Application of a Novel Quantitative Trait Locus Combination to Improve Grain Shape without Yield Loss in Rice (Oryza sativa L. spp. japonica)
Abstract
1. Introduction
2. Results
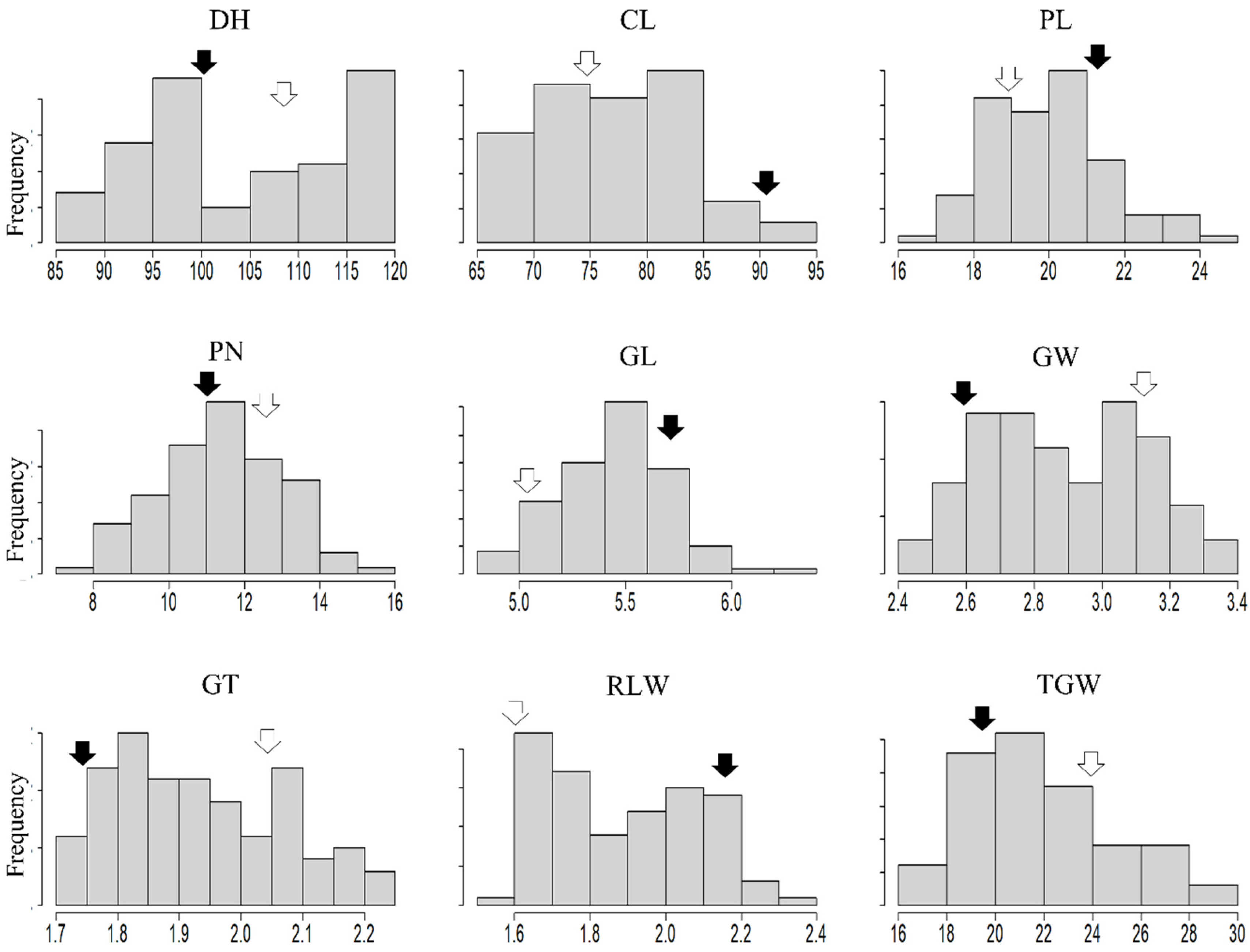
2.1. Evaluation of Tested Traits in the RIL Population
2.2. Correlation Analysis of the Five Traits
2.3. QTL Analysis
2.4. Effect of QTLs
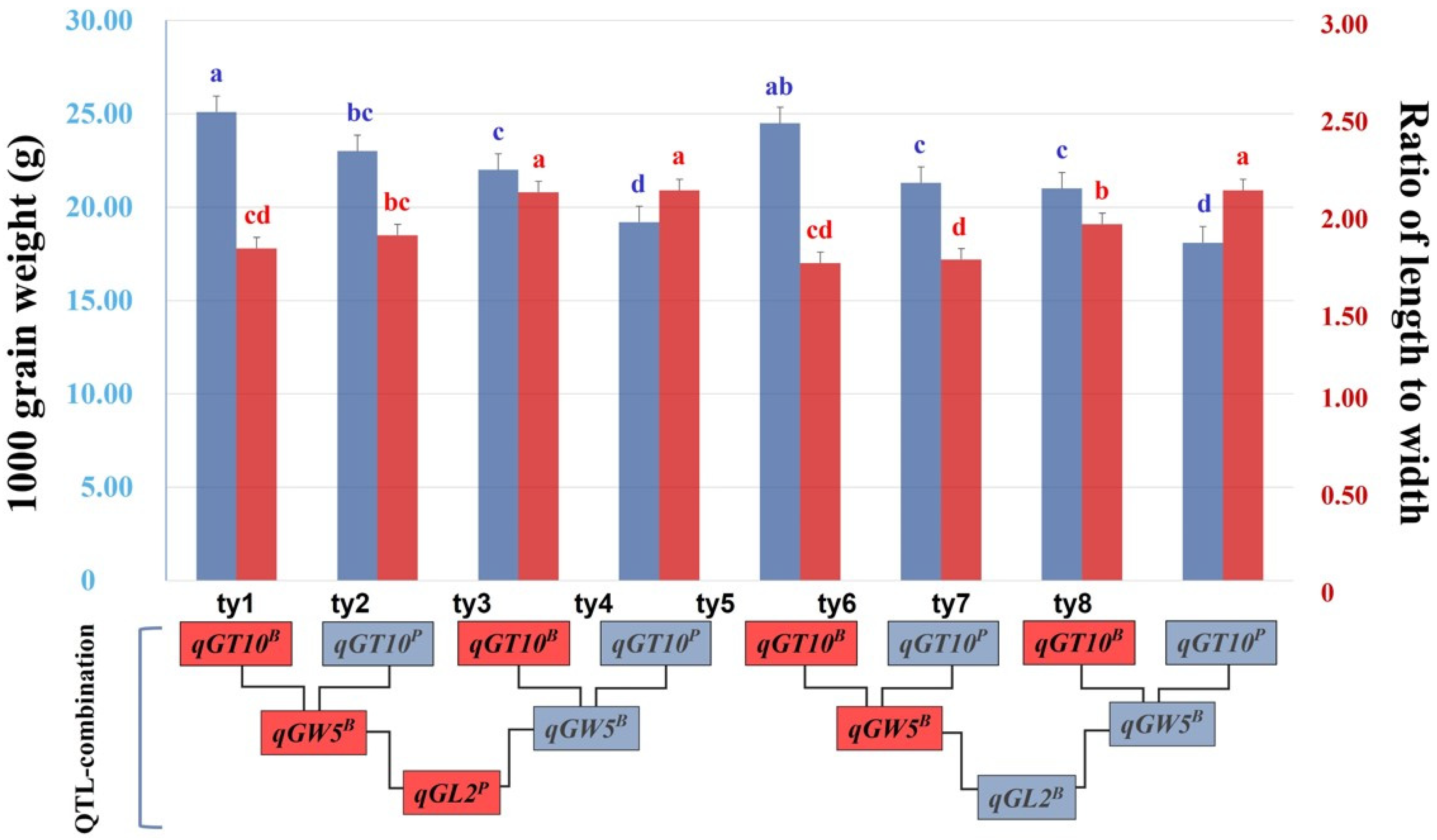
2.5. Determining an Ideal QTL Combination for Grain Shape
2.6. Validation Tests for Grain Shape-Related Genes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Materials and Mapping Population
5.2. Assessments of Traits Associated with Grain Shape
5.3. Genetic Map Construction
5.4. QTL Mapping
5.5. Data Analyses
5.6. Haplotype Test
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Calingacion, M.; Laborte, A.; Nelson, A.; Resurreccion, A.; Concepcion, J.C.; Daygon, V.D.; Mumm, R.; Reinke, R.; Dipti, S.; Bassinello, P.Z.; et al. Diversity of Global Rice Markets and the Science Required for Consumer-Targeted Rice Breeding. PLoS ONE 2014, 9, e85106. [Google Scholar] [CrossRef]
- Russinga, M.A. Correlation Studies on Yield and Yield Contributing Traits in Rice (Oryza sativa L.). Indian J. Pure Appl. Biosci. 2020, 8, 531–538. [Google Scholar] [CrossRef]
- Khush, G.S. What It Will Take to Feed 5.0 Billion Rice Consumers in 2030. Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Xu, J.; Zeng, D.; Zhang, B.; Geng, M.; Zhang, G.; Huang, K.; Huang, L.; Xu, R.; Ge, S.; et al. Natural Variation in the Promoter of GSE5 Contributes to Grain Size Diversity in Rice. Mol. Plant 2017, 10, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, H.; Gu, Y.; Xia, D.; Wu, B.; Gao, G.; Zhang, Q.; He, Y. Mapping and Verification of Grain Shape QTLs Based on High-Throughput SNP Markers in Rice. Mol. Breed. 2019, 39, 42. [Google Scholar] [CrossRef]
- Tan, Y.F.; Xing, Y.Z.; Li, J.X.; Yu, S.B.; Xu, C.G.; Zhang, Q. Genetic Bases of Appearance Quality of Rice Grains in Shanyou 63, an Elite Rice Hybrid. Theor. Appl. Genet. 2000, 101, 823–829. [Google Scholar] [CrossRef]
- Fitzgerald, M.A.; McCouch, S.R.; Hall, R.D. Not Just a Grain of Rice: The Quest for Quality. Trends Plant Sci. 2009, 14, 133–139. [Google Scholar] [CrossRef]
- Aslam, K.; Naveed, S.A.; Sabar, M.; Shabir, G.; Shah, S.M.; Khan, A.R.; Shah, M.M.; Fiaz, S.; Xu, J.; Arif, M. Identification of QTLs for Rice Grain Size and Weight by High-Throughput SNP Markers in the IR64 x Sadri Population. Front. Genet. 2022, 13, 955347. [Google Scholar] [CrossRef]
- Bai, X.; Luo, L.; Yan, W.; Kovi, M.R.; Zhan, W.; Xing, Y. Genetic Dissection of Rice Grain Shape Using a Recombinant Inbred Line Population Derived from Two Contrasting Parents and Fine Mapping a Pleiotropic Quantitative Trait Locus QGL7. BMC Genom. 2010, 11, 16. [Google Scholar] [CrossRef]
- Park, H.-S.; Baek, M.-K.; Nam, J.-K.; Shin, W.-C.; Lee, G.-M.; Park, S.-G.; Lee, C.-M.; Kim, C.-S.; Cho, Y.-C. Development and Characterization of japonica Rice Line with Long and Spindle-Shaped Grain. Korean J. Breed. Sci. 2018, 50, 116–130. [Google Scholar] [CrossRef]
- Park, H.-S.; Baek, M.-K.; Nam, J.-K.; Shin, W.-C.; Jeong, J.-M.; Lee, G.-M.; Park, S.-G.; Kim, C.-S.; Cho, Y.-C.; Kim, B.-K. Development and Characterization of Breeding Materials with Diverse Grain Size and Shape in japonica Rice. Korean J. Breed. Sci. 2017, 49, 369–389. [Google Scholar] [CrossRef]
- Zuo, J.; Li, J. Molecular Genetic Dissection of Quantitative Trait Loci Regulating Rice Grain Size. Annu. Rev. Genet. 2014, 48, 99–118. [Google Scholar] [CrossRef]
- Huang, R.; Jiang, L.; Zheng, J.; Wang, T.; Wang, H.; Huang, Y.; Hong, Z. Genetic Bases of Rice Grain Shape: So Many Genes, so Little Known. Trends Plant Sci. 2013, 18, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.; Zhang, Q. GS3, a Major QTL for Grain Length and Weight and Minor QTL for Grain Width and Thickness in Rice, Encodes a Putative Transmembrane Protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Song, J.X.; Huang, W.; Shi, M.; Zhu, M.Z.; Lin, H.X. A QTL for Rice Grain Width and Weight Encodes a Previously Unknown RING-Type E3 Ubiquitin Ligase. Nat. Genet. 2007, 39, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Gu, S.; Wan, X.; Gao, H.; Guo, T.; Su, N.; Lei, C.; Zhang, X.; Cheng, Z.; Guo, X.; et al. Isolation and Initial Characterization of GW5 a Major QTL Associated with Rice Grain Width and Weight. Cell Res. 2008, 18, 1199–1209. [Google Scholar] [CrossRef]
- Shomura, A.; Izawa, T.; Ebana, K.; Ebitani, T.; Kanegae, H.; Konishi, S.; Yano, M. Deletion in a Gene Associated with Grain Size Increased Yields during Rice Domestication. Nat. Genet. 2008, 40, 1023–1028. [Google Scholar] [CrossRef]
- Li, Y.; Fan, C.; Xing, Y.; Jiang, Y.; Luo, L.; Sun, L.; Shao, D.; Xu, C.; Li, X.; Xiao, J.; et al. Natural Variation in GS5 Plays an Important Role in Regulating Grain Size and Yield in Rice. Nat. Genet. 2011, 43, 1266–1269. [Google Scholar] [CrossRef]
- Qi, P.; Lin, Y.S.; Song, X.J.; Shen, J.B.; Huang, W.; Shan, J.X.; Zhu, M.Z.; Jiang, L.; Gao, J.P.; Lin, H.X. The Novel Quantitative Trait Locus GL3.1 Controls Rice Grain Size and Yield by Regulating Cyclin-T1;3. Cell Res. 2012, 22, 1666–1680. [Google Scholar] [CrossRef]
- Wang, S.; Wu, K.; Yuan, Q.; Liu, X.; Liu, Z.; Lin, X.; Zeng, R.; Zhu, H.; Dong, G.; Qian, Q.; et al. Control of Grain Size, Shape and Quality by OsSPL16 in Rice. Nat. Genet. 2012, 44, 950–954. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Liu, Q.; Wu, K.; Zhang, J.; Wang, S.; Wang, Y.; Chen, X.; Zhang, Y.; Gao, C.; et al. The OsSPL16-GW7 Regulatory Module Determines Grain Shape and Simultaneously Improves Rice Yield and Grain Quality. Nat. Genet. 2015, 47, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Che, R.; Tong, H.; Shi, B.; Liu, Y.; Fang, S.; Liu, D.; Xiao, Y.; Hu, B.; Liu, L.; Wang, H.; et al. Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat. Plants 2016, 2, 15195, Erratum in Nat. Plants 2016, 2, 16002. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Y.; Fang, Y.; Zeng, L.; Xu, J.; Yu, H.; Shi, Z.; Pan, J.; Zhang, D.; Kang, S.; et al. A Rare Allele of GS2 Enhances Grain Size and Grain Yield in Rice. Mol. Plant 2015, 8, 1455–1465. [Google Scholar] [CrossRef]
- Duan, P.; Rao, Y.; Zeng, D.; Yang, Y.; Xu, R.; Zhang, B.; Dong, G.; Qian, Q.; Li, Y. SMALL GRAIN 1, Which Encodes a Mitogen-activated Protein Kinase Kinase 4 Influences. Plant J. 2014, 77, 547–557. [Google Scholar] [CrossRef]
- Na Fang, N.; Xu, R.; Huang, L.; Zhang, B.; Duan, P.; Na Li, N.; Luo, Y.; Li, Y. SMALL GRAIN 11 Controls Grain Size, Grain Number and Grain Yield in Rice. Rice 2016, 9, 64. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, S.; Sun, S.; Zhang, Y.; Li, J.; You, J.; Su, T.; Chen, W.; Ling, Y.; He, G.; et al. Analysis of QTL for Grain Size in a Rice Chromosome Segment Substitution Line Z1392 with Long Grains and Fine Mapping of QGL-6. Rice 2020, 13, 40. [Google Scholar] [CrossRef]
- Shao, G.; Wei, X.; Chen, M.; Tang, S.; Luo, J.; Jiao, G.; Xie, L.; Hu, P. Allelic Variation for a Candidate Gene for GS7, Responsible for Grain Shape in Rice. Theor. Appl. Genet. 2012, 125, 1303–1312. [Google Scholar] [CrossRef]
- Qiu, X.; Gong, R.; Tan, Y.; Yu, S. Mapping and Characterization of the Major Quantitative Trait Locus QSS7 Associated with Increased Length and Decreased Width of Rice Seeds. Theor. Appl. Genet. 2012, 125, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, A.K.; Sharma, T.R.; Singh, A.; Singh, N.K. Fine Mapping of Grain Length QTLs on Chromosomes 1 and 7 in Basmati Rice (Oryza sativa L.). J. Plant Biochem. Biotechnol. 2011, 21, 157–166. [Google Scholar] [CrossRef]
- Wan, X.Y.; Wan, J.M.; Jiang, L.; Wang, J.K.; Zhai, H.Q.; Weng, J.F.; Wang, H.L.; Lei, C.L.; Zhang, X.; Cheng, Z.J.; et al. QTL Analysis for Rice Grain Length and Fine Mapping of an Identified QTL with Stable and Major Effects. Theor. Appl. Genet. 2006, 112, 1258–1270. [Google Scholar] [CrossRef]
- Kato, T.; Segami, S.; Toriyama, M.; Kono, I.; Ando, T.; Yano, M.; Kitano, H.; Miura, K.; Iwasaki, Y. Detection of QTLs for Grain Length from Large Grain Rice (Oryza sativa L.). Breed. Sci. 2011, 61, 269–274. [Google Scholar] [CrossRef]
- Ishimaru, K.; Hirotsu, N.; Madoka, Y.; Murakami, N.; Hara, N.; Onodera, H.; Kashiwagi, T.; Ujiie, K.; Shimizu, B.-I.; Onishi, A.; et al. Loss of Function of the IAA-Glucose Hydrolase Gene TGW6 Enhances Rice Grain Weight and Increases Yield. Nat. Genet. 2013, 45, 707–711. [Google Scholar] [CrossRef]
- Gao, F.Y.; Zeng, L.H.; Ling, Q.I.; Lu, X.J.; Ren, J.S.; Wu, X.T.; Su, X.W.; Gao, Y.M.; Ren, G.J. QTL mapping of grain appearance quality traits and grain weight using a recombinant inbred population in rice (Oryza sativa L.). J. Integr. Agric. 2016, 15, 1693–1702. [Google Scholar] [CrossRef]
- Singh, N.; Majumder, S.; Singh, O.N.; Vikram, P.; Singh, A.K.; Singh, S. A Large-Effect QTL for Grain Weight in Rice on Chromosome 10. Aust. J. Crop Sci. 2015, 9, 372–377. [Google Scholar]
- Yaduraju, J.S.; Mishra, N.T. Sedges in Rice Culture and Their Management; Singh, Y., Singh, V.P., Chauhan, B., Orr, A., Mortimer, A.M., Hohnson, D.E., Hardy, B., Eds.; International Rice Research Institute: Los Banos, Philippines; Directorate of Experiment Station, G.B. Pant University of Agriculture and Technology: Pantnagar, India, 2008; ISBN 9789712202360. [Google Scholar]
- Lee, C.-M.; Lee, K.-M.; Baek, M.-K.; Kim, W.-J.; Suh, J.-P.; Jeong, O.-Y.; Cho, Y.-C.; Park, H.-S.; Kim, S.-M. Characterization of Traits Related to Grain Shape in Korean Rice Varieties. Korean J. Crop Sci. 2020, 65, 199–213. [Google Scholar] [CrossRef]
- RDA (Rural Development Administration). Manual for Standard Evaluation Method in Agricultural Experiment and Research; RDA Press: Suwon, Republic of Korea, 2012; ISBN 978-89-480-1649-9 93520.
- Alvarez-Fernandez, A.; Bernal, M.J.; Fradejas, I.; Ramírez, A.M.; Yusuf, N.A.M.; Lanza, M.; Hisam, S.; de Ayala, A.P.; Rubio, J.M. KASP: A Genotyping Method to Rapid Identification of Resistance in Plasmodium Falciparum. Malar. J. 2021, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated Software for Genetic Linkage Map Construction and Quantitative Trait Locus Mapping in Biparental Populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef]




| GL | GW | GT | RLW | TGW | |
|---|---|---|---|---|---|
| GL | |||||
| GW | −0.28 ** | ||||
| GT | −0.24 * | 0.87 *** | |||
| RLW | 0.68 *** | −0.89 *** | −0.77 *** | ||
| TGW | 0.14 ns | 0.83 *** | 0.86 *** | −0.57 *** |
| Traits | QTL | Chr. | Flanking Markers | Mena LOD (Range) | % of Mean PVE | Mean Add |
|---|---|---|---|---|---|---|
| Grain length (GL) | qGL2 | 2 | KJ02_45–KJ02_47 | 8.32 (7.98–8.41) | 33.9 | −0.15 |
| Grain width (GW) | qGW5 | 5 | KJ05_13–KJ05_17 | 29.32 (283.60–30.04) | 64.4 | 0.17 |
| qGW10 | 10 | KJ10_35–KJ10_39 | 10.20 (9.88–10.52) | 13.6 | 0.07 | |
| Grain thickness (GT) | qGT5 | 5 | KJ05_13–KJ05_17 | 18.64 (18.00–19.23) | 49.2 | 0.09 |
| qGT10 | 10 | KJ10_39–KJ10_41 | 7.97 (7.66–8.23) | 16.5 | 0.05 | |
| Ratio of length to width (RLW) | qRW5 | 5 | KJ05_13–KJ05_17 | 26.77 (21.90–23.64) | 72.6 | −0.15 |
| 1000-grain weight (TGW) | qTW5 | 5 | KJ05_13–KJ05_17 | 15.91 (14.85–16.97) | 35.0 | 1.58 |
| qTW10 | 10 | KJ10_43–KJ10_49 | 11.00 (10.50–11.50) | 22.3 | 1.26 |
| Gene | Trait | Parents | Allele Type (Effect) a | Reference | |
|---|---|---|---|---|---|
| Boramchan (P1) | Pecos (P2) | ||||
| GW2 | Grain width and yield | gw2WY3 (+) | [15] | ||
| GW2 | GW2 | GW2FAZ1 (N) | |||
| GS3 | Grain length and weight | gs3Cuuane (−) | [14] | ||
| GS3hetero-allele (M) | |||||
| GS3 | GS3 | GS3Minghui63 (+) | |||
| GL3.1 | Grain size and yield | qgl3WY3 (+) | [19] | ||
| qGL3 | qGL3 | qGL3FAZ1 (N) | |||
| qSW5 | Grain width | qsw5_N | qsw5_NNipponbare (+) | [17] | |
| qsw5_SSL22 (M) | |||||
| qSW5_K | qSW5Kasalath (N) | ||||
| GS5 | Grain length and weight | gs5 | gs5 | gs5H94 (N) | [18] |
| GS5Zhensan97 (+) | |||||
| TGW6 | Grain weight | tgw6Kasalath (+) | [32] | ||
| TGW6 | TGW6 | TGW6Nipponbare (N) | |||
| GW7 | Grain width (slenderness) | gw7 | gw7 | gw7Nipponbare (N) | [21] |
| GW7TFA1 (+) | |||||
| GW8 | Grain width (slenderness) | gw8Basmati385 (+) | [20] | ||
| GW8 | GW8 | GW8HJX741 (N) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-S.; Lee, C.-M.; Baek, M.-K.; Jeong, O.-Y.; Kim, S.-M. Application of a Novel Quantitative Trait Locus Combination to Improve Grain Shape without Yield Loss in Rice (Oryza sativa L. spp. japonica). Plants 2023, 12, 1513. https://doi.org/10.3390/plants12071513
Park H-S, Lee C-M, Baek M-K, Jeong O-Y, Kim S-M. Application of a Novel Quantitative Trait Locus Combination to Improve Grain Shape without Yield Loss in Rice (Oryza sativa L. spp. japonica). Plants. 2023; 12(7):1513. https://doi.org/10.3390/plants12071513
Chicago/Turabian StylePark, Hyun-Su, Chang-Min Lee, Man-Kee Baek, O-Young Jeong, and Suk-Man Kim. 2023. "Application of a Novel Quantitative Trait Locus Combination to Improve Grain Shape without Yield Loss in Rice (Oryza sativa L. spp. japonica)" Plants 12, no. 7: 1513. https://doi.org/10.3390/plants12071513
APA StylePark, H.-S., Lee, C.-M., Baek, M.-K., Jeong, O.-Y., & Kim, S.-M. (2023). Application of a Novel Quantitative Trait Locus Combination to Improve Grain Shape without Yield Loss in Rice (Oryza sativa L. spp. japonica). Plants, 12(7), 1513. https://doi.org/10.3390/plants12071513





