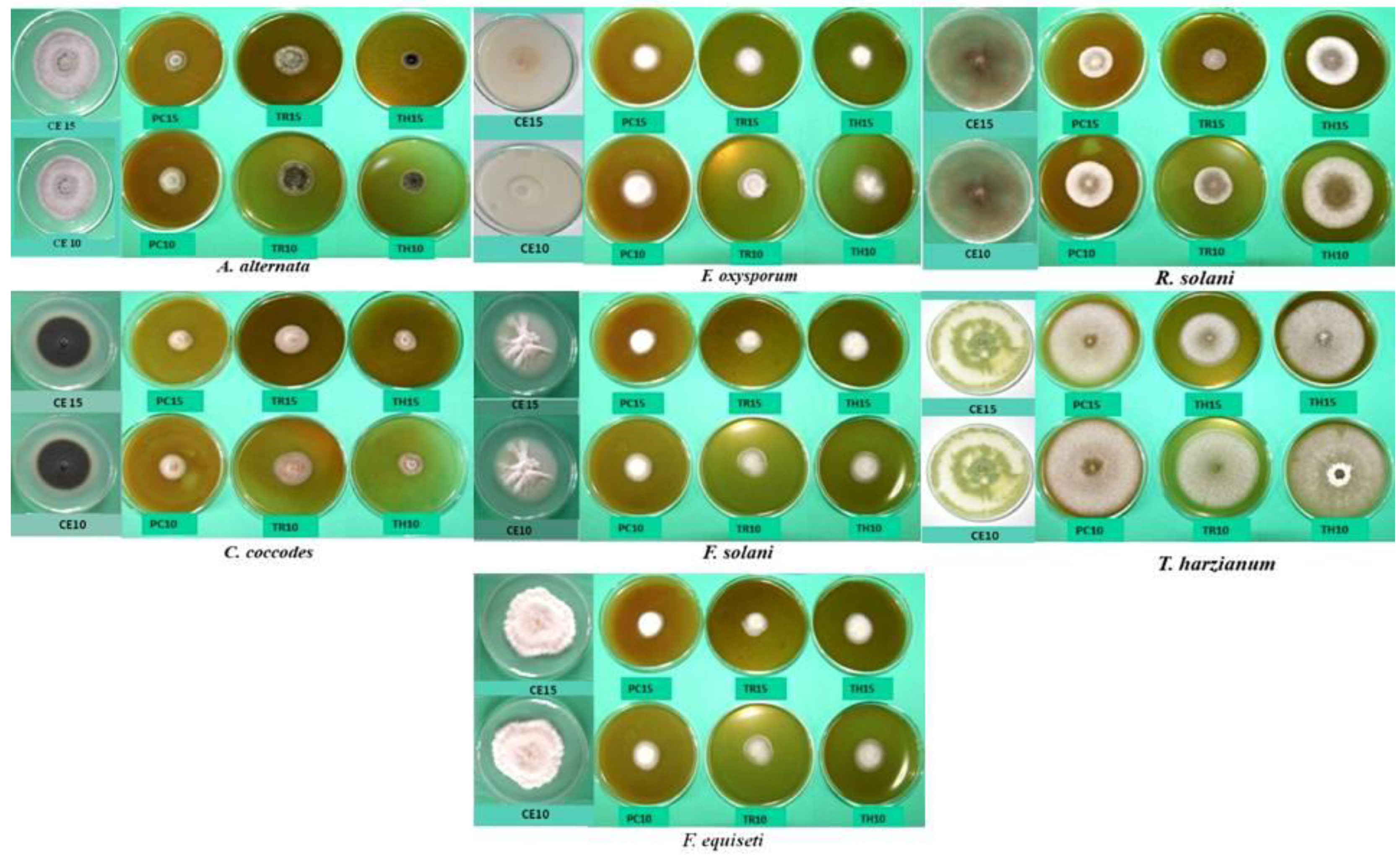
| Fungus Species | Experimental Combination | Mycelium Surface and Structure | Obverse | Reverse | Presence of Spores |
|---|---|---|---|---|---|
| A. alternata | PC10, PC15 | Aerial, regular | White and gray | Black | No aleuroconidia |
| PH10, PH15 | Aerial, regular | White and gray | Black | No aleuroconidia | |
| TH10, TH15 | Aerial, regular, fluffy | Gray | Black | Sparse or no aleuroconidia | |
| TR10, TR15 | Poor growth, low mycelium slightly compacted, regular | Grey and black; gray | Black | Numerous deformed aleuroconidia | |
| CE10, CE15 | fluffy, regular growth | Gray | Black | Sparse aleuroconidia | |
| C. coccodes | PC10, PC15 | Substrate, regular | White and salmon; microsclerotia in the center | Colorless | Sparse conidia |
| PH10, PH15 | Substrate, regular | White and salmon; no microsclerotia | Colorless | No conidia | |
| TH10, TH15 | Aerial, regular | White and orange; sparse black microsclerotia in the center | Colorless | No conidia | |
| TR10, TR15 | Aerial, regular | White and orange; sparse black microsclerotia in the center | Colorless | No conidia | |
| CE10, CE15 | Substrate | Light white; sparse black microsclerotia | Colorless | Sparse conidia | |
| PC10, PC15 | Substrate, restricted aerial | White; green sporulation on the edge | Colorless | Very numerous conidia | |
| PH10, PH15 | Substrate, restricted aerial | White; green sporulation in the center | Colorless | Very numerous conidia | |
| T. harzianum | TH10, TH15 | Substrate, restricted aerial | White; high green sporulation | Colorless | Very numerous conidia |
| TR10, TR15 | Substrate, restricted aerial | White; high dark green sporulation | Colorless | Very numerous conidia | |
| CE10, CE15 | Aerial, regular | White; light green sporulation | Colorless | Sparse conidia | |
| PC10, PC15 | Aerial, regular | White and creamy; creamy and beige | Colorless; creamy and brown | Moderately numerous conidia | |
| PH10, PH15 | Aerial, regular | White and creamy; creamy and beige | Colorless; creamy and brown | Moderately numerous conidia | |
| F. equiseti | TH10, TH15 | Abundant aerial mycelium | Creamy and white | Colorless | Sparse conidia |
| TR10, TR15 | Mainly substrate, restricted aerial | Creamy and white | Colorless | Sparse conidia | |
| CE10, CE15 | Aerial, regular | Creamy and white | Colorless | Numerous microconidia and sparse macroconidia | |
| PC10, PC15 | Regular growth | White | Colorless | Medium-sized microconidia | |
| PH10, PH15 | Regular growth | White; pink and white | Colorless | Medium-sized microconidia | |
| F. oxysporum | TH10, TH15 | Regular growth | White | Purple and red | Microconidia |
| TR10, TR15 | Regular growth | White | Purple and red | Microconidia | |
| CE10, CE15 | Regular growth | White | Colorless | Macro- and microconidia | |
| F. solani | PC10, PC15 | Aerial, regular, abundant | White | Colorless | No conidia |
| PH10, PH15 | Aerial, regular, abundant | White | Colorless | No conidia | |
| TH10, TH15 | Aerial fine, substrate | White | Creamy | Sparse conidia | |
| TR10, TR15 | Aerial fine, substrate | White | Creamy | Sparse conidia | |
| CE10, CE15 | Aerial regular | White | Colorless | Very numerous conidia | |
| R. solani | PC10, PC15 | Aerial, fluffy, abundant | White | Colorless | - |
| PH10, PH15 | Aerial, fluffy, abundant | White and creamy | Colorless | - | |
| TH10, TH15 | Aerial, fluffy, abundant | White and creamy | Colorless | - | |
| TR10, TR15 | Aerial, fluffy, abundant | White and creamy | Colorless | - | |
| CE10, CE15 | Aerial, fluffy, abundant | Creamy and brown | Creamy | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamiołkowska, A.; Skwaryło-Bednarz, B.; Kowalski, R.; Yildirim, I.; Patkowska, E. Antifungal Potency of Amaranth Leaf Extract: An In Vitro Study. Plants 2023, 12, 1723. https://doi.org/10.3390/plants12081723
Jamiołkowska A, Skwaryło-Bednarz B, Kowalski R, Yildirim I, Patkowska E. Antifungal Potency of Amaranth Leaf Extract: An In Vitro Study. Plants. 2023; 12(8):1723. https://doi.org/10.3390/plants12081723
Chicago/Turabian StyleJamiołkowska, Agnieszka, Barbara Skwaryło-Bednarz, Radosław Kowalski, Ismet Yildirim, and Elżbieta Patkowska. 2023. "Antifungal Potency of Amaranth Leaf Extract: An In Vitro Study" Plants 12, no. 8: 1723. https://doi.org/10.3390/plants12081723
APA StyleJamiołkowska, A., Skwaryło-Bednarz, B., Kowalski, R., Yildirim, I., & Patkowska, E. (2023). Antifungal Potency of Amaranth Leaf Extract: An In Vitro Study. Plants, 12(8), 1723. https://doi.org/10.3390/plants12081723








