Chromium Toxicity in Plants: Signaling, Mitigation, and Future Perspectives
Abstract
1. Introduction
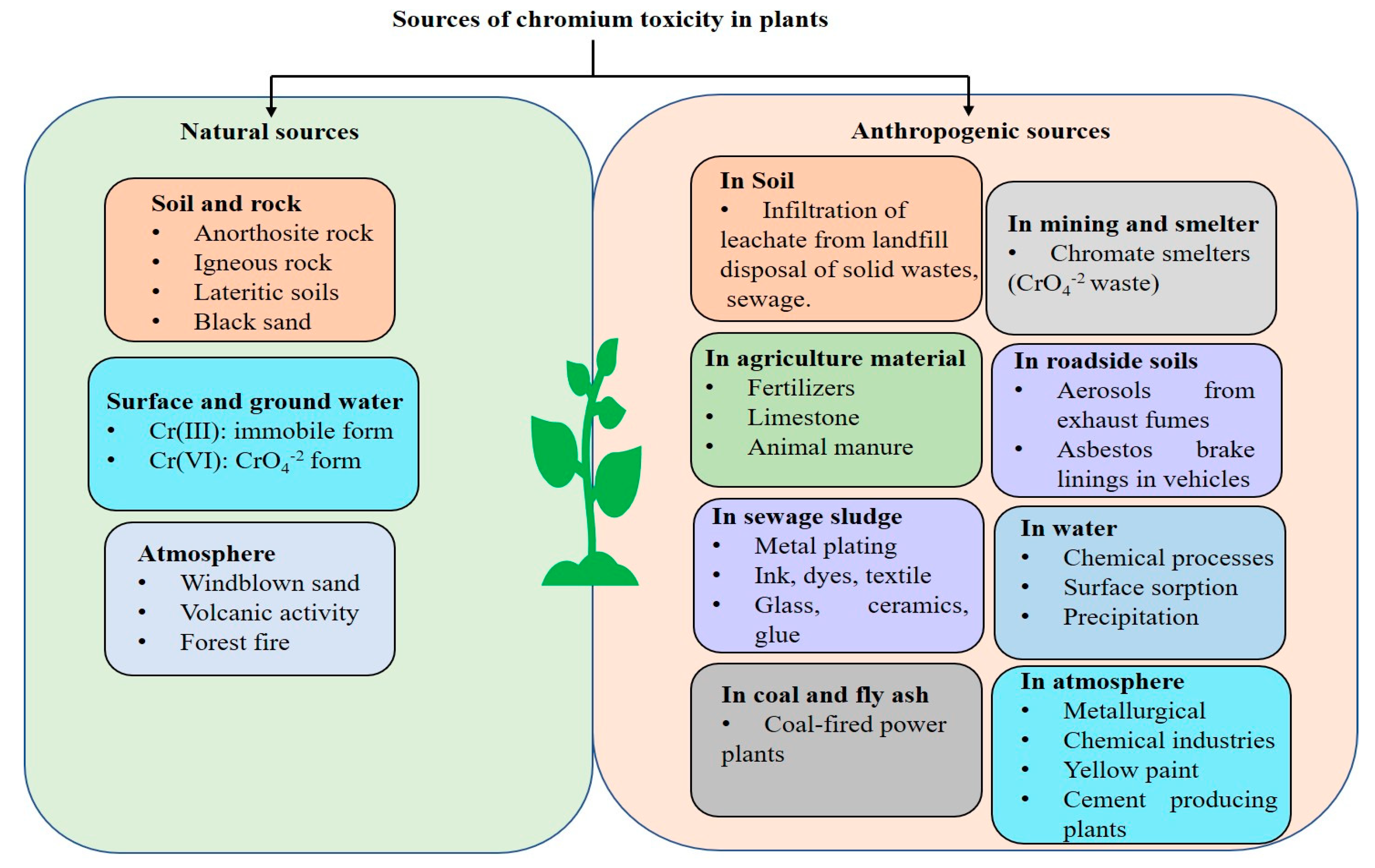
2. Sources of Chromium
3. Chromium Uptake and Translocation
4. Impact of Chromium Toxicity on Different Plant Traits
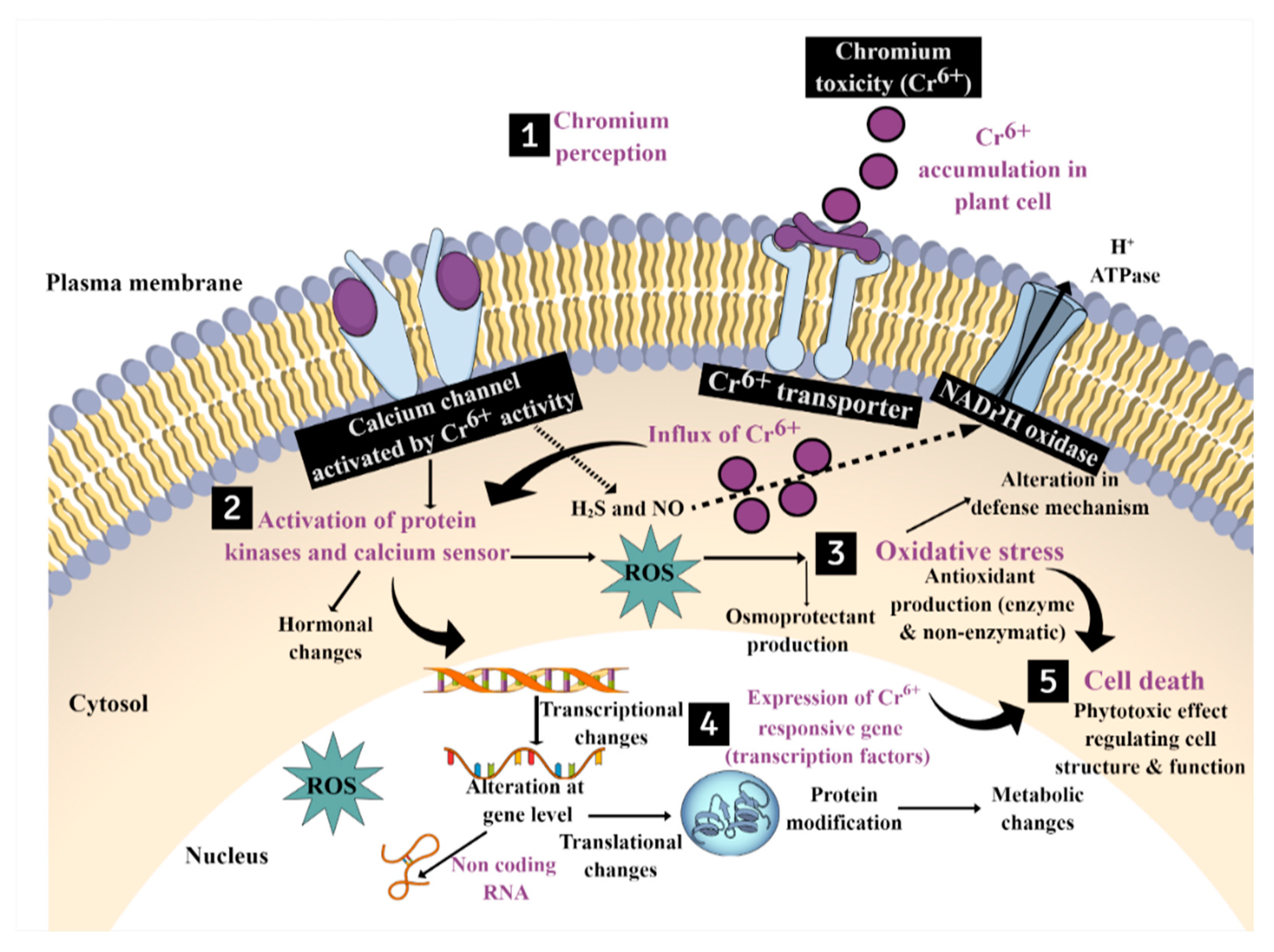
5. Molecular Mechanisms and Signal Transduction in Regulating Chromium Stress in Plants
6. Mitigation of Chromium Toxicity in Sustainable Agriculture
6.1. Microbe-Mediated Mitigation for Chromium Toxicity
6.2. Chemical Priming of Plants to Alleviate Chromium Toxicity
6.3. Nano-Priming as Pilot Strategy to Alleviate Chromium Toxicity in Plants
6.4. Biotechnological Approaches for Mitigating Chromium Stress in Plants
6.5. Breeding of Chromium-Safe Cultivars
7. Conclusion and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neilson, S.; Rajakaruna, N. Phytoremediation of Agricultural Soils: Using Plants to Clean Metal-Contaminated Arable Land. In Phytoremediation: Management of Environmental Contaminants; Springer: Cham, Switzerland, 2015; Volume 1, pp. 159–168. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Singh, V.P. The Relative Impact of Toxic Heavy Metals (THMs) (Arsenic (As), Cadmium (Cd), Chromium (Cr)(VI), Mercury (Hg), and Lead (Pb)) on the Total Environment: An Overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42. IARC Monogr. Eval. Carcinog. Risk Chem. Hum. Suppl. 1987, 7, 1–440.
- Kimbrough, D.E.; Cohen, Y.; Winer, A.M.; Creelman, L.; Mabuni, C. A Critical Assessment of Chromium in the Environment. Crit. Rev. Environ. Sci. Technol. 1999, 29, 1–46. [Google Scholar] [CrossRef]
- Shrivastava, R.; Upreti, R.; Seth, P.; Chaturvedi, U. Effects of Chromium on the Immune System. FEMS Immunol. Med. Microbiol. 2002, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Khalique, G.; Irfan, M.; Wani, A.S.; Tripathi, B.N.; Ahmad, A. Physiological Changes Induced by Chromium Stress in Plants: An Overview. Protoplasma 2012, 249, 599–611. [Google Scholar] [CrossRef]
- Huang, L.; Yu, C.H.; Hopke, P.K.; Shin, J.Y.; Fan, Z. (Tina) Trivalent Chromium Solubility and Its Influence on Quantification of Hexavalent Chromium in Ambient Particulate Matter Using EPA Method 6800. J. Air Waste Manag. Assoc. 2014, 64, 1439–1445. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, D.; Proshad, R.; Uwiringiyimana, E.; Wang, Z. Assessment of the pollution levels of potential toxic elements in urban vegetable gardens in southwest China. Sci. Rep. 2021, 11, 22824. [Google Scholar] [CrossRef]
- Zeng, F.; Wu, X.; Qiu, B.; Wu, F.; Jiang, L.; Zhang, G. Physiological and proteomic alterations in rice (Oryza sativa L.) seedlings under hexavalent chromium stress. Planta 2014, 240, 291–308. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A.H.; Wirtz, M.; Hell, R.; Malagoli, M. Interactions between Chromium and Sulfur Metabolism in Brassica juncea. J. Environ. Qual. 2008, 37, 1536–1545. [Google Scholar] [CrossRef]
- Zayed, A.M.; Terry, N. Chromium in the Environment: Factors Affecting Biological Remediation. Plant Soil 2003, 249, 139–156. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, H.; Chen, X.; Zhu, C.; Li, X. Effect of Incineration Temperature on Chromium Speciation in Real Chromium-Rich Tannery Sludge under Air Atmosphere. Environ. Res. 2020, 183, 109159. [Google Scholar] [CrossRef] [PubMed]
- Quantin, C.; Ettler, V.; Garnier, J.; Šebek, O. Sources and Extractibility of Chromium and Nickel in Soil Profiles Developed on Czech Serpentinites. C. R.-Geosci. 2008, 340, 872–882. [Google Scholar] [CrossRef]
- Babula, P.; Adam, V.; Opatrilova, R.; Zehnalek, J.; Havel, L.; Kizek, R. Uncommon Heavy Metals, Metalloids and Their Plant Toxicity: A Review. Environ. Chem. Lett. 2008, 6, 189–213. [Google Scholar] [CrossRef]
- Cervantes, C.; Campos-García, J.; Devars, S.; Gutiérrez-Corona, F.; Loza-Tavera, H.; Torres-Guzmán, J.C.; Moreno-Sánchez, R. Interactions of Chromium with Microorganisms and Plants. FEMS Microbiol. Rev. 2001, 25, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.; Soufi, S.M.; Cha, J.W.; Romney, E.M. Some Effects of Chromium Toxicity on Bush Bean Plants Grown in Soil. Plant Soil 1976, 44, 471–473. [Google Scholar] [CrossRef]
- de Oliveira, L.M.; Gress, J.; De, J.; Rathinasabapathi, B.; Marchi, G.; Chen, Y.; Ma, L.Q. Sulfate and Chromate Increased Each Other’s Uptake and Translocation in As-Hyperaccumulator Pteris vittata. Chemosphere 2016, 147, 36–43. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.M.; Ma, L.Q.; Santos, J.A.G.; Guilherme, L.R.G.; Lessl, J.T. Effects of Arsenate, Chromate, and Sulfate on Arsenic and Chromium Uptake and Translocation by Arsenic Hyperaccumulator Pteris vittata L. Environ. Pollut. 2014, 184, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium Toxicity in Plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Sharma, N.L.; Singh, C.K.; Kumar Sarkar, S.; Singh, I.; Lal Dotaniya, M. Effect of Chromium (VI) Toxicity on Morpho-Physiological Characteristics, Yield, and Yield Components of Two Chickpea (Cicer arietinum L.) Varieties. PLoS ONE 2020, 15, e0243032. [Google Scholar] [CrossRef]
- Cary, E.E.; Allaway, W.H.; Olson, O.E. Control of chromium concentrations in food plants. 2. Chemistry of chromium in soils and its availability to plants. J. Agric. Food Chem. 1977, 25, 305–309. [Google Scholar] [CrossRef]
- Oliveira, H. Chromium as an Environmental Pollutant: Insights on Induced Plant Toxicity. J. Bot. 2012, 2012, 8. [Google Scholar] [CrossRef]
- Wu, Z.; McGrouther, K.; Chen, D.; Wu, W.; Wang, H. Subcellular Distribution of Metals within Brassica chinensis L. in Response to Elevated Lead and Chromium Stress. J. Agric. Food Chem. 2013, 61, 4715–4722. [Google Scholar] [CrossRef]
- Shukla, O.P.; Dubey, S.; Rai, U.N. Preferential Accumulation of Cadmium and Chromium: Toxicity in Bacopa Monnieri L. under Mixed Metal Treatments. Bull. Environ. Contam. Toxicol. 2007, 78, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium Speciation, Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System: A Review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Dotaniya, M.L.; Thakur, J.K.; Meena, V.D.; Jajoria, D.K.; Rathor, G. Chromium Pollution: A Threat to Environment—A Review. Agric. Rev. 2014, 35, 153. [Google Scholar] [CrossRef]
- Ugwu, E.I.; Agunwamba, J.C. A Review on the Applicability of Activated Carbon Derived from Plant Biomass in Adsorption of Chromium, Copper, and Zinc from Industrial Wastewater. Environ. Monit. Assess. 2020, 192, 240. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.K.; Pradhan, C.; Patra, H.K. Chromium Bioaccumulation, Oxidative Stress Metabolism and Oil Content in Lemon Grass Cymbopogon Flexuosus (Nees Ex Steud.) W. Watson Grown in Chromium Rich over Burden Soil of Sukinda Chromite Mine, India. Chemosphere 2019, 218, 1082–1088. [Google Scholar] [CrossRef]
- Wakeel, A.; Ali, I.; Wu, M.; Raza Kkan, A.; Jan, M.; Ali, A.; Liu, Y.; Ge, S.; Wu, J.; Liu, B.; et al. Ethylene Mediates Dichromate-Induced Oxidative Stress and Regulation of the Enzymatic Antioxidant System-Related Transcriptome in Arabidopsis Thaliana. Environ. Exp. Bot. 2019, 161, 166–179. [Google Scholar] [CrossRef]
- Wakeel, A.; Xu, M.; Gan, Y. Chromium-Induced Reactive Oxygen Species Accumulation by Altering the Enzymatic Antioxidant System and Associated Cytotoxic, Genotoxic, Ultrastructural, and Photosynthetic Changes in Plants. Int. J. Mol. Sci. 2020, 21, 728. [Google Scholar] [CrossRef]
- Habiba, U.; Ali, S.; Rizwan, M.; Ibrahim, M.; Hussain, A.; Shahid, M.R.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Alleviative Role of Exogenously Applied Mannitol in Maize Cultivars Differing in Chromium Stress Tolerance. Environ. Sci. Pollut. Res. 2019, 26, 5111–5121. [Google Scholar] [CrossRef] [PubMed]
- Trinh, N.N.; Huang, T.L.; Chi, W.C.; Fu, S.F.; Chen, C.C.; Huang, H.J. Chromium Stress Response Effect on Signal Transduction and Expression of Signaling Genes in Rice. Physiol. Plant. 2014, 150, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Huang, L.Y.; Fu, S.F.; Trinh, N.N.; Huang, H.J. Genomic Profiling of Rice Roots with Short- and Long-Term Chromium Stress. Plant Mol. Biol. 2014, 86, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.A.H.; Shang, S.; Zhang, M.; Zheng, W.; Zhang, G.; Wang, T.Z.; Shamsi, I.H.; Wu, F. Genome-Wide Identification of Chromium Stress-Responsive Micro RNAs and Their Target Genes in Tobacco (Nicotiana Tabacum) Roots. Environ. Toxicol. Chem. 2015, 34, 2573–2582. [Google Scholar] [CrossRef]
- Zeng, F.; Qiu, B.; Wu, X.; Niu, S.; Wu, F.; Zhang, G. Glutathione-Mediated Alleviation of Chromium Toxicity in Rice Plants. Biol. Trace Elem. Res. 2012, 148, 255–263. [Google Scholar] [CrossRef]
- Dubey, S.; Misra, P.; Dwivedi, S.; Chatterjee, S.; Bag, S.K.; Mantri, S.; Asif, M.H.; Rai, A.; Kumar, S.; Shri, M.; et al. Transcriptomic and Metabolomic Shifts in Rice Roots in Response to Cr (VI) Stress. BMC Genom. 2010, 11, 648. [Google Scholar] [CrossRef]
- Nyer, E.K. Groundwater Treatment Technology; John Wiley & Sons: Hoboken, NJ, USA, 1992; ISBN 0471284149. [Google Scholar]
- Princy, S.; Sathish, S.S.; Cibichakravarthy, B.; Prabagaran, S.R. Hexavalent Chromium Reduction by Morganella Morganii (1Ab1) Isolated from Tannery Effluent Contaminated Sites of Tamil Nadu, India. Biocatal. Agric. Biotechnol. 2020, 23, 101469. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Xu, R.; Wang, Y.N.; Sun, Y.; Bian, R.; Li, W. Remediation of Cr (VI)-contaminated soil by combined chemical reduction and microbial stabilization: The role of biogas solid residue (BSR). Ecotoxicol. Environ. Saf. 2022, 231, 113198. [Google Scholar] [CrossRef]
- Kuppusamy, S.; Thavamani, P.; Venkateswarlu, K.; Lee, Y.B.; Naidu, R.; Megharaj, M. Remediation Approaches for Polycyclic Aromatic Hydrocarbons (PAHs) Contaminated Soils: Technological Constraints, Emerging Trends and Future Directions. Chemosphere 2017, 168, 944–968. [Google Scholar] [CrossRef]
- Kumar, M.; Saini, H.S. Reduction of Hexavalent Chromium (VI) by Indigenous Alkaliphilic and Halotolerant MicrobacteriumS p. M5: Comparative Studies under Growth and Nongrowth Conditions. J. Appl. Microbiol. 2019, 127, 1057–1068. [Google Scholar] [CrossRef]
- Unz, R.F.; Shuttleworth, K.L. Microbial Mobilization and Immobilization of Heavy Metals. Curr. Opin. Biotechnol. 1996, 7, 307–310. [Google Scholar] [CrossRef]
- Dvorak, G.J. Transformation field analysis of inelastic composite materials. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1992, 437, 311–327. [Google Scholar]
- Focardi, S.; Pepi, M.; Landi, G.; Gasperini, S.; Ruta, M.; Di Biasio, P.; Focardi, S.E. Hexavalent Chromium Reduction by Whole Cells and Cell Free Extract of the Moderate Halophilic Bacterial Strain Halomonas Sp. TA-04. Int. Biodeterior. Biodegrad. 2012, 66, 63–70. [Google Scholar] [CrossRef]
- González, P.S.; Ambrosio, L.F.; Paisio, C.E.; Talano, M.A.; Medina, M.I.; Agostini, E. Chromium (VI) Remediation by a Native Strain: Effect of Environmental Conditions and Removal Mechanisms Involved. Environ. Sci. Pollut. Res. 2014, 21, 13551–13559. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, D.C.; Bolaños-Benitez, V.; Sivry, Y.; Gelabert, A.; Riotte, J.; Subramanian, S. Mechanistic Studies on the Bioremediation of Cr(VI) Using Sphingopyxis Macrogoltabida SUK2c, a Cr(VI) Tolerant Bacterial Isolate. Biochem. Eng. J. 2019, 150, 107292. [Google Scholar] [CrossRef]
- Sandaña, P.; Kalazich, J. Ecophysiological Determinants of Tuber Yield as Affected by Potato Genotype and Phosphorus Availability. Field Crops Res. 2015, 180, 21–28. [Google Scholar] [CrossRef]
- Shi, L.; Xue, J.; Liu, B.; Dong, P.; Wen, Z.; Shen, Z.; Chen, Y. Hydrogen Ions and Organic Acids Secreted by Ectomycorrhizal Fungi, Pisolithus Sp1, Are Involved in the Efficient Removal of Hexavalent Chromium from Waste Water. Ecotoxicol. Environ. Saf. 2018, 161, 430–436. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, B.; Cai, Q.T.; Li, X.X.; Liu, M.; Hu, D.; Guo, D.B.; Wang, J.; Fan, C. Bioremediation of Hexavalent Chromium Pollution by Sporosarcina Saromensis M52 Isolated from Offshore Sediments in Xiamen, China. Biomed. Environ. Sci. 2016, 29, 127–136. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Hexavalent Chromium Stress Response, Reduction Capability and Bioremediation Potential of Trichoderma Sp. Isolated from Electroplating Wastewater. Ecotoxicol. Environ. Saf. 2019, 185, 109734. [Google Scholar] [CrossRef]
- Antony, G.S.; Manna, A.; Baskaran, S.; Puhazhendi, P.; Ramchary, A.; Niraikulam, A.; Ramudu, K.N. Non-Enzymatic Reduction of Cr (VI) and It’s Effective Biosorption Using Heat-Inactivated Biomass: A Fermentation Waste Material. J. Hazard. Mater. 2020, 392, 122257. [Google Scholar] [CrossRef]
- Li, J.; Tang, C.; Zhang, M.; Fan, C.; Guo, D.; An, Q.; Wang, G.; Xu, H.; Li, Y.; Zhang, W.; et al. Exploring the Cr(VI) Removal Mechanism of Sporosarcina Saromensis M52 from a Genomic Perspective. Ecotoxicol. Environ. Saf. 2021, 225, 112767. [Google Scholar] [CrossRef] [PubMed]
- Dadrasnia, A.; Ismail, S. Biosurfactant Production by Bacillus Salmalaya for Lubricating Oil Solubilization and Biodegradation. Int. J. Environ. Res. Public Health 2015, 12, 9848–9863. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Nahar, N.; Nawani, N.N.; Jass, J.; Hossain, K.; Saud, Z.A.; Saha, A.K.; Ghosh, S.; Olsson, B.; Mandal, A. Bioremediation of Hexavalent Chromium (VI) by a Soil-Borne Bacterium, Enterobacter Cloacae B2-DHA. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2015, 50, 1136–1147. [Google Scholar] [CrossRef]
- Li, H.; Huang, S.; Zhang, Y. Cr(VI) Removal from Aqueous Solution by Thermophilic Denitrifying Bacterium Chelatococcus Daeguensis TAD1 in the Presence of Single and Multiple Heavy Metals. J. Microbiol. 2016, 54, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Congeevaram, S.; Dhanarani, S.; Park, J.; Dexilin, M.; Thamaraiselvi, K. Biosorption of Chromium and Nickel by Heavy Metal Resistant Fungal and Bacterial Isolates. J. Hazard. Mater. 2007, 146, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Pulimi, M.; Jayaraman, G. Statistical Optimization on Chromium (vi) Reduction by Marine Bacteria, Planococcus sp. Vitp21 Using Cane Sugar as Carbon Source. Int. J. Chem. Sci. 2014, 12, 169–180. [Google Scholar]
- El-Naggar, N.E.A.; El-khateeb, A.Y.; Ghoniem, A.A.; El-Hersh, M.S.; Saber, W.E.I.A. Innovative Low-Cost Biosorption Process of Cr6+ by Pseudomonas Alcaliphila NEWG-2. Sci. Rep. 2020, 10, 14043. [Google Scholar] [CrossRef]
- Kalola, V.; Desai, C. Biosorption of Cr(VI) by Halomonas Sp. DK4, a Halotolerant Bacterium Isolated from Chrome Electroplating Sludge. Environ. Sci. Pollut. Res. 2020, 27, 27330–27344. [Google Scholar] [CrossRef]
- Hossain, S.; De Silva, B.C.J.; Dahanayake, P.S.; Heo, G.J. Phylogenetic Relationships, Virulence and Antimicrobial Resistance Properties of Klebsiella Sp. Isolated from Pet Turtles in Korea. Lett. Appl. Microbiol. 2020, 70, 71–78. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Gupta, A.; Gupte, A.; Desai, N. Biotransformation of Chromium by Root Nodule Bacteria Sinorhizobium sp. SAR1. PLoS ONE 2019, 14, e0219387. [Google Scholar] [CrossRef]
- Kang, C.; Wu, P.; Li, Y.; Ruan, B.; Zhu, N.; Dang, Z. Estimates of heavy metal tolerance and chromium(VI) reducing ability of Pseudomonas aeruginosa CCTCC AB93066: Chromium(VI) toxicity and environmental parameters optimization. World J. Microbiol. Biotechnol. 2014, 30, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, J.; Li, W.; Zhang, S.; Wang, F. Hexavalent chromium removal by a resistant strain Bacillus cereus ZY-2009. Environ. Technol. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Malaviya, P. Bioremediation of Tannery Wastewater by Chromium Resistant Novel Fungal Consortium. Ecol. Eng. 2016, 91, 419–425. [Google Scholar] [CrossRef]
- Taştan, B.E.; Ertuǧrul, S.; Dönmez, G. Effective Bioremoval of Reactive Dye and Heavy Metals by Aspergillus versicolor. Bioresour. Technol. 2010, 101, 870–876. [Google Scholar] [CrossRef]
- Mishra, A.; Malik, A. Novel Fungal Consortium for Bioremediation of Metals and Dyes from Mixed Waste Stream. Bioresour. Technol. 2014, 171, 217–226. [Google Scholar] [CrossRef]
- Kumaran, M.D.B.; Prasathkum, M.; Kumar, D.J.M.; Kalaichelv, P.T. Utilization of Aspergillus Terreus for the Biosorption of Hexavalent Chromium Ions. Asian J. Biol. Sci. 2013, 6, 312–321. [Google Scholar] [CrossRef]
- Dao, T.S.; Le, N.-H.-S.; Vo, M.-T.; Vo, T.-M.-C.; Phan, T.-H. Growth and Metal Uptake Capacity of Microalgae under Exposure to Chromium. J. Vietnam. Environ. 2018, 9, 38–43. [Google Scholar] [CrossRef]
- Jaafari, J.; Yaghmaeian, K. Optimization of Heavy Metal Biosorption onto Freshwater Algae (Chlorella Coloniales) Using Response Surface Methodology (RSM). Chemosphere 2019, 217, 447–455. [Google Scholar] [CrossRef]
- Indhumathi, P.; Syed Shabudeen, P.S.; Shoba, U.S.; Saraswathy, C.P. The Removal of Chromium from Aqueous Solution by Using Green Micro Algae. J. Chem. Pharm. Res. 2014, 6, 799–808. [Google Scholar]
- Ayele, A.; Getachew, D.; Kamaraj, M.; Suresh, A. Phycoremediation of Synthetic Dyes: An Effective and Eco-Friendly Algal Technology for the Dye Abatement. J. Chem. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Park, C.H.; Keyhan, M.; Wielinga, B.; Fendorf, S.; Matin, A. Purification to Homogeneity and Characterization of a Novel Pseudomonas Putida Chromate Reductase. Appl. Environ. Microbiol. 2000, 66, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, E.V.; Lialikova, N.N. Crocoite Reduction by a Culture of Pseudomonas Chromatophila sp. Nov. Mikrobiologiia 1979, 48, 517–522. [Google Scholar] [PubMed]
- Learman, D.R.; Ahmad, Z.; Brookshier, A.; Henson, M.W.; Hewitt, V.; Lis, A.; Morrison, C.; Robinson, A.; Todaro, E.; Wologo, E.; et al. Comparative Genomics of 16 Microbacterium spp. That Tolerate Multiple Heavy Metals and Antibiotics. PeerJ 2019, 2019, e6258. [Google Scholar] [CrossRef] [PubMed]
- Sau, G.B.; Chatterjee, S.; Mukherjee, S.K. Chromate Reduction by Cell-Free Extract of Bacillus Firmus KUCr1. Polish J. Microbiol. 2010, 59, 185–190. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Rasheed, R.; Hussain, I.; Iqbal, M.; Farooq, M.U.; Saleem, M.H.; Ali, S. Taurine Modulates Dynamics of Oxidative Defense, Secondary Metabolism, and Nutrient Relation to Mitigate Boron and Chromium Toxicity in Triticum aestivum L. Plants. Environ. Sci. Pollut. Res. 2022, 29, 45527–45548. [Google Scholar] [CrossRef] [PubMed]
- Bharagava, R.N.; Mishra, S. Hexavalent Chromium Reduction Potential of Cellulosimicrobium Sp. Isolated from Common Effluent Treatment Plant of Tannery Industries. Ecotoxicol. Environ. Saf. 2018, 147, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Wani, P.A.; Wahid, S.; Singh, R.; Kehinde, A.M. Antioxidant and Chromium Reductase Assisted Chromium (VI) Reduction and Cr (III) Immobilization by the Rhizospheric Bacillus Helps in the Remediation of Cr (VI) and Growth Promotion of Soybean Crop. Rhizosphere 2018, 6, 23–30. [Google Scholar] [CrossRef]
- Megharaj, M.; Avudainayagam, S.; Naidu, R. Toxicity of Hexavalent Chromium and Its Reduction by Bacteria Isolated from Soil Contaminated with Tannery Waste. Curr. Microbiol. 2003, 47, 51–54. [Google Scholar] [CrossRef]
- Ramírez, V.; Baez, A.; López, P.; Bustillos, R.; Villalobos, M.Á.; Carreño, R.; Contreras, J.L.; Muñoz-Rojas, J.; Fuentes, L.E.; Martínez, J.; et al. Chromium Hyper-Tolerant Bacillus sp. Mh778713 Assists Phytoremediation of Heavy Metals by Mesquite Trees (Prosopis Laevigata). Front. Microbiol. 2019, 10, 1833. [Google Scholar] [CrossRef]
- Baldiris, R.; Acosta-Tapia, N.; Montes, A.; Hernández, J.; Vivas-Reyes, R. Reduction of Hexavalent Chromium and Detection of Chromate Reductase (ChrR) in Stenotrophomonas maltophilia. Molecules 2018, 23, 406. [Google Scholar] [CrossRef]
- Sampedro, M.A.; Blanco, A.; Llama, M.J.; Serra, J.L. Sorption of Heavy Metals to Phormidium laminosum Biomass. Biotechnol. Appl. Biochem. 1995, 22, 355–366. [Google Scholar]
- Wilde, E.W.; Benemann, J.R. Bioremoval of Heavy Metals by the Use of Microalgae. Biotechnol. Adv. 1993, 11, 781–812. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, F.; da Rocha Ferreira, G.L.; Antoniosi Filho, N.R. Biosorption of Hexavalent Chromium by Microorganisms. Int. Biodeterior. Biodegrad. 2017, 119, 87–95. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Duca, G.; Cepoi, L.; Chiriac, T.; Rudi, L.; Mitina, T.; Frontasyeva, M.V.; Pavlov, S.; Gundorina, S.F. Biotechnology of Metal Removal from Industrial Wastewater: Zinc Case Study. Clean Soil Air Water 2015, 43, 112–117. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and Biotransformation of Hexavalent Chromium [Cr(VI)]: A Comprehensive Review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, M.; de la Calle-Vaquero, M.; Yubero, C. Cultural Heritage and Urban Tourism: Historic City Centres under Pressure. Sustainability 2017, 9, 1346. [Google Scholar] [CrossRef]
- Tian, X.R.; Lei, Y.B. Physiological Responses of Wheat Seedlings to Drought and UV-B Radiation. Effect of Exogenous Sodium Nitroprusside Application. Russ. J. Plant Physiol. 2007, 54, 676–682. [Google Scholar] [CrossRef]
- Conrath, U. Priming of Induced Plant Defense Responses. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2009; Volume 51, pp. 361–395. [Google Scholar]
- Paredes, S.D.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Reiter, R.J. Phytomelatonin: A Review. J. Exp. Bot. 2009, 60, 57–69. [Google Scholar] [CrossRef]
- Becquer, T.; Quantin, C.; Sicot, M.; Boudot, J.P. Chromium Availability in Ultramafic Soils from New Caledonia. Sci. Total Environ. 2003, 301, 251–261. [Google Scholar] [CrossRef]
- Bamagoos, A.A.; Mallhi, Z.I.; El-Esawi, M.A.; Rizwan, M.; Ahmad, A.; Hussain, A.; Alharby, H.F.; Alharbi, B.M.; Ali, S. Alleviating Lead-Induced Phytotoxicity and Enhancing the Phytoremediation of Castor Bean (Ricinus communis L.) by Glutathione Application: New Insights into the Mechanisms Regulating Antioxidants, Gas Exchange and Lead Uptake. Int. J. Phytoremediat. 2022, 24, 933–944. [Google Scholar] [CrossRef]
- Xie, C.; Pu, S.; Xiong, X.; Chen, S.; Peng, L.; Fu, J.; Sun, L.; Guo, B.; Jiang, M.; Li, X. Melatonin-Assisted Phytoremediation of Pb-Contaminated Soil Using Bermudagrass. Environ. Sci. Pollut. Res. 2021, 28, 44374–44388. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Pu, L.; Li, A.; Zhu, X.; Zhao, P.; Xu, X.; Lei, N.; Chen, J. Implication of Exogenous Abscisic Acid (ABA) Application on Phytoremediation: Plants Grown in Co-Contaminated Soil. Environ. Sci. Pollut. Res. 2022, 29, 8684–8693. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Y.; Wang, Y.S.; Ying, G.G. Cadmium-Inducible BgMT2, a Type 2 Metallothionein Gene from Mangrove Species (Bruguiera gymnorrhiza), Its Encoding Protein Shows Metal-Binding Ability. J. Exp. Mar. Biol. Ecol. 2011, 405, 128–132. [Google Scholar] [CrossRef]
- Chen, L.; Ren, F.; Zhong, H.; Jiang, W.; Li, X. Identification and Expression Analysis of Genes in Response to High-Salinity and Drought Stresses in Brassica napus. Acta Biochim. Biophys. Sin. 2010, 42, 154–164. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, X.; Zhao, Y.; Jiao, K.; Herbert, S.J.; Hao, L. Salicylic Acid, Hydrogen Peroxide and Calcium-Induced Saline Tolerance Associated with Endogenous Hydrogen Peroxide Homeostasis in Naked Oat Seedlings. Plant Growth Regul. 2008, 54, 249–259. [Google Scholar] [CrossRef]
- Hu, Y.; Ge, Y.; Zhang, C.; Ju, T.; Cheng, W. Cadmium Toxicity and Translocation in Rice Seedlings Are Reduced by Hydrogen Peroxide Pretreatment. Plant Growth Regul. 2009, 59, 51–61. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef]
- Basit, F.; Bhat, J.A.; Hu, J.; Kaushik, P.; Ahmad, A.; Guan, Y.; Ahmad, P. Brassinosteroid Supplementation Alleviates Chromium Toxicity in Soybean (Glycine max L.) via Reducing Its Translocation. Plants 2022, 11, 2292. [Google Scholar] [CrossRef]
- Hao, L.H.; He, P.Q.; Liu, C.Y.; Chen, K.S.; Li, G.Y. Physiological Effects of Taurine on the Growth of Wheat (Triticum aestivum L.) Seedlings. J. Plant Physiol. Mol. Biol. 2004, 30, 595–598. [Google Scholar]
- Ahmad, R.; Ali, S.; Abid, M.; Rizwan, M.; Ali, B.; Tanveer, A.; Ahmad, I.; Azam, M.; Ghani, M.A. Glycinebetaine Alleviates the Chromium Toxicity in Brassica oleracea L. by Suppressing Oxidative Stress and Modulating the Plant Morphology and Photosynthetic Attributes. Environ. Sci. Pollut. Res. 2020, 27, 1101–1111. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, L.Y.; Di Hu, K.; He, Y.D.; Wang, S.H.; Luo, J.P. Hydrogen Sulfide Promotes Wheat Seed Germination and Alleviates Oxidative Damage against Copper Stress. J. Integr. Plant Biol. 2008, 50, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ye, Y.-K.; Wang, S.-H.; Luo, J.-P.; Tang, J.; Ma, D.-F. Hydrogen Sulfide Counteracts Chlorophyll Loss in Sweetpotato Seedling Leaves and Alleviates Oxidative Damage against Osmotic Stress. Plant Growth Regul. 2009, 58, 243–250. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Li, X.; Xin, C.; Si, J.; Li, S.; Li, Y.; Zheng, X.; Li, H.; Wei, X.; et al. Nano-ZnO Priming Induces Salt Tolerance by Promoting Photosynthetic Carbon Assimilation in Wheat. Arch. Agron. Soil Sci. 2020, 66, 1259–1273. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Hussain, S.; Yasmeen, T.; Abbasi, G.H.; Zhang, G. Alleviation of Chromium Toxicity by Hydrogen Sulfide in Barley. Environ. Toxicol. Chem. 2013, 32, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, X.; Shi, C.; Guo, J.; Ma, P.; Ren, X.; Wei, T.; Liu, H.; Li, J. Hydrogen Sulfide Decreases Cd Translocation from Root to Shoot through Increasing Cd Accumulation in Cell Wall and Decreasing Cd2+ Influx in Isatis Indigotica. Plant Physiol. Biochem. 2020, 155, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Li, X.; Huang, R.; Nian, H. Application of Exogenous Glutathione Decreases Chromium Translocation and Alleviates Its Toxicity in Soybean (Glycine max L.). Ecotoxicol. Environ. Saf. 2022, 234, 113405. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Inhibitory Activities of Soluble and Bound Millet Seed Phenolics on Free Radicals and Reactive Oxygen Species. J. Agric. Food Chem. 2011, 59, 428–436. [Google Scholar] [CrossRef]
- Barrameda-Medina, Y.; Montesinos-Pereira, D.; Romero, L.; Blasco, B.; Ruiz, J.M. Role of GSH Homeostasis under Zn Toxicity in Plants with Different Zn Tolerance. Plant Sci. 2014, 227, 110–121. [Google Scholar] [CrossRef]
- Gill, R.A.; Ali, B.; Islam, F.; Farooq, M.A.; Gill, M.B.; Mwamba, T.M.; Zhou, W. Physiological and Molecular Analyses of Black and Yellow Seeded Brassica napus Regulated by 5-Aminolivulinic Acid under Chromium Stress. Plant Physiol. Biochem. 2015, 94, 130–143. [Google Scholar] [CrossRef]
- Fang, Z.; Hu, Z.; Yin, X.; Song, G.; Cai, Q. Exogenous Glutathione Alleviation of Cd Toxicity in Italian Ryegrass (Lolium multiflorum) by Modulation of the Cd Absorption, Subcellular Distribution, and Chemical Form. Int. J. Environ. Res. Public Health 2020, 17, 8143. [Google Scholar] [CrossRef]
- Nakamura, S.-i.; Suzui, N.; Yin, Y.G.; Ishii, S.; Fujimaki, S.; Kawachi, N.; Rai, H.; Matsumoto, T.; Sato-Izawa, K.; Ohkama-Ohtsu, N. Effects of Enhancing Endogenous and Exogenous Glutathione in Roots on Cadmium Movement in Arabidopsis thaliana. Plant Sci. 2020, 290, 110304. [Google Scholar] [CrossRef] [PubMed]
- Adrees, M.; Ali, S.; Iqbal, M.; Aslam Bharwana, S.; Siddiqi, Z.; Farid, M.; Ali, Q.; Saeed, R.; Rizwan, M. Mannitol Alleviates Chromium Toxicity in Wheat Plants in Relation to Growth, Yield, Stimulation of Anti-Oxidative Enzymes, Oxidative Stress and Cr Uptake in Sand and Soil Media. Ecotoxicol. Environ. Saf. 2015, 122, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Askari, S.H.; Ashraf, M.A.; Ali, S.; Rizwan, M.; Rasheed, R. Menadione Sodium Bisulfite Alleviated Chromium Effects on Wheat by Regulating Oxidative Defense, Chromium Speciation, and Ion Homeostasis. Environ. Sci. Pollut. Res. 2021, 28, 36205–36225. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Jamil, H.M.A.; Hayat, M.T.; Mahmood, Q.; Ali, S. Use of Phytohormones to Improve Abiotic Stress Tolerance in Wheat. In Wheat Production in Changing Environments; Springer: Singapore, 2019; pp. 465–479. [Google Scholar]
- Mir, R.A.; Bhat, B.A.; Yousuf, H.; Islam, S.T.; Raza, A.; Rizvi, M.A.; Charagh, S.; Albaqami, M.; Sofi, P.A.; Zargar, S.M. Multidimensional Role of Silicon to Activate Resilient Plant Growth and to Mitigate Abiotic Stress. Front. Plant Sci. 2022, 13, 819658. [Google Scholar] [CrossRef]
- Mumtaz, M.A.; Hao, Y.; Mehmood, S.; Shu, H.; Zhou, Y.; Jin, W.; Chen, C.; Li, L.; Altaf, M.A.; Wang, Z. Physiological and Transcriptomic Analysis Provide Molecular Insight into 24-Epibrassinolide Mediated Cr(VI)-Toxicity Tolerance in Pepper Plants. Environ. Pollut. 2022, 306, 119375. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Kanwar, M.; Bhardwaj, R.; Yu, J.Q.; Tran, L.S.P. Chromium Stress Mitigation by Polyamine-Brassinosteroid Application Involves Phytohormonal and Physiological Strategies in Raphanus sativus L. PLoS ONE 2012, 7, e33210. [Google Scholar] [CrossRef]
- Gangwar, S.; Singh, V.P. Indole Acetic Acid Differently Changes Growth and Nitrogen Metabolism in Pisum sativum L. Seedlings under Chromium (VI) Phytotoxicity: Implication of Oxidative Stress. Sci. Hortic. 2011, 129, 321–328. [Google Scholar] [CrossRef]
- Husain, T.; Suhel, M.; Prasad, S.M.; Singh, V.P. Ethylene and Hydrogen Sulphide Are Essential for Mitigating Hexavalent Chromium Stress in Two Pulse Crops. Plant Biol. 2022, 24, 652–659. [Google Scholar] [CrossRef]
- Kamran, M.; Wang, D.; Alhaithloul, H.A.S.; Alghanem, S.M.; Aftab, T.; Xie, K.; Lu, Y.; Shi, C.; Sun, J.; Gu, W.; et al. Jasmonic Acid-Mediated Enhanced Regulation of Oxidative, Glyoxalase Defense System and Reduced Chromium Uptake Contributes to Alleviation of Chromium (VI) Toxicity in Choysum (Brassica parachinensis L.). Ecotoxicol. Environ. Saf. 2021, 208, 111758. [Google Scholar] [CrossRef]
- Jan, S.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Siddique, K.H.; Ahmad, P. Interactive Effect of 24-Epibrassinolide and Silicon Alleviates Cadmium Stress via the Modulation of Antioxidant Defense and Glyoxalase Systems and Macronutrient Content in Pisum sativum L. Seedlings. BMC Plant Biol. 2018, 18, 146. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal Oxides as Photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ulhassan, Z.; Shah, A.M.; Khan, A.R.; Azhar, W.; Hamid, Y.; Hussain, S.; Sheteiwy, M.S.; Salam, A.; Zhou, W. Salicylic Acid Underpins Silicon in Ameliorating Chromium Toxicity in Rice by Modulating Antioxidant Defense, Ion Homeostasis and Cellular Ultrastructure. Plant Physiol. Biochem. 2021, 166, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.D.E.S.; Oliveira, H.C.; Fraceto, L.F.; Santaella, C. Nanotechnology Potential in Seed Priming for Sustainable Agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Mattos, B.D.; Antunes, D.R.; Forini, M.M.L.; Monikh, F.A.; Rojas, O.J. Foliage Adhesion and Interactions with Particulate Delivery Systems for Plant Nanobionics and Intelligent Agriculture. Nano Today 2021, 37, 101078. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, F.; Wang, W.; Zhang, S.; Wang, F. Remediation of Cr(VI)-Contaminated Soil by Nano-Zero-Valent Iron in Combination with Biochar or Humic Acid and the Consequences for Plant Performance. Toxics 2020, 8, 26. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, S.; Tripathi, D.K.; Prasad, S.M.; Chauhan, D.K. Plant Responses to Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 3030367398. [Google Scholar]
- Saleh, M.M.; Alnaddaf, L.M.; Almuhammady, A.K.; Salem, K.F.M.; Alloosh, M.T.; Al-Khayri, J.M. Applications of Plant-Derived Nanomaterials in Mitigation of Crop Abiotic Stress. In Nanobiotechnology; Springer: Cham, Switzerland, 2021; pp. 201–238. [Google Scholar]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Abdullah Alsahli, A.; Jan, S.; Ahmad, P. Seed Priming with Titanium Dioxide Nanoparticles Enhances Seed Vigor, Leaf Water Status, and Antioxidant Enzyme Activities in Maize (Zea mays L.) under Salinity Stress. J. King Saud Univ. Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Ye, Y.; Cota-Ruiz, K.; Hernández-Viezcas, J.A.; Valdés, C.; Medina-Velo, I.A.; Turley, R.S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Manganese Nanoparticles Control Salinity-Modulated Molecular Responses in Capsicum annuum L. Through Priming: A Sustainable Approach for Agriculture. ACS Sustain. Chem. Eng. 2020, 8, 1427–1436. [Google Scholar] [CrossRef]
- Khalaki, M.A.; Ghorbani, A.; Dadjou, F. Influence of Nano-Priming on Festuca Ovina Seed Germination and Early Seedling Traits under Drought Stress, in Laboratory Condition. Ecopersia 2019, 7, 133–139. [Google Scholar]
- El-Badri, A.M.A.; Batool, M.; Mohamed, I.A.A.; Khatab, A.; Sherif, A.; Wang, Z.; Salah, A.; Nishawy, E.; Ayaad, M.; Kuai, J.; et al. Modulation of Salinity Impact on Early Seedling Stage via Nano-Priming Application of Zinc Oxide on Rapeseed (Brassica napus L.). Plant Physiol. Biochem. 2021, 166, 376–392. [Google Scholar] [CrossRef]
- Ismail, G.; Abou-Zeid, H. The Role of Priming with Biosynthesized Silver Nanoparticles in the Response of Triticum aestivum L. to Salt Stress. Egypt. J. Bot. 2018, 58, 73–85. [Google Scholar] [CrossRef]
- Yousefi, S.; Kartoolinejad, D.; Naghdi, R. Effects of Priming with Multi-Walled Carbon Nanotubes on Seed Physiological Characteristics of Hopbush (Dodonaeaviscosa L.) under Drought Stress. Int. J. Environ. Stud. 2017, 74, 528–539. [Google Scholar] [CrossRef]
- Sharma, A.; Vishwakarma, K.; Singh, N.K.; Prakash, V.; Ramawat, N.; Prasad, R.; Sahi, S.; Singh, V.P.; Tripathi, D.K.; Sharma, S. Synergistic Action of Silicon Nanoparticles and Indole Acetic Acid in Alleviation of Chromium (CrVI) Toxicity in Oryza sativa Seedlings. J. Biotechnol. 2022, 343, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Jasim, B.; Thomas, R.; Mathew, J.; Radhakrishnan, E.K. Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharm. J. 2017, 25, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Mfarrej, M.F.B.; El-Esawi, M.A.; Waseem, M.; Alatawi, A.; Nafees, M.; Saleem, M.H.; Rizwan, M.; Yasmeen, T.; Anayat, A.; et al. Chromium-Resistant Staphylococcus aureus Alleviates Chromium Toxicity by Developing Synergistic Relationships with Zinc Oxide Nanoparticles in Wheat. Ecotoxicol. Environ. Saf. 2022, 230, 113142. [Google Scholar] [CrossRef]
- Rao, S.; Shekhawat, G.S. Toxicity of ZnO Engineered Nanoparticles and Evaluation of Their Effect on Growth, Metabolism and Tissue Specific Accumulation in Brassica juncea. J. Environ. Chem. Eng. 2014, 2, 105–114. [Google Scholar] [CrossRef]
- Malik, Z.; Afzal, S.; Dawood, M.; Abbasi, G.H.; Khan, M.I.; Kamran, M.; Zhran, M.; Hayat, M.T.; Aslam, M.N.; Rafay, M. Exogenous Melatonin Mitigates Chromium Toxicity in Maize Seedlings by Modulating Antioxidant System and Suppresses Chromium Uptake and Oxidative Stress. Environ. Geochem. Health 2022, 44, 1451–1469. [Google Scholar] [CrossRef]
- Kharbech, O.; Sakouhi, L.; Mahjoubi, Y.; Ben Massoud, M.; Debez, A.; Zribi, O.T.; Djebali, W.; Chaoui, A.; Mur, L.A.J. Nitric Oxide Donor, Sodium Nitroprusside Modulates Hydrogen Sulfide Metabolism and Cysteine Homeostasis to Aid the Alleviation of Chromium Toxicity in Maize Seedlings (Zea mays L.). J. Hazard. Mater. 2022, 424, 127302. [Google Scholar] [CrossRef]
- Singh, D.; Singh, C.K.; Singh, D.; Sarkar, S.K.; Prasad, S.K.; Sharma, N.L.; Singh, I. Glycine Betaine Modulates Chromium (VI)-Induced Morpho-Physiological and Biochemical Responses to Mitigate Chromium Toxicity in Chickpea (Cicer arietinum L.) Cultivars. Sci. Rep. 2022, 12, 8005. [Google Scholar] [CrossRef]
- Singh, S.; Dubey, N.K.; Singh, V.P. Nitric Oxide and Hydrogen Peroxide Independently Act in Mitigating Chromium Stress in Triticum aestivum L. Seedlings: Regulation of Cell Death, Chromium Uptake, Antioxidant System, Sulfur Assimilation and Proline Metabolism. Plant Physiol. Biochem. 2022, 183, 76–84. [Google Scholar] [CrossRef]
- Ilyas, N.; Akhtar, N.; Yasmin, H.; Sahreen, S.; Hasnain, Z.; Kaushik, P.; Ahmad, A.; Ahmad, P. Efficacy of Citric Acid Chelate and Bacillus Sp. in Amelioration of Cadmium and Chromium Toxicity in Wheat. Chemosphere 2022, 290, 133342. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Yousaf, H.S.; Malik, A.; Abbas, Z.; Rizwan, M.; Abualreesh, M.H.; Alatawi, A.; Wang, X. Combined Application of Zinc and Iron-Lysine and Its Effects on Morpho-Physiological Traits, Antioxidant Capacity and Chromium Uptake in Rapeseed (Brassica napus L.). PLoS ONE 2022, 17, e0262140. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Alshaya, H.; Okla, M.K.; Alwasel, Y.A.; Chen, F.; Adrees, M.; Hussain, A.; Hameed, S.; Shahid, M.J. Application of Cerium Dioxide Nanoparticles and Chromium-Resistant Bacteria Reduced Chromium Toxicity in Sunflower Plants. Front. Plant Sci. 2022, 13, 876119. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.F.; Ali, S. Combined Role of Fe Nanoparticles (Fe NPs) and Staphylococcus aureus L. in the Alleviation of Chromium Stress in Rice Plants. Life 2022, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Prakash, V.; Rai, P.; Sharma, N.C.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Sahi, S. Application of Zinc Oxide Nanoparticles as Fertilizer Boosts Growth in Rice Plant and Alleviates Chromium Stress by Regulating Genes Involved in Oxidative Stress. Chemosphere 2022, 303, 134554. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Shahid, M.; Ahmed, T.; Tahir, M.; Naqqash, T.; Muhammad, S.; Song, F.; Abid, H.M.A.; Aslam, Z. Green Copper Nanoparticles from a Native Klebsiella Pneumoniae Strain Alleviated Oxidative Stress Impairment of Wheat Plants by Reducing the Chromium Bioavailability and Increasing the Growth. Ecotoxicol. Environ. Saf. 2020, 192, 110303. [Google Scholar] [CrossRef] [PubMed]
- López-Luna, J.; Silva-Silva, M.J.; Martinez-Vargas, S.; Mijangos-Ricardez, O.F.; González-Chávez, M.C.; Solís-Domínguez, F.A.; Cuevas-Díaz, M.C. Magnetite Nanoparticle (NP) Uptake by Wheat Plants and Its Effect on Cadmium and Chromium Toxicological Behavior. Sci. Total Environ. 2015, 565, 941–950. [Google Scholar] [CrossRef]
- Mohammadi, H.; Hatami, M.; Feghezadeh, K.; Ghorbanpour, M. Mitigating Effect of Nano-Zerovalent Iron, Iron Sulfate and EDTA against Oxidative Stress Induced by Chromium in Helianthus annuus L. Acta Physiol. Plant. 2018, 40, 69. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Khan, I.; Hussain, M.; Khan, A.R.; Hamid, Y.; Hussain, S.; Allakhverdiev, S.I.; Zhou, W. Efficacy of Metallic Nanoparticles in Attenuating the Accumulation and Toxicity of Chromium in Plants: Current Knowledge and Future Perspectives. Environ. Pollut. 2022, 315, 120390. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. Ionomic Approaches for Discovery of Novel Stress-resilient Genes in Plants. Int. J. Mol. Sci. 2021, 22, 7182. [Google Scholar] [CrossRef]
- Tyagi, A.; Sharma, S.; Srivastava, H.; Singh, A.; Kaila, T.; Ali, S.; Gaikwad, A.B.; Singh, N.K.; Gaikwad, D. Transcriptome Profiling of Two Contrasting Pigeon Pea (Cajanus cajan) Genotypes in Response to Waterloging Stress. Front. Genet. 2023, 13, 3757. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Singh, N.; Ali, S.; Srivastava, H.; Mushtaq, M.; Mir, Z.A. Metabolomic Approaches to Study Nutritional Aspects in Cereal Crops. In Biofortification in Cereals: Progress and Prospects; Springer: Berlin/Heidelberg, Germany, 2023; pp. 127–148. [Google Scholar]
- Goupil, P.; Souguir, D.; Ferjani, E.; Faure, O.; Hitmi, A.; Ledoigt, G. Expression of Stress-Related Genes in Tomato Plants Exposed to Arsenic and Chromium in Nutrient Solution. J. Plant Physiol. 2009, 166, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Ali, B.; Cui, P.; Shen, E.; Farooq, M.A.; Islam, F.; Ali, S.; Mao, B.; Zhou, W. Comparative Transcriptome Profiling of Two Brassica napus Cultivars under Chromium Toxicity and Its Alleviation by Reduced Glutathione. BMC Genom. 2016, 17, 885. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Marsoni, M.; Bracale, M.; Sestili, S.; Ficcadenti, N.; Speranza, A.; Crinelli, R.; Carloni, E.; Scoccianti, V. Proteomic Changes and Molecular Effects Associated with Cr(III) and Cr(VI) Treatments on Germinating Kiwifruit Pollen. Phytochemistry 2011, 72, 1786–1795. [Google Scholar] [CrossRef]
- Sharmin, S.A.; Alam, I.; Kim, K.H.; Kim, Y.G.; Kim, P.J.; Bahk, J.D.; Lee, B.H. Chromium-Induced Physiological and Proteomic Alterations in Roots of Miscanthus sinensis. Plant Sci. 2012, 187, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Fulekar, M.H.; Singh, A.; Bhaduri, A.M. Genetic Engineering Strategies for Enhancing Phytoremediation of Heavy Metals. Afr. J. Biotechnol. 2009, 8, 529–535. [Google Scholar]
- Mushtaq, M.; Bhat, J.A.; Mir, Z.A.; Sakina, A.; Ali, S.; Singh, A.K.; Tyagi, A.; Salgotra, R.K.; Dar, A.A.; Bhat, R. CRISPR/Cas Approach: A New Way of Looking at Plant-Abiotic Interactions. J. Plant Physiol. 2018, 224, 156–162. [Google Scholar] [CrossRef]
- Tyagi, A.; Sharma, S.; Vats, S.; Ali, S.; Kumar, S.; Gulzar, N.; Deshmukh, R. Translational Research Using CRISPR/Cas. In CRISPR/Cas Genome Editing: Strategies and Potential for Crop Improvement; Springer: Cham, Switzerland, 2020; pp. 165–191. [Google Scholar]
- Mushtaq, M.; Dar, A.A.; Skalicky, M.; Tyagi, A.; Bhagat, N.; Basu, U.; Bhat, B.A.; Zaid, A.; Ali, S.; Dar, T.U.H.; et al. Crispr-Based Genome Editing Tools: Insights into Technological Breakthroughs and Future Challenges. Genes 2021, 12, 797. [Google Scholar] [CrossRef]
- Mushtaq, M.; Dar, A.A.; Basu, U.; Bhat, B.A.; Mir, R.A.; Vats, S.; Dar, M.S.; Tyagi, A.; Ali, S.; Bansal, M.; et al. Integrating CRISPR-Cas and Next Generation Sequencing in Plant Virology. Front. Genet. 2021, 12, 1914. [Google Scholar] [CrossRef]
- Basu, U.; Riaz Ahmed, S.; Bhat, B.A.; Anwar, Z.; Ali, A.; Ijaz, A.; Gulzar, A.; Bibi, A.; Tyagi, A.; Nebapure, S.M.; et al. A CRISPR Way for Accelerating Cereal Crop Improvement: Progress and Challenges. Front. Genet. 2023, 13, 866976. [Google Scholar] [CrossRef]
- Pérez-Palacios, P.; Agostini, E.; Ibáñez, S.G.; Talano, M.A.; Rodríguez-Llorente, I.D.; Caviedes, M.A.; Pajuelo, E. Removal of Copper from Aqueous Solutions by Rhizofiltration Using Genetically Modified Hairy Roots Expressing a Bacterial Cu-Binding Protein. Environ. Technol. 2017, 38, 2877–2888. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Tyagi, A.; Mushtaq, M.; Al-Mahmoudi, H.; Bae, H. Harnessing Plant Microbiome for Mitigating Arsenic Toxicity in Sustainable Agriculture. Environ. Pollut. 2022, 300, 118940. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Tyagi, A.; Park, S.; Mir, R.A.; Mushtaq, M.; Bhat, B.; Al-Mahmoudi, H.; Bae, H. Deciphering the Plant Microbiome to Improve Drought Tolerance: Mechanisms and Perspectives. Environ. Exp. Bot. 2022, 201, 104933. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. Plant Microbiome: An Ocean of Possibilities for Improving Disease Resistance in Plants. Microorganisms 2023, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation Techniques for Removal of Heavy Metals from the Soil Contaminated through Different Sources: A Review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation Techniques for Heavy Metal-Contaminated Soils: Principles and Applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Wang, K.R. Tolerance of Cultivated Plants to Cadmium and Their Utilization in Polluted Farmland Soils. Acta Biotechnol. 2002, 22, 189–198. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, F.S.; Li, H.F.; Jiang, R.F. Accumulation of Cadmium in the Edible Parts of Six Vegetable Species Grown in Cd-Contaminated Soils. J. Environ. Manag. 2009, 90, 1117–1122. [Google Scholar] [CrossRef]
- Feng, R.W.; Zhao, P.P.; Zhu, Y.M.; Yang, J.G.; Wei, X.Q.; Yang, L.; Liu, H.; Rensing, C.; Ding, Y.Z. Application of Inorganic Selenium to Reduce Accumulation and Toxicity of Heavy Metals (Metalloids) in Plants: The Main Mechanisms, Concerns, and Risks. Sci. Total Environ. 2021, 771, 144776. [Google Scholar] [CrossRef]
- Guo, J.; Tan, X.; Fu, H.-L.; Chen, J.-X.; Lin, X.-X.; Ma, Y.; Yang, Z.-Y. Selection for Cd Pollution-Safe Cultivars of Chinese Kale (Brassica alboglabra L. H. Bailey) and Biochemical Mechanisms of the Cultivar-Dependent Cd Accumulation Involving in Cd Subcellular Distribution. J. Agric. Food Chem. 2018, 66, 1923–1934. [Google Scholar] [CrossRef]
- Gill, R.A.; Zang, L.; Ali, B.; Farooq, M.A.; Cui, P.; Yang, S.; Ali, S.; Zhou, W. Chromium-Induced Physio-Chemical and Ultrastructural Changes in Four Cultivars of Brassica napus L. Chemosphere 2015, 120, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Mao, Y.; Cheng, W.; Wu, F.; Zhang, G. Genotypic and Environmental Variation in Chromium, Cadmium and Lead Concentrations in Rice. Environ. Pollut. 2008, 153, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Almas, F.; Hassan, A.; Bibi, A.; Ali, M.; Lateef, S.; Mahmood, T.; Rasheed, A.; Quraishi, U.M. Identification of Genome-Wide Single-Nucleotide Polymorphisms (SNPs) Associated with Tolerance to Chromium Toxicity in Spring Wheat (Triticum aestivum L.). Plant Soil 2018, 422, 371–384. [Google Scholar] [CrossRef]
- Zeb, A.; Liu, W.; Lian, Y.; Zheng, Z.; Meng, L.; Chen, C.; Song, X. Selection and Breeding of Pollution-Safe Cultivars (PSCs)—An Eco-Friendly Technology for Safe Utilization of Heavy Metal(Loid) Contaminated Soils. Environ. Technol. Innov. 2022, 25, 102142. [Google Scholar] [CrossRef]
- Haider, F.U.; Wang, X.; Farooq, M.; Hussain, S.; Cheema, S.A.; ul Ain, N.; Virk, A.L.; Ejaz, M.; Janyshova, U.; Liqun, C. Biochar Application for the Remediation of Trace Metals in Contaminated Soils: Implications for Stress Tolerance and Crop Production. Ecotoxicol. Environ. Saf. 2022, 230, 113165. [Google Scholar] [CrossRef]
- Nath, K.; Singh, D.; Shyam, S.; Sharma, Y.K. Phytotoxic Effects of Chromium and Tannery Effluent on Growth and Metabolism of Phaseolus mungo Roxb. J. Environ. Biol. 2009, 30, 227–234. [Google Scholar]
- Arthur, E.; Crews, H.; Morgan, C. Optimizing Plant Genetic Strategies for Minimizing Environmental Contamination in the Food Chain: Report on the MAFF Funded Joint JIC/CSL Workshop Held at the John Innes Centre, October 21–23, 1998. Int. J. Phytoremediat. 2000, 2, 1–21. [Google Scholar] [CrossRef]
- Jun, R.; Ling, T.; Guanghua, Z. Effects of Chromium on Seed Germination, Root Elongation and Coleoptile Growth in Six Pulses. Int. J. Environ. Sci. Technol. 2009, 6, 571–578. [Google Scholar] [CrossRef]
- Gouda, G.; Gupta, M.K.; Donde, R.; Mohapatra, T.; Vadde, R.; Behera, L. Marker-Assisted Selection for Grain Number and Yield-Related Traits of Rice (Oryza sativa L.). Physiol. Mol. Biol. Plants 2020, 26, 885–898. [Google Scholar] [CrossRef]
- Qiu, B.; Zhou, W.; Xue, D.; Zeng, F.; Ali, S.; Zhang, G. Identification of Cr-Tolerant Lines in a Rice (Oryza sativa) DH Population. Euphytica 2010, 174, 199–207. [Google Scholar] [CrossRef]

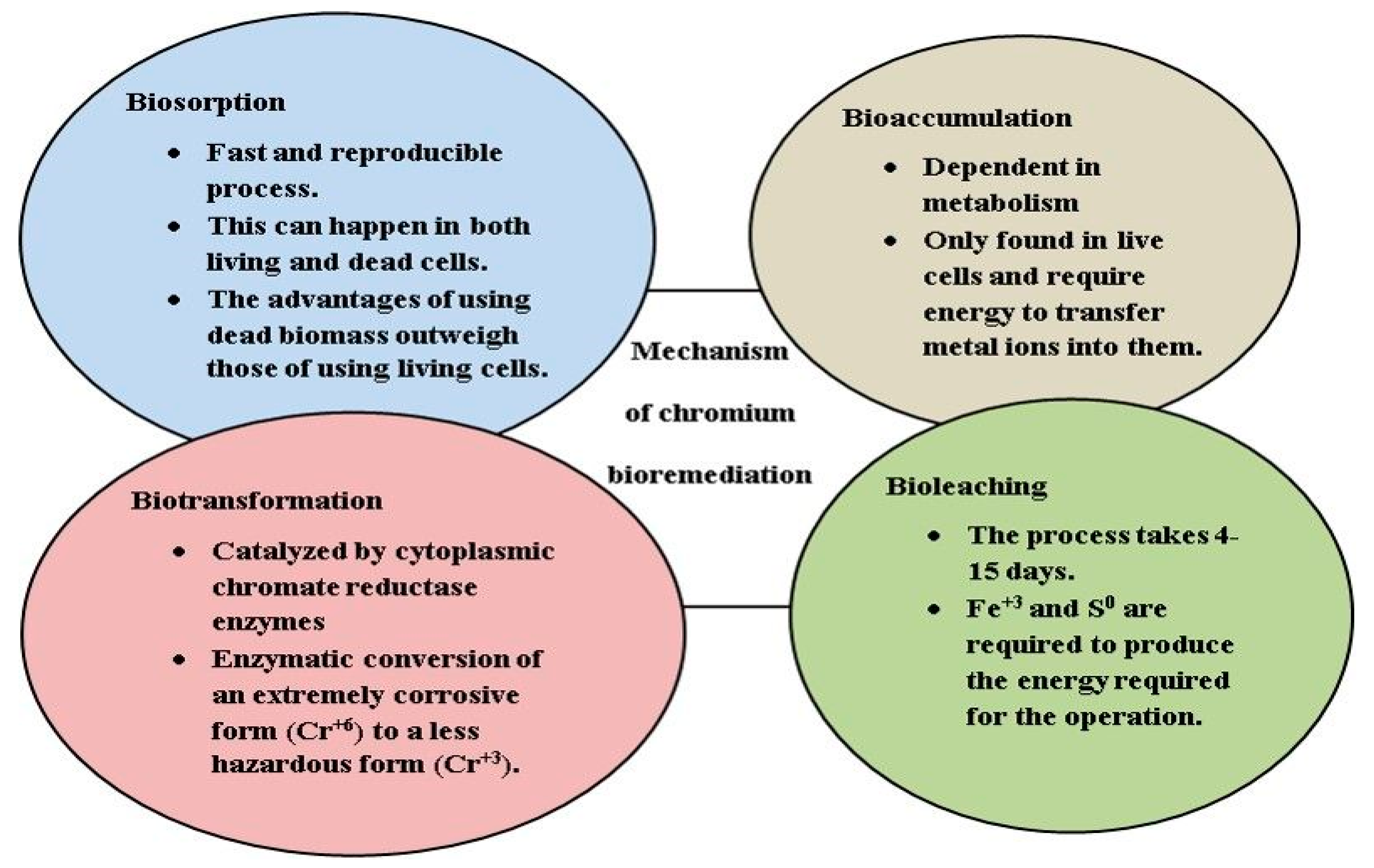
| Microorganisms | pH | Mechanisms | References |
|---|---|---|---|
| Bacteria | |||
| Serratia sp. C8 | 6–8 | Bioreduction | [46] |
| Sphingopyxis macrogoltabida SUK2c | 7 | Bioreduction, Biosorption | [47] |
| Bacillus methylotrophicus | 7 | Bioreduction | [48] |
| Pisolithus sp1 | 5–6 | Bioreduction, Biosorption | [49] |
| Sporosarcina saromensis M52 | 7–8.5 | Bioreduction | [50] |
| Asperillus flavus CR500 | 6.5 | Bioreduction, Biosorption | [51] |
| Leiotrametes flavida | 6 | Biosorption | [52] |
| Sporosarcina saromensis M52 | 2 | Biosorption | [53] |
| Bacillus salmalaya | 3 | Biosorption | [54] |
| Enterobacter cloacae | Biosorption | [55] | |
| Chelatococcus daeguensis | 7 | Biosorption | [56] |
| Micrococcus spp. | 7 | Biosorption | [57] |
| Planococcus sp. VITP21 | 6.8 | Biosorption | [58] |
| Pseudomonas alcaliphila NEWG-2 | 7 | Biosorption | [59] |
| Halomonas sp. DK4 | 6 | Biosorption | [60] |
| Klebsiella spp. | 9 | Biosorption | [61] |
| Sinorhizobium sp. SAR1 | 1 | Biosorption | [62] |
| Pseudomonas aeruginosa CCTCC AB93066 | 7.0 | Biosorption | [63] |
| Bacillus cereus ZY-2009 | 7.0 | Bioreduction | [64] |
| Fungi | |||
| Paecilomyces lilacinus, Penicillium commune, Fusarium equiseti, and Cladosporium perangustum | 4 | Biosorption | [65] |
| Aspergillus versicolor | 6 | Biosorption | [66] |
| Consortium of Rhizopus oryzae, Aspergillus lentulus, and Aspergillus terreus | 6.5 | Biosorption | [67] |
| Aspergillus terreus | Biosorption | [68] | |
| Microalgae | |||
| Pseudanabaena mucicola | 2 | Biosorption | [69] |
| Chlorella colonials | Biosorption | [70] | |
| Chlorella vulagris | 3 | Biosorption | [71] |
| Chlamydomonas spp. | 4 | Biosorption | [72] |
| Name of Compound | Effect on Chromium Toxicity | Alleviated Physiological Effects under Chromium Toxicity | Crop Plant under Investigation | References |
|---|---|---|---|---|
| Chemicals Used for Alleviating Cr Toxicity | ||||
| Menadione sodium bisulfite (MSB) | Considerably reduces accumulation and transport |
| Wheat | [116] |
| Melatonin (MT) (N-acetyl-5-methoxytryptamine) | Detoxification of Cr toxicity |
| Maize | [143] |
| Taurine | Lesser accumulation of Cr in aerial parts of plants |
| Wheat | [77] |
| Hydrogen sulfide | Restriction of uptake |
| Rice Wheat Barley | [107,122] |
| Indole acetic acid | Restriction of uptake |
| Rice | [139] |
| Brassinosteroid | Decreases Cr-induced phytotoxicity by lowering Cr uptake, accumulation, and translocation |
| Soybean | [101] |
| Sodium nitroprusside (SNP) | Restriction of uptake |
| Maize | [144] |
| Glutathione | Increases Cr accumulation Improves Cr tolerance Decreases Cr toxicity |
| Soybean | [109] |
| Glycine betaine | Reduces accumulation of Cr |
| Chickpea | [145] |
| Hydrogen peroxide (H2O2) |
| WheatRice | [99,146] | |
| Citric acid chelate | Reduces accumulation of Cr |
| Wheat | [147] |
| Iron (Fe)–lysine (lys) | Reduces accumulation of Cr |
| Rapeseed | [148] |
| Nitric oxide (NO) | Reduces uptake and accumulation of Cr in roots |
| Wheat | [146] |
| Nanoparticles for Alleviating Cr Toxicity | ||||
| SiNPs | Reduces the uptake and accumulation of Cr |
| RicePea | [124,139] |
| Cerium dioxide nanoparticles (CeO2) | Reduces the uptake and accumulation of Cr6+ and Cr3+ |
| Sunflower plants | [149] |
| Fe nanoparticles (Fe NPs) | Reduces the uptake and accumulation of Cr |
| Rice | [150] |
| Zinc oxide nanoparticles (ZnO NPs) | Detoxification of Cr |
| Wheat Rice | [141,151] |
| Green copper nanoparticles | Immobilizes Cr in the soil |
| Wheat | [152] |
| Citrate-coated magnetite nanoparticles (NPs) | Diminishes the toxicity effects of Cr |
| Wheat | [153] |
| Nano-zerovalent iron Nanoparticles | Decreases Cr uptake and buildup |
| Sunflower | [154] |
| Metallic nanoparticles | Reduces the uptake and toxicity of Cr |
| Rapeseed Rice | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Mir, R.A.; Tyagi, A.; Manzar, N.; Kashyap, A.S.; Mushtaq, M.; Raina, A.; Park, S.; Sharma, S.; Mir, Z.A.; et al. Chromium Toxicity in Plants: Signaling, Mitigation, and Future Perspectives. Plants 2023, 12, 1502. https://doi.org/10.3390/plants12071502
Ali S, Mir RA, Tyagi A, Manzar N, Kashyap AS, Mushtaq M, Raina A, Park S, Sharma S, Mir ZA, et al. Chromium Toxicity in Plants: Signaling, Mitigation, and Future Perspectives. Plants. 2023; 12(7):1502. https://doi.org/10.3390/plants12071502
Chicago/Turabian StyleAli, Sajad, Rakeeb A. Mir, Anshika Tyagi, Nazia Manzar, Abhijeet Shankar Kashyap, Muntazir Mushtaq, Aamir Raina, Suvin Park, Sandhya Sharma, Zahoor A. Mir, and et al. 2023. "Chromium Toxicity in Plants: Signaling, Mitigation, and Future Perspectives" Plants 12, no. 7: 1502. https://doi.org/10.3390/plants12071502
APA StyleAli, S., Mir, R. A., Tyagi, A., Manzar, N., Kashyap, A. S., Mushtaq, M., Raina, A., Park, S., Sharma, S., Mir, Z. A., Lone, S. A., Bhat, A. A., Baba, U., Mahmoudi, H., & Bae, H. (2023). Chromium Toxicity in Plants: Signaling, Mitigation, and Future Perspectives. Plants, 12(7), 1502. https://doi.org/10.3390/plants12071502






