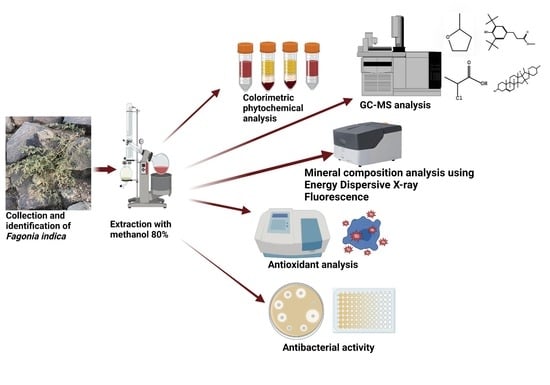
Unveiling Chemical, Antioxidant and Antibacterial Properties of Fagonia indica Grown in the Hail Mountains, Saudi Arabia
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Screening of Fagonia indica
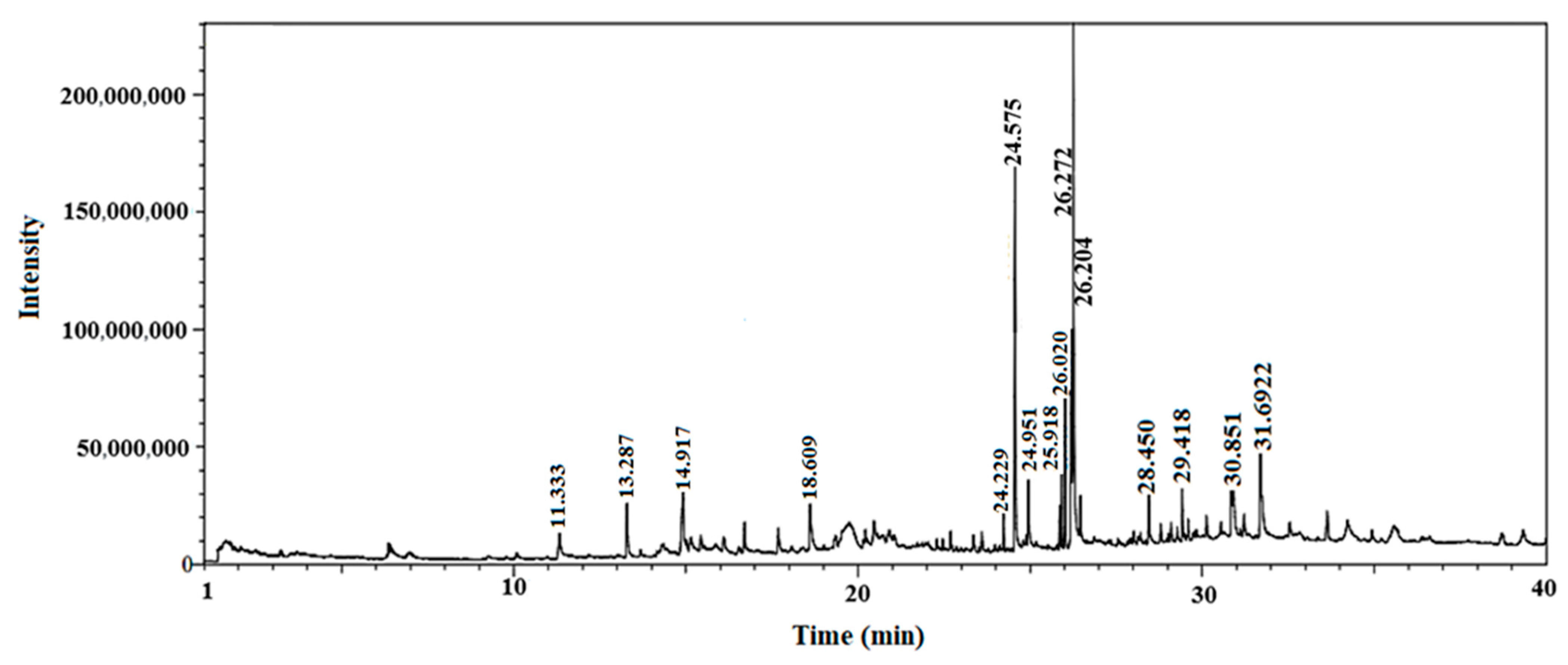
2.2. GC–MS Analysis of Fagonia indica
2.3. Energy Dispersive X-ray Fluorescence Analysis
2.4. Antioxidant Activities
2.5. Antibacterial Activity
3. Materials and Methods
3.1. Sample Collection
3.2. Chemicals
3.3. Preparation of Plant Extract
3.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
3.5. GC–MS Analysis Conditions
3.6. Phytochemical Profile
3.7. Energy Dispersive X-ray Fluorescence Measurements
3.8. Determination of Total Phenolic Content (TPC)
3.9. Determination of Total Flavonoid Content (TFC)
3.10. Determination of Total Tannin Content (TTC)
3.11. DPPH Radical Scavenging
3.12. ABTS Radical Scavenging Activity Assay
3.13. β-Carotene/Linoleic Acid Method
3.14. Bacterial Strains
3.15. Agar Disc-Diffusion Method
3.16. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.17. Anti-Biofilm Assays
3.18. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdallah, E.M. Plants: An alternative source for antimicrobials. J. Appl. Pharm. Sci. 2011, 1, 16–20. [Google Scholar]
- Waziri, H.M. Plants as antiviral agents. J. Plant Pathol. Microbiol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, L.; Singh, S. A review on medicinal plants having antioxidant potential. Indian J. Res. Pharm. Biotechnol. 2013, 1, 404. [Google Scholar]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.; Roy, J. Antimicrobial and chemopreventive properties of herbs and spices. Curr. Med. Chem. 2004, 11, 1451–1460. [Google Scholar] [CrossRef]
- Parekh, J.; Chanda, S. Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. Afr. J. Biomed. Res. 2007, 10, 175–181. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Jaouadi, I.; Zeouk, I.; Ghchime, R.; El Menyiy, N.; El Omari, N.; Balahbib, A.; Al-Mijalli, S.H.; Abdallah, E.M.; El-Shazly, M. Biological and Pharmacological Properties of Myrtenol: A Review. Curr. Pharm. Des. 2022; Online ahead of print. [Google Scholar]
- Patel, P.; Patel, N.; Patel, D.; Desai, S.; Meshram, D. Phytochemical analysis and antifungal activity of Moringa oleifera. Int. J. Pharm. Pharm. Sci. 2014, 6, 144–147. [Google Scholar]
- Kapoor, R.; Sharma, B.; Kanwar, S. Antiviral phytochemicals: An overview. Biochem. Physiol. 2017, 6, 7. [Google Scholar] [CrossRef]
- Lewington, A. A Review of the Importation of Medicinal Plants and Plant Extracts into Europe; Traffic International: Cambridge, UK, 1993. [Google Scholar]
- Yoshikawa, T.; Naito, Y. What is oxidative stress? Jpn. Med. Assoc. J. 2002, 45, 271–276. [Google Scholar]
- Sati, S.C.; Nitin, S.; Rawat, U.; Sati, O. Medicinal plants as a source of antioxidants. Res. J. Phytochem. 2010, 4, 213–224. [Google Scholar] [CrossRef]
- Aati, H.; El-Gamal, A.; Shaheen, H.; Kayser, O. Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. J. Ethnobiol. Ethnomed. 2019, 15, 2. [Google Scholar] [CrossRef]
- Rahman, M.A.; Mossa, J.S.; Al-Said, M.S.; Al-Yahya, M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: A report on seven plant families. Fitoterapia 2004, 75, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Nazzal, Y.; Zaidi, F. Groundwater pollution risk mapping using modified DRASTIC model in parts of Hail region of Saudi Arabia. Environ. Eng. Res. 2018, 23, 84–91. [Google Scholar] [CrossRef]
- Ali, K.; Khan, H. Fagonia indica; A review on chemical constituents, traditional uses and pharmacological activities. Curr. Pharm. Des. 2021, 27, 2648–2660. [Google Scholar] [CrossRef]
- Azam, F.; Sheikh, N.; Ali, G.; Tayyeb, A. Fagonia indica repairs hepatic damage through expression regulation of toll-like receptors in a liver injury model. J. Immunol. Res. 2018, 2018, 7967135. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Sohn, E.Y.; Lee, I.-J. Folk medicinal knowledge and conservation status of some economically valued medicinal plants of District Swat, Pakistan. Lyonia 2006, 11, 101–113. [Google Scholar]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Veeramuthu, D.; Muniappan, A.; Savarimuthu, I. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 2006, 6, 35. [Google Scholar] [CrossRef]
- Sadeek, A.; Abdallah, E.M. Phytochemical compounds as antibacterial agents a mini review. Saudi Arab. Glob. J. Pharm. Sci. 2019, 53, 131–136. [Google Scholar]
- Espinosa-Pérez, E.N.; Ramírez-Vallejo, P.; Crosby-Galván, M.M.; Estrada-Gómez, J.A.; Lucas-Florentino, B.; Chávez-Servia, J.L. Clasificación de poblaciones nativas de frijol común del centro-sur de México por morfología de semilla. Rev. Fitotec. Mex. 2015, 38, 29–38. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, J.; Xiao, A.; Liu, L. Antibacterial activity of polyphenols: Structure-activity relationship and influence of hyperglycemic condition. Molecules 2017, 22, 1913. [Google Scholar] [CrossRef] [PubMed]
- Talib, F.; Aftab, T. FTIR, HPLC, GC-MS analysis and investigation of hypoglycemic effects of leaves extracts of Fagonia indica. Pharmacogn. Commun. 2021, 11, 109–118. [Google Scholar]
- Rahman, L.; Mukhtar, A.; Ahmad, S.; Rahman, L.; Ali, M.; Saeed, M.; Shinwari, Z.K. Endophytic bacteria of Fagonia indica Burm. f revealed to harbour rich secondary antibacterial metabolites. PLoS ONE 2022, 17, e0277825. [Google Scholar] [CrossRef]
- Anil, P.; Nikhil, B.; Manoj, G.; Prakash, N. Phytochemicals and biological activities of Fagonia indica. Int. Res. J. Pharm. 2012, 3, 56–59. [Google Scholar]
- Farheen, R.; Siddiqui, B.S.; Mahmood, I.; Simjee, S.U.; Majeed, S. Triterpenoids and triterpenoid saponins from the aerial parts of Fagonia indica Burm. Phytochem. Lett. 2015, 13, 256–261. [Google Scholar] [CrossRef]
- Idriss, H.; Siddig, B.; Maldonado, P.G.; Elkhair, H.; Alakhras, A.; Abdallah, E.M.; Torres, P.H.S.; Elzupir, A.O. Phytochemical Discrimination, Biological Activity and Molecular Docking of Water-Soluble Inhibitors from Saussurea costus Herb against Main Protease of SARS-CoV-2. Molecules 2022, 27, 4908. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef]
- Dikkala, P.K.; Kakarlapudi, J.; Rokalla, P.; Vedantam, S.K.; Kaur, A.; Kaur, K.; Sharma, M.; Sridhar, K. Computational screening of phytochemicals for anti-diabetic drug discovery. In Phytochemistry, Computational Tools and Databases in Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 285–311. [Google Scholar]
- Sreekumari, K.R.; Sato, Y.; Kikuchi, Y. Antibacterial Metals—A Viable Solution for Bacterial Attachment and Microbiologically Influenced Corrosion—. Mater. Trans. 2005, 46, 1636–1645. [Google Scholar] [CrossRef]
- Tschinkel, P.F.; Melo, E.S.; Pereira, H.S.; Silva, K.; Arakaki, D.G.; Lima, N.V.; Fernandes, M.R.; Leite, L.; Melo, E.S.; Melnikov, P. The hazardous level of heavy metals in different medicinal plants and their decoctions in water: A public health problem in Brazil. BioMed Res. Int. 2020, 2020, 1465051. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, E.R.; Abdallah, E.M.; Shiboob, M.H.; Alghanem, S. Phytochemical, antioxidant and antibacterial potential of Ducrosia anethifolia in Northern border region of Saudi Arabia. J. Pharm Res. Int. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Abdallah, E.M. Antibacterial activity of Hibiscus sabdariffa L. calyces against hospital isolates of multidrug resistant Acinetobacter baumannii. J. Acute Dis. 2016, 5, 512–516. [Google Scholar] [CrossRef]
- Mustafa, G.; Ahmed, S.; Ahmed, N.; Jamil, A. Phytochemical and antibacterial activity of some unexplored medicinal plants of Cholistan desert. Pak. J. Bot. 2016, 48, 2057–2062. [Google Scholar]
- Shehab, N.G.; Mahdy, A.; Khan, S.; Noureddin, S. Chemical constituents and biological activities of Fagonia indica Burm F. Res. J. Med. Plant 2011, 5, 531–546. [Google Scholar] [CrossRef]
- Rios, J.-L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Nwozo, O.S.; Effiong, E.M.; Aja, P.M.; Awuchi, C.G. Antioxidant, phytochemical, and therapeutic properties of medicinal plants: A review. Int. J. Food Prop. 2023, 26, 359–388. [Google Scholar] [CrossRef]
- Sajid, B.; Alia, E.; Rizwana, K.; Uzma, S.; Alamgeer, H.M. Phytochemical screening and antimicrobial activity of Fagonia cretica plant extracts against selected microbes. J. Pharm Res. 2011, 4, 962–963. [Google Scholar]
- Thetwar, L.; Seema, A.S.T.; Augor, M.; Tandon, R.; Deshmukh, N. Antimicrobial efficacy of methanolic extracts of Fagonia cretica. Asian J. Chem. 2006, 18, 743. [Google Scholar]
- Zain, W.Z.W.M.; Kassim, J.; Karim, S.A. Antimicrobial activity of plant extracts against Bacillus subtilis, Staphylococcus aureus and Escherichia coli. E-J. Chem. 2011, 8 (Suppl. S1), S282. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Agents that inhibit bacterial biofilm formation. Future Med. Chem. 2015, 7, 647–671. [Google Scholar] [CrossRef]
- Chung, P.Y.; Khanum, R. Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria. J. Microbiol. Immunol. Infect. 2017, 50, 405–410. [Google Scholar] [CrossRef]
- Brackman, G.; Coenye, T. Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des. 2015, 21, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Salminen, J.-P.; Taube, F.; Malisch, C.S. Large inter-and intraspecies variability of polyphenols and proanthocyanidins in eight temperate forage species indicates potential for their exploitation as nutraceuticals. J. Agric. Food Chem. 2021, 69, 12445–12455. [Google Scholar] [CrossRef] [PubMed]
- Neves, F.S.; Silva, J.O.; Espírito-Santo, M.M.; Fernandes, G.W. Insect herbivores and leaf damage along successional and vertical gradients in a tropical dry forest. Biotropica 2014, 46, 14–24. [Google Scholar] [CrossRef]
- Al-Aamri, M.S.; Al-Abousi, N.M.; Al-Jabri, S.S.; Alam, T.; Khan, S.A. Chemical composition and in-vitro antioxidant and antimicrobial activity of the essential oil of Citrus aurantifolia L. leaves grown in Eastern Oman. J. Taibah Univ. Med. Sci. 2018, 13, 108–112. [Google Scholar] [CrossRef]
- Idriss, H.; Siddig, B.; González-Maldonado, P.; Elkhair, H.; Alakhras, A.I.; Abdallah, E.M.; Elzupir, A.O.; Sotelo, P.H. Inhibitory Activity of Saussurea costus Extract against Bacteria, Candida, Herpes, and SARS-CoV-2. Plants 2023, 12, 460. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Elgaaly, G.A.; Ibrahim, M.M. Identification of bioactive phytochemical from two Punica species using GC–MS and estimation of antioxidant activity of seed extracts. Saudi J. Biol. Sci. 2018, 25, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Raaman, N. Phytochemical Techniques; New India Publishing: New Deli, India, 2006. [Google Scholar]
- Banso, A.; Adeyemo, S. Phytochemical screening and antimicrobial assessment of Abutilon mauritianum, Bacopa monnifera and Datura stramonium. Biokemistri 2006, 18, 56390. [Google Scholar] [CrossRef]
- Ismail, M.; Abdallah, E.; Elsharkawy, E. Physico-chemical properties, antioxidant, and antimicrobial activity of five varieties of honey from Saudi Arabia. Asia Pac. J. Mol. Biol. Biotechnol. 2021, 29, 27–34. [Google Scholar] [CrossRef]
- Shaltout, A.A.; Hassan, F.A. Seasonal variability of elemental composition and phytochemical analysis of Moringa oleifera leaves using energy-dispersive X-ray fluorescence and other related methods. Biol. Trace Elem. Res. 2021, 199, 4319–4329. [Google Scholar] [CrossRef]
- Kumar, V.D.R.; Jayanthi, G.; Gurusamy, K.; Gowri, S. Antioxidant and anticancer activities of ethanolic extract of ficus glomerata roxb. in dmba induced rats. Int. J. Pharm. Sci. Res. 2013, 4, 3087. [Google Scholar] [CrossRef]
- Zhao, L.-J.; Liu, W.; Xiong, S.-H.; Tang, J.; Lou, Z.-H.; Xie, M.-X.; Xia, B.-H.; Lin, L.-M.; Liao, D.-F. Determination of total flavonoids contents and antioxidant activity of Ginkgo biloba leaf by near-infrared reflectance method. Int. J. Anal. Chem. 2018, 2018, 8195784. [Google Scholar] [CrossRef] [PubMed]
- Selvi, A.T.; Anjugam, E.; Devi, R.A.; Madhan, B.; Kannappan, S.; Chandrasekaran, B. Isolation and characterization of bacteria from tannery effluent treatment plant and their tolerance to heavy metals and antibiotics. Asian J. Exp. Biol. Sci. 2012, 3, 34–41. [Google Scholar]
- Chakraborty, K.; Paulraj, R. Sesquiterpenoids with free-radical-scavenging properties from marine macroalga Ulva fasciata Delile. Food Chem. 2010, 122, 31–41. [Google Scholar] [CrossRef]
- Ikram, E.H.K.; Eng, K.H.; Jalil, A.M.M.; Ismail, A.; Idris, S.; Azlan, A.; Nazri, H.S.M.; Diton, N.A.M.; Mokhtar, R.A.M. Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J. Food Compos. Anal. 2009, 22, 388–393. [Google Scholar] [CrossRef]
- Ali, A.M.; Abdallah, E.M.; Mujawah, A.A.; Avdeeva, E.Y. Chemical composition, larvicidal and antibacterial activity of the essential oil of trachyspermum ammi fruit. J. Nat. Remedies 2019, 19, 64–73. [Google Scholar] [CrossRef]
- Hamad Al-Mijalli, S.; ELsharkawy, E.R.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Alshahrani, M.M.; Mrabti, H.N.; Bouyahya, A. Determination of Volatile Compounds of Mentha piperita and Lavandula multifida and Investigation of Their Antibacterial, Antioxidant, and Antidiabetic Properties. Evid.-Based Complement. Altern. Med. 2022, 2022, 9306251. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Mujawah, A.; Al Mijalli, S.H. GC-MS and antibacterial potential of methanolic extract hyphaene thebaica L fruit pulp against antibiotics-resistant pathogens. J. Pure Appl. Microbiol. 2021, 15, 1655–1664. [Google Scholar] [CrossRef]
- Venkatramanan, M.; Sankar Ganesh, P.; Senthil, R.; Akshay, J.; Veera Ravi, A.; Langeswaran, K.; Vadivelu, J.; Nagarajan, S.; Rajendran, K.; Shankar, E.M. Inhibition of quorum sensing and biofilm formation in Chromobacterium violaceum by fruit extracts of Passiflora edulis. ACS Omega 2020, 5, 25605–25616. [Google Scholar] [CrossRef] [PubMed]



| Plant Extract | Saponins | Terpenes | Flavonoids | Phenols | Tannins | Alkaloids (Dragendorf) | Alkaloids (Mayer) | Cardiac Glycosides |
|---|---|---|---|---|---|---|---|---|
| Fogonia indica Methanol extract 80% v/v | + | + | + | + | + | − | − | + |
| No | Compound | Chemical Structure | Molecular Formula | R-Time | Area % |
|---|---|---|---|---|---|
| 1 | 2-Chloropropanoic acid | 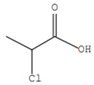 | C3H5ClO2 | 1.4 | 18.5 |
| 2 | Tridecanoic acid, 12-methyl-, methyl ester | 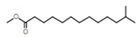 | C15H30O2 | 22.9 | 2.2 |
| 3 | Hexadecanoic acid, methyl ester | 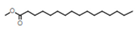 | C17H34O2 | 24.2 | 8.6 |
| 4 | Methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate | 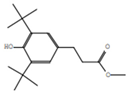 | C18H28O3 | 24.3 | 13.4 |
| 5 | Methyl linoleate | 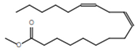 | C19H34O2 | 25.9 | 7 |
| 6 | Petroselinic acid methyl ester |  | C19H36O2 | 25.9 | 15 |
| 7 | Erucylamide | 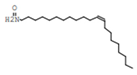 | C22H43NO | 31.7 | 6.7 |
| 8 | Tetrahydro-2-methylfuran | 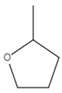 | C5H10O | 1.6 | 20.1 |
| 9 | Diosgenin | 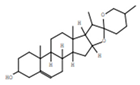 | C27H42O3 | 1.4 | 8.5 |
| Compound | Concentration (Mass Percent, m/m%) | Std Err | Element | Concentration (Mass Percent, m/m%) | Std Err |
|---|---|---|---|---|---|
| CaO | 48.28 | 0.25 | Ca | 34.52 | 0.18 |
| SO3 | 17.96 | 0.19 | S | 7.19 | 0.08 |
| K2O | 13.37 | 0.17 | K | 11.10 | 0.14 |
| Al2O3 | 6.68 | 0.15 | AL | 3.54 | 0.08 |
| Cl | 5.45 | 0.11 | Cl | 5.45 | 0.11 |
| SiO2 | 3.10 | 0.13 | Si | 1.45 | 0.06 |
| P2O5 | 2.03 | 0.18 | P | 0.884 | 0.08 |
| F2O3 | 1.31 | 0.06 | Fe | 0.919 | 0.04 |
| MgO | 0.88 | 0.16 | Mg | 0.533 | 0.09 |
| Na2O | 0.53 | 0.15 | Na | 0.39 | 0.11 |
| TiO2 | 0.166 | 0.008 | Ti | 0.099 | 0.005 |
| SrO | 0.110 | 0.005 | Sr | 0.093 | 0.005 |
| ZnO | 0.051 | 0.003 | Zn | 0.041 | 0.002 |
| MnO | 0.043 | 0.0041 | Mn | 0.033 | 0.003 |
| Test System | Extract | Butylated Hydroxytoluene | Ascorbic Acid |
|---|---|---|---|
| Phytochemical compounds | |||
| 1. Total Phenols (mg GAE/g Extract) | 3.72 ± 0.05 | - | - |
| 2. Total Tannins (mg TAE/g Extract) | 24.14 ± 0.65 | - | - |
| 3. Total Flavonoids (mg QE/g Extract) | 136.05 ± 0.45 | - | - |
| Antioxidant Assays | |||
| 1. DPPH IC50 (mg/mL) | 0.06 ± 0.003 | 0.023 ± 3 × 104 | 0.022 ± 5 × 104 |
| 2. ABTS IC50 (mg/mL) | 0.07 ± 0.001 | 0.018 ± 4 × 104 | 0.021 ± 0.001 |
| 3. β-carotene IC50 (mg/mL) | 2.34 ± 0.04 | 0.042 ± 3.5 × 103 | 0.017 ± 0.001 |
| Bacteria Tested | 1 mg/disc | 2 mg/disc | 3 mg/disc | Ampicillin 10 μg/disc |
|---|---|---|---|---|
| Bacillus subtilis (MTCC121) | 12.0 ± 1.52 | 14.00 ± 1.5 | 15.00 ± 1.5 | 12.0 ± 1.0 |
| Pseudomonas aeruginosa (MTCC741) | 10.0 ± 1.0 | 11.0 ± 1.15 | 12.0 ± 1.0 | 6.0 ± 0.0 |
| Microorganisms Tested | Fogonia indica Methanol Extract (80% v/v) | MBC/MIC | |
|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | ||
| B subtilis (MTCC121) | 125 | 250 | 4 |
| P. aeruginosa (MTCC741). | 250 | 500 | 16 |
| B. subtilis | Mean | SD | % Inhibition | |||
| Control | 0.62 | 0.65 | 0.69 | 0.65 | 0.034 | |
| ½ MIC | 0.33 | 0.46 | 0.39 | 0.33 | 0.06 | 40.19 |
| ¼ MIC | 0.42 | 0.52 | 0.59 | 0.51 | 0.08 | 21.60 |
| P. aeruginosa | Mean | SD | % Inhibition | |||
| Control | 0.85 | 0.85 | 0.88 | 0.86 | 0.01 | |
| ½ MIC | 0.51 | 0.57 | 0.63 | 0.57 | 0.06 | 33.69 |
| ¼ MIC | 0.68 | 0.72 | 0.73 | 0.70 | 0.03 | 17.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulieman, A.M.E.; Alanaizy, E.; Alanaizy, N.A.; Abdallah, E.M.; Idriss, H.; Salih, Z.A.; Ibrahim, N.A.; Ali, N.A.; Ibrahim, S.E.; Abd El Hakeem, B.S. Unveiling Chemical, Antioxidant and Antibacterial Properties of Fagonia indica Grown in the Hail Mountains, Saudi Arabia. Plants 2023, 12, 1354. https://doi.org/10.3390/plants12061354
Sulieman AME, Alanaizy E, Alanaizy NA, Abdallah EM, Idriss H, Salih ZA, Ibrahim NA, Ali NA, Ibrahim SE, Abd El Hakeem BS. Unveiling Chemical, Antioxidant and Antibacterial Properties of Fagonia indica Grown in the Hail Mountains, Saudi Arabia. Plants. 2023; 12(6):1354. https://doi.org/10.3390/plants12061354
Chicago/Turabian StyleSulieman, Abdel Moneim E., Eida Alanaizy, Naimah A. Alanaizy, Emad M. Abdallah, Hajo Idriss, Zakaria A. Salih, Nasir A. Ibrahim, Nahid Abdelraheem Ali, Salwa E. Ibrahim, and Bothaina S. Abd El Hakeem. 2023. "Unveiling Chemical, Antioxidant and Antibacterial Properties of Fagonia indica Grown in the Hail Mountains, Saudi Arabia" Plants 12, no. 6: 1354. https://doi.org/10.3390/plants12061354
APA StyleSulieman, A. M. E., Alanaizy, E., Alanaizy, N. A., Abdallah, E. M., Idriss, H., Salih, Z. A., Ibrahim, N. A., Ali, N. A., Ibrahim, S. E., & Abd El Hakeem, B. S. (2023). Unveiling Chemical, Antioxidant and Antibacterial Properties of Fagonia indica Grown in the Hail Mountains, Saudi Arabia. Plants, 12(6), 1354. https://doi.org/10.3390/plants12061354