Response of Maize (Zea mays L.) to Drought under Salinity and Boron Stress in the Atacama Desert
Abstract
1. Introduction
2. Results
2.1. Meteorological and Soil Conditions
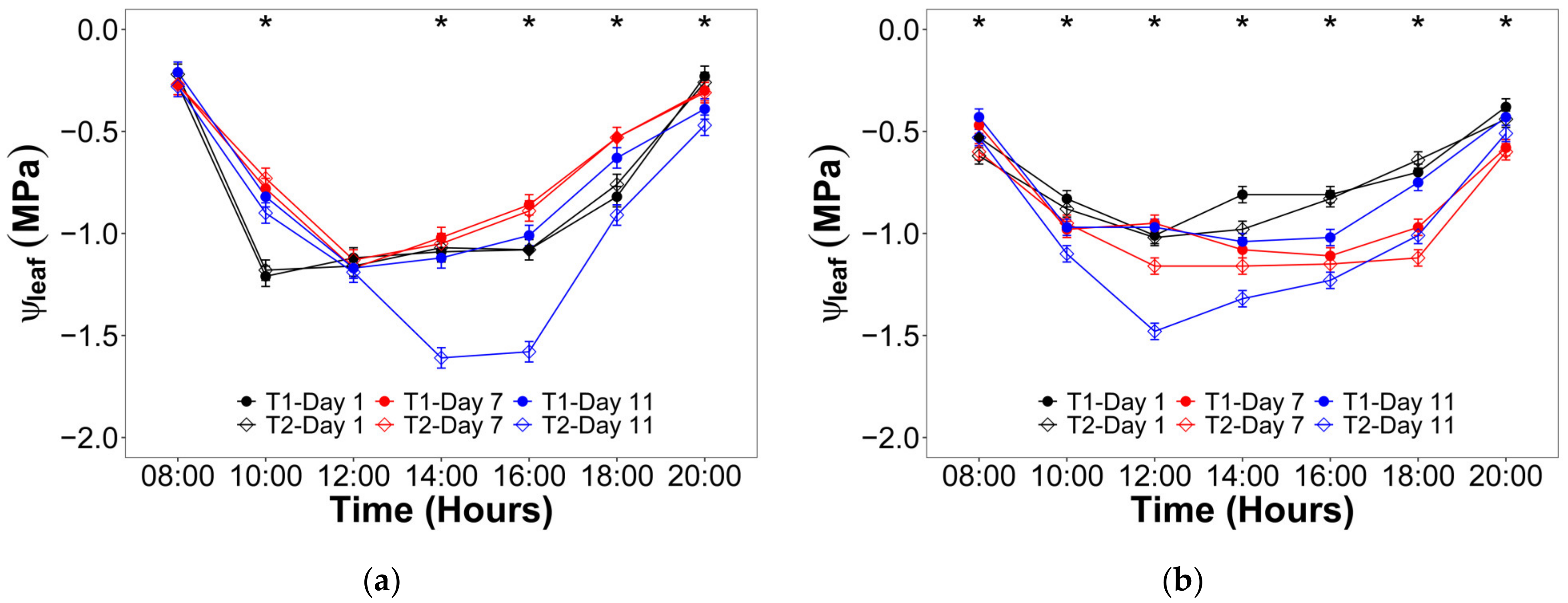
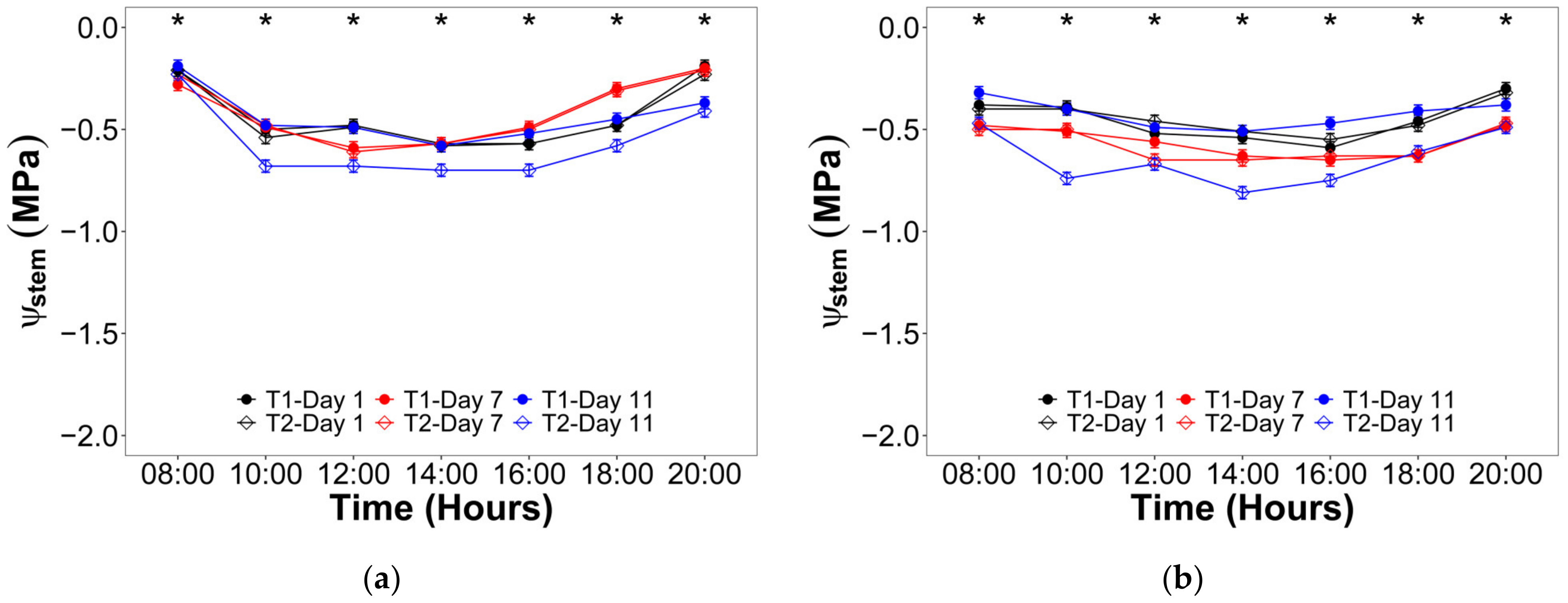
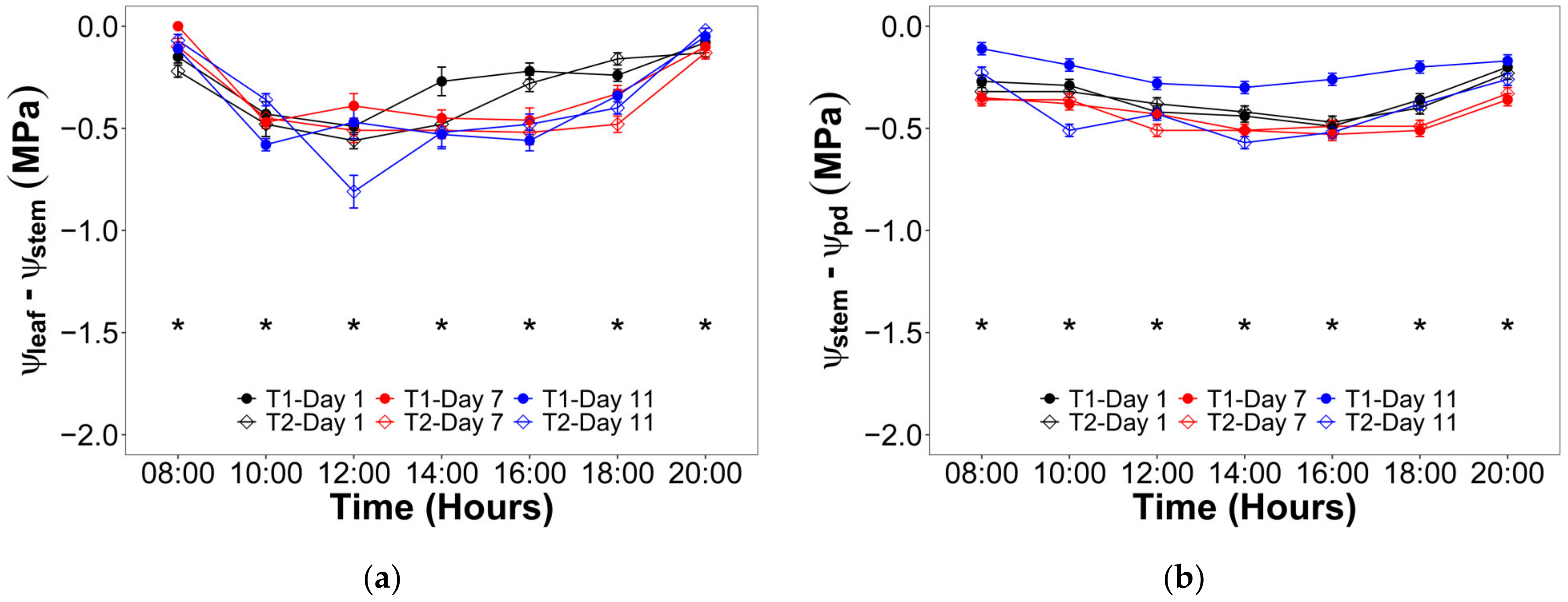
2.2. Water Status
2.3. Leaf Gas Exchange
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Measurements
4.2.1. Meteorological Measurements
4.2.2. Soil Water Content
4.2.3. Plant Water Status
4.2.4. Leaf Gas Exchange
4.2.5. Yield Response
4.3. Experimental Design
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nawaz, M.; Ishaq, S.; Ishaq, H.; Khan, N.; Iqbal, N.; Ali, S.; Rizwan, M.; Alsahli, A.A.; Alyemeni, M.N. Salicylic Acid Improves Boron Toxicity Tolerance by Modulating the Physio-Biochemical Characteristics of Maize (Zea mays L.) at an Early Growth Stage. Agronomy 2020, 10, 2013. [Google Scholar] [CrossRef]
- Kaya, C.; Akram, N.A.; Ashraf, M. Kinetin and Indole Acetic Acid Promote Antioxidant Defense System and Reduce Oxidative Stress in Maize (Zea mays L.) Plants Grown at Boron Toxicity. J. Plant Growth Regul. 2018, 37, 1258–1266. [Google Scholar] [CrossRef]
- Mamani-Huarcaya, B.M.; González-Fontes, A.; Navarro-Gochicoa, M.T.; Camacho-Cristóbal, J.J.; Ceacero, C.J.; Herrera-Rodríguez, M.B.; Cutire, Ó.F.; Rexach, J. Characterization of Two Peruvian Maize Landraces Differing in Boron Toxicity Tolerance. Plant Physiol. Biochem. 2022, 185, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Esim, N.; Tiryaki, D.; Karadagoglu, O.; Atici, O. Toxic Effects of Boron on Growth and Antioxidant System Parameters of Maize (Zea Mays L.) Roots. Toxicol. Ind. Health 2013, 29, 800–805. [Google Scholar] [CrossRef]
- Pandey, A.; Khan, M.K.; Hakki, E.E.; Gezgin, S.; Hamurcu, M. Combined Boron Toxicity and Salinity Stress—An Insight into Its Interaction in Plants. Plants 2019, 8, 364. [Google Scholar] [CrossRef]
- Naz, T.; Akhtar, J.; Iqbal, M.M.; ul Haq, M.A.; Saqib, M. Boron Toxicity in Salt-Affected Soils and Effects on Plants. In Soil Science: Agricultural and Environmental Prospectives; Hakeem, K.R., Akhtar, J., Sabir, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 259–286. ISBN 978-3-319-34451-5. [Google Scholar]
- Akram, M.A.; Wahid, A.; Abrar, M.; Manan, A.; Naeem, S.; Zahid, M.A.; Gilani, M.M.; Paudyal, R.; Gong, H.Y.; Ran, J.Z.; et al. Comparative Study of Six Maize (Zea mays L.) Cultivars Concerning Cadmium Uptake, Partitioning and Tolerance. Appl. Ecol. Environ. Res. 2021, 19, 2305–2331. [Google Scholar] [CrossRef]
- Freitas, F.O.; Bendel, G.; Allaby, R.G.; Brown, T.A. DNA from Primitive Maize Landraces and Archaeological Remains: Implications for the Domestication of Maize and Its Expansion into South America. J. Archaeol. Sci. 2003, 30, 901–908. [Google Scholar] [CrossRef]
- Pope, K.S.; Da Silva, D.; Brown, P.H.; DeJong, T.M. A Biologically Based Approach to Modeling Spring Phenology in Temperate Deciduous Trees. Agric. For. Meteorol. 2014, 198–199, 15–23. [Google Scholar] [CrossRef]
- Li, X.; Gentine, P.; Lin, C.; Zhou, S.; Sun, Z.; Zheng, Y.; Liu, J.; Zheng, C. A Simple and Objective Method to Partition Evapotranspiration into Transpiration and Evaporation at Eddy-Covariance Sites. Agric. For. Meteorol. 2019, 265, 171–182. [Google Scholar] [CrossRef]
- Katerji, N.; van Hoorn, J.W.; Hamdy, A.; Mastrorilli, M. Comparison of Corn Yield Response to Plant Water Stress Caused by Salinity and by Drought. Agric. Water Manag. 2003, 65, 95–101. [Google Scholar] [CrossRef]
- Çakir, R. Effect of Water Stress at Different Development Stages on Vegetative and Reproductive Growth of Corn. Field Crop. Res. 2004, 89, 1–16. [Google Scholar] [CrossRef]
- Fuertes-Mendizábal, T.; Bastías, E.I.; González-Murua, C.; González-Moro, M.B. Nitrogen Assimilation in the Highly Salt- and Boron-Tolerant Ecotype Zea mays L. Amylacea. Plants 2020, 9, 322. [Google Scholar] [CrossRef]
- Bastías, E.I.; González-Moro, M.B.; González-Murua, C. Zea Mays L. Amylacea from the Lluta Valley (Arica-Chile) Tolerates Salinity Stress When High Levels of Boron Are Available. Plant Soil 2004, 267, 73–84. [Google Scholar] [CrossRef]
- Bastías, E.; Díaz, M.M.; Pacheco, C.P.; Bustos, P.R.; Hurtado, C.E. Caracterización Del Maíz “Lluteño” (Zea mays L. tipo amylacea) Proveniente Del Norte de Chile, Tolerante a NaCl y Exceso de Boro, Como Una Alternativa Para La Producción de Bioenergía. Idesia (Arica) 2011, 29, 7–16. [Google Scholar] [CrossRef]
- Bastías, E.; González-Moro, M.B.; González-Murua, C. Interactive Effects of Excess Boron and Salinity on Histological and Ultrastructure Leaves of Zea Mays Amylacea from Lluta Valley (Arica-Chile). Cienc. Investig. Agrar. 2013, 40, 581–595. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; He, Q.; Zhou, H. Stomatal Limitations to Photosynthesis and Their Critical Water Conditions in Different Growth Stages of Maize under Water Stress. Agric. Water Manag. 2020, 241, 106330. [Google Scholar] [CrossRef]
- Buckley, T.N.; Mott, K.A. Modelling Stomatal Conductance in Response to Environmental Factors. Plant Cell Environ. 2013, 36, 1691–1699. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Kang, S.; Shi, W.; Zhang, J. An Improved Water-Use Efficiency for Maize Grown under Regulated Deficit Irrigation. Field Crop. Res. 2000, 67, 207–214. [Google Scholar] [CrossRef]
- Liao, Q.; Gu, S.; Kang, S.; Du, T.; Tong, L.; Wood, J.D.; Ding, R. Mild Water and Salt Stress Improve Water Use Efficiency by Decreasing Stomatal Conductance via Osmotic Adjustment in Field Maize. Sci. Total Environ. 2022, 805, 150364. [Google Scholar] [CrossRef]
- Nissanka, S.P.; Dixon, M.A.; Tollenaar, M. Canopy Gas Exchange Response to Moisture Stress in Old and New Maize Hybrid. Crop Sci. 1997, 37, 172–181. [Google Scholar] [CrossRef]
- Cochard, H. Xylem Embolism and Drought-Induced Stomatal Closure in Maize. Planta 2002, 215, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Atteya, A.M. Alteration of Water Relations and Yield of Corn Genotypes in Response To Drought Stress. Bulg. J. Plant Physiol. 2003, 29, 63–76. [Google Scholar]
- Gao, G.; Zhang, X.; Yu, T.; Liu, B. Comparison of Three Evapotranspiration Models with Eddy Covariance Measurements for a Populus euphratica Oliv. Forest in an Arid Region of Northwestern China. J. Arid. Land 2016, 8, 146–156. [Google Scholar] [CrossRef]
- Schussler, J.R.; Westgate, M.E. Maize Kernel Set at Low Water Potential: II. Sensitivity to Reduced Assimilates at Pollination. Crop Sci. 1991, 31, 1196–1203. [Google Scholar] [CrossRef]
- Acevedo, E.; Fereres, E.; Hsiao, T.C.; Henderson, D.W. Diurnal Growth Trends, Water Potential, and Osmotic Adjustment of Maize and Sorghum Leaves in the Field. Plant Physiol. 1979, 64, 476–480. [Google Scholar] [CrossRef]
- Godfrey, L.D.; Norman, J.M.; Holtzer, T.O. Interactive Effects of European Corn Borer (Lepidoptera: Pyralidae) Tunneling and Drought Stress on Field Corn Water Relations. Environ. Entomol. 1992, 21, 1060–1071. [Google Scholar] [CrossRef]
- Bhattarai, B.; Singh, S.; West, C.P.; Ritchie, G.L.; Trostle, C.L. Effect of Deficit Irrigation on Physiology and Forage Yield of Forage Sorghum, Pearl Millet, and Corn. Crop Sci. 2020, 60, 2167–2179. [Google Scholar] [CrossRef]
- Creek, D.; Blackman, C.J.; Brodribb, T.J.; Choat, B.; Tissue, D.T. Coordination between Leaf, Stem, and Root Hydraulics and Gas Exchange in Three Arid-Zone Angiosperms during Severe Drought and Recovery. Plant Cell Environ. 2018, 41, 2869–2881. [Google Scholar] [CrossRef]
- D, Q.; Zhao, X.-H.; Xia, L.; Jiang, C.-J.; Wang, X.-G.; Han, Y.; Wang, J.; Yu, H.-Q. Effects of Potassium Deficiency on Photosynthesis, Chloroplast Ultrastructure, ROS, and Antioxidant Activities in Maize (Zea mays L.). J. Integr. Agric. 2019, 18, 395–406. [Google Scholar] [CrossRef]
- Bhusal, B.; Poudel, M.R.; Rishav, P.; Regmi, R.; Neupane, P.; Bhattarai, K.; Maharjan, B.; Bigyan, K.C.; Acharya, S. A Review on Abiotic Stress Resistance in Maize (Zea mays L.): Effects, Resistance Mechanisms and Management. J. Biol. Today’s World 2021, 10, 1–3. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of Drought Stress on Photosynthetic Response, Leaf Water Potential, and Stem Sap Flow in Two Cultivars of Bi-Leader Apple Trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Tiwari, Y.K.; Yadav, S.K. High Temperature Stress Tolerance in Maize (Zea mays L.): Physiological and Molecular Mechanisms. J. Plant Biol. 2019, 62, 93–102. [Google Scholar] [CrossRef]
- Huang, C.; Ma, S.; Gao, Y.; Liu, Z.; Qin, A.; Zhao, B.; Ning, D.; Duan, A.; Liu, X.; Chen, H.; et al. Response of Summer Maize Growth and Water Use to Different Irrigation Regimes. Agronomy 2022, 12, 768. [Google Scholar] [CrossRef]
- Oktem, A.; Simsek, M.; Oktem, A.G. Deficit Irrigation Effects on Sweet Corn (Zea mays saccharata Sturt) with Drip Irrigation System in a Semi-Arid Region. I. Water-yield relationship. Agric. Water Manag. 2003, 61, 63–74. [Google Scholar] [CrossRef]
- Gomaa, M.A.; Kandil, E.E.; El-Dein, A.A.M.Z.; Abou-Donia, M.E.M.; Ali, H.M.; Abdelsalam, N.R. Increase Maize Productivity and Water Use Efficiency through Application of Potassium Silicate under Water Stress. Sci. Rep. 2021, 11, 224. [Google Scholar] [CrossRef]
- Strasser, M.; Schlunegger, F. Erosional Processes, Topographic Length-Scales and Geomorphic Evolution in Arid Climatic Environments: The ‘Lluta Collapse’, Northern Chile. Int. J. Earth Sci. 2005, 94, 433–446. [Google Scholar] [CrossRef]
- Chacón Cruz, G.; Román Osorio, L.F.; Morales Salinas, L.; Escobar Avaria, C.; Morales Campaña, F. Atlas Zonificación Agroclimática de La Región de Arica y Parinacota; INIA: Santiago, Chile, 2016. [Google Scholar]
- Rámila, C.D.P.; Leiva, E.D.; Bonilla, C.A.; Pastén, P.A.; Pizarro, G.E. Boron Accumulation in Puccinellia Frigida, an Extremely Tolerant and Promising Species for Boron Phytoremediation. J. Geochem. Explor. 2015, 150, 25–34. [Google Scholar] [CrossRef]
- Torres, H.A.; Acevedo, H.E. El Problema de Salinidad En Los Recursos Suelo y Agua Que Afectan El Riego y Cultivos En Los Valles de Lluta y Azapa En El Norte de Chile. Idesia (Arica) 2008, 26, 31–44. [Google Scholar] [CrossRef]
- INDERCO Ltda. Compilación y Evaluación de Los Antecedentes Del Valle Del Río Lluta; Dirección General de Aguas, Ministerio de Obras Públicas: Santiago, Chile. 1980. Available online: https://snia.mop.gob.cl/repositoriodga/handle/20.500.13000/4531 (accessed on 1 August 2021).
- Luzio, L.W.; Casanova, P.M.; Seguel, S.Ó. Suelos de Chile; Universidad de Chile: Santiago, Chile, 2010; ISBN 978-956-19-0648-8. [Google Scholar]
- Díaz, V.C.; Avilés, S.C.; Meléndez, A.E.; Nogueira, C.A.; Valdés, F.A.; León, R.L. Reconocimiento de Suelos Del Valle Del Rio Lluta. Agric. Técnica 1958, 18, 305–354. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; Volume 300, p. 6541. [Google Scholar]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils; Pearson Press: Upper Saddle River, NJ, USA, 2017. [Google Scholar]
- DeJonge, K.C.; Taghvaeian, S.; Trout, T.J.; Comas, L.H. Comparison of Canopy Temperature-Based Water Stress Indices for Maize. Agric. Water Manag. 2015, 156, 51–62. [Google Scholar] [CrossRef]
- Zúñiga, M.; Ortega-Farías, S.; Fuentes, S.; Riveros-Burgos, C.; Poblete-Echeverría, C. Effects of Three Irrigation Strategies on Gas Exchange Relationships, Plant Water Status, Yield Components and Water Productivity on Grafted Carménère Grapevines. Front. Plant Sci. 2018, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Ramazan, S.; Bhat, H.A.; Zargar, M.A.; Ahmad, P.; John, R. Combined Gas Exchange Characteristics, Chlorophyll Fluorescence and Response Curves as Selection Traits for Temperature Tolerance in Maize Genotypes. Photosynth. Res. 2021, 150, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, T.T.; Mahmood, J.; Kamal, A.M. Effectiveness of Farmyard Manure, Poultry Manure and Nitrogen for Corn (Zea mays L.) Productivity. Int. J. Agric. Biol. 2004, 2, 260–263. [Google Scholar]
- Schütte, F.; Meier, U. Entwicklungsstadien Des Mais. Biol. Bundesanstalt für Land- und Forstwirtschaft; Springer: Braunschweig, Germany, 1981; Volume 27, p. 10. [Google Scholar]
- Gökmen, S.; Sencar, O.; Sakin, M.A. Response of Popcorn (Zea mays everta) to Nitrogen Rates and Plant Densities. Turk. J. Agric. For. 2001, 25, 15–23. [Google Scholar] [CrossRef]
- Rahman, M.M.; Govindarajulu, Z. A Modification of the Test of Shapiro and Wilk for Normality. J. Appl. Stat. 1997, 24, 219–236. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 9th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Tablada, M.; Robledo, C.W. Infostat Versión 2017. Grupo Infostat, FCA, Universidad Nacional de Córdoba, Argentina. Available online: http://www.infostat.com.ar (accessed on 31 January 2021).


| A (µmol CO2 m−2 s−1) | gs (mol H2O m−2 s−1) | E (mmol H2O m−2 s−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V10 | VT | V10 | VT | V10 | VT | |||||||
| Irrigation | ||||||||||||
| T1 | 36.6 | 32.2 | 0.12 | 0.17 | 2.63 | 4.58 | ||||||
| T2 | 34.8 | 24.4 | 0.09 | 0.13 | 2.10 | 3.82 | ||||||
| Time | ||||||||||||
| 1 | 38.2 | 30.3 | 0.13 a | 0.16 | 1.93 b | 4.17 | ||||||
| 11 | 33.2 | 26.4 | 0.08 b | 0.14 | 2.80 a | 4.23 | ||||||
| Irrigation × Time | 1 | 11 | 1 | 11 | 1 | 11 | 1 | 11 | 1 | 11 | 1 | 11 |
| T1 | 32.1 b | 41.1 a | 31.7 a | 32.7 a | 0.15 | 0.10 | 0.17 a | 0.17 a | 2.09 | 3.17 | 4.11 b | 5.05 a |
| T2 | 34.4 b | 35.2 b | 28.8 a | 20.1 b | 0.11 | 0.07 | 0.16 a | 0.10 b | 1.76 | 2.43 | 4.23 b | 3.40 c |
| Significances | ||||||||||||
| Irrigation | 0.49 | 0.03 | 0.20 | 0.09 | 0.18 | 0.08 | ||||||
| Time | 0.02 | 0.02 | 0.02 | 0.03 | <0.01 | 0.75 | ||||||
| Irrigation × Time | 0.05 | <0.01 | 0.83 | 0.02 | 0.37 | <0.01 | ||||||
| Treatment | Yield (kg ha−1) | Harvest Index (%) | ||
|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | |
| T1 | 5350 | 650 | 20.0 a | 4.0 |
| T2 | 5337 | 489 | 13.0 b | 2.0 |
| Parameter | Soil | Water | ||
|---|---|---|---|---|
| Median | Standard Deviation | Median | Standard Deviation | |
| ECe (mS cm−1) | 5.5 | 0.5 | 2.3 | 0.3 |
| pH (dimensionless) | 7.2 | 0.2 | 7.5 | 0.4 |
| MO (%) | 1.0 | 0.5 | - | - |
| Ca2+ (meq L−1) | 22.0 | 1.6 | 9.0 | 1.3 |
| Mg2+ (meq L−1) | 10.3 | 1.1 | 2.5 | 0.2 |
| K+ (meq L−1) | 2.1 | 0.2 | 0.9 | 0.1 |
| Na+ (meq L−1) | 17.8 | 3.6 | 11.0 | 1.2 |
| Cl− (meq L−1) | 17.6 | 6.0 | 13.4 | 3.4 |
| SO42− (meq L−1) | 32.0 | 6.3 | 8.6 | 0.5 |
| B (meq L−1) | 21.1 | 4.1 | 15.3 | 1.7 |
| PSI (%) | 15.7 | 7.8 | - | - |
| SAR (dimensionless) | - | - | 4.6 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riveros-Burgos, C.; Bustos-Peña, R.; Esteban-Condori, W.; Bastías, E. Response of Maize (Zea mays L.) to Drought under Salinity and Boron Stress in the Atacama Desert. Plants 2023, 12, 1519. https://doi.org/10.3390/plants12071519
Riveros-Burgos C, Bustos-Peña R, Esteban-Condori W, Bastías E. Response of Maize (Zea mays L.) to Drought under Salinity and Boron Stress in the Atacama Desert. Plants. 2023; 12(7):1519. https://doi.org/10.3390/plants12071519
Chicago/Turabian StyleRiveros-Burgos, Camilo, Richard Bustos-Peña, Wladimir Esteban-Condori, and Elizabeth Bastías. 2023. "Response of Maize (Zea mays L.) to Drought under Salinity and Boron Stress in the Atacama Desert" Plants 12, no. 7: 1519. https://doi.org/10.3390/plants12071519
APA StyleRiveros-Burgos, C., Bustos-Peña, R., Esteban-Condori, W., & Bastías, E. (2023). Response of Maize (Zea mays L.) to Drought under Salinity and Boron Stress in the Atacama Desert. Plants, 12(7), 1519. https://doi.org/10.3390/plants12071519






