Impact of Postharvest Putrescine Treatments on Phenolic Compounds, Antioxidant Capacity, Organic Acid Contents and Some Quality Characteristics of Fresh Fig Fruits during Cold Storage
Abstract
1. Introduction
2. Results and Discussion
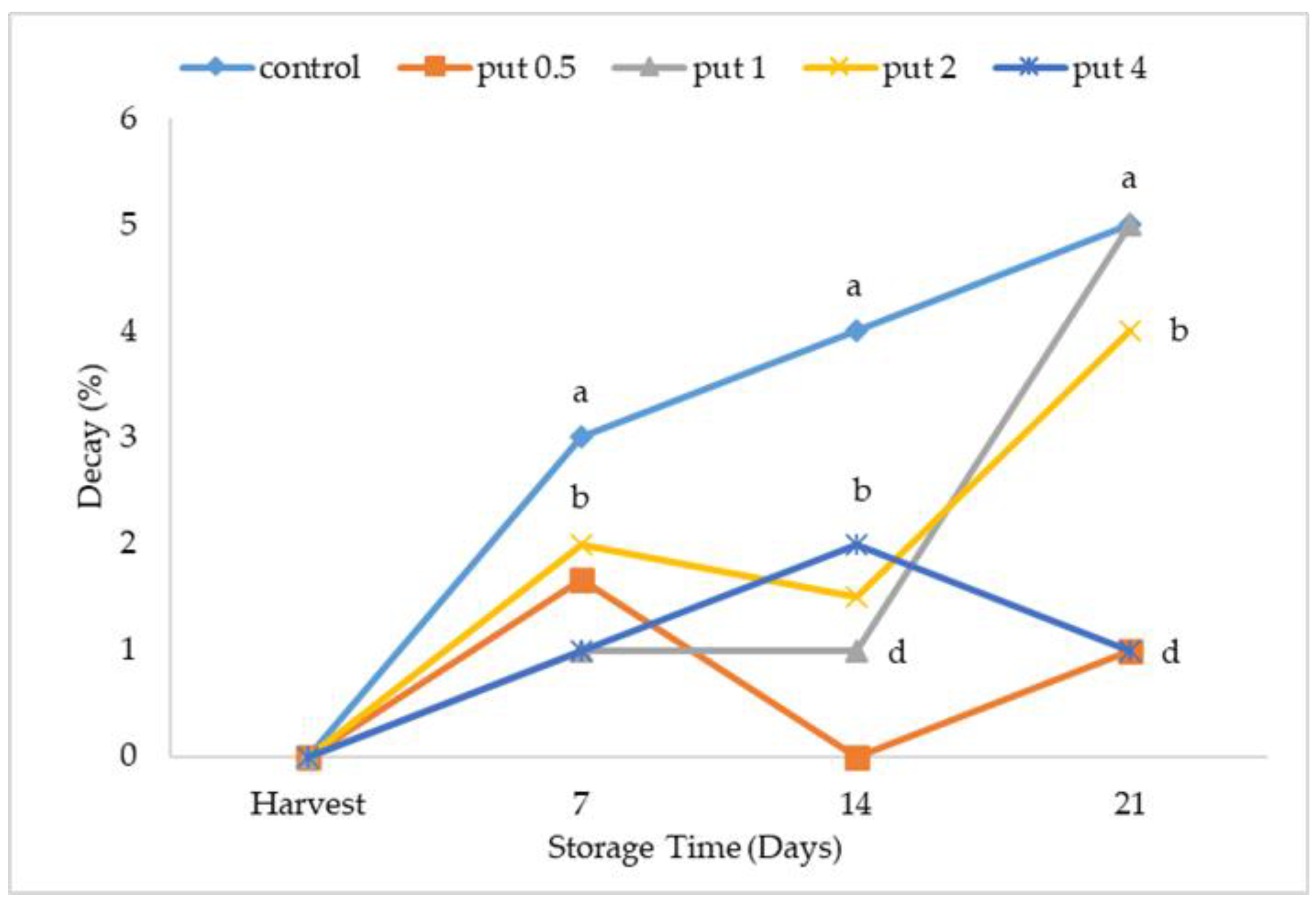
2.1. Weight Loss, Decay Ratio, and Fruit Firmness
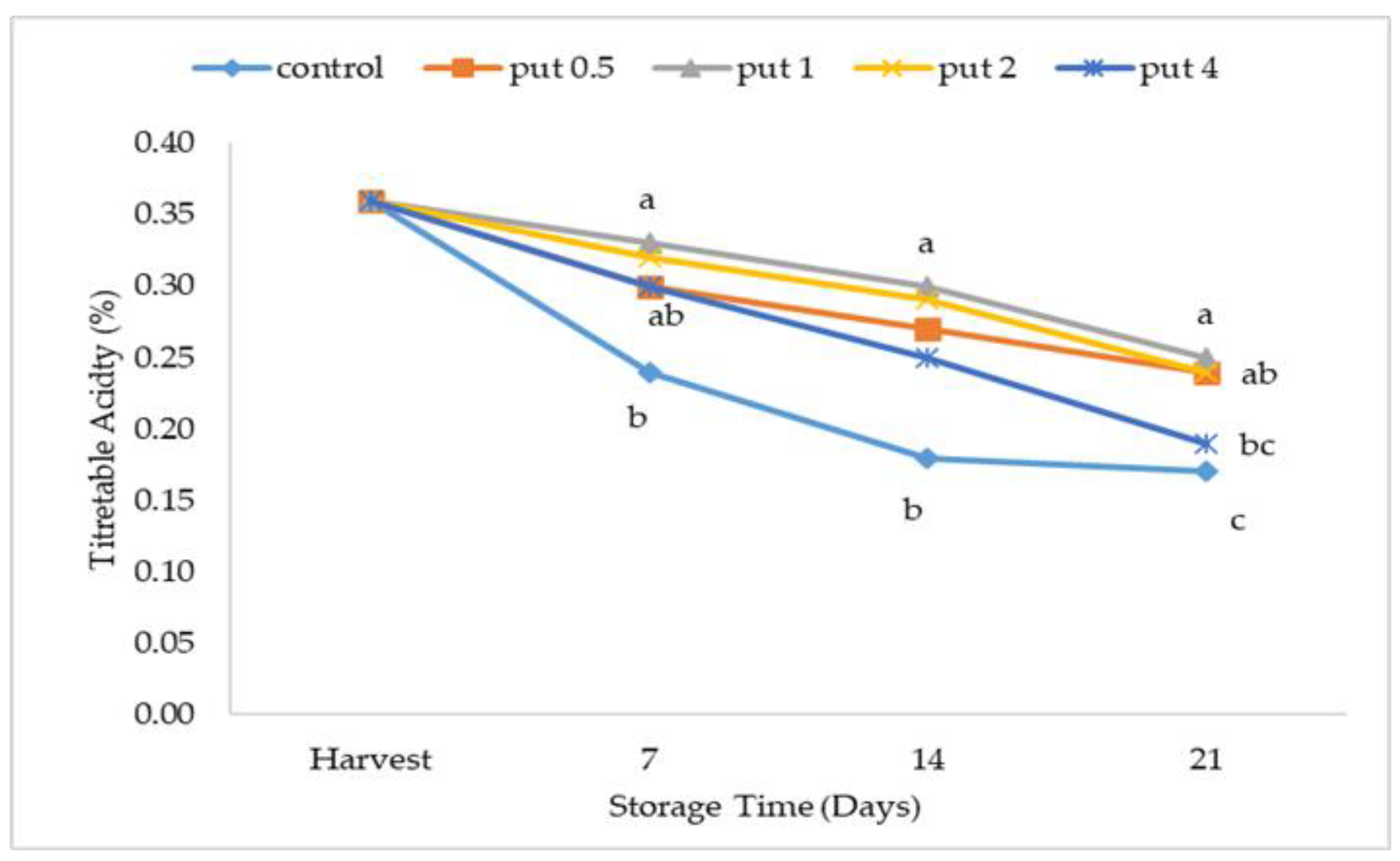
2.2. Soluble Solids Content and Titratable Acidity
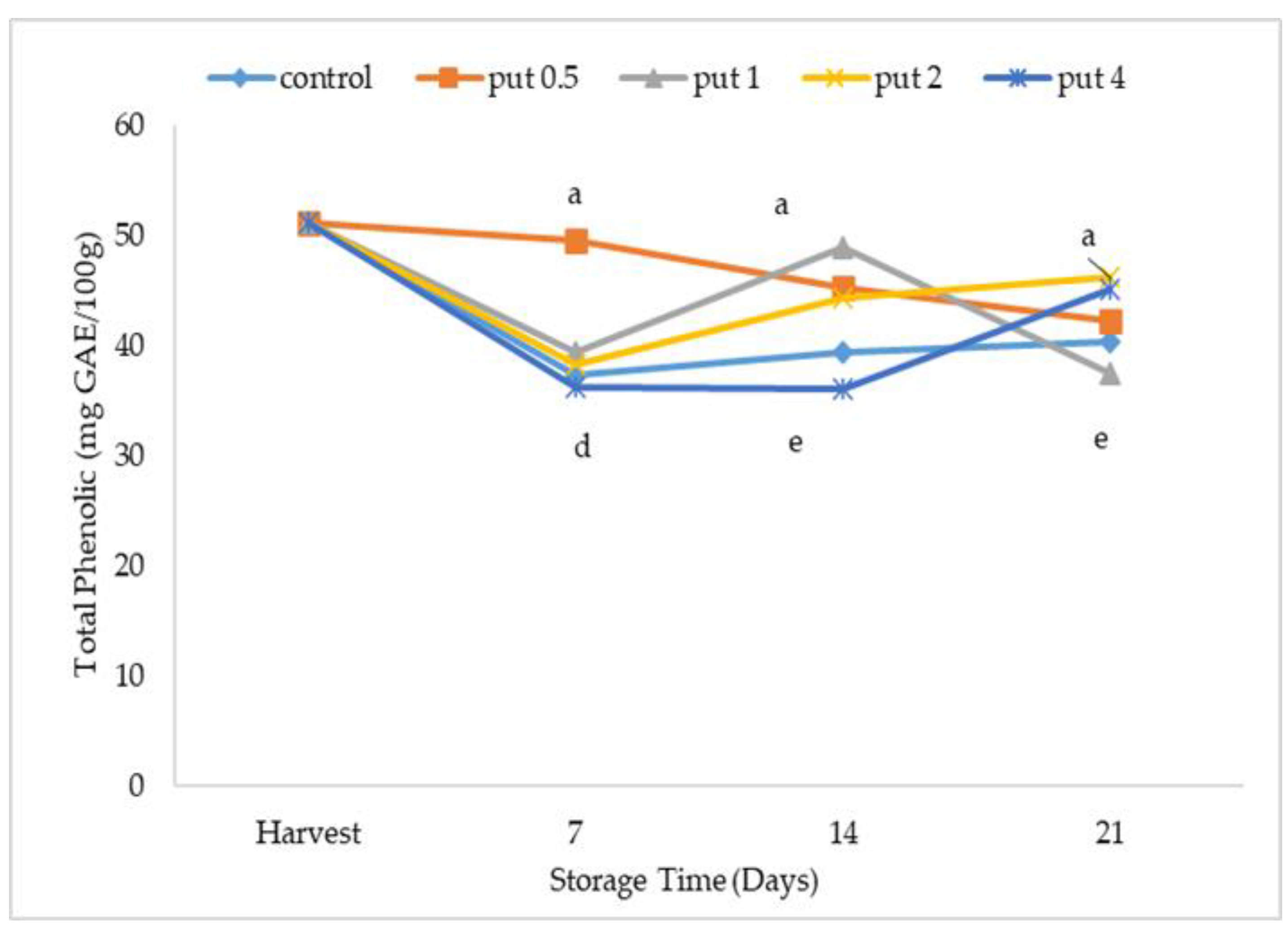
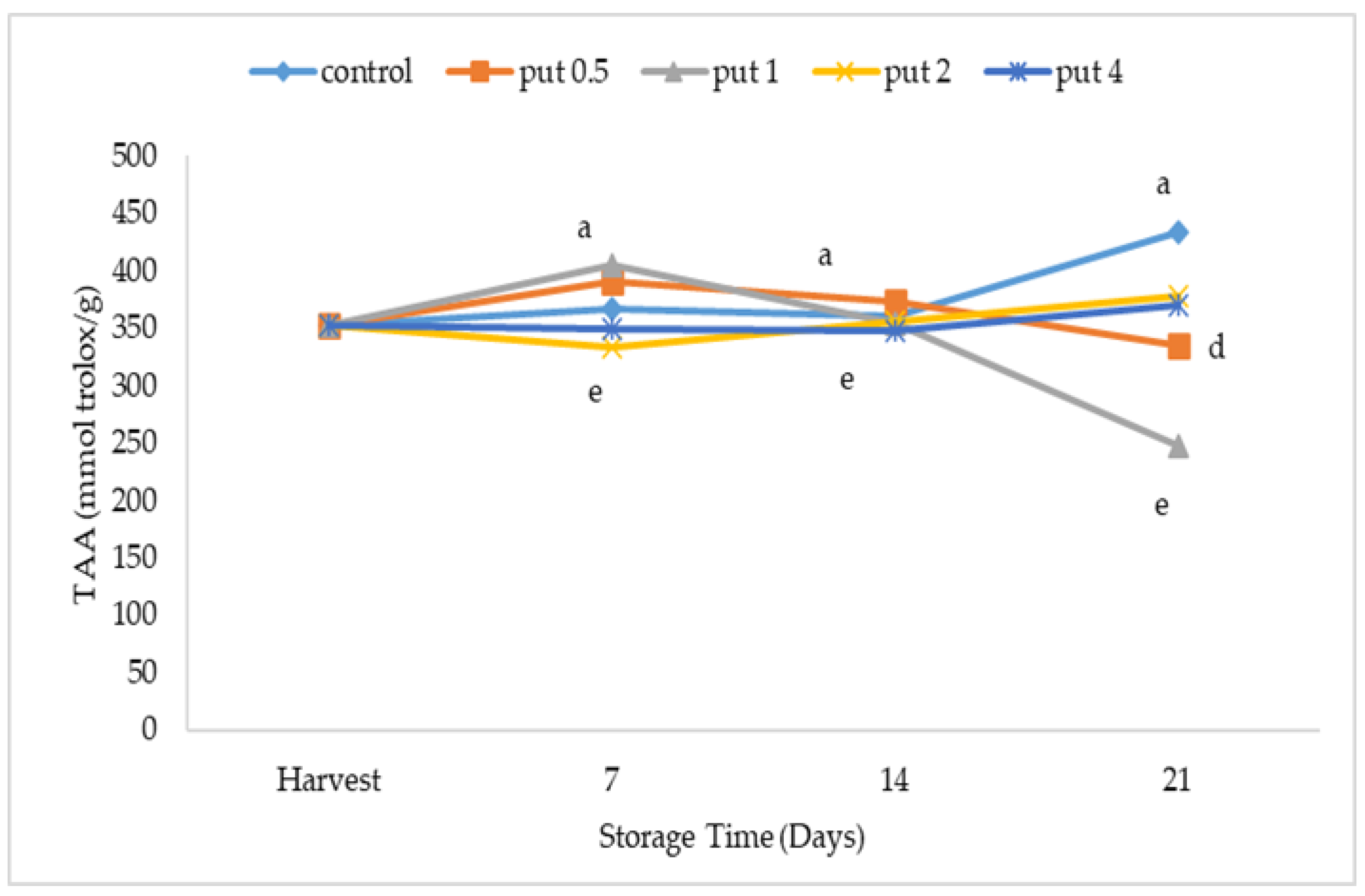
2.3. Total Phenolics and Total Antioxidant Activity
2.4. Specific Phenolic Compounds
2.5. Organic Acids
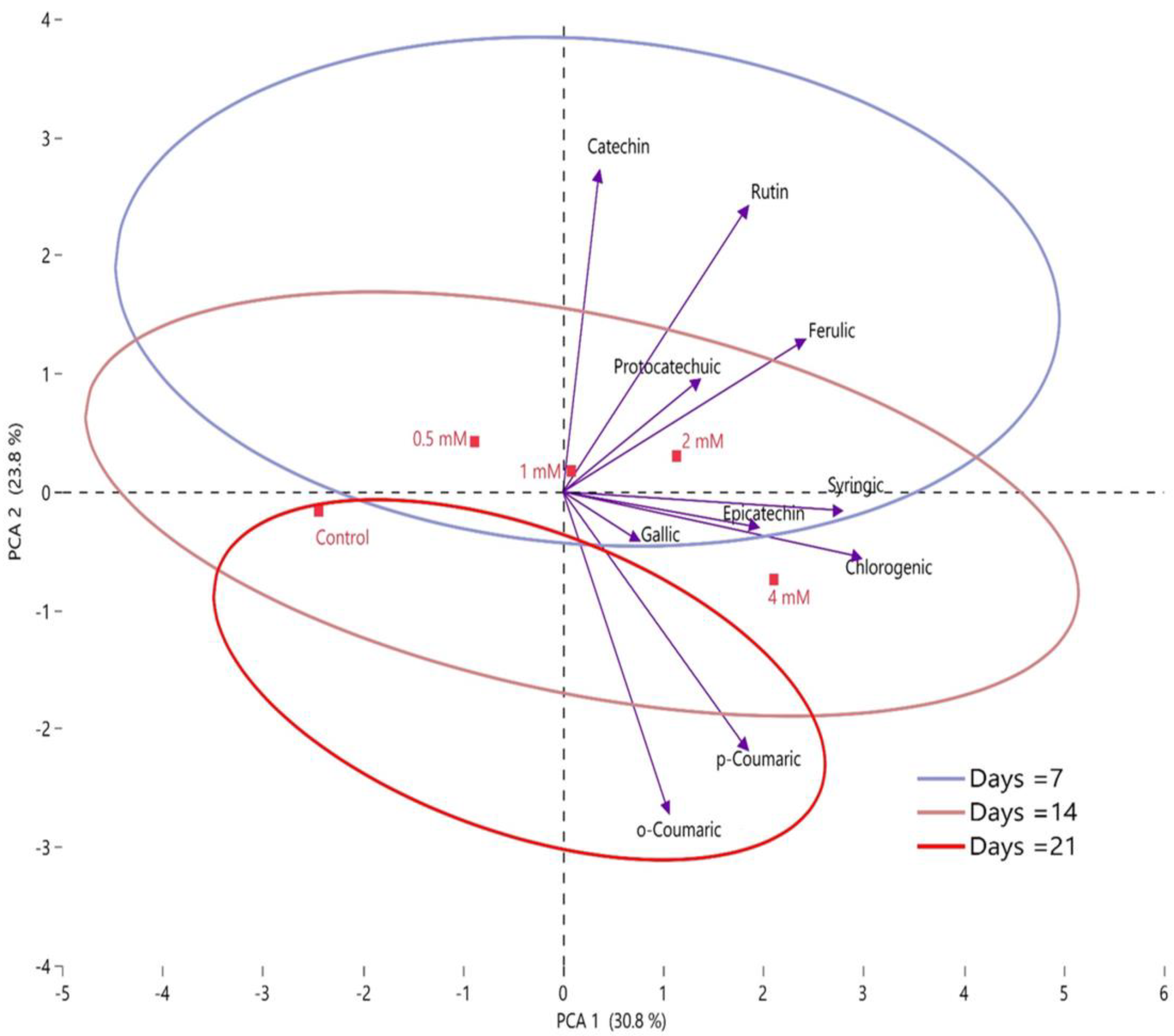
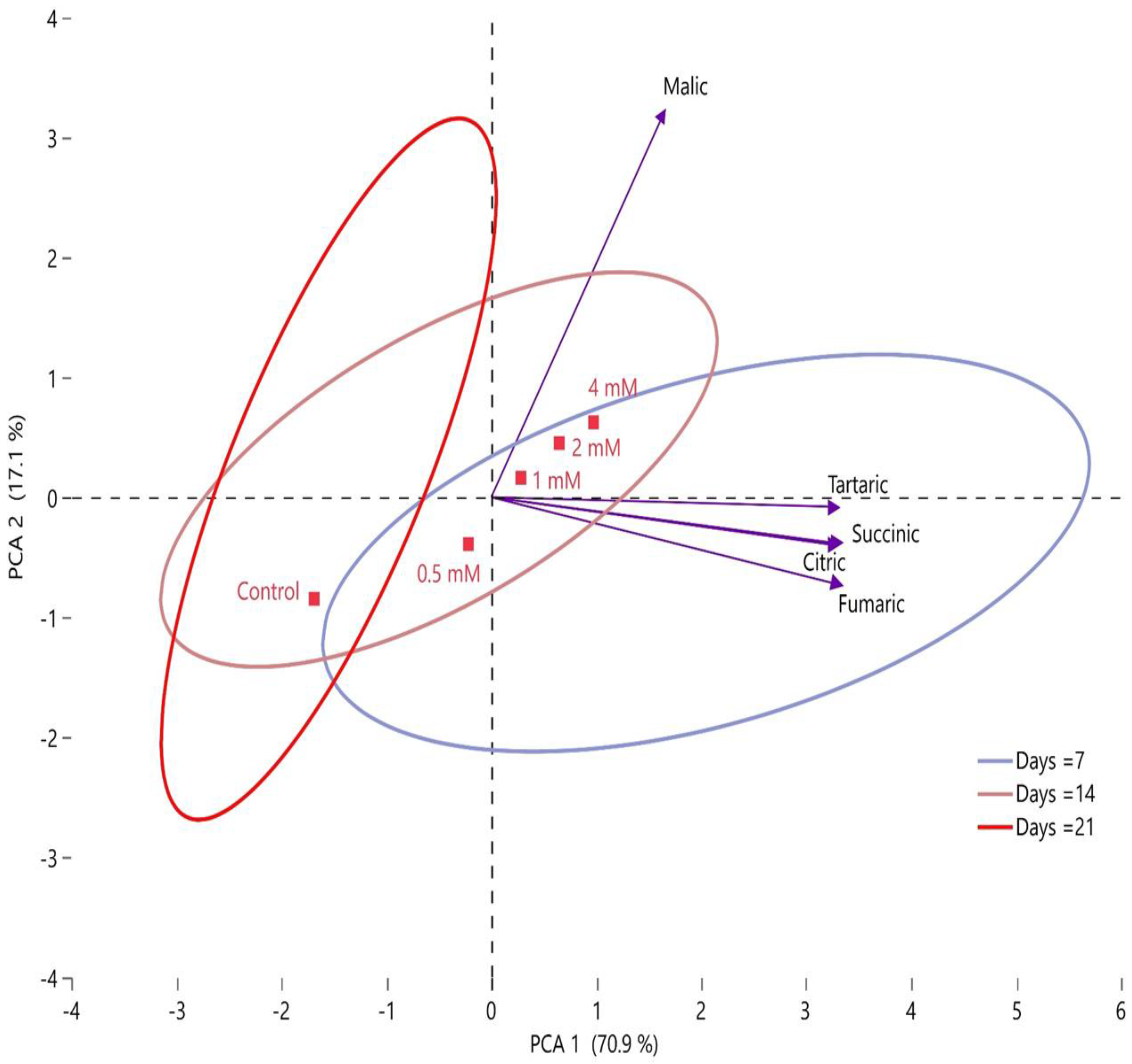
2.6. Correlation between Postharvest Putrescine Treatments and Quality Characteristics of Fruits, Organic Acids, and Phenolic Compounds by PCA
3. Materials and Methods
3.1. Weight Loss
3.2. Decay Rate
3.3. Fruit Firmness
3.4. Soluble Solids Content, Titratable Acidity
3.5. Total Phenolics and Antioxidant Capacity
3.6. Specific Phenolic Compounds
3.7. Organic Acids
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slatnar, A.; Klancar, U.; Stampar, F.; Veberic, R. Effect of drying of figs (Ficus carica L.) on the contents of sugars, organic acids, and phenolic compounds. J. Agric. Food Chem. 2011, 59, 11696–11702. [Google Scholar] [CrossRef]
- Marpudi, S.L.; Ramachandran, P.; Srividya, N. Aloe vera gel coating for postharvest quality maintenance of fresh fig fruits. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 878–886. [Google Scholar]
- Irfan, P.K.; Vanjakshi, V.; Keshava Prakash, M.N.; Ravi, R.; Kudachikar, V.B. Calcium chloride extends the keeping quality of fig fruit (Ficus carica L.) during storage and shelf-life. Postharvest Biol. Technol. 2013, 82, 70–75. [Google Scholar] [CrossRef]
- Karabulut, O.A.; Ilhan, K.; Arslan, U.; Vardar, C. Evaluation of the use of chlorine dioxide by fogging for decreasing postharvest decay of fig. Postharvest Biol. Technol. 2009, 52, 313–315. [Google Scholar]
- Hung, D.V.; Tanaka, F.; Uchino, T.; Hiruma, N. Using nanomist humidifier to maintain postharvest quality of fig (Ficus carica L.) fruit in high humidity storage environment. J. Fac. Agric. Kyushu Univ. 2011, 56, 361–365. [Google Scholar] [CrossRef]
- Luna-Guzman, I.; Cantwell, M.; Barret, D.M. Fresh-cut cantaloupe: Effects of CaCl2 dips and heat treatments on firmness and metabolic activity. Postharvest Biol. Technol. 1999, 17, 201–213. [Google Scholar] [CrossRef]
- Cohen, S.S. A Guide to the Polyamines; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Kaur-Sawhney, R.; Tiburcio, A.F.; Altabella, T.; Galston, A.W. Polyamines in plants: An overview. J. Cell Mol. Biol. 2003, 2, 1–12. [Google Scholar]
- Slocum, R.D.; Flores, H.E. Biochemistry and Physiology of Polyamines in Plants; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Cassol, T.; Mattoo, A.K. Do polyamines and ethylene interact to regulate plant growth, development and senescence. In Molecular Insight in Plant Biology; Nath, P., Mattoo, A.K., Ranade, S.A., Weil, J.H., Eds.; Science Publishers Inc.: Enfield, NH, USA, 2003; pp. 121–132. [Google Scholar]
- Janne, J.; Alhonen, L.; Pietila, M.; Keinanen, T.A. Genetic approaches to the cellular functions of polyamines in mammals. Eur. J. Biochem. 2004, 271, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Martinez Romero, D.; Serrano, M. The role of polyamines in the improvement of shelf life of fruits. Trends Food Sci. Technol. 2002, 13, 228–234. [Google Scholar] [CrossRef]
- Wang, T.; Hu, M.; Yuan, D.; Yun, Z.; Gao, Z.; Su, Z.; Zhang, Z. Melatonin alleviates pericarp browning in litchi fruit by regulating membrane lipid and energy metabolisms. Postharvest Biol. Technol. 2020, 160, 111066. [Google Scholar] [CrossRef]
- Liu, S.; Huang, H.; Huber, D.J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of ripening and softening in ’Guifei’ mango fruit by postharvest application of melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Ma, Q.; Lin, X.; Wei, Q.; Yang, X.; Zhang, Y.N.; Chen, J. Melatonin treatment delays postharvest senescence and maintains the organoleptic quality of ‘Newhall’navel orange (Citrus sinensis (L.) Osbeck) by inhibiting respiration and enhancing antioxidant capacity. Sci. Hortic. 2021, 286, 110236. [Google Scholar] [CrossRef]
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin treatment reduces chilling injury in peach fruit through its regulation of membrane fatty acid contents and phenolic metabolism. Food Chem. 2018, 245, 659–666. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Li, L.; Aghdam, M.S.; Wei, X.; Liu, J.; Luo, Z. Elevated CO2 delayed the chlorophyll degradation and anthocyanin accumulation in postharvest strawberry fruit. Food Chem. 2019, 285, 163–170. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Khadivi, A. Morphological variability of Prunus lycioides Spach germplasm using multivariate analysis. Sci. Hortic. 2020, 261, 108973. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Wu, T.; Zhai, R.; Yang, C.; Wang, Z. Effects of melatonin treatment of postharvest pear fruit on aromatic volatile biosynthesis. Molecules 2019, 24, 4233. [Google Scholar] [CrossRef]
- Jannatizadeh, A. Exogenous melatonin applying confers chilling tolerance in pomegranate fruit during cold storage. Sci. Hortic. 2019, 246, 544–549. [Google Scholar] [CrossRef]
- Bal, E. Physicochemical changes in ‘Santa Rosa’ plum fruit treated with melatonin during cold storage. J. Food Meas. Charact. 2019, 13, 1713–1720. [Google Scholar] [CrossRef]
- Hu, M.; Li, J.; Rao, J. Effect of melatonin on ripening and senescence of postharvest kiwifruits. Food Sci. 2018, 39, 226–232. [Google Scholar]
- Candir, E.; Ozdemir, A.E.; Aksoy, M.C. Effects of chitosan coating and modified atmosphere packaging on postharvest quality and bioactive compounds of pomegranate fruit cv. ‘Hicaznar’. Sci. Hortic. 2018, 235, 235–243. [Google Scholar] [CrossRef]
- Rasouli, M.; Saba, M.K.; Ramezanian, A. Inhibitory effect of salicylic acid and Aloe vera gel edible coating on microbial load and chilling injury of orange fruit. Sci. Hortic. 2019, 247, 27–34. [Google Scholar] [CrossRef]
- Byeon, S.; Lee, J. Differential responses of fruit quality and major targeted metabolites in three different cultivars of cold-stored figs (Ficus carica L.). Sci. Hortic. 2020, 260, 108877. [Google Scholar] [CrossRef]
- Ozturk, B.; Yildiz, M.; Yildiz, K.; Gun, S. Maintaining the postharvest quality and bioactive compounds of jujube (Ziziphus jujuba Mill. Cv. ‘Li’) fruit by applying 1-methylcyclopropene. Sci. Hortic. 2021, 275, 109671. [Google Scholar] [CrossRef]
- Kader, A.A.; Yahia, E.M. Postharvest biology of tropical and subtropical fruits. In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 79–111. [Google Scholar]
- Sandhya, K.V.K. Modified atmosphere packaging of fresh produce: Current status and future needs. LWT Food Sci. Technol. 2010, 43, 381–392. [Google Scholar] [CrossRef]
- Patel, N.; Gantait, S.; Panigrahi, J. Extension of postharvest shelf-life in green bell pepper (Capsicum annuum L.) using exogenous application of polyamines (spermidine and putrescine). Food Chem. 2019, 275, 681–687. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F.; et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Onik, J.C.; Wai, S.C.; Li, A.; Lin, Q.; Sun, Q.; Wang, Z. Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chem. 2020, 337, 127753. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, S.; Khankahdani, H.H.; Rahimzadeh, M. Effects of melatonin treatment on the biochemical changes and antioxidant enzyme activity of mango fruit during storage. Sci. Hortic. 2020, 259, 108835. [Google Scholar] [CrossRef]
- Fawole, O.A.; Atukuri, J.; Arendse, E.; Opara, U.O. Postharvest physiological responses of pomegranate fruit (cv. Wonderful) to exogenous putrescine treatment and effects on physico-chemical and phytochemical properties. Food Sci. Hum. Wellness 2020, 9, 146–161. [Google Scholar] [CrossRef]
- Kibar, H.; Tas, A.; Gundogdu, M. Evaluation of biochemical changes and quality in peach fruit: Effect of putrescine treatments and storage. J. Food Compos. Anal. 2021, 102, 104048. [Google Scholar] [CrossRef]
- Hanif, A.; Ahmad, S.; Jaskani, M.J.; Ahmad, R. Papaya treatment with putrescine maintained the overall quality and promoted the antioxidative enzyme activities of the stored fruit. Sci. Hortic. 2020, 268, 109367. [Google Scholar] [CrossRef]
- Zokaee Khosroshahi, M.R.; Esna-Ashari, M. Effect of exogenous putrescine treatment on the quality and storage life of peach (Prunus persica L.) fruit. Int. J. Postharvest Technol. Innov. 2008, 1, 278–287. [Google Scholar] [CrossRef]
- Barman, K.; Asrey, R.; Pal, R.K. Putrescine and carnauba wax pretreatments alleviate chilling injury, enhance shelf life and preserve pomegranate fruit quality during cold storage. Sci. Hortic. 2011, 130, 795–800. [Google Scholar] [CrossRef]
- Koushesh Saba, M.; Arzani, K.; Barzegar, M. Postharvest polyamine application alleviates chilling injury and affects apricot storage ability. J. Agric. Food Chem. 2012, 60, 8947–8953. [Google Scholar] [CrossRef]
- Mannozzi, C.; Tylewicz, U.; Chinnici, F.; Siroli, L.; Rocculi, P.; Rosa, M.D.; Romani, S. Effects of chitosan based coatings enriched with procyanidin by-product on quality of fresh blueberries during storage. Food Chem. 2018, 251, 18–24. [Google Scholar] [CrossRef]
- Ozturk, B.; Kucuker, E.; Karaman, S.; Ozkan, Y. The effects of cold storage and aminoethoxyvinylglycine (AVG) on bioactive compounds of plum fruit (Prunus salicina Lindell cv. ‘Black Amber’). Postharvest Biol. Technol. 2012, 72, 35–41. [Google Scholar] [CrossRef]
- Cheng, S.B.; Yu, Y.; Guo, J.Y.; Chen, G.G.; Guo, M.R. Effect of 1-methylcyclopropene and chitosan treatment on the storage quality of jujube fruit and its related enzyme activities. Sci. Hortic. 2020, 265, 109281. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Q.; Chen, Z.; Li, T.; Zhang, Z.; Gao, H. Changes in pericarp metabolite profiling of four litchi cultivars during browning. Food Res. Int. 2019, 120, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Koc Guler, S.; Karakaya, O.; Karakaya, M.; Ozturk, B.; Aglar, E.; Yarılgac, T.; Sefa, G. Combined treatments of modified at-mosphere packaging with aminoethoxyvinylglycine maintained fruit quality in sweet cherry throughout cold storage and shelf life. Acta Sci. Pol. Hortorum Cultus 2019, 18, 13–26. [Google Scholar] [CrossRef]
- Ozturk, B.; Karakaya, O.; Yildiz, K.; Saracoglu, O. Effects of Aloe vera gel and MAP on bioactive compounds and quality attributes of cherry laurel fruit during cold storage. Sci. Hortic. 2019, 249, 31–37. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Bremer, V.; Ferguson, L.; Crisosto, G.M. Evaluating quality attributes of four fresh fig (Ficus carica L.) cultivars harvested at two maturity stages. HortScience 2010, 45, 707–710. [Google Scholar] [CrossRef]
- Freiman, Z.E.; Rodov, V.; Yablovitz, Z.; Horev, B.; Flaishman, M.A. Preharvest application of 1-methylcyclopropene inhibits ripening and improves keeping quality of ‘Brown Turkey’ figs (Ficus carica L.). Sci. Hortic. 2012, 138, 266–272. [Google Scholar] [CrossRef]
- Bahar, A.; Lichter, A. Effect of controlled atmosphere on the storage potential of Ottomanit fig fruit. Sci. Hortic. 2018, 227, 196–201. [Google Scholar] [CrossRef]
- Khan, A.S.; Singh, Z.; Abbasi, N.A.; Swinny, E.E. Pre-or post-harvest applications of putrescine and low temperature storage affect fruit ripening and quality of ‘Angelino’ plum. J. Sci. Food Agric. 2008, 88, 1686–1695. [Google Scholar] [CrossRef]
- Serrano, M.; Martinez-Romero, D.; Guillen, F.; Valero, D. Effects of exogenous putrescine on improving shelf life of four plum cultivars. Postharvest Biol. Technol. 2003, 30, 259–271. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, A. Improvement in storability and quality of peach cv. Flordaprince with postharvest application of various chemicals. J. Pharmacogn. Phytochem. 2019, 8, 460–464. [Google Scholar]
- Abd El-Gawad, M.G.; Zaki, Z.A.; Ekbal, Z.A. Effect of some postharvest treatments on quality of ‘Alphonse’ mango fruits during cold storage. Middle East J. Agric. Res. 2019, 8, 1067–1079. [Google Scholar]
- Abbasi, N.A.; Ali, I.; Hafiz, I.A.; Alenazi, M.M.; Shafiq, M. Effects of putrescine application on peach fruit during storage. Sus-tainability 2019, 11, 2013. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, H.; Guo, X.; Bi, X.; Liu, X.; Xu, Q.; Zheng, Y. Effect of chitosan/ Nano-TiO2 composite coatings on the postharvest quality and physico chemical characteristics of mango fruits. Sci. Hortic. 2020, 263, 109–135. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, W.; Liu, Y.; Sang, Y.; Yang, W.; Guo, M.; Cheng, S.; Chen, G. Effect of composite coating treatment and low-temperature storage on the quality and antioxidant capacity of Chinese jujube (Zizyphus jujuba cv. Junzao). Sci. Hortic. 2021, 288, 110372. [Google Scholar] [CrossRef]
- Davarynejad, G.H.; Zarei, M.; Nasrabadi, M.E.; Ardakani, E. Effects of salicylic acid and putrescine on storability, quality at-tributes and antioxidant activity of plum cv. ‘Santa Rosa’. J. Food Sci. Technol. 2015, 52, 2053–2062. [Google Scholar] [CrossRef]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate fruit as a rich source of biologically active compounds. Hindawi Publ. Corp. BioMed Res. Int. 2014, 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, J.; Fan, X.; Jiang, W. Applications of nitric oxide and melatonin in improving postharvest fruit quality and the separate and crosstalk biochemical mechanisms. Trends Food Sci. Technol. 2020, 99, 531–541. [Google Scholar] [CrossRef]
- Xu, L.; Yue, Q.; Xiang, G.; Bian, F.E.; Yao, Y. Melatonin promotes ripening of grape berry via increasing the levels of ABA, H2O2, and particularly ethylene. Hortic. Res. 2018, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Shen, Y.; Shen, T.; Wang, X.; Zhang, X.; Hu, P. Melatonin accumulation in sweet cherry and its influence on fruit quality and antioxidant properties. Molecules 2020, 25, 753. [Google Scholar] [CrossRef]
- Pang, L.; Wu, Y.; Pan, Y.; Ban, Z.; Li, L.; Li, X. Insights into exogenous melatonin associated with phenylalanine metabolism in postharvest strawberry. Postharvest Biol. Technol. 2020, 168, 111244. [Google Scholar] [CrossRef]
- Kiralan, M.; Gundogdu, M. Dut türlerine ait meyvelerin organik asit ve c vitamini içerikleri üzerine farklı kurutma teknikle-rinin etkisi. Uluslararası Tarım Ve Yaban Hayatı Bilim. Derg. 2021, 7, 404–411. [Google Scholar] [CrossRef]
- Veberic, R.; Colaric, M.; Stampar, F. Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chem. 2008, 106, 153–157. [Google Scholar] [CrossRef]
- Sedaghat, S.; Rahemi, M. Effects of physio-chemical changes during fruit development on nutritional quality of fig (Ficus carica L. var. ‘Sabz’) under rain-fed condition. Sci. Hortic. 2018, 237, 44–50. [Google Scholar] [CrossRef]
- Celikel, F.G.; Karacal, I. Effects of harvest maturity and precooling on fruit quality and longevity of ‘Bursa Siyah’ figs (Ficus carica L.). Acta Hortic. 1998, 480, 283–288. [Google Scholar] [CrossRef]
- Tijero, V.; Muoz, P.; Munńe-Bosch, S. Melatonin as an inhibitor of sweet cherries ripening in orchard trees. Plant Physiol. Biochem. 2019, 140, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kaskoniene, V.; Bimbiraite-Surviliene, K.; Kaskonas, P.; Tiso, N.; Cesoniene, L.; Daubaras, R.; Maruska, A.S. Changes in the biochemical compounds of Vaccinium myrtillus, Vaccinium vitis-idaea, and forest litter collected from various forest types. Turk. J. Agric. For. 2020, 44, 557–566. [Google Scholar] [CrossRef]
- Gundogu, M. Effect of rootstocks on phytochemical properties of apricot fruit. Turk. J. Agric. For. 2019, 43, 1–10. [Google Scholar] [CrossRef]
- Khosroshahi, M.R.Z.; Esna-Ashari, M.; Ershadi, A. Effect of exogenous putrescine on post-harvest life of strawberry (Fragaria ananassa Duch.) fruit, cultivar Selva. Sci. Hortic. 2007, 114, 27–32. [Google Scholar] [CrossRef]
- Bregoli, A.M.; Scaramagli, S.; Costa, G.; Sabatini, E.; Ziosi, V.; Biondi, S.; Torrigiani, P. Peach (Prunus persica L.) fruit ripening: Aminoethoxyvinylglycine (AVG) and exogenous polyamines affect ethylene emission and flesh firmness. Physiol. Plant. 2022, 114, 472–481. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.A.; Malovaná, S.; Pérez, J.P.; Borges, T.; García Montelongo, F.J. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorimetric detection. J. Chromatogr. A 2001, 912, 249–257. [Google Scholar] [CrossRef]
- Bevilacqua, A.E.; Califano, A.N. Determination of organic acids in dairy products by high performance liquid chromatography. J. Food Sci. 1989, 54, 1076. [Google Scholar] [CrossRef]




| Phenolic Compounds (mg/100 g FW) | Putrescine Treatment (mM) | Storage Time (Days) | |||
|---|---|---|---|---|---|
| Harvest | 7 | 14 | 21 | ||
| Gallic acid | Control | 1.52 | 1.50 bc | 1.31 b | 1.40 c |
| 0.5 | 1.37 c | 1.36 b | 1.70 bc | ||
| 1 | 2.25 a | 1.93 a | 1.77 ba | ||
| 2 | 1.70 ac | 0.64 c | 2.06 a | ||
| 4 | 1.03 ba | 1.57 ba | 1.75 ba | ||
| p-Coumaric acid | Control | 0.18 | 0.10 a | 0.13 c | 0.20 b |
| 0.5 | 0.19 a | 0.18 c | 0.40 ba | ||
| 1 | 0.23 a | 0.31 c | 0.71 a | ||
| 2 | 0.25 a | 0.65 b | 0.74 a | ||
| 4 | 0.10 a | 0.89 a | 0.75 a | ||
| Chlorogenic acid | Control | 2.08 | 1.25 d | 1.51 d | 2.15 a |
| 0.5 | 1.31 d | 1.72 dc | 2.72 a | ||
| 1 | 2.74 c | 1.97 c | 2.32 a | ||
| 2 | 3.22 b | 2.63 b | 2.80 a | ||
| 4 | 3.98 a | 4.04 a | 2.82 a | ||
| Syringic acid | Control | 0.28 | 0.17 b | 0.27 c | 0.09 b |
| 0.5 | 0.23 b | 0.32 c | 0.18 b | ||
| 1 | 0.31 b | 0.82 b | 0.56 a | ||
| 2 | 0.33 b | 0.85 b | 0.66 a | ||
| 4 | 1.88 a | 1.66 a | 0.15 b | ||
| Ferulic acid | Control | 0.41 | 1.16 c | 1.34 c | 0.37 d |
| 0.5 | 1.82 b | 1.94 b | 0.77 c | ||
| 1 | 1.91 ba | 1.90 b | 0.88 cb | ||
| 2 | 1.93 ba | 2.05 b | 1.62 a | ||
| 4 | 2.17 a | 2.85 a | 1.22 b | ||
| Protocatechuic acid | Control | 3.02 | 2.16 b | 1.59 b | 2.73 cb |
| 0.5 | 4.69 a | 2.86 ba | 3.18 b | ||
| 1 | 3.15 b | 2.49 ba | 5.07 a | ||
| 2 | 5.50 a | 3.48 a | 2.29 c | ||
| 4 | 4.49 a | 2.62 ba | 4.62 a | ||
| o-Coumaric acid | Control | 0.35 | 0.04 c | 0.08 a | 0.33 a |
| 0.5 | 0.07 cb | 0.14 a | 0.36 a | ||
| 1 | 0.08 cb | 0.16 a | 0.38 a | ||
| 2 | 0.12 c | 0.20 a | 0.52 a | ||
| 4 | 0.34 a | 0.26 a | 0.53 a | ||
| Flavonoid Acid Contents (mg/100 g FW) | Putrescine Treatment (mM) | Storage Time (Days) | |||
|---|---|---|---|---|---|
| Harvest | 7 | 14 | 21 | ||
| Rutin | Control | 5.88 | 5.79 d | 2.20 d | 1.36 c |
| 0.5 | 7.64 c | 3.71 c | 2.70 b | ||
| 1 | 8.57 cb | 3.72 c | 3.50 b | ||
| 2 | 9.01 b | 4.62 b | 3.11 b | ||
| 4 | 10.56 a | 5.38 a | 4.88 a | ||
| Catechin | Control | 2.31 | 0.73 cb | 2.25 c | 0.14 b |
| 0.5 | 076 cb | 0.49 bac | 0.49 a | ||
| 1 | 0.85 b | 0.56 ba | 0.46 a | ||
| 2 | 1.47 a | 0.70 a | 0.47 a | ||
| 4 | 0.34 c | 0.26 bc | 0.09 b | ||
| Epicatechin | Control | 0.71 | 0.17 d | 0.33 c | 0.67 c |
| 0.5 | 0.66 c | 2.34 b | 1.62 b | ||
| 1 | 1.36 b | 1.78 b | 1.70 b | ||
| 2 | 1.81 a | 4.03 a | 2.26 a | ||
| 4 | 1.29 b | 2.22 b | 0.72 c | ||
| Organic Acids | Putrescine Treatment (mM) | Storage Time (Days) | |||
|---|---|---|---|---|---|
| Harvest | 7 | 14 | 21 | ||
| Citric acid | Control | 14.21 | 12.20 b | 7.19 b | 8.02 b |
| 0.5 | 19.00 a | 8.25 b | 11.13 a | ||
| 1 | 19.05 a | 12.14 a | 12.22 a | ||
| 2 | 20.02 a | 12.67 a | 12.36 a | ||
| 4 | 19.96 a | 13.50 a | 8.56 b | ||
| Succinic acid | Control | 0.61 | 0.55 b | 0.12 c | 0.05 c |
| 0.5 | 0.74 b | 0.18 c | 0.06 c | ||
| 1 | 1.56 a | 0.25 c | 0.25 a | ||
| 2 | 1.62 a | 0.44 b | 0.28 a | ||
| 4 | 1.30 a | 0.69 a | 0.17 b | ||
| Fumaric acid | Control | 1.03 | 0.92 b | 0.70 b | 0.30 c |
| 0.5 | 1.86 a | 1.19 ba | 0.67 a | ||
| 1 | 1.87 a | 0.86 ba | 0.69 a | ||
| 2 | 1.92 | 0.93 ba | 0.43 c | ||
| 4 | 1.99 | 1.28 a | 0.33 c | ||
| Tartaric acid | Control | 12.21 | 11.29 c | 9.14 b | 7.66 c |
| 0.5 | 13.35 b | 11.28 b | 9.22 ba | ||
| 1 | 13.39 b | 10.74 b | 8.36 bc | ||
| 2 | 14.89 b | 11.25 b | 9.40 ba | ||
| 4 | 17.57 a | 14.07 a | 10.14 a | ||
| Time (min) | Dissolvent A (%) | Dissolvent B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 15 | 85 | 15 |
| 25 | 50 | 50 |
| 35 | 15 | 85 |
| 45 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucuker, E.; Aglar, E.; Sakaldaş, M.; Şen, F.; Gundogdu, M. Impact of Postharvest Putrescine Treatments on Phenolic Compounds, Antioxidant Capacity, Organic Acid Contents and Some Quality Characteristics of Fresh Fig Fruits during Cold Storage. Plants 2023, 12, 1291. https://doi.org/10.3390/plants12061291
Kucuker E, Aglar E, Sakaldaş M, Şen F, Gundogdu M. Impact of Postharvest Putrescine Treatments on Phenolic Compounds, Antioxidant Capacity, Organic Acid Contents and Some Quality Characteristics of Fresh Fig Fruits during Cold Storage. Plants. 2023; 12(6):1291. https://doi.org/10.3390/plants12061291
Chicago/Turabian StyleKucuker, Emine, Erdal Aglar, Mustafa Sakaldaş, Fatih Şen, and Muttalip Gundogdu. 2023. "Impact of Postharvest Putrescine Treatments on Phenolic Compounds, Antioxidant Capacity, Organic Acid Contents and Some Quality Characteristics of Fresh Fig Fruits during Cold Storage" Plants 12, no. 6: 1291. https://doi.org/10.3390/plants12061291
APA StyleKucuker, E., Aglar, E., Sakaldaş, M., Şen, F., & Gundogdu, M. (2023). Impact of Postharvest Putrescine Treatments on Phenolic Compounds, Antioxidant Capacity, Organic Acid Contents and Some Quality Characteristics of Fresh Fig Fruits during Cold Storage. Plants, 12(6), 1291. https://doi.org/10.3390/plants12061291







