Dynamics of Endogenous Auxin and Its Role in Somatic Embryogenesis Induction and Progression in Cork Oak
Abstract
1. Introduction
2. Results
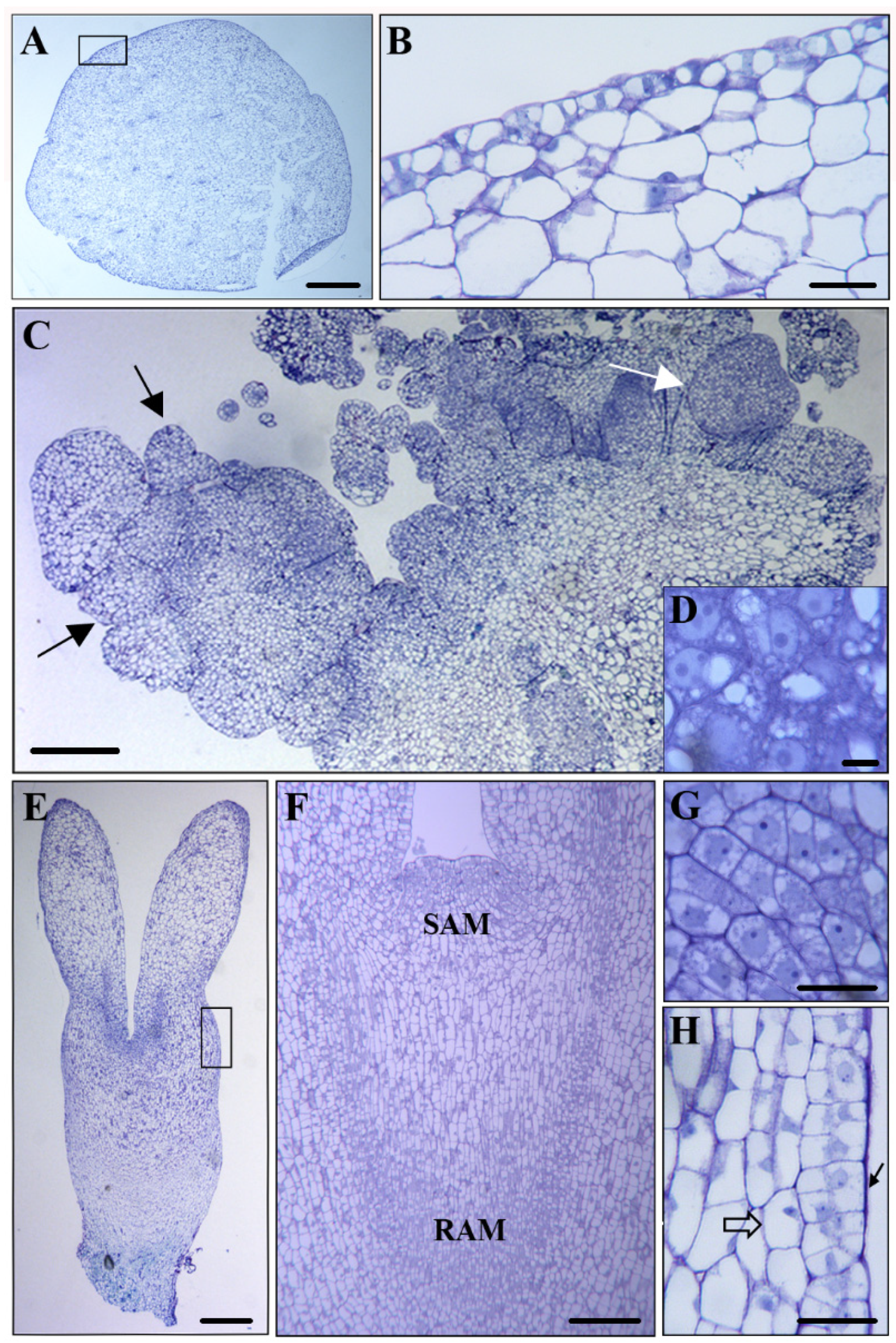
2.1. Auxin Localization and Accumulation during SE
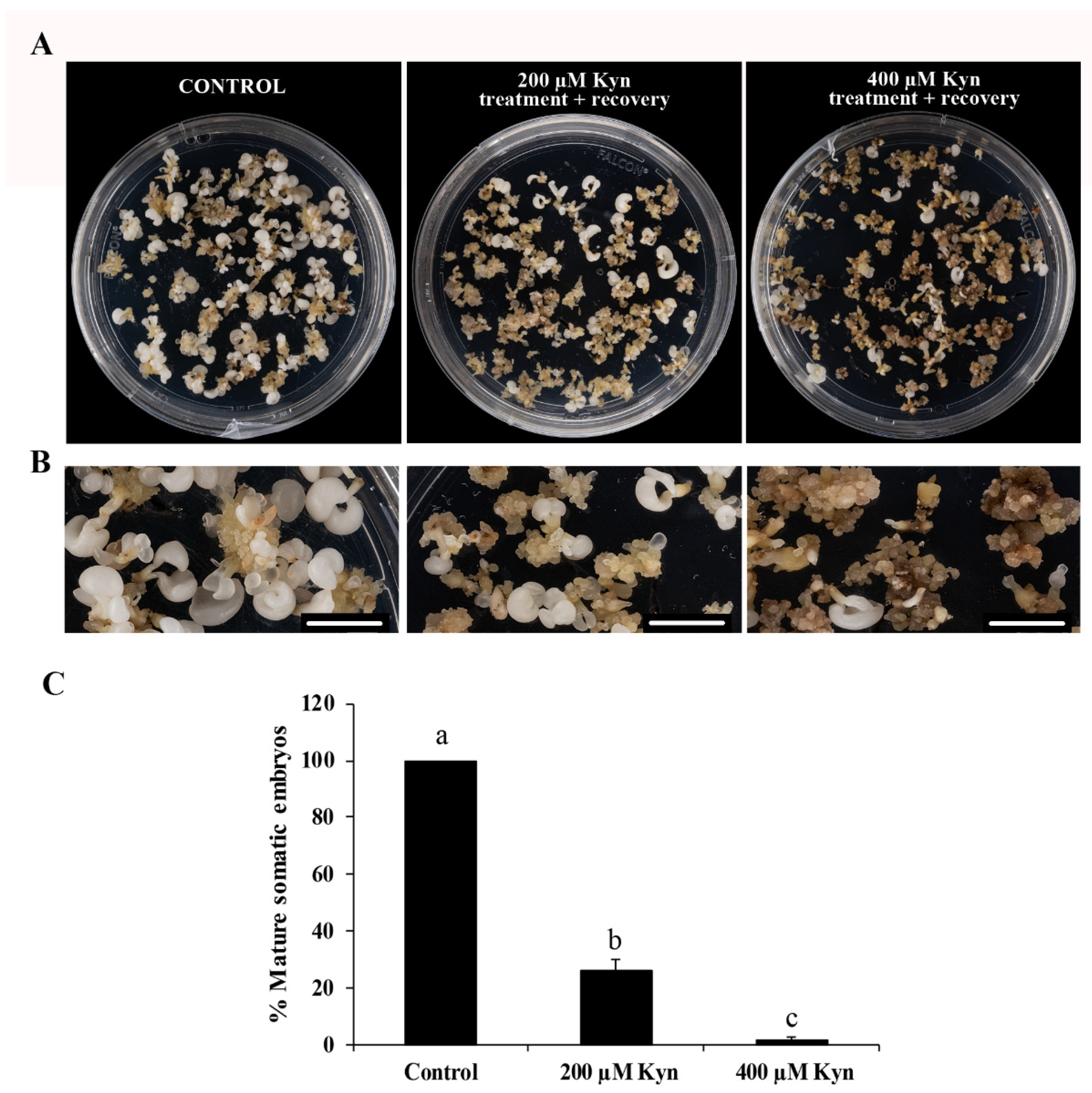
2.2. Effect of the Inhibitor of Auxin Biosynthesis Kynurenine on SE
2.3. Expression of Auxin Biosynthesis and Signaling Genes QsTAR2 and QsARF5 during SE and after Treatment with the Inhibitor of Auxin Biosynthesis
3. Discussion
3.1. Endogenous Auxin Accumulation Accompanies SE Induction and Embryo Development
3.2. Endogenous Auxin Biosynthesis Is Needed for Proliferation of Embryogenic Cells and SE Progression
3.3. Activation of Auxin Biosynthesis and Signaling Genes Are Required for SE Induction, Proliferation, and Progression
4. Materials and Methods
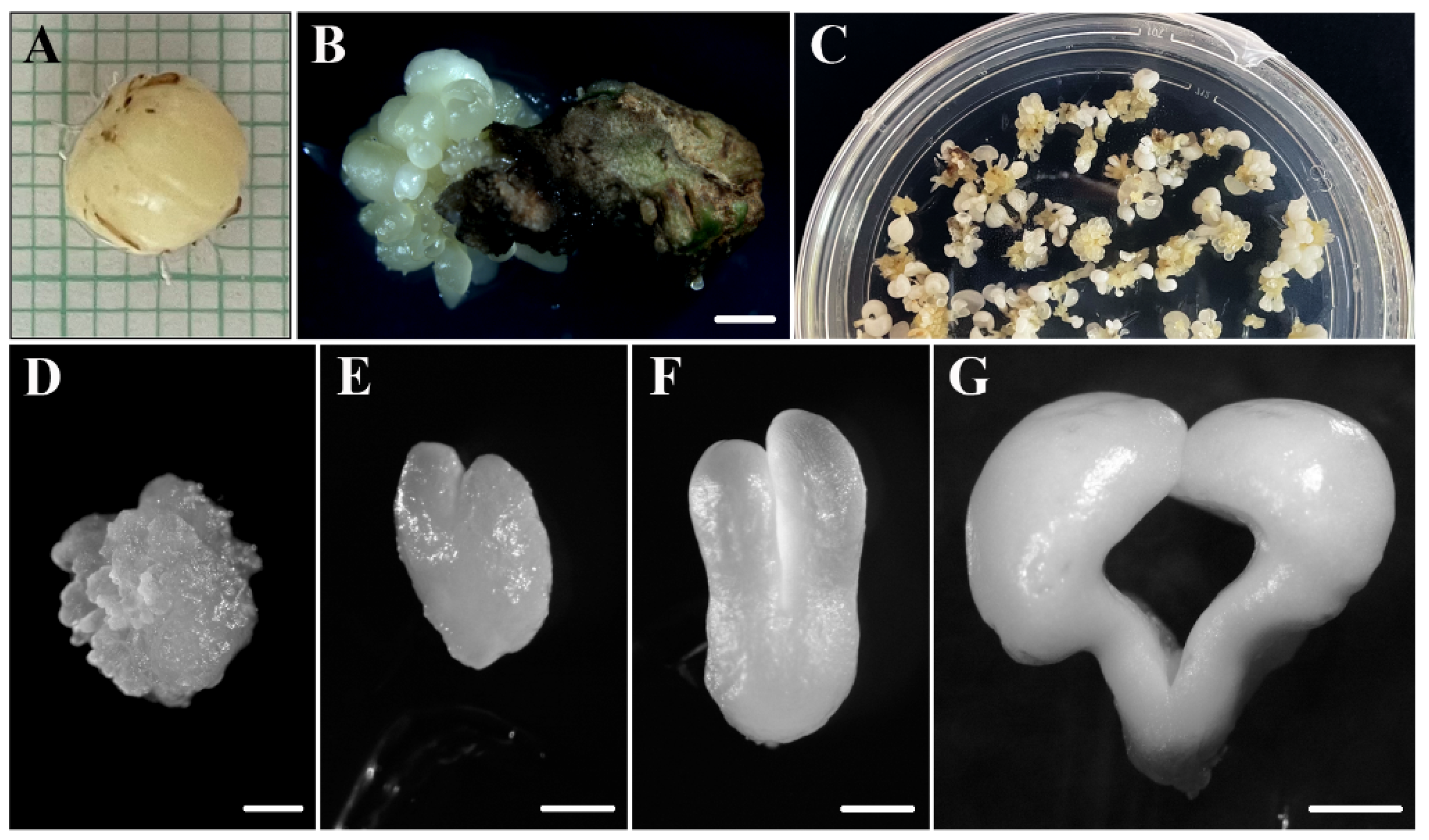
4.1. Somatic Embryogenesis Cultures
4.2. Sample Fixation and Processing for Microscopy
4.3. Immunofluorescence and Laser Confocal Microscopy Analysis
4.4. Treatment with the Inhibitor of Auxin Biosynthesis Kynurenine
4.5. Gene Expression Analysis by RT-qPCR
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Germanà, M.A.; Chiancone, B.; Padoan, D.; Bárány, I.; Risueño, M.C.; Testillano, P.S. First stages of microspore reprogramming to embryogenesis through anther culture in Prunus armeniaca L. Environ. Exp. Bot. 2011, 71, 152–157. [Google Scholar] [CrossRef]
- Guan, Y.; Li, S.G.; Fan, X.F.; Su, Z.H. Application of somatic embryogenesis in woody plants. Front. Plant Sci. 2016, 7, 938. [Google Scholar] [CrossRef] [PubMed]
- Germanà, M.A.; Lambardi, M. In Vitro Embryogenesis in Higher Plants; Humana Press: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. (Eds.) Plant Cell Culture Protocols; Humana Press: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Mohan Jain, S.; Gupta, P. (Eds.) Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants; Springer International Publishing: Berlin, Germany, 2018. [Google Scholar]
- von Arnold, S.; Sabala, I.; Bozhkov, P.; Dyachok, J.; Filonova, L.H. Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult. 2002, 69, 233–249. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Trontin, J.F.; Becwar, M.R.; Devillard, C.; Park, Y.S.; Lelu-Walter, M.A. Recent progress in somatic embryogenesis of four Pinus spp. Tree For. Sci. Biotechnol. 2007, 1, 11–25. [Google Scholar]
- Walter, C.; Find, J.I.; Grace, L.J. Somatic Embryogenesis and Genetic Transformation in Pinus radiata. In Protocol for Somatic Embryogenesis in Woody Plants. Forestry Sciences; Jain, S.M., Gupta, P.K., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 77. [Google Scholar] [CrossRef]
- Gao, C. Genome engineering for crop improvement and future agriculture. Cell 2021, 184, 1621–1635. [Google Scholar] [CrossRef]
- Bueno, M.A.; Astorga, R.; Manzanera, J.A. Plant regeneration through somatic embryogenesis in Quercus suber L. Physiol. Plant. 1992, 85, 30–34. [Google Scholar] [CrossRef]
- Testillano, P.S.; Gómez-Garay, A.; Pintos, B.; Risueño, M.C. Somatic Embryogenesis from Immature Zygotic Embryos in Quercus suber L. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Humana Press: New York, NY, USA, 2018. [Google Scholar]
- Hernández, I.; Celestino, C.; Toribio, M. Vegetative propagation of Quercus suber L. by somatic embryogenesis. Factors affecting the induction in leaves from mature cork oak trees. Plant Cell Rep. 2003, 21, 759–764. [Google Scholar] [CrossRef]
- Lelu-Walter, M.A.; Thompson, D.; Harvengt, L.; Sanchez, L.; Toribio, M.; Pâques, L.E. Somatic embryogenesis in forestry with a focus on Europe: State-of-the-art, benefits, challenges and future direction. Tree Genet. Genomes 2013, 9, 883–899. [Google Scholar] [CrossRef]
- Ballester, A.; Corredoira, E.; Vieitez, A.M. Limitations of Somatic Embryogenesis in Hardwood Trees. In Vegetative Propagation of Forest Trees; Park, Y.-S., Bonga, J.M., Moon, H.-K., Eds.; National Institute of Forest Science (Nifos): Seoul, Republic of Korea, 2016; pp. 56–74. [Google Scholar]
- Bonga, J.M. Can explant choice help resolve recalcitrance problems in in vitro propagation, a problem still acute especially for adult conifers? Trees 2017, 31, 781–789. [Google Scholar] [CrossRef]
- Feher, A. Somatic embryogenesis—stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta 2015, 1849, 385–402. [Google Scholar] [CrossRef]
- Díaz-Sala, C. Molecular dissection of the regenerative capacity of forest tree species: Special focus on conifers. Front. Plant. Sci. 2018, 9, 1943. [Google Scholar] [CrossRef]
- Ibáñez, S.; Carneros, E.; Testillano, P.S.; Pérez-Pérez, J.M. Advances in plant regeneration: Shake, rattle and roll. Plants 2020, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Testillano, P.S. Microspore embryogenesis: Targeting the determinant factors of stress induced cell reprogramming for crop improvement. J. Exp. Bot. 2019, 70, 2965–2978. [Google Scholar] [CrossRef] [PubMed]
- Solís, M.T.; Pintos, B.; Prado, M.J.; Bueno, M.A.; Raska, I.; Risueño, M.C.; Testillano, P.S. Early markers of in vitro microspore reprogramming to embryogenesis in olive (Olea europaea L.). Plant Sci. 2008, 174, 597–605. [Google Scholar] [CrossRef]
- Germanà, M.A. Anther culture for haploid and doubled haploid production. Plant Cell Tissue Organ Cult. 2011, 104, 283–300. [Google Scholar] [CrossRef]
- Enders, T.A.; Strader, L.C. Auxin activity: Past, present, and future. Am. J. Bot. 2015, 102, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Paque, S.; Weijers, D. Q&A: Auxin: The plant molecule that influences almost anything. BMC Biol. 2016, 14, 67. [Google Scholar]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signaling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Grones, P.; Friml, J. Auxin transporters and binding proteins at a glance. J. Cell Sci. 2015, 128, 1–7. [Google Scholar] [CrossRef]
- Weijers, D.; Nemhauser, J.; Yang, Z. Auxin: Small molecule, big impact. J. Exp. Bot. 2018, 69, 133–136. [Google Scholar] [CrossRef]
- Chen, D.; Ren, Y.; Deng, Y.; Zhao, J. Auxin polar transport is essential for the development of zygote and embryo in Nicotiana tabacum L. and correlated with ABP1 and PM H+-ATPase activities. J. Exp. Bot. 2010, 61, 1853–1867. [Google Scholar] [CrossRef]
- Möller, B.; Weijers, D. Auxin control of embryo patterning. Cold Spring Harb. Perspect. Biol. 2009, 1, a001545. [Google Scholar] [CrossRef]
- Rademacher, E.H.; Lokerse, A.S.; Schlereth, A.; Llavata-Peris, C.I.; Bayer, M.; Kientz, M.; FreireRios, A.; Borst, J.W.; Lukowitz, W.; Jürgens, G.; et al. Different auxin response machineries control distinct cell fates in the early plant embryo. Dev. Cell 2012, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin Biosynthesis. Arabidopsis Book 2014, 12, e0173. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Ma, W.; Zhao, X.; Hu, M.; He, X.; Teng, W.; Li, H.; Tong, Y. The auxin biosynthetic TRYPTOPHAN AMINOTRANSFERASE RELATED TaTAR2.1-3A increases grain yield of wheat. Plant Physiol. 2017, 174, 2274–2288. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, A.N.; Yun, J.; Robles, L.M.; Novak, O.; He, W.; Guo, H.; Ljung, K.; Alonso, J.M. The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 2011, 23, 3961–3973. [Google Scholar] [CrossRef]
- Brumos, J.; Alonso, J.M.; Stepanova, A.N. Genetic aspects of auxin biosynthesis and its regulation. Physiol. Plant. 2014, 151, 3–12. [Google Scholar] [CrossRef]
- Karami, O.; Philipsen, C.; Rahimi, A.; Nurillah, A.R.; Boutilier, K.; Offringa, R. Endogenous auxin maintains embryonic cell identity and promotes somatic embryo development in Arabidopsis. Plant J. 2023, 113, 7–22. [Google Scholar] [CrossRef]
- Lavy, M.; Estelle, M. Mechanisms of auxin signaling. Development 2016, 143, 3226–3229. [Google Scholar] [CrossRef]
- Weijers, D.; Wagner, D. Transcriptional Responses to the Auxin Hormone. Annu. Rev. Plant Biol. 2016, 67, 539–574. [Google Scholar] [CrossRef]
- Rademacher, E.H.; Möller, B.; Lokerse, A.S.; Llavata-Peris, C.I.; van Den Berg, W.; Weijers, D. A cellular expression map of the Arabidopsis AUXIN RESPONSE FACTOR gene family. Plant J. 2011, 68, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Wójcikowska, B.; Gaj, M.D. Expression profiling of AUXIN RESPONSE FACTOR genes during somatic embryogenesis induction in Arabidopsis. Plant Cell Rep. 2017, 36, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Nic-Can, G.I.; Loyola-Vargas, V.M. The Role of the Auxins during Somatic Embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 171–182. [Google Scholar]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-La-Peña, C.; Loyola-Vargas, V.M. Signaling overview of plant somatic embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef]
- Prem, D.; Solís, M.T.; Bárány, I.; Rodríguez-Sanz, H.; Risueño, M.C.; Testillano, P.S. A new microspore embryogenesis system under low temperature which mimics zygotic embryogenesis initials, expresses auxin and efficiently regenerates doubled-haploid plants in Brassica napus. BMC Plant Biol. 2012, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sanz, H.; Solís, M.; López, M.; Gómez-Cadenas, A.; Risueño, M.; Testillano, P.S. Auxin biosynthesis, accumulation, action and transport are involved in stress-induced microspore embryogenesis initiation and progression in Brassica napus. Plant Cell Physiol. 2015, 56, 1401–1417. [Google Scholar] [CrossRef]
- Pérez-Pérez, Y.; El-Tantawy, A.-A.; Solís, M.T.; Risueño, M.C.; Testillano, P.S. Stress-induced microspore embryogenesis requires endogenous auxin synthesis and polar transport in barley. Front. Plant Sci. 2019, 10, 1200. [Google Scholar] [CrossRef]
- Hakman, I.; Hallberg, H.; Palovaara, J. The polar auxin transport inhibitor NPA impairs morphology and increases the expression of an auxin efflux facilitator protein PIN during Picea abies somatic embryo development. Tree Physiol. 2009, 29, 483–496. [Google Scholar] [CrossRef]
- Vondráková, Z.; Eliášová, K.; Fischerová, L.; Vágner, M. The role of auxins in somatic embryogenesis of Abies alba. Cent. Eur. J. Biol. 2011, 4, 587–596. [Google Scholar] [CrossRef]
- Corredoira, E.; Cano, V.; Bárány, I.; Solís, M.T.; Rodríguez, H.; Vieitez, A.M.; Risueño, M.C.; Testillano, P.S. Initiation of leaf somatic embryogenesis involves high pectin esterification, auxin accumulation and DNA demethylation in Quercus alba. J. Plant Physiol. 2017, 213, 42–54. [Google Scholar] [CrossRef]
- García-Martin, G.; Manzanera, J.A.; González-Benito, M.E. Effect of exogenous ABA on embryo maturation and quantification of endogenous levels of ABA and IAA in Quercus suber somatic embryos. Plant Cell Tissue Organ Cult. 2005, 80, 171–177. [Google Scholar] [CrossRef]
- Rodríguez-Sanz, H.; Manzanera, J.A.; Solís, M.T.; Gómez-Garay, A.; Pintos, B.; Risueño, M.C.; Testillano, P.S. Early markers are present in both embryogenesis pathways from microspores and immature zygotic embryos in cork oak, Quercus suber L. BMC Plant Biol. 2014, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.; Sitbon, F.; Ljung, K.; von Arnold, S. Inhibited polar auxin transport results in aberrant embryo development in Norway spruce. New Phytol. 2007, 177, 356–366. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Brumos, J.; Li, H.; Ji, Y.; Ke, M.; Gong, X.; Zeng, Q.; Li, W.; Zhang, X.; An, F. A small-molecule screen identifies L-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 2011, 23, 3944–3960. [Google Scholar] [CrossRef]
- de Wit, M.; Ljung, K.; Fankhauser, C. Contrasting growth responses in lamina and petiole during neighbor detection depend on differential auxin responsiveness rather than different auxin levels. New Phytol. 2015, 208, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Itouga, M.; Kojima, M.; Kato, Y.; Sakakibara, H.; Hasezawa, S. Copper mediates auxin signaling to control cell differentiation in the copper moss Scopelophila cataractae. J. Exp. Bot. 2015, 66, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Roosjen, M.; Paque, S.; Weijers, D. Auxin Response Factors: Output control in auxin biology. J. Exp. Bot. 2018, 69, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Elhiti, M.; Tahir, M.; Gulden, R.H.; Khamiss, K.; Stasolla, C. Modulation of embryo-forming capacity in culture through the expression of Brassica genes involved in the regulation of the shoot apical meristem. J. Exp. Bot. 2010, 61, 4069–4085. [Google Scholar] [CrossRef] [PubMed]
- San-José, M.C.; Corredoira, E.; Martínez, M.T.; Vidal, N.; Valladares, S.; Mallón, R.; Vieitez, A.M. Shoot apex explants for induction of somatic embryogenesis in mature Quercus robur L. trees. Plant Cell Rep. 2010, 29, 661–671. [Google Scholar] [CrossRef]
- Raza, G.; Singh, M.B.; Bhalla, P.L. Somatic Embryogenesis and Plant Regeneration from Commercial Soybean Cultivars. Plants 2019, 9, 38. [Google Scholar] [CrossRef]
- Márquez-López, R.E.; Pérez-Hernández, C.; Ku-González, A.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M. Localization and transport of indole-3- acetic acid during somatic embryogenesis in Coffea canephora. Protoplasma 2018, 255, 695–708. [Google Scholar] [CrossRef]
- Awada, R.; Campa, C.; Gibault, E.; Déchamp, E.; Georget, F.; Lepelley, M.; Abdallah, C.; Erban, A.; Martinez-Seidel, F.; Kopka, J.; et al. Unraveling the metabolic and hormonal machinery during key steps of somatic embryogenesis: A case study in coffee. Int. J. Mol. Sci. 2019, 20, 4665. [Google Scholar] [CrossRef] [PubMed]
- Careiro, A.; Careiro, S.; Correia, S.; Canhoto, J. Induction of somatic embryogenesis in Tamarillo (Solanum Betaceum Cav.) involves increases in the endogenous auxin indole-3-acetic acid. Plants 2022, 11, 1347. [Google Scholar] [CrossRef] [PubMed]
- Vondrakova, Z.; Dobrev, P.I.; Pesek, B.; Fischerova, L.; Vagner, M.; Motyka, V. Profiles of endogenous phytohormones over the course of Norway spruce somatic embryogenesis. Front. Plant Sci. 2018, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.K.; Liao, C.-K.; Ho, Y.L. Maturation of somatic embryos in two embryonic cultures of Picea morrisonicola Hayata as affected by alternation of endogenous IAA content. Plant Cell Tissue Organ Cult. 2008, 93, 257–268. [Google Scholar] [CrossRef]
- Stasolla, C.; Yeung, E.C. Recent advances in conifer somatic embryogenesis: Improving somatic embryo quality. Plant Cell Tissue Organ Cult. 2003, 74, 15–35. [Google Scholar] [CrossRef]
- Businge, E.; Brackmann, K.; Moritz, T.; Egertsdotter, U. Metabolite profiling reveals clear metabolic changes during somatic embryo development of Norway spruce (Picea abies). Tree Physiol. 2012, 32, 232–244. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Robertson-Hoyt, J.; Yun, J.J.; Benavente, L.M.; Xie, D.-Y.; Dolezal, K.; Schlereth, A.; Jürgens, G.; Alonso, J.M. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 2008, 133, 177–191. [Google Scholar] [CrossRef]
- Robert, S.H.; Grones, P.; Stepanova, A.N.; Robles, L.M.; Lokerse, A.S.; Alonso, J.M.; Weijers, D.; Friml, J. Local auxin sources orient the apical-basal axis in Arabidopsis embryos. Curr. Biol. 2013, 23, 2506–2512. [Google Scholar] [CrossRef]
- Robert, S.H.; Khaitova, L.C.; Mroue, S.; Benková, E. The importance of localized auxin production for morphogenesis of reproductive organs and embryos in Arabidopsis. J. Exp. Bot. 2015, 66, 5029–5042. [Google Scholar] [CrossRef]
- Wójcikowska, B.; Jaskóła, K.; Gasiorek, P.; Meus, M.; Nowak, K.; Gaj, M.D. LEAFY COTYLEDON2 (LEC2) promotes embryogenic induction in somatic tissues of Arabidopsis, via YUCCA-mediated auxin biosynthesis. Planta 2013, 238, 425–440. [Google Scholar] [CrossRef]
- Ayil-Gutiérrez, B.; Galaz-Avalos, R.M.; Peña-Cabrera, E.; Loyola-Vargas, V.M. Dynamics of the concentration of IAA and some of its conjugates during the induction of somatic embryogenesis in Coffea canephora. Plant Signal. Behav. 2013, 8, e26998. [Google Scholar] [CrossRef] [PubMed]
- Capote, T.; Usié, A.; Barbosa, P.; Ramos, M.; Morais-Celcílio, L.; Gonçalves, S. Transcriptome dynamics of cork oak (Quercus suber) somatic embryogenesis reveals active gene players in transcription regulation and phytohormone homeostasis of embryo development. Tree Genet. Genom. 2019, 15, 52. [Google Scholar] [CrossRef]
- Rozen, S.; Skaletsky, H.J. Primer3 on the www for General Users and for Biologist Programmers. In Bioinformatics Methods and Protocols; Krawetz, S., Misener, S., Eds.; Humana Press: Totowa, NJ, USA, 2000. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneros, E.; Sánchez-Muñoz, J.; Pérez-Pérez, Y.; Pintos, B.; Gómez-Garay, A.; Testillano, P.S. Dynamics of Endogenous Auxin and Its Role in Somatic Embryogenesis Induction and Progression in Cork Oak. Plants 2023, 12, 1542. https://doi.org/10.3390/plants12071542
Carneros E, Sánchez-Muñoz J, Pérez-Pérez Y, Pintos B, Gómez-Garay A, Testillano PS. Dynamics of Endogenous Auxin and Its Role in Somatic Embryogenesis Induction and Progression in Cork Oak. Plants. 2023; 12(7):1542. https://doi.org/10.3390/plants12071542
Chicago/Turabian StyleCarneros, Elena, Jorge Sánchez-Muñoz, Yolanda Pérez-Pérez, Beatriz Pintos, Aránzazu Gómez-Garay, and Pilar S. Testillano. 2023. "Dynamics of Endogenous Auxin and Its Role in Somatic Embryogenesis Induction and Progression in Cork Oak" Plants 12, no. 7: 1542. https://doi.org/10.3390/plants12071542
APA StyleCarneros, E., Sánchez-Muñoz, J., Pérez-Pérez, Y., Pintos, B., Gómez-Garay, A., & Testillano, P. S. (2023). Dynamics of Endogenous Auxin and Its Role in Somatic Embryogenesis Induction and Progression in Cork Oak. Plants, 12(7), 1542. https://doi.org/10.3390/plants12071542






