A Modeling Framework to Frame a Biological Invasion: Impatiens glandulifera in North America
Abstract
1. Introduction
2. Materials and Methods
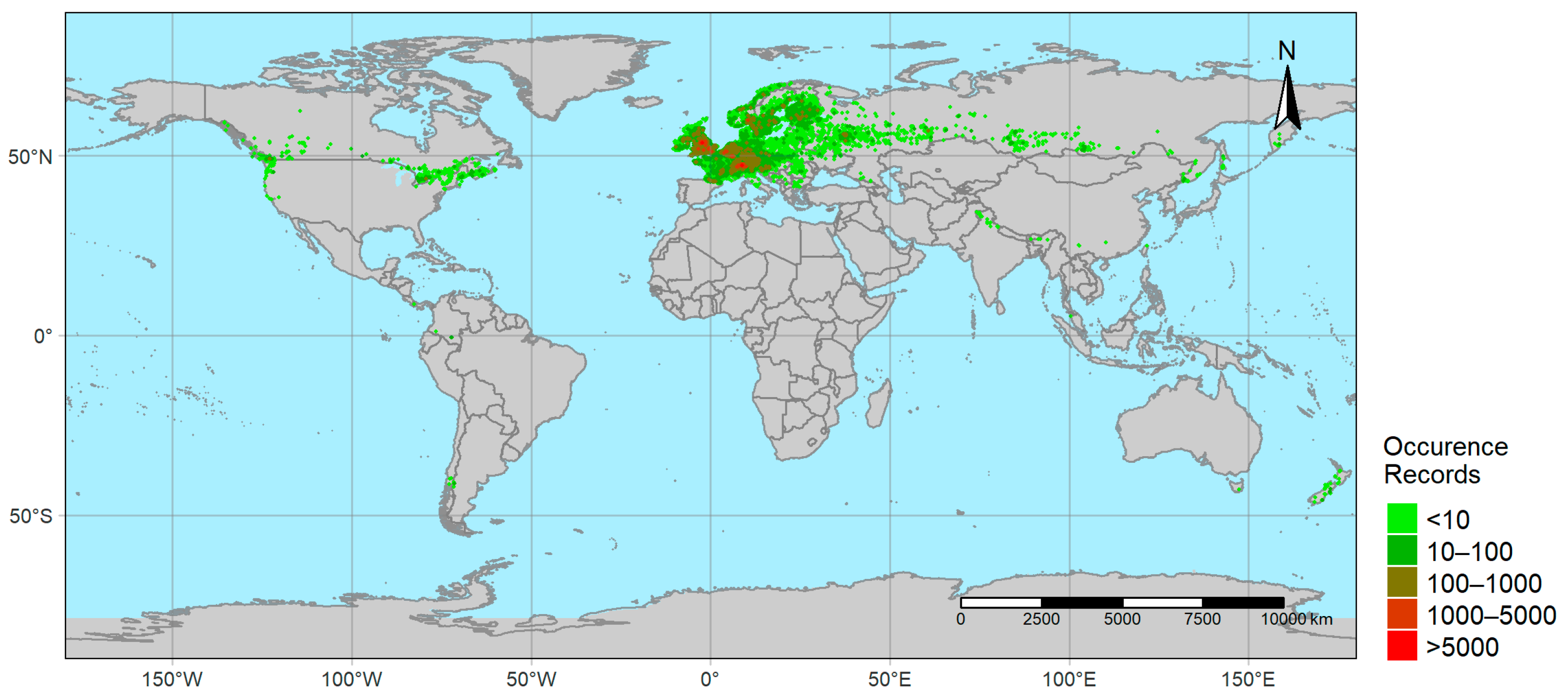
2.1. Occurrence Data
2.2. Environmental Data Layers
2.2.1. Climatic Data Layers
2.2.2. Land Use Data Layers
2.2.3. Elevation and Slope Data Layers
2.2.4. Soil pH Data Layers
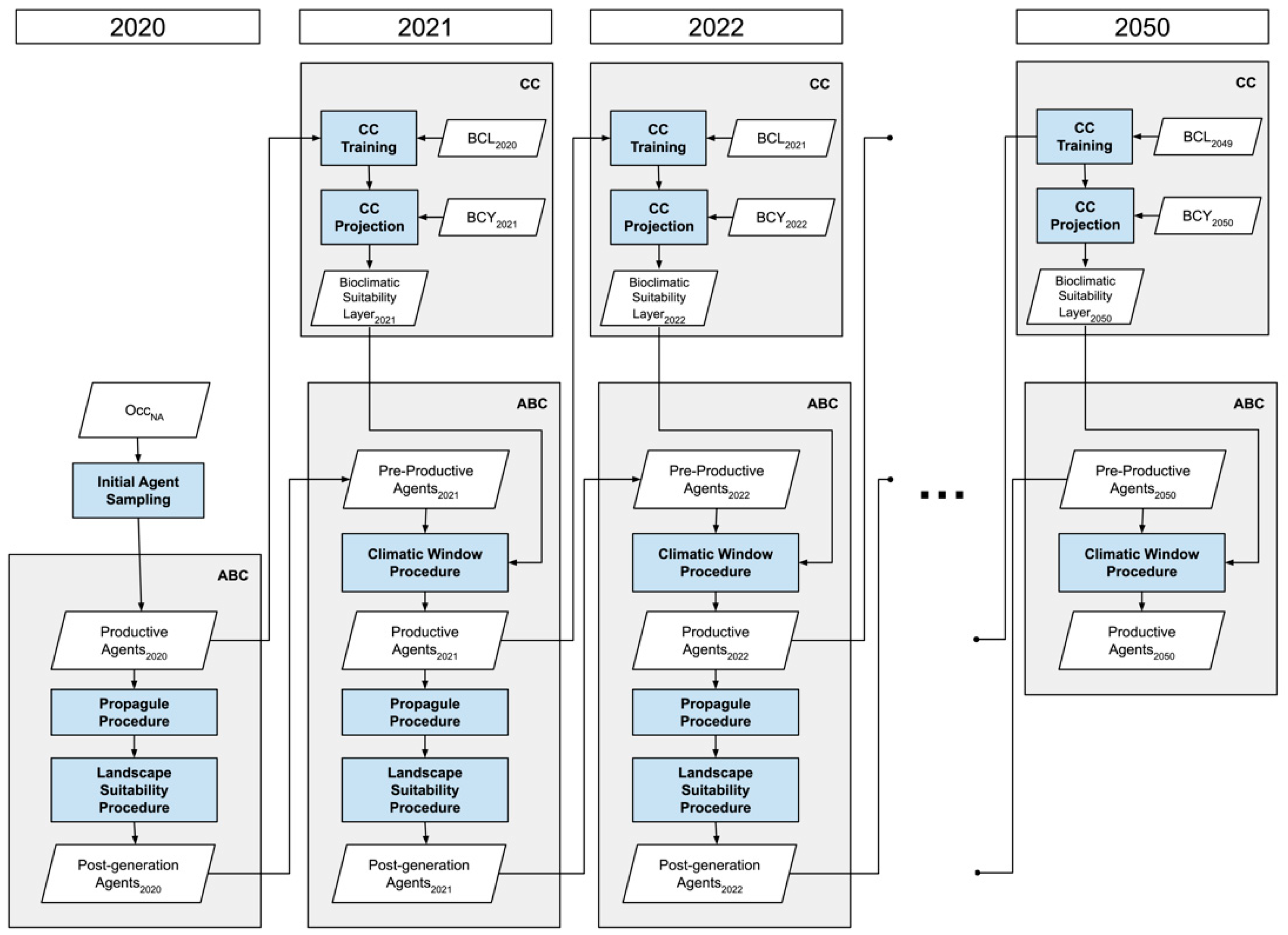
2.3. Structure of the Framework
2.3.1. Correlative Component
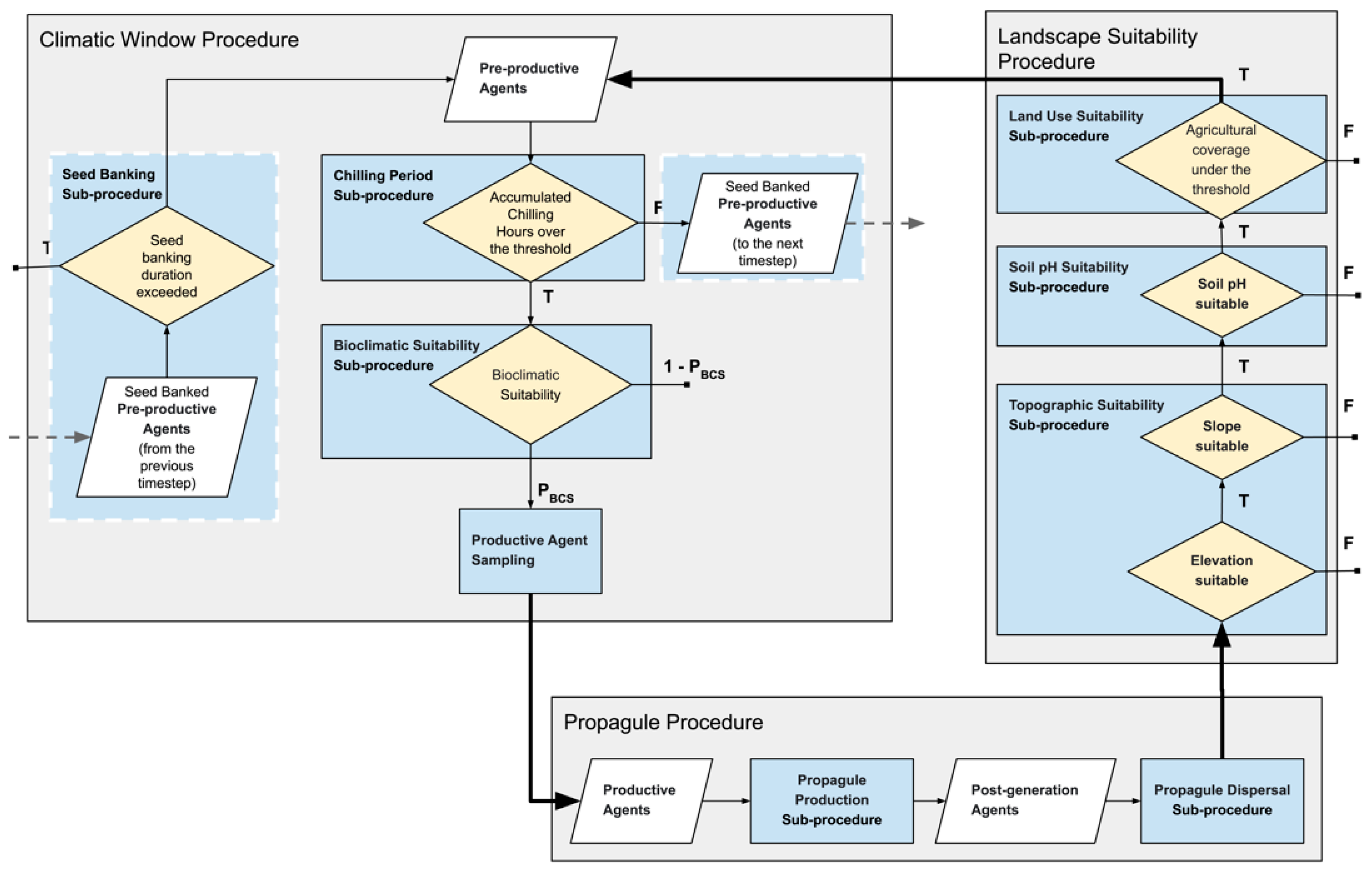
2.3.2. Agent Based Component
Climatic Window Procedure
Propagule Procedure
Landscape Suitability Procedure
2.4. Initialization
2.5. Output
3. Results
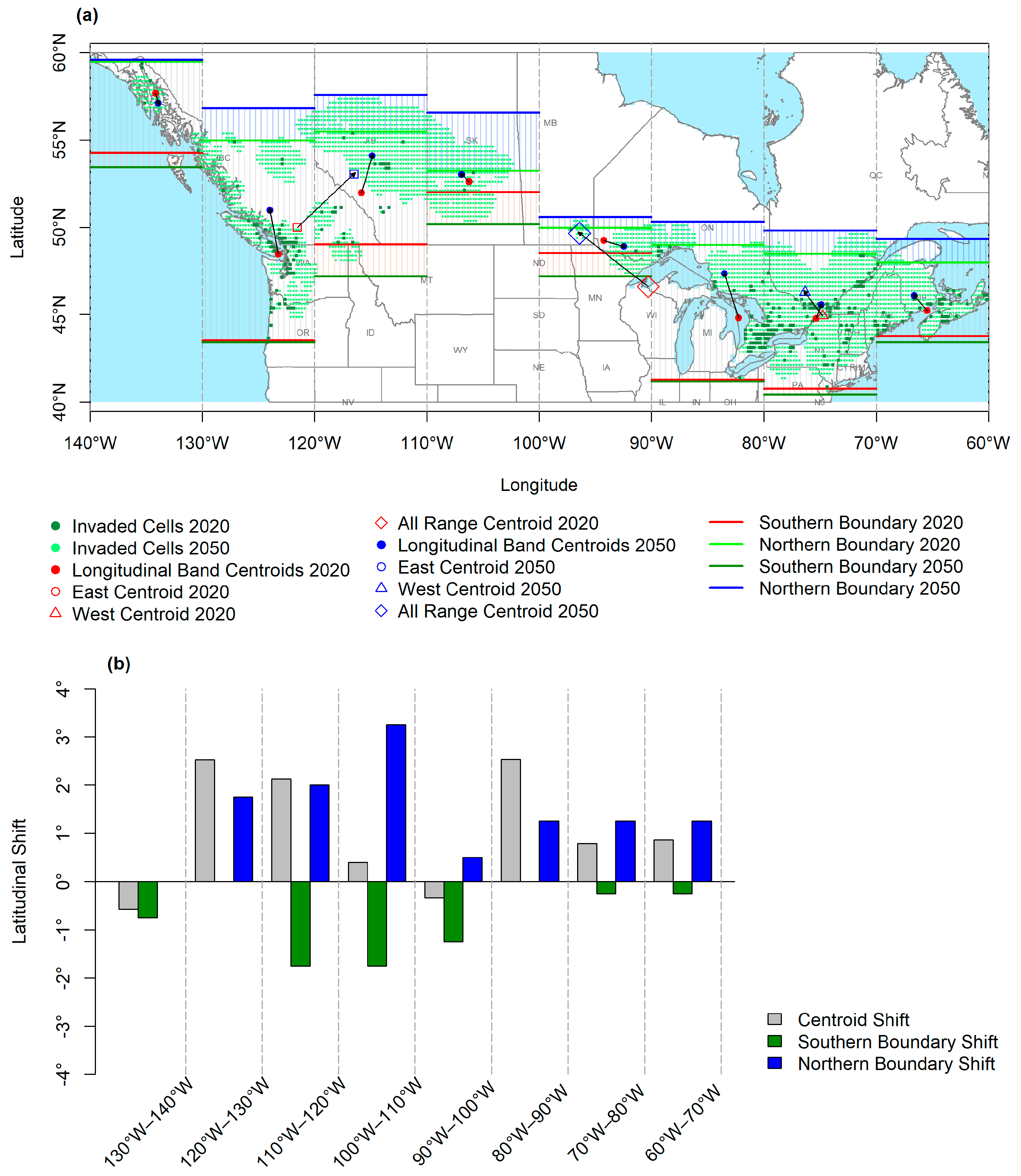
3.1. Geographic Overview
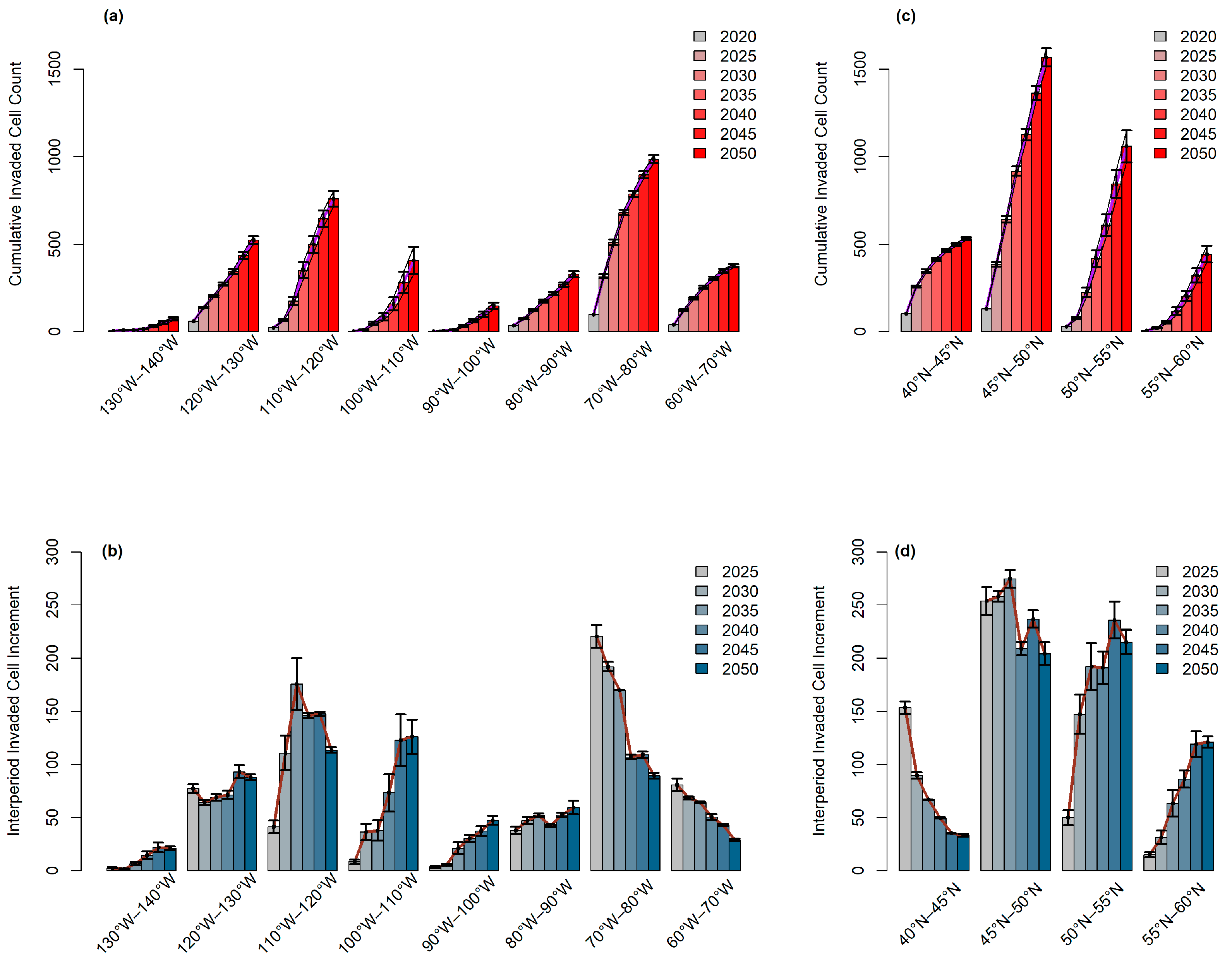
3.2. Longitudinal and Latitudinal Gradients
3.3. Latitudinal Shifts
3.4. Predictive Performance of Correlative Component
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitousek, P.M. Beyond global warming: Ecology and global change. Ecology 1994, 75, 1861–1876. [Google Scholar] [CrossRef]
- Mainka, S.A.; Howard, G.W. Climate. change and invasive species: Double jeopardy. Integr. Zool. 2010, 5, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Masters, G.; Norgrove, L. Climate Change and Invasive Alien Species; CABI Working Paper 1; CABI: Wallingford, UK, 2010; p. 30. [Google Scholar]
- Vitousek, P.M. Global environmental change: An introduction. Annu. Rev. Ecol. Syst. 1992, 23, 1–14. [Google Scholar] [CrossRef]
- Novacek, M.J.; Cleland, E.E. The current biodiversity extinction event: Scenarios for mitigation and recovery. Proc. Natl. Acad. Sci. USA 2001, 98, 5466–5470. [Google Scholar] [CrossRef]
- Evans, E.A. Economic dimensions of invasive species. Choices 2003, 18, 5–9. [Google Scholar] [CrossRef]
- Ziska, L.H.; Blumenthal, D.M.; Runion, G.B.; Hunt, E.R.; Diaz-Soltero, H. Invasive species and climate change: An agronomic perspective. Clim. Chang. 2011, 105, 13–42. [Google Scholar] [CrossRef]
- IUCN. Invasive Alien Species and Climate Change. 2021. Available online: https://www.iucn.org/resources/issues-briefs/invasive-alien-species-and-climate-change (accessed on 7 December 2021).
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Annual review of ecology, evolution, and systematics. Annu. Rev. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Rahel, F.J. Homogenization of freshwater faunas. Annu. Rev. Ecol. Syst. 2002, 33, 291–315. [Google Scholar] [CrossRef]
- Olden, J.D.; Lockwood, J.L.; Parr, C.L. Biological invasions and the homogenization of faunas and floras. Conserv. Biogeogr. 2011, 9, 224–244. [Google Scholar] [CrossRef]
- Brooks, M.L.; Pyke, D.A. Invasive plants and fire in the deserts of North America. In Proceedings of the Invasive Species Workshop: The Role of Fire in the Control and Spread of Invasive Species. Fire Conference 2000: The First National Congress on Fire Ecology, Prevention and Management, San Diego, CA, USA, 27 November–1 December 2000; Miscellaneous Publication No. 11. Galley, K.E.M., Wilson, T.P., Eds.; Tall Timbers Research Station: Tallahassee, FL, USA, 2001; pp. 1–14. [Google Scholar]
- Brooks, M.L.; D’antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of invasive alien plants on fire regimes. BioScience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire and invasive species in Mediterranean-climate ecosystems of California. In Proceedings of the Invasive Species Workshop: The Role of Fire in the Control and Spread of Invasive Species. Fire Conference 2000: The First National Congress on Fire Ecology, Prevention and Management, San Diego, CA, USA, 27 November–1 December 2000; Miscellaneous publication: 11. Galley, K.E.M., Wilson, T.P., Eds.; Tall Timbers Research Station: Tallahassee, FL, USA, 2001; pp. 81–94. [Google Scholar]
- Simberloff, D. How common are invasion-induced ecosystem impacts? Biol. Invasions 2011, 13, 1255–1268. [Google Scholar] [CrossRef]
- Crystal-Ornelas, R.; Hudgins, E.J.; Cuthbert, R.N.; Haubrock, P.J.; Fantle-Lepczyk, J.; Angulo, E.; Kramer, A.M.; Ballesteros-Mejia, L.; Leroy, B.; Leung, B.; et al. Economic costs of biological invasions within North America. NeoBiota 2021, 67, 485. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.C.; Fraser, R.W.; Paini, D.R.; Warden, A.C.; Lonsdale, W.M.; De Barro, P.J. Biosecurity and yield improvement technologies are strategic complements in the fight against food insecurity. PLoS ONE 2011, 6, e26084. [Google Scholar] [CrossRef] [PubMed]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef] [PubMed]
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Thomas, S.M.; Moloney, K.A. Combining the effects of surrounding land-use and propagule pressure to predict the distribution of an invasive plant. Biol. Invasions 2015, 17, 477–495. [Google Scholar] [CrossRef]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W. Climate change and the ecologist. Nature 2007, 448, 550–552. [Google Scholar] [CrossRef]
- Ladle, R.J.; Malhado, A.C.; Correia, R.A.; dos Santos, J.G.; Santos, A.M. Research trends in biogeography. J. Biogeogr. 2015, 42, 2270–2276. [Google Scholar] [CrossRef]
- Soberón, J.; Peterson, T. Biodiversity informatics: Managing and applying primary biodiversity data. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Blair, G.S.; Henrys, P.; Leeson, A.; Watkins, J.; Eastoe, E.; Jarvis, S.; Young, P.J. Data science of the natural environment: A research roadmap. Front. Environ. Sci. 2019, 7, 121. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Peterson, A.T.; Soberón, J.; Overton, J.M.; Aragón, P.; Lobo, J.M. Use of niche models in invasive species risk assessments. Biol. Invasions 2011, 13, 2785–2797. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Márcia Barbosa, A.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Jarnevich, C.S.; Pearse, I.S.; Smyth, R.L.; Auer, S.; Cook, G.L.; Edwards, T.C., Jr.; Guala, G.F.; Howard, T.G.; Morisette, J.T.; et al. Development and delivery of species distribution models to inform decision-making. BioScience 2019, 69, 544–557. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Beale, C.M.; Lennon, J.J. Incorporating uncertainty in predictive species distribution modelling. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Werkowska, W.; Márquez, A.L.; Real, R.; Acevedo, P. A practical overview of transferability in species distribution modeling. Environ. Rev. 2017, 25, 127–133. [Google Scholar] [CrossRef]
- Bradley, B.A.; Wilcove, D.S.; Oppenheimer, M. Climate change increases risk of plant invasion in the Eastern United States. Biol. Invasions 2010, 12, 1855–1872. [Google Scholar] [CrossRef]
- Padalia, H.; Srivastava, V.; Kushwaha, S.P.S. Modeling potential invasion range of alien invasive species, Hyptis suaveolens (L.) Poit. in India: Comparison of MaxEnt and GARP. Ecol. Inform. 2014, 22, 36–43. [Google Scholar] [CrossRef]
- Mainali, K.P.; Warren, D.L.; Dhileepan, K.; McConnachie, A.; Strathie, L.; Hassan, G.; Karki, D.; Shrestha, B.B.; Parmesan, C. Projecting future expansion of invasive species: Comparing and improving methodologies for species distribution modeling. Glob. Chang. Biol. 2015, 21, 4464–4480. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef]
- Jeschke, J.M.; Strayer, D.L. Usefulness of bioclimatic models for studying climate change and invasive species. Ann. N. Y. Acad. Sci. 2008, 1134, 1–24. [Google Scholar] [CrossRef]
- Guillaumot, C.; Belmaker, J.; Buba, Y.; Fourcy, D.; Dubois, P.; Danis, B.; Le Moan, E.; Saucède, T. Classic or hybrid? The performance of next generation ecological models to study the response of Southern Ocean species to changing environmental conditions. Divers. Distrib. 2022, 28, 2286–2302. [Google Scholar] [CrossRef]
- Parrott, L. Hybrid modelling of complex ecological systems for decision support: Recent successes and future perspectives. Ecol. Inform. 2011, 6, 44–49. [Google Scholar] [CrossRef]
- 41] Gallien, L.; Münkemüller, T.; Albert, C.H.; Boulangeat, I.; Thuiller, W. Predicting potential distributions of invasive species: Where to go from here? Divers. Distrib. 2010, 16, 331–342. [Google Scholar] [CrossRef]
- Srivastava, V.; Lafond, V.; Griess, V.C. Species distribution models (SDM): Applications, benefits and challenges in invasive species management. CAB Rev. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Engler, R.; Guisan, A. MigClim: Predicting plant distribution and dispersal in a changing climate. Divers. Distrib. 2009, 15, 590–601. [Google Scholar] [CrossRef]
- Smolik, M.; Dullinger, S.; Essl, F.; Kleinbauer, I.; Leitner, M.; Peterseil, J.; Stadler, L.-M.; Vogl, G. Integrating species distribution models and interacting particle systems to predict the spread of an invasive alien plant. J. Biogeogr. 2010, 37, 411–422. [Google Scholar] [CrossRef]
- Williams, R.J.; Dunn, A.M.; Mendes da Costa, L.; Hassall, C. Climate and habitat configuration limit range expansion and patterns of dispersal in a non-native lizard. Ecol. Evol. 2021, 11, 3332–3346. [Google Scholar] [CrossRef] [PubMed]
- Meier, E.S.; Dullinger, S.; Zimmermann, N.E.; Baumgartner, D.; Gattringer, A.; Hülber, K. Space matters when defining effective management for invasive plants. Divers. Distrib. 2014, 20, 1029–1043. [Google Scholar] [CrossRef]
- DeAngelis, D.L.; Grimm, V. Individual-based models in ecology after four decades. F1000Prime Rep. 2014, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Buckley, Y.M.; Briese, D.T.; Rees, M. Demography and management of the invasive plant species Hypericum perforatum. II. Construction and use of an individual-based model to predict population dynamics and the effects of management strategies. J. Appl. Ecol. 2003, 40, 494–507. [Google Scholar] [CrossRef]
- Rebaudo, F.; Crespo-Pérez, V.; Silvain, J.F.; Dangles, O. Agent-based modeling of human-induced spread of invasive species in agricultural landscapes: Insights from the potato moth in Ecuador. J. Artif. Soc. Soc. Simul. 2011, 14, 7. [Google Scholar] [CrossRef]
- Fraser, E.J.; Lambin, X.; Travis, J.M.; Harrington, L.A.; Palmer, S.C.; Bocedi, G.; Macdonald, D.W. Range expansion of an invasive species through a heterogeneous landscape–the case of American mink in Scotland. Divers. Distrib. 2015, 21, 888–900. [Google Scholar] [CrossRef]
- Aurambout, J.P.; Endress, A.G. A model to simulate the spread and management cost of kudzu (Pueraria montana var. lobata) at landscape scale. Ecol. Inform. 2018, 43, 146–156. [Google Scholar] [CrossRef]
- Pattison, Z.; Vallejo-Marín, M.; Willby, N. Riverbanks as battlegrounds: Why does the abundance of native and invasive plants vary? Ecosystems 2019, 22, 578–586. [Google Scholar] [CrossRef]
- Pyšek, P.; Prach, K. Invasion dynamics of Impatiens glandulifera—A century of spreading reconstructed. Biol. Conserv. 1995, 74, 41–48. [Google Scholar] [CrossRef]
- Millane, M.; Caffrey, J.M. Risk Assessment of Impatiens Glandulifera. Inland Fisheries Ireland; National Biodiversity Centre: Dublin, Ireland, 2014; 29p. Available online: http://nonnativespecies.ie/wp–content/uploads/2014/03/Impatiens-glandulifera-Himalayan-balsam1.pdf (accessed on 7 August 2022).
- Drescher, A.; Prots, B. Warum breitet sich das Drüsen-Springkraut (Impatiens glandulifera Royle) in den Alpen aus? Wulfenia 2000, 7, 5–26. [Google Scholar]
- Čuda, J.; Skálová, H.; Pyšek, P. Spread of Impatiens glandulifera from riparian habitats to forests and its associated impacts: Insights from a new invasion. Weed Res. 2020, 60, 8–15. [Google Scholar] [CrossRef]
- Čuda, J.; Rumlerová, Z.; Brůna, J.; Skálová, H.; Pyšek, P. Floods affect the abundance of invasive Impatiens glandulifera and its spread from river corridors. Divers. Distrib. 2017, 23, 342–354. [Google Scholar] [CrossRef]
- Coakley, S.; Petti, C. Impacts of the invasive Impatiens glandulifera: Lessons learned from one of Europe’s top invasive species. Biology 2021, 10, 619. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P. What is the impact of Impatiens glandulifera on species diversity of invaded riparian vegetation? Biol. Conserv. 2006, 132, 143–152. [Google Scholar] [CrossRef]
- Čuda, J.; Vítková, M.; Albrechtová, M.; Guo, W.Y.; Barney, J.N.; Pyšek, P. Invasive herb Impatiens glandulifera has minimal impact on multiple components of temperate forest ecosystem function. Biol. Invasions 2017, 19, 3051–3066. [Google Scholar] [CrossRef]
- Diekmann, M.; Effertz, H.; Baranowski, M.; Dupré, C. Weak effects on plant diversity of two invasive Impatiens species. Plant Ecol. 2016, 217, 1503–1514. [Google Scholar] [CrossRef]
- Hulme, P.E.; Bremner, E.T. Assessing the impact of Impatiens glandulifera on riparian habitats: Partitioning diversity components following species removal. J. Appl. Ecol. 2006, 43, 43–50. [Google Scholar] [CrossRef]
- Gaggini, L.; Rusterholz, H.P.; Baur, B. The invasive plant Impatiens glandulifera affects soil fungal diversity and the bacterial community in forests. Appl. Soil Ecol. 2018, 124, 335–343. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Jamin, J.; Diehl, D.; Meyer, M.; David, J.; Schaumann, G.E.; Buchmann, C. Physico-Chemical Soil Properties Affected by Invasive Plants in Southwest Germany (Rhineland-Palatinate)—A Case Study. Soil Syst. 2022, 6, 93. [Google Scholar] [CrossRef]
- Kiełtyk, P.; Delimat, A. Impact of the alien plant Impatiens glandulifera on species diversity of invaded vegetation in the northern foothills of the Tatra Mountains, Central Europe. Plant Ecol. 2019, 220, 1–12. [Google Scholar] [CrossRef]
- Dassonville, N.; Vanderhoeven, S.; Vanparys, V.; Hayez, M.; Gruber, W.; Meerts, P. Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 2008, 157, 131–140. [Google Scholar] [CrossRef]
- Rusterholz, H.P.; Küng, J.; Baur, B. Experimental evidence for a delayed response of the above-ground vegetation and the seed bank to the invasion of an annual exotic plant in deciduous forests. Basic Appl. Ecol. 2017, 20, 19–30. [Google Scholar] [CrossRef]
- Greenwood, P.; Kuhn, N.J. Does the invasive plant, Impatiens glandulifera, promote soil erosion along the riparian zone? An investigation on a small watercourse in northwest Switzerland. J. Soils Sediments 2014, 14, 637–650. [Google Scholar] [CrossRef]
- Clements, D.R.; Feenstra, K.R.; Jones, K.; Staniforth, R. The biology of invasive alien plants in Canada. 9. Impatiens glandulifera Royle. Can. J. Plant Sci. 2008, 88, 403–417. [Google Scholar] [CrossRef]
- Burkhart, K.; Nentwig, W. Control of Impatiens glandulifera (Balsaminaceae) by antagonists in its invaded range. Invasive Plant Sci. Manag. 2008, 1, 352–358. [Google Scholar] [CrossRef]
- Tanner, R.A.; Jin, L.; Shaw, R.; Murphy, S.T.; Gange, A.C. An Ecological Assessment of Impatiens glandulifera in its Introduced and Native Range and the Potential for its Classical Biological Control. Ph.D. Thesis, Royal Holloway, University of London, London, UK, 2012. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility (GBIF). Impatiens Glandulifera Royle. 2022. Available online: https://www.gbif.org/species/2891770 (accessed on 12 August 2022).
- GLANSIS. Impatiens Glandulifera Royle. Available online: https://nas.er.usgs.gov/queries/GreatLakes/FactSheet.aspx?Species_ID=2695 (accessed on 10 August 2022).
- CABI Digital Library. Impatiens Glandulifera (Himalayan Balsam). Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.28766 (accessed on 12 August 2022).
- USDA. Impatiens Glandulifera Royle. Available online: https://plants.usda.gov/home/plantProfile?symbol=IMGL (accessed on 13 August 2022).
- Invasive Species Centre. Himalayan Balsam (Impatiens Glandulifera). Available online: https://www.invasivespeciescentre.ca/invasive-species/meet-the-species/invasive-plants/himalayan-balsam/ (accessed on 12 August 2022).
- IPANE. Impatiens Glandulifera. Available online: https://www.invasive.org/weedcd/pdfs/ipane/Impatiensglandulifera.pdf (accessed on 14 August 2022).
- Toney, J.C.; Rice, P.M.; Forecella, F. Exotic plant records in the northwest United States 1950–1996: An ecological assessment. Northwest Sci. 1998, 72, 198–213. [Google Scholar] [CrossRef]
- Tabak, N.M.; von Wettberg, E. Native and introduced jewelweeds of the Northeast. Northeast. Nat. 2008, 15, 159–176. [Google Scholar] [CrossRef]
- Mills, E.L.; Leach, J.H.; Carlton, J.T.; Secor, C.L. Exotic species in the Great Lakes: A history of biotic crises and anthropogenic introductions. J. Great Lakes Res. 1993, 19, 1–54. [Google Scholar] [CrossRef]
- Alaska Center for Conservation Science (ACCS). Available online: https://accs.uaa.alaska.edu/invasive-species/non-native-plant-species-list/ (accessed on 11 December 2022).
- Adamowski, W. Status of Impatiens Genus in Mexico—Research Proposal. 2019. Available online: https://www.researchgate.net/publication/331210846_Status_of_Impatiens_genus_in_Mexico_-_research_proposal (accessed on 1 October 2022).
- Kurtto, A. Impatiens glandulifera (Balsaminaceae) as an ornamental and escape in Finland, with notes on the other Nordic countries. Acta Univ. Ups. Symb. Bot. Ups. 1996, 31, 221–228. [Google Scholar]
- Prots, B.; Drescher, A. Role of dispersal agents for the spread of Impatiens glandulifera in Transcarpathia. J. Biol. Syst. 2010, 2, 42–46. [Google Scholar]
- Perrins, J.; Fitter, A.; Williamson, M. Population biology and rates of invasion of three introduced Impatiens species in the British Isles. J. Biogeogr. 1993, 20, 33–44. [Google Scholar] [CrossRef]
- Helsen, K.; Diekmann, M.; Decocq, G.; De Pauw, K.; Govaert, S.; Graae, B.J.; Hagenblad, J.; Liira, J.; Orczewska, A.; Sanczuk, P.; et al. Biological flora of Central Europe: Impatiens glandulifera Royle. Perspect. Plant Ecol. Evol. Syst. 2021, 50, 125609. [Google Scholar] [CrossRef]
- Vorstenbosch, T.; Essl, F.; Lenzner, B. An uphill battle? The elevational distribution of alien plant species along rivers and roads in the Austrian Alps. NeoBiota 2020, 63, 1–24. [Google Scholar] [CrossRef]
- Carlton, J.T. Pattern, process, and prediction in marine invasion ecology. Biol. Conserv. 1996, 78, 97–106. [Google Scholar] [CrossRef]
- Johnstone, I.M. Plant invasion windows: A time-based classification of invasion potential. Biol. Rev. 1986, 61, 369–394. [Google Scholar] [CrossRef]
- Crawley, M.J. Chance and timing in biological invasions. In Biological Invasions: A Global Perspective; Wiley: New York, NY, USA, 1989; pp. 407–424. [Google Scholar]
- Ehrlich, P. Attributes of invaders and the invading processes vertebrates. In Biological Invasions: A Global Perspective; Wiley: New York, NY, USA, 1989; pp. 315–328. [Google Scholar]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 22 April 2018).
- Phillips, S.J.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (Version 3.4.1). 2022. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 3 December 2018).
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. R-Package Version 1.3-3. 2020. Available online: https://CRAN.R-project.org/package=dismo (accessed on 5 December 2018).
- GBIF.org. GBIF Occurrence Download. 2022. Available online: https://www.gbif.org/occurrence/download/0265702-210914110416597 (accessed on 5 May 2022).
- Hersbach, H.; Bell, B.; Berrisford, P.; Biavati, G.; Horányi, A.; Muñoz Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Rozum, I.; et al. ERA5 Hourly Data on Single Levels from 1940 to Present. Copernicus Climate Change Service (C3S) Climate Data Store (CDS). 2021. Available online: https://cds.climate.copernicus.eu/cdsapp#!/dataset/reanalysis-era5-single-levels?tab=overview (accessed on 10 June 2021).
- ERA5-Land Monthly Averaged Data from 1950 to Present. Available online: https://cds.climate.copernicus.eu/cdsapp#!/dataset/reanalysis-era5-land-monthly-means?tab=overview (accessed on 15 June 2021).
- Watanabe, M.; Suzuki, T.; O’ishi, R.; Komuro, Y.; Watanabe, S.; Emori, S.; Takemura, T.; Chikira, M.; Ogura, T.; Sekiguchi, M.; et al. Improved climate simulation by MIROC5: Mean states, variability, and climate sensitivity. J. Clim. 2010, 23, 6312–6335. [Google Scholar] [CrossRef]
- Thrasher, B.; Maurer, E.P.; McKellar, C.; Duffy, P.B. Bias correcting climate model simulated daily temperature extremes with quantile mapping. Hydrol. Earth Syst. Sci. 2012, 16, 3309–3314. [Google Scholar] [CrossRef]
- NASA Earth Exchange Global Daily Downscaled Projections (NEX-GDDP). Available online: https://ds.nccs.nasa.gov/thredds/catalog/NEX-GDDP/IND/BCSD/rcp45/day/atmos/catalog.html (accessed on 11 February 2018).
- Teutschbein, C.; Seibert, J. Bias correction of regional climate model simulations for hydrological climate-change impact studies: Review and evaluation of different methods. J. Hydrol. 2012, 456, 12–29. [Google Scholar] [CrossRef]
- Santos, C.A.; Rocha, F.; Ramos, T.B.; Alves, L.M.; Mateus, M.; Oliveira, R.P.D.; Neves, R. Using a hydrologic model to assess the performance of regional climate models in a semi-arid watershed in Brazil. Water 2019, 11, 170. [Google Scholar] [CrossRef]
- Phillips, S.J. A brief tutorial on Maxent. ATT Res. 2005, 190, 231–259. [Google Scholar]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Luedeling, E. chillR: Statistical Methods for Phenology Analysis in Temperate Fruit Trees. R Package (Version 0.54, 2013). 2020. Available online: https://CRAN.R-project.org/package=chillR (accessed on 16 February 2021).
- Hurtt, G.C.; Chini, L.; Sahajpal, R.; Frolking, S.; Bodirsky, B.L.; Calvin, K.; Doelman, J.C.; Fisk, J.; Fujimori, S.; Klein Goldewijk, K.; et al. Harmonization of global land use change and management for the period 850–2100 (LUH2) for CMIP6. Geosci. Model Dev. 2020, 13, 5425–5464. [Google Scholar] [CrossRef]
- Land-Use Harmonization 2. Available online: https://luh.umd.edu/data.shtml (accessed on 30 May 2019).
- Danielson, J.J.; Gesch, D.B. Global Multi-Resolution Terrain Elevation Data 2010 (GMTED2010); US Department of the Interior, Geological Survey: Washington, DC, USA, 2011; p. 26. [CrossRef]
- Global Multi-Resolution Terrain Elevation Data (GMTED2010). Available online: http://edcintl.cr.usgs.gov/downloads/sciweb1/shared/topo/downloads/GMTED/Grid_ZipFiles/md15_grd.zip (accessed on 5 December 2022).
- Hijmans, R.J.; van Etten, J. Raster: Geographic Data Analysis and Modeling. R Package Version, 2. 2016. Available online: https://CRAN.R-project.org/package=raster (accessed on 5 December 2018).
- Poggio, L.; De Sousa, L.M.; Batjes, N.H.; Heuvelink, G.; Kempen, B.; Ribeiro, E.; Rossiter, D. SoilGrids 2.0: Producing soil information for the globe with quantified spatial uncertainty. Soil 2021, 7, 217–240. [Google Scholar] [CrossRef]
- International Soil Reference and Information Centre (ISRIC) Soil Data Hub. Available online: https://data.isric.org/geonetwork/srv/eng/catalog.search#/metadata/4c59ee58-a24e-4154-912e-0ff18395ac0d (accessed on 25 December 2022).
- Jouret, M.F. Écologie de la dormance séminale et de la germination chez diverses espèces du genre Impatiens. L. In Bulletin de la Société Royale de Botanique de Belgique; Société Royale de Botanique de Belgique: Brussels, Belgium, 1976; pp. 213–225. [Google Scholar]
- Beerling, D.J.; Perrins, J.M. Impatiens glandulifera royle (impatiens Roylei Walp.). J. Ecol. 1993, 81, 367–382. [Google Scholar] [CrossRef]
- Mumford, P.M. Alleviation and induction of dormancy by temperature in Impatiens glandulifera Royle. New Phytol. 1988, 109, 107–110. [Google Scholar] [CrossRef]
- Grime, J.P.; Mason, G.; Curtis, A.V.; Rodman, J.; Band, S.R. A comparative study of germination characteristics in a local flora. J. Ecol. 1981, 69, 1017–1059. [Google Scholar] [CrossRef]
- Andrews, M.; Maule, H.G.; Hodge, S.; Cherrill, A.; Raven, J.A. Seed dormancy, nitrogen nutrition and shade acclimation of Impatiens glandulifera: Implications for successful invasion of deciduous woodland. Plant Ecol. Divers. 2009, 2, 145–153. [Google Scholar] [CrossRef]
- Skálová, H.; Moravcová, L.; Čuda, J.; Pyšek, P. Seed-bank dynamics of native and invasive Impatiens species during a five-year field experiment under various environmental conditions. NeoBiota 2019, 50, 75. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Hunter-Ayad, J.; Hassall, C. An empirical, cross-taxon evaluation of landscape-scale connectivity. Biodivers. Conserv. 2020, 29, 1339–1359. [Google Scholar] [CrossRef]
- Willis, S.G.; Hulme, P.E. Does temperature limit the invasion of Impatiens glandulifera and Heracleum mantegazzianum in the UK? Funct. Ecol. 2002, 16, 530–539. [Google Scholar] [CrossRef]
- Willis, S.G.; Hulme, P.E. Environmental severity and variation in the reproductive traits of Impatiens glandulifera. Funct. Ecol. 2004, 18, 887–898. [Google Scholar] [CrossRef]
- Kollmann, J.; Bañuelos, M.J. Latitudinal trends in growth and phenology of the invasive alien plant Impatiens glandulifera (Balsaminaceae). Divers. Distrib. 2004, 10, 377–385. [Google Scholar] [CrossRef]
- Nasir, Y.J.; Rafiq, R.A.; Roberts, T.J. Wild Flowers of Pakistan; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Global Invasive Species Database. Species Profile: Impatiens Glandulifera. 2022. Available online: http://www.iucngisd.org/gisd/species.php?sc=942 (accessed on 11 December 2022).
- Ennos, A.R.; Crook, M.J.; Grimshaw, C. A comparative study of the anchorage systems of Himalayan balsam Impatiens glandulifera and mature sunflower Helianthus annuus. J. Exp. Bot. 1993, 44, 133–146. [Google Scholar] [CrossRef]
- Pacanoski, Z.; Saliji, A. The invasive Impatiens glandulifera Royle (Himalayan balsam) in the Republic of Macedonia: First record and forecast. EPPO Bull. 2014, 44, 87–93. [Google Scholar] [CrossRef]
- Ten Broeke, G.; Van Voorn, G.; Ligtenberg, A. Which sensitivity analysis method should I use for my agent-based model? J. Artif. Soc. Soc. Simul. 2016, 19, 5. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef]
- Clements, D.R.; Ditommaso, A. Climate change and weed adaptation: Can evolution of invasive plants lead to greater range expansion than forecasted? Weed Res. 2011, 51, 227–240. [Google Scholar] [CrossRef]
- Wang, A.; Melton, A.E.; Soltis, D.E.; Soltis, P.S. Potential distributional shifts in North America of allelopathic invasive plant species under climate change models. Plant Divers. 2022, 44, 11–19. [Google Scholar] [CrossRef]
- Chai, S.L.; Zhang, J.; Nixon, A.; Nielsen, S. Using risk assessment and habitat suitability models to prioritise invasive species for management in a changing climate. PLoS ONE 2016, 11, e0165292. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, R.; Leikola, N.; Fronzek, S.; Lampinen, R.; Toivonen, H. Predicting distribution patterns and recent northward range shift of an invasive aquatic plant: Elodea canadensis in Europe. BioRisk 2009, 2, 1–32. [Google Scholar] [CrossRef]
- Guan, B.C.; Guo, H.J.; Chen, S.S.; Li, D.M.; Liu, X.; Gong, X.I.; Ge, G. Shifting ranges of eleven invasive alien plants in China in the face of climate change. Ecol. Inform. 2020, 55, 101024. [Google Scholar] [CrossRef]
- Beerling, D.J. The impact of temperature on the northern distribution limits of the introduced species Fallopia japonica and Impatiens glandulifera in north-west Europe. J. Biogeogr. 1993, 20, 45–53. [Google Scholar] [CrossRef]
- Goldblum, D.; Rigg, L.S. The deciduous forest–boreal forest ecotone. Geogr. Compass 2010, 4, 701–717. [Google Scholar] [CrossRef]
- Frelich, L.E.; Reich, P.B. Will environmental changes reinforce the impact of global warming on the prairie–forest border of central North America? Front. Ecol. Environ. 2010, 8, 371–378. [Google Scholar] [CrossRef]
- Scheffer, M.; Hirota, M.; Holmgren, M.; Van Nes, E.H.; Chapin III, F.S. Thresholds for boreal biome transitions. Proc. Natl. Acad. Sci. USA 2012, 109, 21384–21389. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Altdorff, D.; Li, P.; Galagedara, L.; Holden, J.; Unc, A. Northward shift of the agricultural climate zone under 21st-century global climate change. Sci. Rep. 2018, 8, 7904. [Google Scholar] [CrossRef] [PubMed]
- Koven, C.D. Boreal carbon loss due to poleward shift in low-carbon ecosystems. Nat. Geosci. 2013, 6, 452–456. [Google Scholar] [CrossRef]
- New Study: As Climate Changes, Boreal Forests to Shift North and Relinquish More Carbon than Expected. Available online: https://newscenter.lbl.gov/2013/05/05/boreal/ (accessed on 20 December 2022).
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.G. Boreal forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef]
- Brice, M.H.; Vissault, S.; Vieira, W.; Gravel, D.; Legendre, P.; Fortin, M.J. Moderate disturbances accelerate forest transition dynamics under climate change in the temperate–boreal ecotone of eastern North America. Glob. Chang. Biol. 2020, 26, 4418–4435. [Google Scholar] [CrossRef]
- Leithead, M.D.; Anand, M.; Silva, L.C. Northward migrating trees establish in treefall gaps at the northern limit of the temperate–boreal ecotone, Ontario, Canada. Oecologia 2010, 164, 1095–1106. [Google Scholar] [CrossRef]
- Andrews, M.; Maule, H.G.; Raven, J.A.; Mistry, A. Extension growth of Impatiens glandulifera at low irradiance: Importance of nitrate and potassium accumulation. Ann. Bot. 2005, 95, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Descamps, C.; Boubnan, N.; Jacquemart, A.L.; Quinet, M. Growing and Flowering in a Changing Climate: Effects of Higher Temperatures and Drought Stress on the Bee-Pollinated Species Impatiens glandulifera Royle. Plants 2021, 10, 988. [Google Scholar] [CrossRef]
- PaiMazumder, D.; Sushama, L.; Laprise, R.; Khaliq, M.N.; Sauchyn, D. Canadian RCM projected changes to short-and long-term drought characteristics over the Canadian Prairies. Int. J. Climatol. 2013, 33, 1409–1423. [Google Scholar] [CrossRef]
- Gamelin, B.L.; Feinstein, J.; Wang, J.; Bessac, J.; Yan, E.; Kotamarthi, V.R. Projected US drought extremes through the twenty-first century with vapor pressure deficit. Sci. Rep. 2022, 12, 8615. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.I.; Sushama, L.; Naveed Khaliq, M. The role of temperature in drought projections over North America. Clim. Chang. 2014, 127, 289–303. [Google Scholar] [CrossRef]
- Seager, R.; Ting, M.; Held, I.; Kushnir, Y.; Lu, J.; Vecchi, G.; Huang, H.-P.; Harnik, N.; Leetmaa, A.; Lau, N.-C.; et al. Model projections of an imminent transition to a more arid climate in southwestern North America. Science 2007, 316, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, M.; Trailovic, M.; Dubljanin, T.; Krivošej, Z.; Nikolic, M.; Nikolic, N. Sewage Pollution Promotes the Invasion-Related Traits of Impatiens glandulifera in an Oligotrophic Habitat of the Sharr Mountain (Western Balkans). Plants 2021, 10, 2814. [Google Scholar] [CrossRef]
- Gray, L.K.; Hamann, A. Tracking suitable habitat for tree populations under climate change in western North America. Clim. Chang. 2013, 117, 289–303. [Google Scholar] [CrossRef]
- Fettig, C.J.; Asaro, C.; Nowak, J.T.; Dodds, K.J.; Gandhi, K.J.; Moan, J.E.; Robert, J. Trends in bark beetle impacts in North America during a period (2000–2020) of rapid environmental change. J. For. 2022, 120, 693–713. [Google Scholar] [CrossRef]
- Ammer, C.; Schall, P.; Wördehoff, R.; Lamatsch, K.; Bachmann, M. Does tree seedling growth and survival require weeding of Himalayan balsam (Impatiens glandulifera)? Eur. J. For. Res. 2011, 130, 107–116. [Google Scholar] [CrossRef]
- Suarez, A.V.; Holway, D.A.; Case, T.J. Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from Argentine ants. Proc. Natl. Acad. Sci. USA 2001, 98, 1095–1100. [Google Scholar] [CrossRef]
- Lenda, M.; Skórka, P.; Knops, J.M.; Moroń, D.; Sutherland, W.J.; Kuszewska, K.; Woyciechowski, M. Effect of the internet commerce on dispersal modes of invasive alien species. PLoS ONE 2014, 9, e99786. [Google Scholar] [CrossRef]
- Drescher, A.; Prots, B. Distribution patterns of himalayan balsam (Impatiens glandulifera royale) in Austria. Kanitzia 2003, 11, 85–96. [Google Scholar]
- Lachman, L.; Šerá, B. Alien plant species growing near traffic line structures in the protected landscape area. Ann. Bot. 2022, 12, 11–22. [Google Scholar]
- Kapitonova, O.A. Additions to the vascular flora of the Tyumen region, Western Siberia. Acta Biol. Sib. 2020, 6, 339. [Google Scholar] [CrossRef]
- Hulme, P.E. Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Walters, L.J.; Brown, K.R.; Stam, W.T.; Olsen, J.L. E-commerce and Caulerpa: Unregulated dispersal of invasive species. Front. Ecol. Environ. 2006, 4, 75–79. [Google Scholar] [CrossRef]
- Humair, F.; Humair, L.; Kuhn, F.; Kueffer, C. E-commerce trade in invasive plants. Conserv. Biol. 2015, 29, 1658–1665. [Google Scholar] [CrossRef]
- Jerardo, A. Floriculture and Nursery crops Situation and Outlook Yearbook; Economic Research Service, United States Department of Agriculture, FLO-2005: Washington, DC, USA, 2005. [Google Scholar]
- Fourcade, Y.; Engler, J.O.; Rödder, D.; Secondi, J. Mapping species distributions with MAXENT using a geographically biased sample of presence data: A performance assessment of methods for correcting sampling bias. PLoS ONE 2014, 9, e97122. [Google Scholar] [CrossRef]
- Jarnevich, C.S.; Stohlgren, T.J.; Kumar, S.; Morisette, J.T.; Holcombe, T.R. Caveats for correlative species distribution modeling. Ecol. Inform. 2015, 29, 6–15. [Google Scholar] [CrossRef]
- Hortal, J.; de Bello, F.; Diniz-Filho, J.A.F.; Lewinsohn, T.M.; Lobo, J.M.; Ladle, R.J. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 523–549. [Google Scholar] [CrossRef]
- Braunisch, V.; Suchant, R. Predicting species distributions based on incomplete survey data: The trade-off between precision and scale. Ecography 2010, 33, 826–840. [Google Scholar] [CrossRef]
- Boakes, E.H.; McGowan, P.J.; Fuller, R.A.; Chang-qing, D.; Clark, N.E.; O’Connor, K.; Mace, G.M. Distorted views of biodiversity: Spatial and temporal bias in species occurrence data. PLoS Biol. 2010, 8, e1000385. [Google Scholar] [CrossRef]
- Václavík, T.; Meentemeyer, R.K. Equilibrium or not? Modelling potential distribution of invasive species in different stages of invasion. Divers. Distrib. 2012, 18, 73–83. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Beck, J.; Ballesteros-Mejia, L.; Nagel, P.; Kitching, I.J. Online solutions and the ‘W allacean shortfall’: What does GBIF contribute to our knowledge of species’ ranges? Divers. Distrib. 2013, 19, 1043–1050. [Google Scholar] [CrossRef]
- Beck, J.; Böller, M.; Erhardt, A.; Schwanghart, W. Spatial bias in the GBIF database and its effect on modeling species’ geographic distributions. Ecol. Inform. 2014, 19, 10–15. [Google Scholar] [CrossRef]
- Gruntman, M.; Pehl, A.K.; Joshi, S.; Tielbörger, K. Competitive dominance of the invasive plant Impatiens glandulifera: Using competitive effect and response with a vigorous neighbour. Biol. Invasions 2014, 16, 141–151. [Google Scholar] [CrossRef]
- Diller, J.G.P.; Hüftlein, F.; Lücker, D.; Feldhaar, H.; Laforsch, C. Allelochemical run-off from the invasive terrestrial plant Impatiens glandulifera decreases defensibility in Daphnia. Sci. Rep. 2023, 13, 1207. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.V.; Maczey, N.; Currie, A.F.; Lowry, A.J.; Rabiey, M.; Ellison, C.A.; Jackson, R.W.; Gange, A.C. Rapid impact of Impatiens glandulifera control on above-and belowground invertebrate communities. Weed Res. 2021, 61, 35–44. [Google Scholar] [CrossRef]
- Oliver, B.W.; Berge, T.W.; Solhaug, K.A.; Fløistad, I.S. Hot water and cutting for control of Impatiens glandulifera. Invasive Plant Sci. Manag. 2020, 13, 84–93. [Google Scholar] [CrossRef]
- Leblanc, M.; Lavoie, C. Controlling purple jewelweed (Impatiens glandulifera): Assessment of feasibility and costs. Invasive Plant Sci. Manag. 2017, 10, 254–261. [Google Scholar] [CrossRef]
- Tanner, R.A.; Gange, A.C. Himalayan balsam, Impatiens glandulifera: Its ecology, invasion and management. Weed Res. 2020, 60, 4–7. [Google Scholar] [CrossRef]
- Bieberich, J.; Müller, S.; Feldhaar, H.; Lauerer, M. Invasive Impatiens glandulifera: A driver of changes in native vegetation? Ecol. Evol. 2021, 11, 1320–1333. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanmaz, O.; Şenel, T.; Dalfes, H.N. A Modeling Framework to Frame a Biological Invasion: Impatiens glandulifera in North America. Plants 2023, 12, 1433. https://doi.org/10.3390/plants12071433
Kanmaz O, Şenel T, Dalfes HN. A Modeling Framework to Frame a Biological Invasion: Impatiens glandulifera in North America. Plants. 2023; 12(7):1433. https://doi.org/10.3390/plants12071433
Chicago/Turabian StyleKanmaz, Oğuzhan, Tuğçe Şenel, and H. Nüzhet Dalfes. 2023. "A Modeling Framework to Frame a Biological Invasion: Impatiens glandulifera in North America" Plants 12, no. 7: 1433. https://doi.org/10.3390/plants12071433
APA StyleKanmaz, O., Şenel, T., & Dalfes, H. N. (2023). A Modeling Framework to Frame a Biological Invasion: Impatiens glandulifera in North America. Plants, 12(7), 1433. https://doi.org/10.3390/plants12071433



