Effects of Multi-Walled Carbon Nanotubes and Nano-Silica on Root Development, Leaf Photosynthesis, Active Oxygen and Nitrogen Metabolism in Maize
Abstract
1. Introduction
2. Results
2.1. Seedling Morphology
2.2. Dry and Fresh Weight of Seedlings
2.3. Relative Water Content of Leaves
2.4. Electrical Conductivity of Leaves
2.5. Root Morphology
2.6. Root Activity
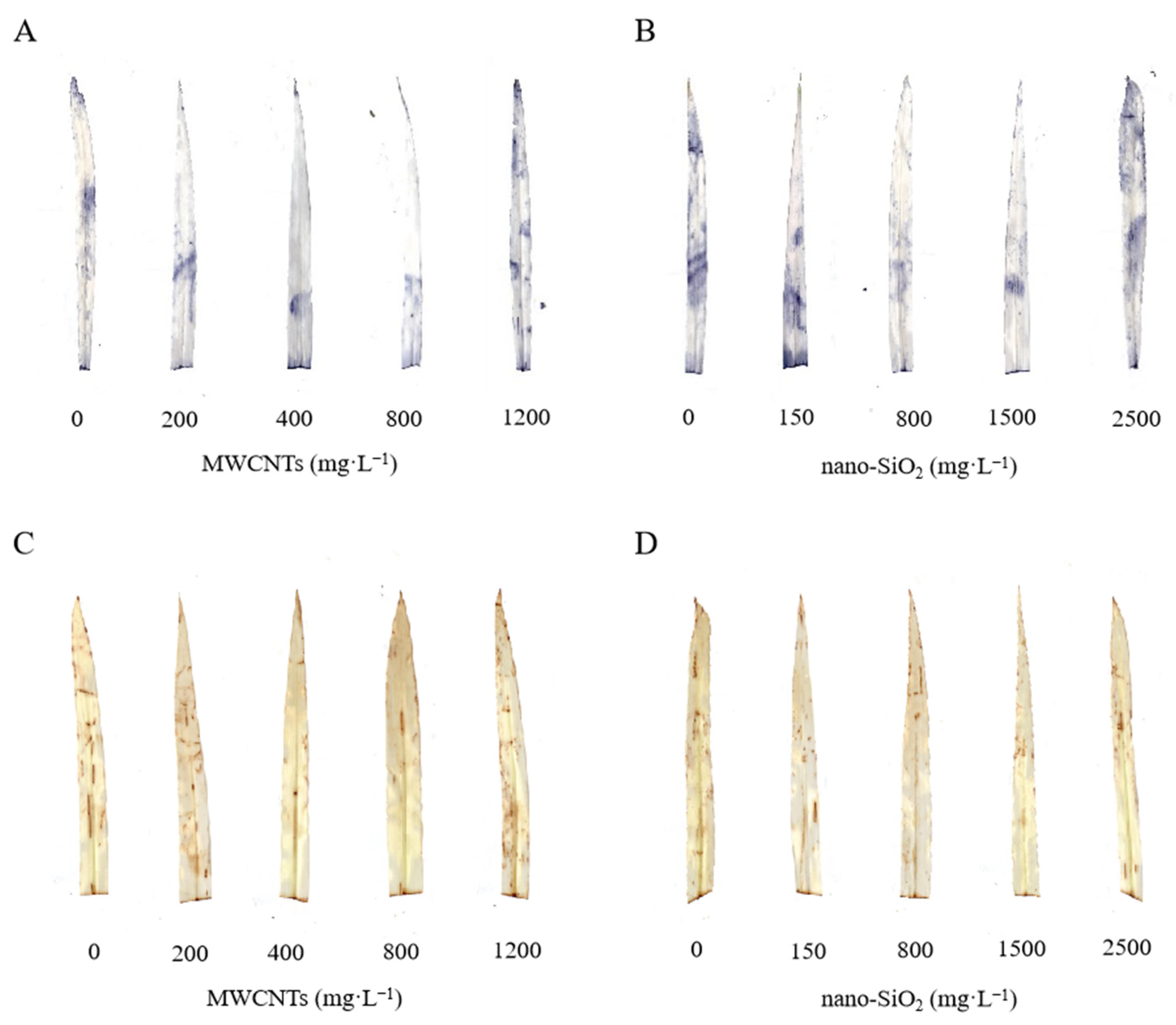
2.7. Detection of Superoxide Anion (O2·−) and Hydrogen Peroxide (H2O2) in Leaves
2.8. Activities of Phosphoenolpyruvate Carboxylase (PEPC), Ribose Diphosphate Carboxylase (Rubisco), Malase (NADP-ME), Fructose Dehydrogenase (NADP-MDH) and Pyruvate Phosphate Double Kinase (PPDK)
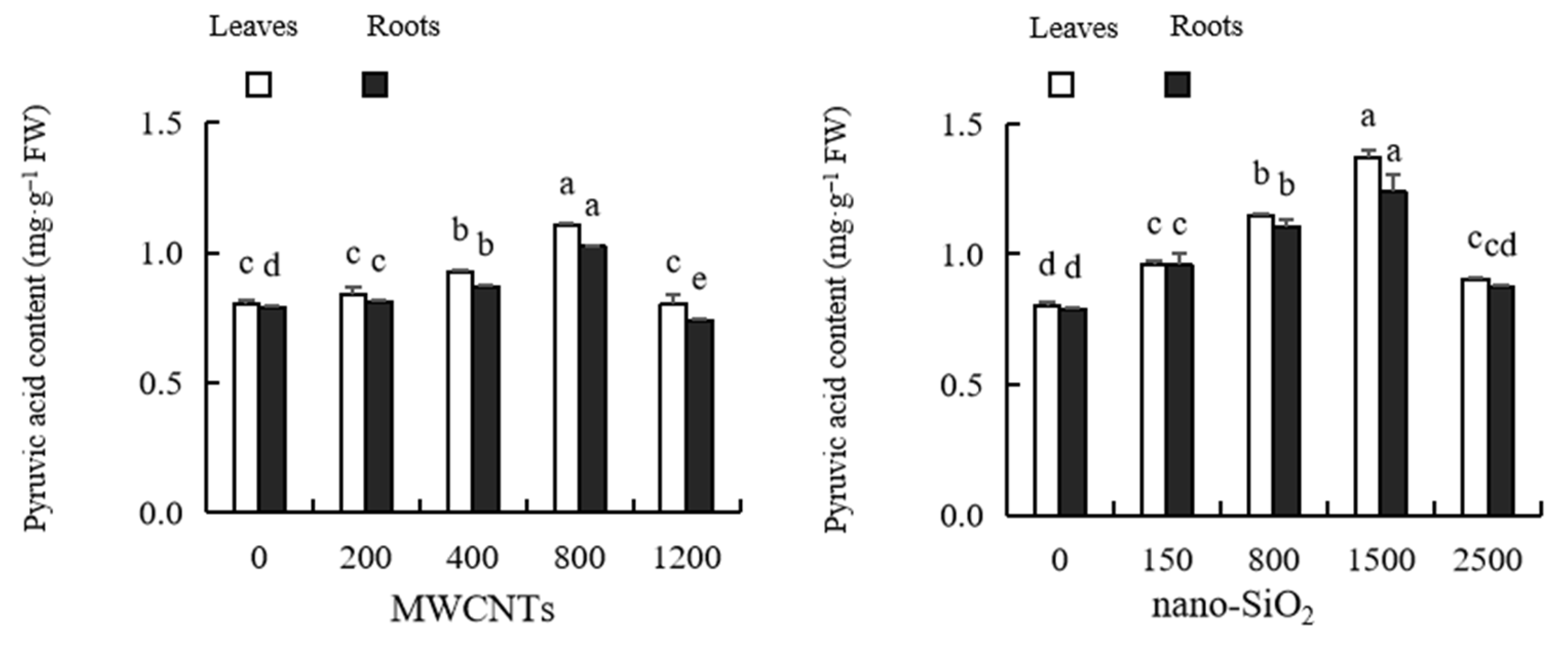
2.9. Pyruvic Acid Content
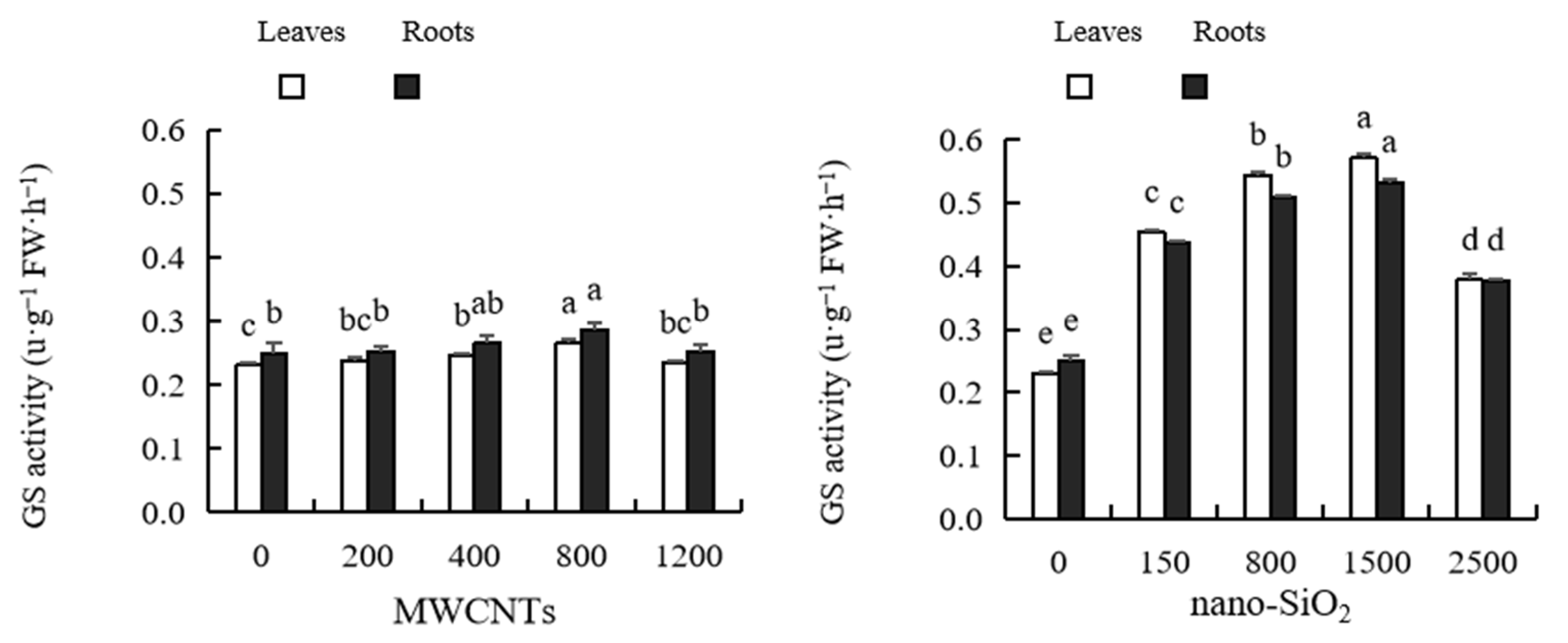
2.10. Glutamine Synthetase (GS) Activity
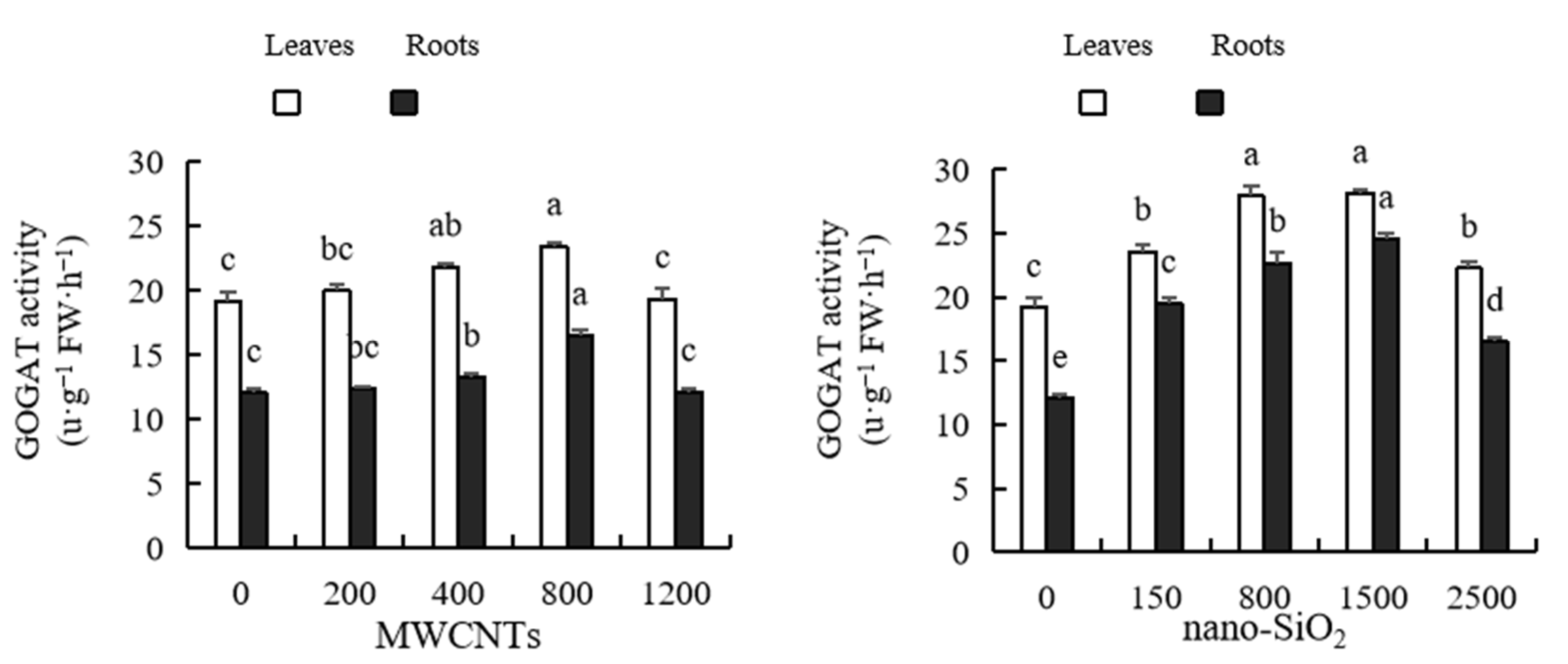
2.11. Glutamic Acid Synthase (GOGAT) Activity
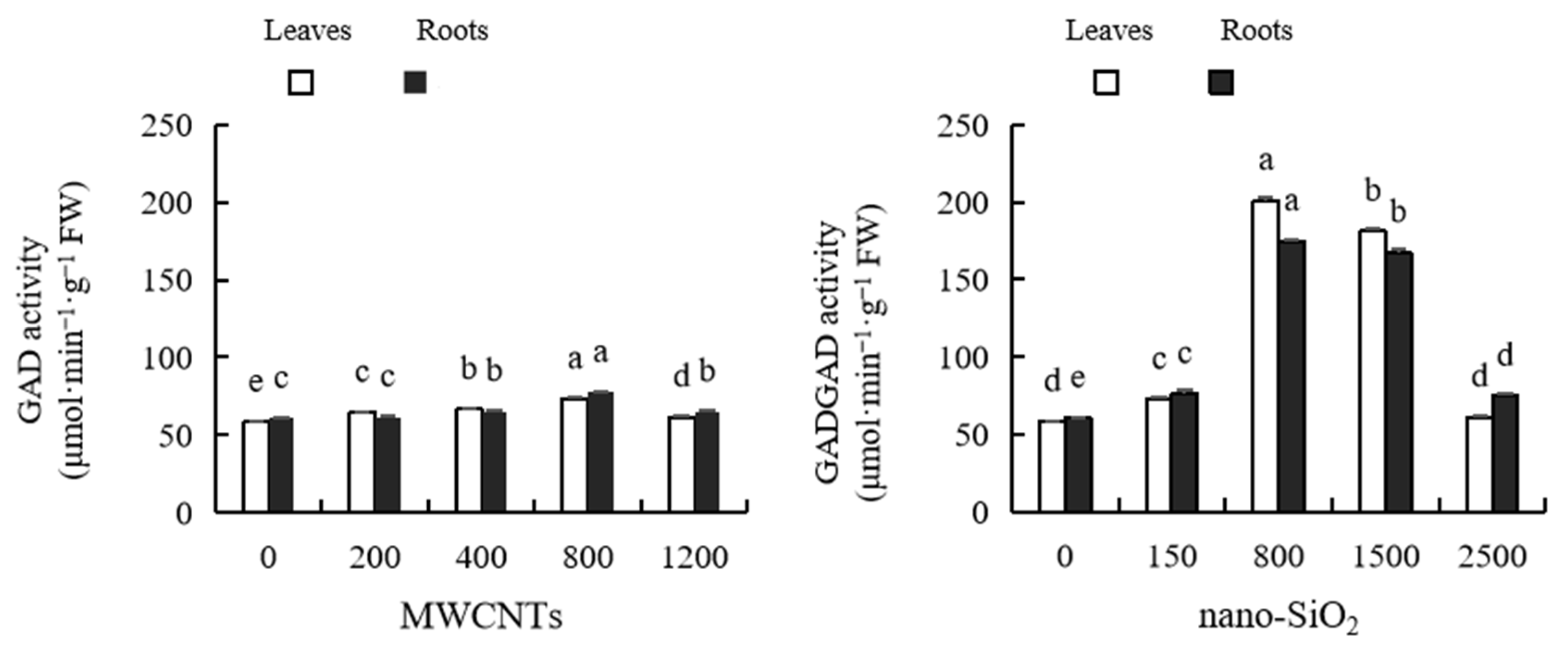
2.12. Glutamic Acid Decarboxylase (GAD) Activity
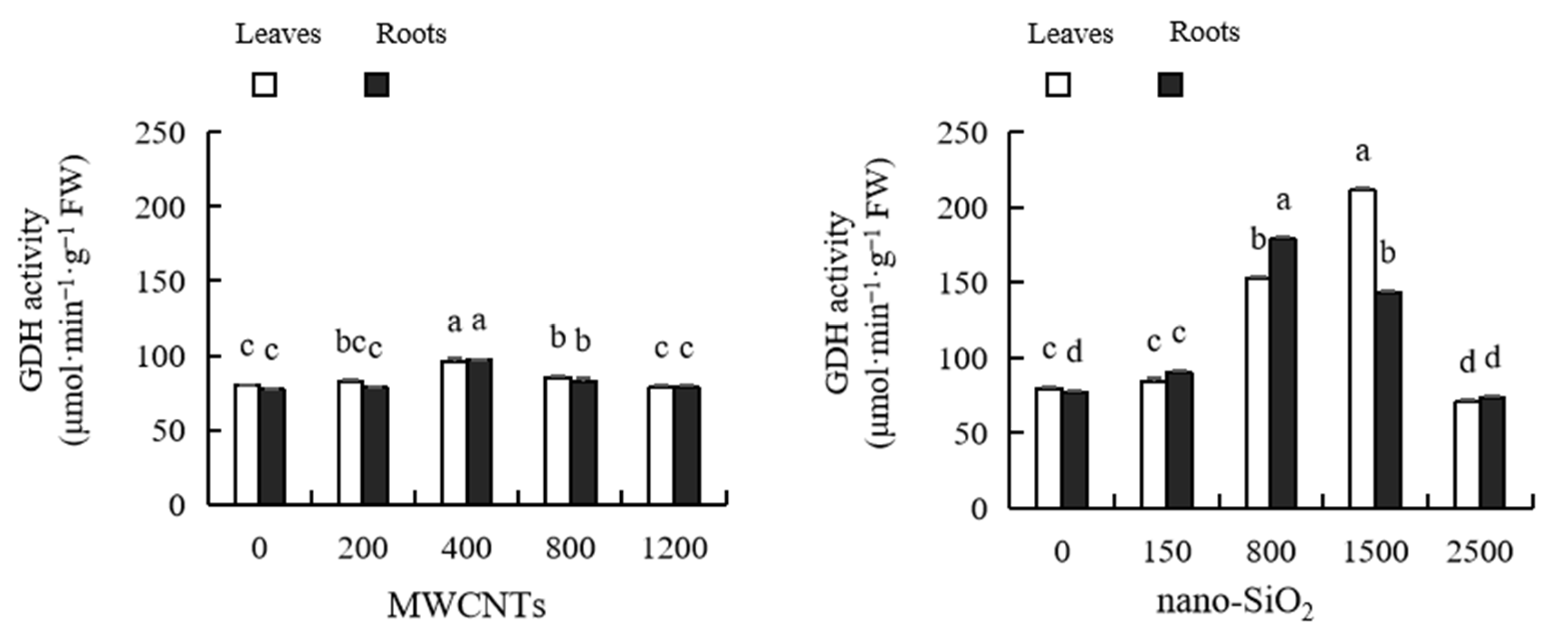
2.13. Glutamic Acid Dehydrogenase (GDH) Activity
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Seedling Morphology and Dry–Fresh Weight
4.3. Relative Water Content of Leaves
4.4. Relative Conductivity of Leaves
4.5. Root Morphological Parameters
4.6. Root Activity
4.7. DAB and NBT Staining Analysis
4.8. Photosynthetic Enzyme Activity of Leaves
4.9. Pyruvic Acid Content
4.10. Enzyme Activity Related to Nitrogen Metabolism
4.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grassini, P.; Thorburn, J.; Burr, C.; Cassman, K.G. High-yield irrigated maize in the Western U.S. Corn Belt: I. On-farm yield, yield potential, and impact of agronomic practices. Field Crops Res. 2011, 120, 142–150. [Google Scholar] [CrossRef]
- Yuan, S. Progress of the development of nanophase materials. J. Guangdong Non-Ferr. Met. 1998, 8, 125–130. [Google Scholar]
- Qi, M.; Liu, Y.; Li, T. Nano-TiO2 Improve the Photosynthesis of Tomato Leaves under Mild Heat Stress. Biol. Trace Elem. Res. 2013, 156, 323–328. [Google Scholar] [CrossRef]
- Rose, P.; Bhat, M.; Vidhani, K.; Gole, A.; Ghaisas, S. Intelligent Informatics Platform for Nano-Agriculture. In Proceedings of the 11th IEEE International Conference on Nanotechnology, Portland, OR, USA, 15–18 August 2011; pp. 916–919. [Google Scholar]
- Demirer, G.S.; Zhang, H.; Matos, J.L.; Chang, R.; Chio, L.; Staskawicz, B.; Landry, M.P. (Invited) High Aspect Ratio Nanomaterials Enable Biomolecule Delivery and Transgene Expression or Silencing in Mature Plants. bioRxiv 2018, 5, 179549. [Google Scholar] [CrossRef]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Sharma, P.; Kumar, S.; Nayan, R.; Khanna, P.K.; Zaidi, M.G.H. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012, 66, 303–310. [Google Scholar] [CrossRef]
- Savithramma, N.; Ankanna, S.; Bhumi, G. Effect of Nanoparticles on Seed Germination and Seedling Growth of Boswellia Ovalifoliolata–An Endemic and Endangered Medicinal Tree Taxon. Nano Vision 2012, 2, 61–68. [Google Scholar]
- Gopinath, K.; Gowri, S.; Karthika, V.; Arumugam, A. Green synthesis of gold nanoparticles from fruit extract of Terminalia arjuna, for the enhanced seed germination activity of Gloriosa superba. J. Nanostructure Chem. 2014, 4, 115. [Google Scholar] [CrossRef]
- Lin, B.; Diao, S.; Li, C.; Fang, L.; Qiao, S.; Yu, M. Effect of TMS (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J. For. Res. 2004, 15, 138–140. [Google Scholar] [CrossRef]
- Jaberzadeh, A.; Moaveni, P.; Moghadam, T.; Zahedi, H. Influence of Bulk and Nanoparticles Titanium Foliar Application on some Agronomic Traits, Seed Gluten and Starch Contents of Wheat Subjected to Water Deficit Stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 201–207. [Google Scholar] [CrossRef]
- Mahmoodzadeh, H.; Nabavi, M.; Kashefi, H. Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus). J. Ornam. Hortic. Plants 2013, 3, 25–32. [Google Scholar]
- Yang, F.; Hong, F.; You, W.; Liu, C.; Gao, F.; Wu, C.; Yang, P. Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006, 110, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, Y.; Zhang, H.; Liu, Z.; Shi, S. Phytotoxicity, Uptake, and Translocation of Fluorescent Carbon Dots in Mung Bean Plants. ACS Appl. Mater. Interfaces 2016, 8, 19939–19945. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Dhoke, S.K.; Khanna, A.S. Effect of Nano-ZnO Particle Suspension on Growth of Mung (Vigna radiata) and Gram (Cicer arietinum) Seedlings Using Plant Agar Method. J. Nanotechnol. 2011, 2011, 696535.1–696535.7. [Google Scholar] [CrossRef]
- Tripathi, S.; Sarkar, S. Influence of water soluble carbon dots on the growth of wheat plant. Appl. Nanosci. 2015, 5, 609–616. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Dasgupta-Schubert, N.; Villaseñor Cendejas, L.M.; Villegas, J.; Carreto Montoya, L.; Borjas García, S.E. Interfacing carbon nanotubes (CNT) with plants: Enhancement of growth, water and ionic nutrient uptake in maize (Zea mays) and implications for nanoagriculture. Appl. Nanosci. 2014, 4, 577–591. [Google Scholar] [CrossRef]
- Villagarcia, H.; Dervishi, E.; de Silva, K.; Biris, A.S.; Khodakovskaya, M.V. Bioresponse to Nanotubes: Surface Chemistry of Carbon Nanotubes Impacts the Growth and Expression of Water Channel Protein in Tomato Plants (Small 15/2012). Small 2012, 8, 2327. [Google Scholar] [CrossRef]
- Villagarcia, H.; Dervishi, E.; de Silva, K.; Biris, A.S.; Khodakovskaya, M.V. Surface Chemistry of Carbon Nanotubes Impacts the Growth and Expression of Water Channel Protein in Tomato Plants. Small 2012, 8, 2328–2334. [Google Scholar] [CrossRef]
- Robert, C.; Odile, E.; Gjergji, S.; Eduardo, P. New types of hydrogen bonds. Science 1998, 282, 2000–2001. [Google Scholar] [CrossRef]
- Hong, F.; Yang, F.; Liu, C.; Gao, Q.; Wan, Z.; Gu, F.; Wu, C.; Ma, Z.; Zhou, J.; Yang, P. Influences of Nano-TiO2 on the chloroplast aging of spinach under light. Biol. Trace Elem. Res. 2005, 104, 249–260. [Google Scholar] [CrossRef]
- Gao, F.; Hong, F.; Liu, C.; Zheng, L.; Su, M.; Wu, X.; Yang, F.; Wu, C.; Yang, P. Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach. Biol. Trace Elem. Res. 2006, 111, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, C.; Qu, C.; Yin, S.; Liu, J.; Gao, F.; Fashui, H. Rubisco Activase mRNA Expression in Spinach: Modulation by Nanoanatase Treatment. Biol. Trace Elem. Res. 2008, 122, 168–178. [Google Scholar] [CrossRef]
- Haghighi, M.; Afifipour, Z.; Mozafarian, M. The Effect of N-Si on Tomato Seed Germination under Salinity Levels. J. Biodivers. Environ. Sci. 2012, 6, 87–90. [Google Scholar]
- Siddiqui, M.; HAl-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef] [PubMed]
- El-Metwally, M. Effect of nanoparticles on biological contamination of in vitro cultures and organogenic regeneration of banana. Aust. J. Crop Sci. 2014, 8, 612–624. [Google Scholar]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef]
- Wang, X.; Han, H.; Liu, X.; Gu, X.; Chen, K.; Lu, D. Multi-walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants. J. Nanoparticle Res. 2012, 14, 841. [Google Scholar] [CrossRef]
- Ghodake, G.; Seo, Y.; Park, D.; Lee, D.S. Phytotoxicity of carbon nanotubes assessed by Brassica juncea and Phaseolus mungo. J. Nanoelectron. Optoelectron. 2010, 5, 157–160. [Google Scholar] [CrossRef]
- Lin, C.; Fugetsu, B.; Su, Y.; Watari, F. Studies on toxicity of multi-walled carbon nanotubes on Arabidopsis T87 suspension cells. J. Hazard. Mater. 2009, 170, 578–583. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Kim, B.S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon nanotubes as plant growth regulators: Effects on tomato growth, reproductive system, and soil microbial community. Small 2012, 9, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, M.; Rizwan, M.S.; Mushtaq, M.A.; Ashraf, M.; Shahzad, S.M.; Yousaf, B.; Saeed, D.A.; Rizwan, M.; Nawaz, M.A.; Mehmood, S.; et al. Silicon occurrence, uptake, transport and mechanisms of heavy metals, minerals and salinity enhanced tolerance in plants with future prospects: A review. J. Environ. Manag. 2016, 183, 521–529. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted Application of Silica Nanoparticles. A Review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, C.; Wen, J.; Wu, G.; Tao, M. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002, 21, 168–172. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Kalteh, M.; Zarrin, T.A.; Shahram, A.; Maryam, M.A.; Alireza, F.N. Effect of silica Nanoparticles on Basil (Ocimum basilicum) Under Salinity Stress. J. Chem. Health Risks 2014, 4, 49–55. [Google Scholar]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-Ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef]
- Dev, A.; Srivastava, A.K.; Karmakar, S. Uptake and Toxicity of Nanomaterials in Plants. Nanosci. Food Agric. 2017, 5, 169–204. [Google Scholar] [CrossRef]
- Giorgetti, L. Chapter 4-Effects of Nanoparticles in Plants: Phytotoxicity and Genotoxicity Assessment. Nanomater. Plants Algae Microorg. 2019, 2, 65–87. [Google Scholar] [CrossRef]
- Mohsenzadeh, S.; Karimi, J. Effects of silicon oxide nanoparticles on growth and physiology of wheat seedlings. Russ. J. Plant Physiol. 2016, 63, 119–123. [Google Scholar]
- Le, V.N.; Rui, Y.; Gui, X.; Li, X.; Liu, S.; Han, Y. Uptake, transport, distribution and Bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J. Nanobiotechnol. 2014, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.J.; Chen, K.M.; Zhao, Z.G.; Chen, G.C.; Zhou, W.J. Effects of silicon on defense of wheat against oxidative stress under drought at different developmental stages. Biol. Plant. 2008, 52, 592–596. [Google Scholar] [CrossRef]
- Liu, P.; Yin, L.; Deng, X.; Wang, S.; Tanaka, K.; Zhang, S. Aquaporin-mediated increase in root hydraulic conductance is involved in silicon-induced improved root water uptake under osmotic stress in Sorghum bicolor L. J. Exp. Bot. 2014, 65, 4747–4756. [Google Scholar] [CrossRef]
- Haghighi, M.; Pessarakli, M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013, 161, 111–117. [Google Scholar] [CrossRef]
- Feizi, H.; Moghaddam, P.R.; Shahtahmassebi, N.; Fotovat, A. Impact of Bulk and Nanosized Titanium Dioxide (TiO2) on Wheat Seed Germination and Seedling Growth. Biol. Trace Elem. Res. 2012, 146, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Owolade, O.F.; Ogunleti, D.O. Effects of Titanium Dioxide on The Diseases, Development and Yield of Edible Cowpea. J. Plant Prot. Res. 2008, 48, 329–336. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of Nanoscale Zinc Oxide Particles on the Germination, Growth and Yield of Peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Rajendran, V.; Kannan, N. Silica Nanoparticles for Increased Silica Availability in Maize (Zea mays L.) Seeds Under Hydroponic Conditions. Curr. Nanosci. 2012, 8, 902–908. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A Meta-Analysis and Review of Plant-Growth Response to Humic Substances: Practical Implications for Agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar] [CrossRef]
- Aguiar, N.O.; Novotny, E.H.; Oliveira, A.L.; Rumjanek, V.M.; Olivares, F.L.; Canellas, L.P. Prediction of humic acids bioactivity using spectroscopy and multivariate analysis. J. Geochem. Explor. 2013, 129, 95–102. [Google Scholar] [CrossRef]
- Collins, D.; Luxton, T.; Kumar, N.; Shah, S.; Walker, V.; Shah, V. Assessing the impact of copper and zinc oxide nanoparticles on soil: A field study. PLoS ONE 2012, 7, e42663. [Google Scholar] [CrossRef]
- Mura, S.; Greppi, G.; Irudayaraj, J.M.K. Nanotechnogy and Plant Sciences: Nanoparticles and Their Impact on Plants; Springer International Publishing: Cham, Switzerland, 2015; pp. 125–151. [Google Scholar]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Kheirizadeh Arough, Y.; Seyed Sharifi, R.; Seyed Sharifi, R. Bio fertilizers and zinc effects on some physiological parameters of triticale under water-limitation condition. J. Plant Interact. 2016, 11, 167–177. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Feng, H.; Jia, H.; Yang, H.; Sun, X. Mechanism of magnesium oxide nanoparticles effectively stimulate the Mg absorption and growth of tomato plants. J. Plant Nutr. Fertil. 2020, 26, 1318–1327. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Karimi, J.; Mohsenzadeh, S.; Sharifi-Rad, M.; Moradgholi, J. Evaluating SiO2 Nanoparticles Effects on Developmental Characteristic and Photosynthetic Pigment Contents of Zea mays L. Bull. Environ. Pharmacol. Life Sci. 2014, 3, 194–201. [Google Scholar]
- Maswada, H.F.; Djanaguiraman, M.; Prasad, P.V.V. Seed treatment with nano-iron (III) oxide enhances germination, seeding growth and salinity tolerance of sorghum. J. Agron. Crop Sci. 2018, 204, 577–587. [Google Scholar] [CrossRef]
- Karami, A.; Sepehri, A. Nano titanium dioxide and nitric oxide alleviate salt induced changes in seedling growth, physiological and photosynthesis attributes of barley. Zemdirb.-Agric. 2018, 105, 123–132. [Google Scholar] [CrossRef]
- Zhang, S. Mechanism of Migration and Transformation of Nano Selenium and Mitigates Cadmium Stress in Plants. Master’s Thesis, Shandong University, Jinan, China, 2019. [Google Scholar]
- Martínez-Ballesta, M.C.; Zapata, L.; Chalbi, N.; Carvajal, M. Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J. Nanobiotechnol. 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal transduction in plants. Curr. Sci. A Fortn. J. Res. 2005, 89. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Wei, B.; Cheng, S.; Zhou, Q.; Ji, S. GABA application improves the mitochondrial antioxidant system and reduces peel browning in ‘Nanguo’ pears after removal from cold storage. Food Chem. 2019, 297, 124903. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver Nanoparticle-Mediated Enhancement in Growth and Antioxidant Status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef]
- Li, J.; Chang, P.; Huang, J.; Wang, Y.; Yuan, H.; Ren, H. Physiological Effects of Magnetic Iron Oxide Nanoparticles Towards Watermelon. J. Nanosci. Nanotechnol. 2013, 13, 5561–5567. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Canas, J.E.; Long, M.; Nations, S.; Vadan, R.; Dai, L.; Luo, M.; Ambikapathi, R.; Lee, E.H.; Olszyk, D. Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species. Environ. Toxicol. Chem. 2008, 27, 1922–1931. [Google Scholar] [CrossRef]
- Pavani, K.V.; Divya, V.; Veena, I.; Aditya, M.; Devakinandan, G.V.S. Influence of bioengineered zinc nanoparticles and zinc metal on cicer arietinum seedlings growth. Asian J. Agri. Biol. 2014, 2, 216–223. [Google Scholar]
- Lee, W.M.; An, Y.J.; Yoon, H.; Kweon, H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants Mung Bean (Phaseolus radiatus) and Wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2008, 27, 1915–1921. [Google Scholar] [CrossRef]
- Ma, Y.; Kuang, L.; He, X.; Bai, W.; Ding, Y.; Zhang, Z.; Zhao, Y.; Chai, Z. Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 2010, 78, 273–279. [Google Scholar] [CrossRef]
- Cui, D.; Zhang, P.; Ma, Y.; He, X.; Li, Y.; Zhang, J.; Zhao, Y.; Zhang, Z. Effect of cerium oxide nanoparticles on asparagus lettuce cultured in an agar medium. Environ. Sci.-Nano 2014, 1, 459–465. [Google Scholar] [CrossRef]
- Ren, H.; Liu, L.; Liu, C.; He, S.; Huang, J.; Li, J.; Zhang, Y.; Huang, X.; Gu, N. Physiological Investigation of Magnetic Iron Oxide Nanoparticles Towards Chinese Mung Bean. J. Biomed. Nanotechnol. 2011, 7, 677–684. [Google Scholar] [CrossRef]
- Jiang, Y.; Hua, Z.; Zhao, Y.; Liu, Q.; Wang, F.; Zhang, Q. The Effect of Carbon Nanotubes on Rice Seed Germination and Root Growth. In Proceedings of the 2012 International Conference on Applied Biotechnology (ICAB 2012); Zhang, T.-C., Ouyang, P., Kaplan, S., Skarnes, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1207–1212. [Google Scholar]
- Hawrylak-Nowak, B.; Matraszek, R.; Szymańska, M. Selenium Modifies the Effect of Short-Term Chilling Stress on Cucumber Plants. Biol. Trace Elem. Res. 2010, 138, 307–315. [Google Scholar] [CrossRef]
- Răcuciu, M.; Creanga, D.E. TMA-OH coated magnetic nanoparticles internalized in vegetal tissue. Rom. J. Phys 2007, 52, 395–402. [Google Scholar]
- Song, G.; Gao, Y.; Wu, H.; Hou, W.; Zhang, C.; Ma, H. Physiological effect of anatase TiO2 nanoparticles on Lemna minor. Environ. Toxicol. Chem. 2012, 31, 2147–2152. [Google Scholar] [CrossRef]
- Alidoust, D.; Isoda, A. Effect of γFe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): Foliar spray versus soil amendment. Acta Physiol. Plant. 2013, 35, 3365–3375. [Google Scholar] [CrossRef]
- Hasanpour, H.; Maali-Amir, R.; Zeinali, H. Effect of TiO2 nanoparticles on metabolic limitations to photosynthesis under cold in chickpea. Russ. J. Plant Physiol. 2015, 62, 779–787. [Google Scholar] [CrossRef]
- Nekrasova, G.F.; Ushakova, O.S.; Ermakov, A.E.; Uimin, M.A.; Byzov, I.V. Effects of copper(II) ions and copper oxide nanoparticles on Elodea densa Planch. Russ. J. Ecol. 2011, 42, 458–463. [Google Scholar] [CrossRef]
- Spreitzer, R.J. Questions about the complexity of chloroplast ribulose-1,5-bisphosphate carboxylase/oxygenase. Photosynth. Res. Int. J. 1999, 60, 29–42. [Google Scholar] [CrossRef]
- Song, Y.; Wynn, J.P.; Li, Y.; Grantham, D.; Ratledge, C. A pre-genetic study of the isoforms of malic enzyme associated with lipid accumulation in Mucorcircinelloides. Microbiology 2001, 147, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Cao, Z.; Wang, Z.; Chen, L.; Zhu, Y.; Zhu, G. Identification and biochemical characterization of a thermostable malate dehydrogenase from mesophile Streptomyces coelicolor A3(2). Biosci. Biotechnol. Biochem. 2010, 74, 2194–2201. [Google Scholar] [CrossRef]
- Gietl, C. Malate dehydrogenase isoenzymes: Cellular locations and role in the flow of metabolites between the cytoplasm and cell organelles. Biochim. Biophys. Acta (BBA)–Bioenerg. 1992, 1100, 217–234. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W. In Biochemistry and Molecular Biology of Plants. Am. Soc. Plant Physiol. 2000, 150. [Google Scholar]
- Nirmaljit, K.; Mukhopadhyaya. Nanotechnology in Soil-Plant System. Plant Nanotechnol. 2016, 329–348. [Google Scholar] [CrossRef]
- Shabbaj, I.I.; Madany, M.M.Y.; Tammar, A.; Balkhyour, M.A.; Abdelgawad, H. Silicon dioxide nanoparticles orchestrate carbon and nitrogen metabolism in pea seedlings to cope with broomrape infection. Environ. Sci. Nano 2021, 8, 1960–1977. [Google Scholar] [CrossRef]
- Li, G.; Chen, R.; Liu, H.; Song, S.; Sun, G. Effects of Nano-Devices on Growth, Quality and Activities of Enzymes in Nitrogen Metabolism of Hydroponic Lettuce. In Key Engineering Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2014; Volume 609–610, pp. 1453–1458. [Google Scholar]
- Yaoyao, W.; Li, M.; Guo, Z.; Ullah, S.; Rui, Y.; Lynch, I. Alleviation of Nitrogen Stress in Rice (Oryza sativa) by Ceria Nanoparticles. Environ. Sci. Nano 2020, 7, 2930–2940. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H.; Singh, S. Plant-nanoparticle interaction: An approach to improve agricultural practices and plant productivity. Int. J. Pharm. Sci. Invent. (IJPSI) 2015, 4, 2319–6718. [Google Scholar]
- Hu, Y.; Zhang, P.; Zhang, X.; Liu, Y.; Feng, S.; Guo, D.; Nadezhda, T.; Song, Z.; Dang, X. Multi-Wall Carbon Nanotubes Promote the Growth of Maize (Zea mays) by Regulating Carbon and Nitrogen Metabolism in Leaves. J. Agric. Food Chem. 2021, 69, 4981–4991. [Google Scholar] [CrossRef] [PubMed]
- Joghatay, A.; Afshari, H.; Masoud, S.J. Effect of Nano Iron and Solupotasse Fertilizers on Accumulation of Nutrient Elements and Quality of Two Onion (Allium cepa) Cultivars. J. Crop Ecophysiol. 2015, 9, 491–502. [Google Scholar]
- Li, L.; Gu, W.; Li, J.; Li, C.; Xie, T.; Qu, D.; Meng, Y.; Li, C.; Wei, S. Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol. Biochem. 2018, 129, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Nayyar, H.; Bains, T.S.; Kumar, S. Chilling stressed chickpea seedlings: Effect of cold acclimation, calcium and abscisic acid on cryoprotective solutes and oxidative damage. Environ. Exp. Bot. 2005, 54, 275–285. [Google Scholar] [CrossRef]
- Orozco-Cardenas, M.; Ryan, C.A. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 6553–6557. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Rodriguez-Serrano, M.; Corpas, F.J.; Gomez, M.; Del Rio, L.A.; Sandalio, L.M. Cadmium-induced subcellular accumulation of O2.- and H2O2 in pea leaves. Plant Cell Environ. 2004, 27, 1122–1134. [Google Scholar] [CrossRef]
- Johnson, H.S.; Hatch, M.D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and ‘malic’ enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem. J. 1970, 119, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Sayre, R.T.; Kennedy, R.A. Photosynthetic Enzyme Activities and Localization in Mollugo verticillata Populations Differing in the Levels of C(3) and C(4) Cycle Operation. Plant Physiol. 1979, 64, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.H.; Iglesias, A.A.; Andreo, C.S. On the Regulation of Phosphoenolpyruvate Carboxylase Activity from Maize Leaves by L-malate. Effect of pH. J. Plant Physiol. 1984, 116, 425–434. [Google Scholar] [CrossRef]
- Hatch, M.; Slack, C.R. Pyruvate, P1 dikinase from leaves. Methods Enzymol. 1975, 42, 212–219. [Google Scholar] [CrossRef]
- Meng, D. Glycolysis Metabolic Mechanism Response Exogenous Salicylic Acid under Low-Temperature of Winter Wheat. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2017. [Google Scholar]
- Li, C.; Ma, F.; Zhao, Y.; Li, W. Effects of Nitrogen Forms on Key Enzyme Activities and Related Products in Sugar and Nitrogen Metabolism of Sugar Beet (Beta vulgaris L.). Acta Agron. Sin. 2003, 29, 128–132. [Google Scholar]
- Miflin, B.J.; Lea, P.J. Amino Acid Metabolism. Annu. Rev. Plant Physiol. 1977, 28, 299–329. [Google Scholar] [CrossRef]
- Zheng, C.; Lin, Z. Rapid determination of glutamate synthase activity. Plant Physiol. Commun. 1985, 4, 43–46. [Google Scholar] [CrossRef]
- Wang, L. The Molecular Physiology and Regulatory Network of Mangrove Kandelia candel in Response to Salt Stress. Ph.D. Thesis, Fujian Agricultural and Forestry University, Fuzhou, China, 2015. [Google Scholar]





| Nanomaterials | Concentrations (mg·L−1) | Seedling Height (cm) | Seedling Fresh Weight (g) | Root Fresh Weight (g) | Root–Shoot Ratio | Root Dry Weight (g) | Shoot Dry Weight (g) |
|---|---|---|---|---|---|---|---|
| MWCNTs | 0 | 32.113 ± 1.169 c | 8.017 ± 0.803 c | 2.321 ± 0.348 c | 0.286 ± 0.016 b | 0.527 ± 0.065 b | 0.081 ± 0.010 c |
| 200 | 35.673 ± 1.116 b | 9.264 ± 0.625 bc | 3.049 ± 0.297 bc | 0.328 ± 0.011 a | 0.607 ± 0.024 ab | 0.104 ± 0.012 bc | |
| 400 | 42.850 ± 0.853 a | 10.076 ± 0.301 ab | 3.389 ± 0.166 ab | 0.336 ± 0.007 a | 0.651 ± 0.015 a | 0.123 ± 0.011 ab | |
| 800 | 45.670 ± 0.616 a | 11.209 ± 0.374 a | 3.933 ± 0.157 a | 0.351 ± 0.002 a | 0.702 ± 0.014 a | 0.154 ± 0.009 a | |
| 1200 | 37.487 ± 1.129 b | 9.782 ± 0.375 abc | 3.265 ± 0.206 ab | 0.333 ± 0.011 a | 0.625 ± 0.021 ab | 0.113 ± 0.009 bc | |
| Nano-SiO2 | 0 | 32.113 ± 1.169 d | 8.017 ± 0.803 b | 2.321 ± 0.348 c | 0.286 ± 0.016 c | 0.527 ± 0.065 b | 0.081 ± 0.010 b |
| 150 | 37.020 ± 0.777 c | 9.316 ± 0.625 b | 2.782 ± 0.206 ab | 0.298 ± 0.011 bc | 0.621 ± 0.032 ab | 0.109 ± 0.012 ab | |
| 800 | 42.590 ± 1.187 b | 10.051 ± 0.301 ab | 3.227 ± 0.142 ab | 0.321 ± 0.007 ab | 0.683 ± 0.030 a | 0.128 ± 0.007 a | |
| 1500 | 47.123 ± 0.845 a | 11.491 ± 0.374 a | 3.778 ± 0.158 a | 0.329 ± 0.002 a | 0.756 ± 0.025 a | 0.144 ± 0.012 a | |
| 2500 | 39.223 ± 0.908 bc | 9.529 ± 0.295 ab | 2.918 ± 0.194 bc | 0.306 ± 0.011 abc | 0.631 ± 0.032 ab | 0.117 ± 0.011 ab |
| Nanomaterials | Concentrations (mg·L−1) | Root Length (cm) | Root Surface Area (cm2) | Average Diameter of the Root (mm) | Root Volume (cm3) | Number of Root Tips |
|---|---|---|---|---|---|---|
| MWCNTs | 0 | 11.19 ± 0.64 c | 3.69 ± 0.36 b | 1.17 ± 0.17 abc | 0.12 ± 0.02 b | 60.33 ± 8.95 c |
| 200 | 11.48 ± 0.66 c | 3.91 ± 0.38 b | 1.48 ± 0.21 a | 0.14 ± 0.02 b | 101.67 ± 11.46 c | |
| 400 | 18.59 ± 0.61 b | 4.81 ± 0.32 ab | 0.86 ± 0.05 bc | 0.18 ± 0.02 ab | 468.33 ± 42.44 b | |
| 800 | 22.16 ± 0.92 a | 5.78 ± 0.19 a | 0.71 ± 0.06 c | 0.22 ± 0.02 a | 810.67 ± 35.48 a | |
| 1200 | 13.11 ± 0.60 c | 4.36 ± 0.37 b | 1.30 ± 0.24 ab | 0.15 ± 0.03 ab | 129.67 ± 14.68 c | |
| nano-SiO2 | 0 | 11.19 ± 0.64 d | 3.69 ± 0.36 c | 1.17 ± 0.17 a | 0.12 ± 0.02 b | 60.33 ± 8.95 c |
| 150 | 13.50 ± 0.73 cd | 4.20 ± 0.51 bc | 1.24 ± 0.15 a | 0.12 ± 0.02 b | 124.67 ± 26.89 bc | |
| 800 | 15.78 ± 0.68 bc | 4.95 ± 0.22 b | 0.77 ± 0.09 ab | 0.18 ± 0.01 ab | 176.00 ± 39.72 b | |
| 1500 | 25.29 ± 1.50 a | 6.20 ± 0.35 a | 0.67 ± 0.06 b | 0.23 ± 0.02 a | 785.33 ± 15.93 a | |
| 2500 | 17.66 ± 0.73 b | 5.30 ± 0.23 ab | 1.10 ± 0.15 ab | 0.13 ± 0.02 b | 146.00 ± 23.80 bc |
| Name | CAS Number | Diameter (nm) | Purity (wt%) | Specific Surface Area (m2·g−1) | Appearance |
|---|---|---|---|---|---|
| MWCNTs | 308068-56-6 | 20–30 | >98.0 | >233 |  |
| nano-SiO2 | 60676-86-0 | 10–20 | >99.8 | >200 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Yu, Y.; Sun, G.; Gong, X.; Jiang, Y.; Lv, G.; Zhang, Y.; Li, L.; Zhao, Y.; Sun, D.; et al. Effects of Multi-Walled Carbon Nanotubes and Nano-Silica on Root Development, Leaf Photosynthesis, Active Oxygen and Nitrogen Metabolism in Maize. Plants 2023, 12, 1604. https://doi.org/10.3390/plants12081604
Hao Y, Yu Y, Sun G, Gong X, Jiang Y, Lv G, Zhang Y, Li L, Zhao Y, Sun D, et al. Effects of Multi-Walled Carbon Nanotubes and Nano-Silica on Root Development, Leaf Photosynthesis, Active Oxygen and Nitrogen Metabolism in Maize. Plants. 2023; 12(8):1604. https://doi.org/10.3390/plants12081604
Chicago/Turabian StyleHao, Yubo, Yang Yu, Guangyan Sun, Xiujie Gong, Yubo Jiang, Guoyi Lv, Yiteng Zhang, Liang Li, Yang Zhao, Dan Sun, and et al. 2023. "Effects of Multi-Walled Carbon Nanotubes and Nano-Silica on Root Development, Leaf Photosynthesis, Active Oxygen and Nitrogen Metabolism in Maize" Plants 12, no. 8: 1604. https://doi.org/10.3390/plants12081604
APA StyleHao, Y., Yu, Y., Sun, G., Gong, X., Jiang, Y., Lv, G., Zhang, Y., Li, L., Zhao, Y., Sun, D., Gu, W., & Qian, C. (2023). Effects of Multi-Walled Carbon Nanotubes and Nano-Silica on Root Development, Leaf Photosynthesis, Active Oxygen and Nitrogen Metabolism in Maize. Plants, 12(8), 1604. https://doi.org/10.3390/plants12081604







