Population Structure, Genetic Diversity and Candidate Genes for the Adaptation to Environmental Stress in Picea koraiensis
Abstract
1. Introduction
2. Results
2.1. Characterization of GBS-Seq Data and SNPs
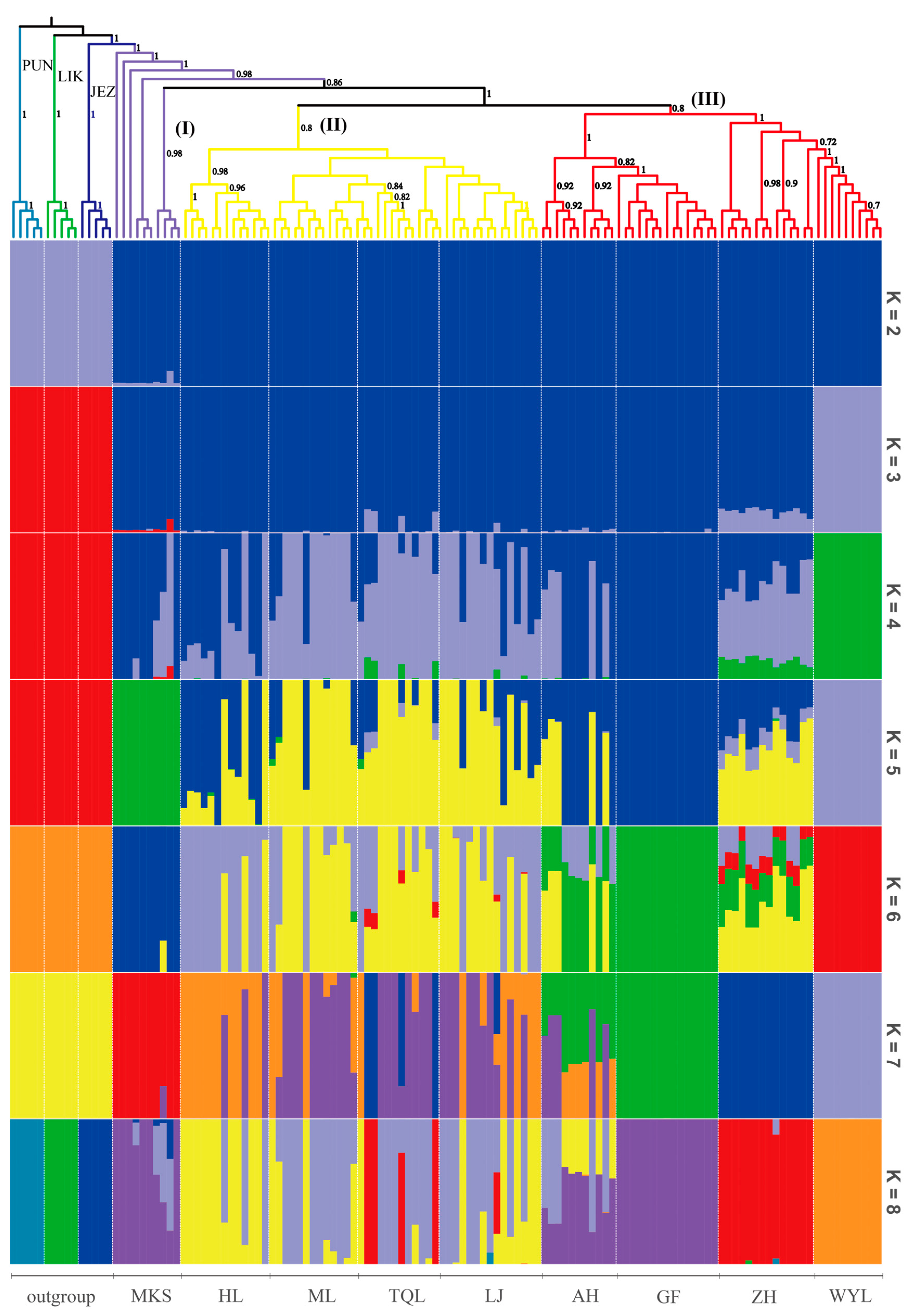
2.2. Phylogenetic and Population Genetic Structure Analysis
2.3. Population Diversity and Selective Sweep Analysis
3. Discussion
3.1. Geographical Isolation, Climate Heterogeneity, and Gene Introgression
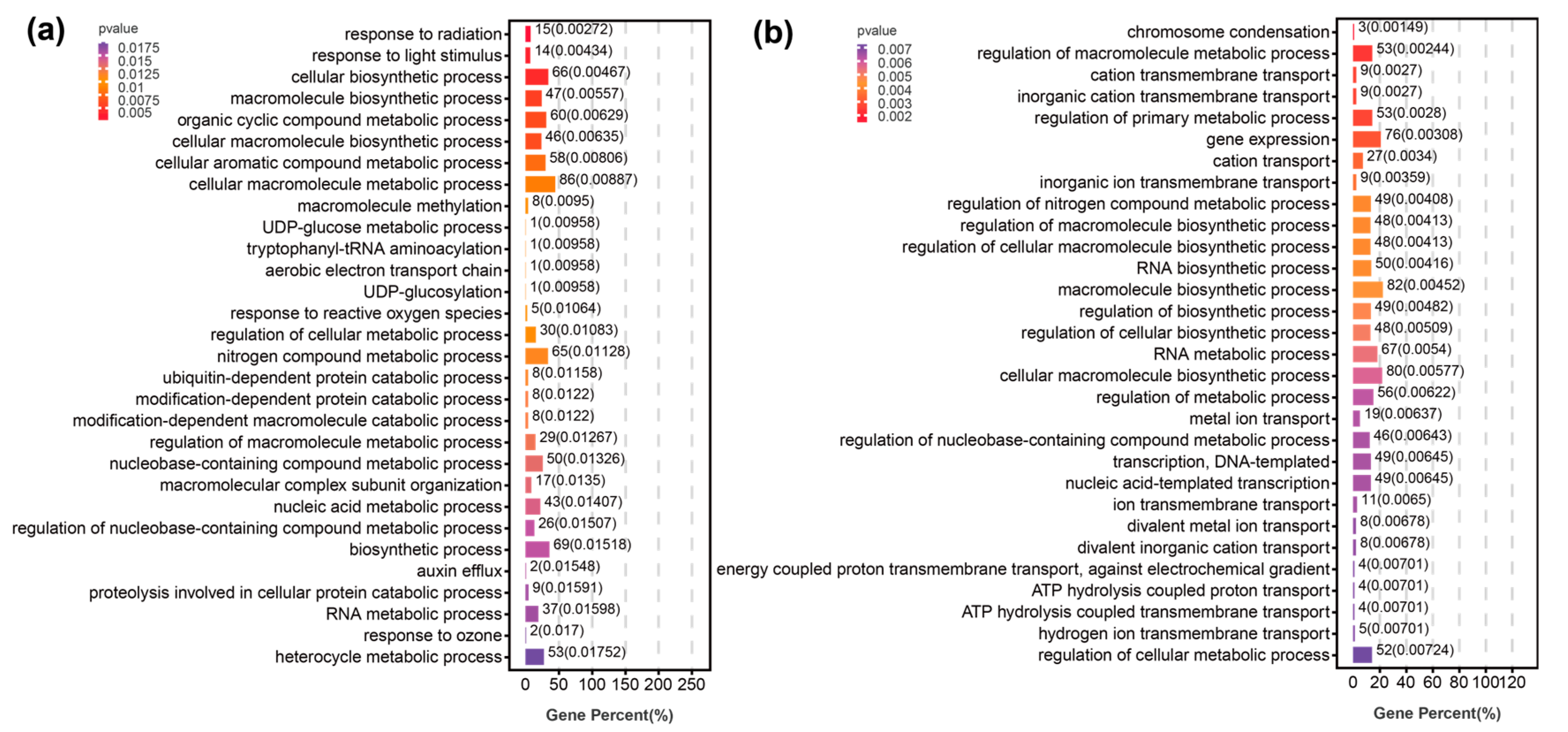
3.2. Molecular Mechanisms of Population Differentiation
4. Materials and Methods
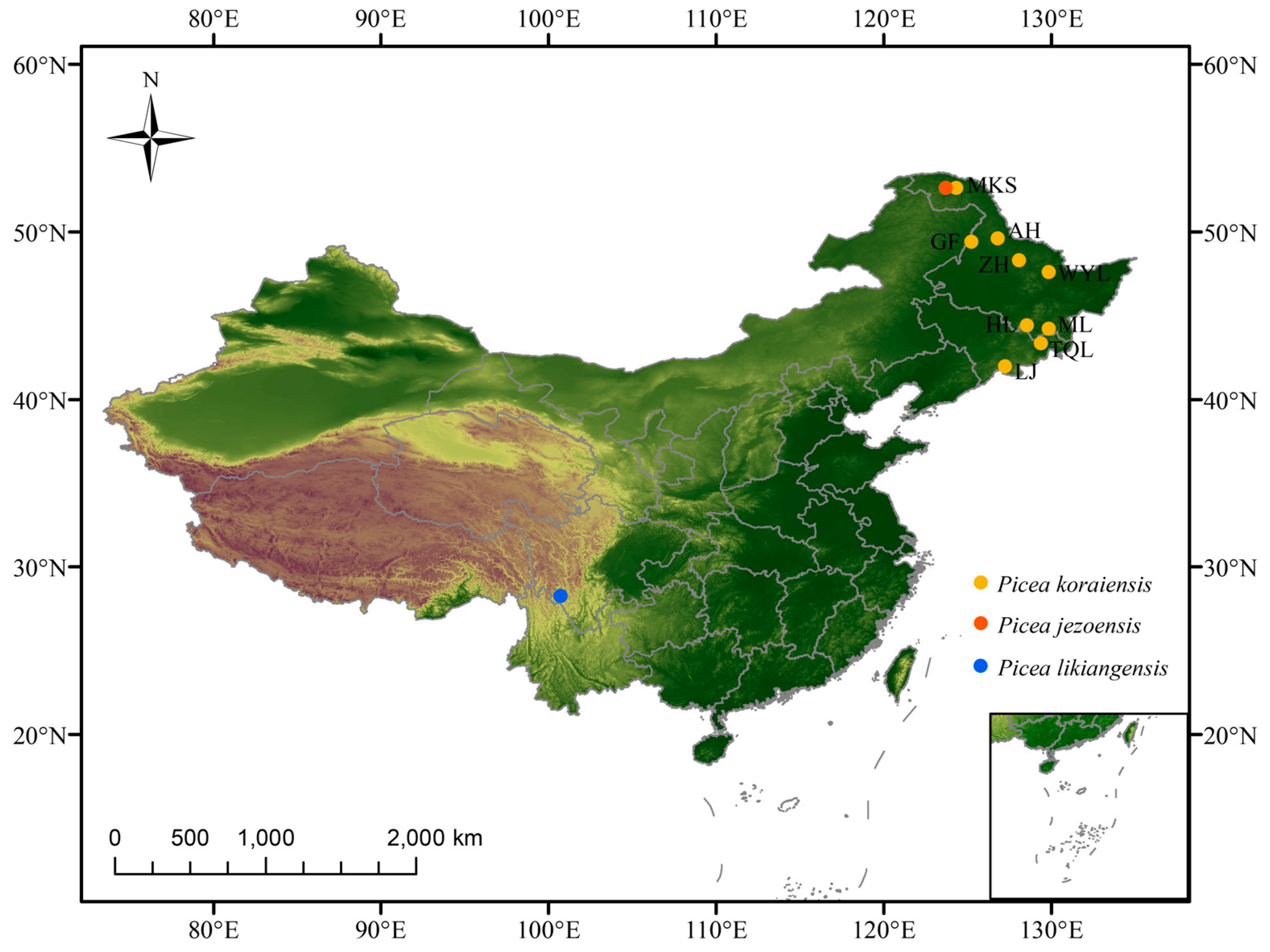
4.1. Sample Collection and GBS Analysis
4.2. Phylogenetic and Population Structure Analyses
4.3. Genetic Diversity and Selective Sweep Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schuster, W.S.F.; Mitton, J.B. Paternity and gene dispersal in limber pine (Pinus flexilis James). Heredity 2000, 84, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.R.; Gill, G.P.; Kuntz, R.J.; Langley, C.H.; Neale, D.B. Nucleotide diversity and linkage disequilibrium in loblolly pine. Proc. Natl. Acad. Sci. USA 2004, 101, 15255–15260. [Google Scholar] [CrossRef]
- Syring, J.; Farrell, K.; Businský, R.; Cronn, R.; Liston, A. Widespread genealogical nonmonophyly in species of pinus subgenus strobus. Syst. Biol. 2007, 56, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Ledig, F.T.; Hodgskiss, P.D.; Krutovskii, K.V.; Neale, D.B.; Eguiluz-Piedra, T. Relationships among the spruces (Picea, Pinaceae) of southwestern North America. Syst. Bot. 2004, 29, 275–295. [Google Scholar] [CrossRef]
- Ran, J.-H.; Shen, T.-T.; Wang, M.-M.; Wang, X.-Q. Phylogenomics resolves the deep phylogeny of seed plants and indicates partial convergent or homoplastic evolution between Gnetales and angiosperms. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181012. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Abbott, R.J.; Lu, Z.; Mao, K.; Zhang, L.; Wang, X.; Ru, D.; Liu, J. Reticulate evolution within a spruce (Picea) species complex revealed by population genomic analysis. Evolution 2018, 72, 2669–2681. [Google Scholar] [CrossRef] [PubMed]
- Du, F.K.; Petit, R.J.; Liu, J.Q. More introgression with less gene flow: Chloroplast vs. mitochondrial DNA in the Picea asperata complex in China, and comparison with other Conifers. Mol. Ecol. 2009, 18, 1396–1407. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Wang, Z.; Shrestha, N.; Liu, J. Species divergence with gene flow and hybrid speciation on the Qinghai–Tibet Plateau. New Phytol. 2022, 234, 392–404. [Google Scholar] [CrossRef]
- Ding, W.-N.; Ree, R.H.; Spicer, R.A.; Xing, Y.-W. Ancient orogenic and monsoon-driven assembly of the world’s richest temperate alpine flora. Science 2020, 369, 578–581. [Google Scholar] [CrossRef]
- Owens, G.L.; Rieseberg, L.H. Hybrid incompatibility is acquired faster in annual than in perennial species of sunflower and tarweed. Evolution 2014, 68, 893–900. [Google Scholar] [CrossRef]
- Feng, S.; Ru, D.; Sun, Y.; Mao, K.; Milne, R.; Liu, J. Trans-lineage polymorphism and nonbifurcating diversification of the genus Picea. New Phytol. 2019, 222, 576–587. [Google Scholar] [CrossRef]
- Ran, J.-H.; Wei, X.-X.; Wang, X.-Q. Molecular phylogeny and biogeography of Picea (Pinaceae): Implications for phylogeographical studies using cytoplasmic haplotypes. Mol. Phylogenetics Evol. 2006, 41, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Sigurgeirsson, A.; Szmidt, A.E. Phylogenetic and biogeographic implications of chloroplast DNA variation in Picea. Nord. J. Bot. 1993, 13, 233–246. [Google Scholar] [CrossRef]
- Lockwood, J.D.; Aleksić, J.M.; Zou, J.; Wang, J.; Liu, J.; Renner, S.S. A new phylogeny for the genus Picea from plastid, mitochondrial, and nuclear sequences. Mol. Phylogenet. Evol. 2013, 69, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.W. Species crossability in spruce in relation to distribution and taxonomy. For. Sci. 1955, 1, 319–349. [Google Scholar]
- Nienstaedt, H. Genetics of White Spruce; US Department of Agriculture: Washington, DC, USA, 1972.
- Ren, Y.G.; Wan, C.B.; Qiao, X.Y.; Jiao, Y.G.; Chi, H.Y.; Zhao, C.B. Palynological assemblages of the Shahezi formation in Changwu area, north of Songliao Basin. J. Jilin Univ. 2014, 33, 406–412. [Google Scholar]
- Wang, R.; Wang, Y.; Chen, Y. Fossil woods from late cretaceous of Heilong-Jiang Province, Northeast China, and their palaeoenviromental implications. J. Integr. Plant Biol. 1997, 39, 10. [Google Scholar]
- Ding, Q.H.; Zhang, W.; Zheng, S.L. Research on fossil woods from the Fuxin formation in West Liaoning. Liaoning Geol. 2000, 17, 284–291. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, L.; Han, J.; Feng, B. Study on the genetic diversity of Picea koraiensis. Zhiwu Yanjiu 2003, 23, 224–229. [Google Scholar]
- Jiang, X.; Gao, X.; Li, Y. Character diversity of mineral nutrition of koyama spruce populations. J. Northeast For. Univ. 2002, 30, 24–29. [Google Scholar]
- Wang, Q.; Ren, X.; Jiang, J. Genetic diversity for the provenance of Picea koraiensis by RAPD markers. J. Northeast For. Univ. 2004, 32, 1–3. [Google Scholar]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Vázquez, L.; Feng, L.; Liu, Z.; Zhao, G. Climatic and soil factors shape the demographical history and genetic diversity of a deciduous oak (Quercus liaotungensis) in Northern China. Front. Plant Sci. 2018, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Costa e Silva, J.; Potts, B.; Harrison, P.A.; Bailey, T. Temperature and rainfall are separate agents of selection shaping population differentiation in a forest tree. Forests 2019, 10, 1145. [Google Scholar] [CrossRef]
- Đurović, S.Z.; Temunović, M.; Niketić, M.; Tomović, G.; Schönswetter, P.; Frajman, B.; Lavergne, S. Impact of Quaternary climatic oscillations on phylogeographic patterns of three habitat-segregated Cerastium taxa endemic to the Dinaric Alps. J. Biogeogr. 2021, 48, 2022–2036. [Google Scholar] [CrossRef]
- Aizawa, M.; Yoshimaru, H.; Saito, H.; Katsuki, T.; Kawahara, T.; Kitamura, K.; Shi, F.; Kaji, M. Phylogeography of a northeast Asian spruce, Picea jezoensis, inferred from genetic variation observed in organelle DNA markers. Mol. Ecol. 2007, 16, 3393–3405. [Google Scholar] [CrossRef]
- Ran, J.-H.; Shen, T.-T.; Liu, W.-J.; Wang, P.-P.; Wang, X.-Q. Mitochondrial introgression and complex biogeographic history of the genus Picea. Mol. Phylogenet. Evol. 2015, 93, 63–76. [Google Scholar] [CrossRef]
- Sullivan, A.R.; Eldfjell, Y.; Schiffthaler, B.; Delhomme, N.; Asp, T.; Hebelstrup, K.H.; Keech, O.; Öberg, L.; Møller, I.M.; Arvestad, L.; et al. The mitogenome of norway spruce and a reappraisal of mitochondrial recombination in plants. Genome Biol. Evol. 2019, 12, 3586–3598. [Google Scholar] [CrossRef]
- Sutton, B.C.S.; Flanagan, D.J.; Gawley, J.R.; Newton, C.H.; Lester, D.T.; El-Kassaby, Y.A. Inheritance of chloroplast and mitochondrial DNA in Picea and composition of hybrids from introgression zones. Theor. Appl. Genet. 1991, 82, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Rajora, O.P.; Dancik, B.P. Population genetic variation, structure, and evolution in Engelmann spruce, white spruce, and their natural hybrid complex in Alberta. Can. J. Bot. 2000, 78, 768–780. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Lexer, C.; Aitken, S.N. Genomic and phenotypic architecture of a spruce hybrid zone (Picea sitchensis × P. glauca). Mol. Ecol. 2013, 22, 827–841. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, A.R.; Lin, Y.-C.; Van de Peer, Y.; Ingvarsson, P.K. Genome-wide analysis reveals diverged patterns of codon bias, gene expression, and rates of sequence evolution in Picea gene families. Genome Biol. Evol. 2015, 7, 1002–1015. [Google Scholar] [CrossRef]
- de Lafontaine, G.; Prunier, J.; Gérardi, S.; Bousquet, J. Tracking the progression of speciation: Variable patterns of introgression across the genome provide insights on the species delimitation between progenitor-derivative spruces (Picea mariana × P. rubens). Mol. Ecol. 2015, 24, 5229–5247. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Chen, J.; Stocks, M.; Källman, T.; Sønstebø, J.H.; Parducci, L.; Semerikov, V.; Sperisen, C.; Politov, D.; Ronkainen, T.; et al. The extent and meaning of hybridization and introgression between Siberian spruce (Picea obovata) and Norway spruce (Picea abies): Cryptic refugia as stepping stones to the west? Mol. Ecol. 2016, 25, 2773–2789. [Google Scholar] [CrossRef]
- Du, F.K.; Peng, X.L.; Liu, J.Q.; Lascoux, M.; Hu, F.S.; Petit, R.J. Direction and extent of organelle DNA introgression between two spruce species in the Qinghai-Tibetan Plateau. New Phytol. 2011, 192, 1024–1033. [Google Scholar] [CrossRef]
- Li, Y.; Stocks, M.; Hemmilä, S.; Källman, T.; Zhu, H.; Zhou, Y.; Chen, J.; Liu, J.; Lascoux, M. Demographic histories of four spruce (Picea) species of the Qinghai-Tibetan Plateau and neighboring areas inferred from multiple nuclear loci. Mol. Biol. Evol. 2010, 27, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Sun, Y.; Li, L.; Wang, G.; Yue, W.; Lu, Z.; Wang, Q.; Liu, J. Population genetic evidence for speciation pattern and gene flow between Picea wilsonii, P. morrisonicola and P. neoveitchii. Ann. Bot. 2013, 112, 1829–1844. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Uchiyama, K.; Ueno, S.; Ishizuka, W.; Tsuyama, I.; Goto, S. Geographical gradients of genetic diversity and differentiation among the southernmost marginal populations of Abies sachalinensis revealed by EST-SSR polymorphism. Forests 2020, 11, 233. [Google Scholar] [CrossRef]
- Tóth, E.G.; Tremblay, F.; Housset, J.M.; Bergeron, Y.; Carcaillet, C. Geographic isolation and climatic variability contribute to genetic differentiation in fragmented populations of the long-lived subalpine conifer Pinus cembra L. in the western Alps. BMC Evol. Biol. 2019, 19, 190. [Google Scholar] [CrossRef] [PubMed]
- Mengoni, A.; Barabesi, C.; Gonnelli, C.; Galardi, F.; Gabbrielli, R.; Bazzicalupo, M. Genetic diversity of heavy metal-tolerant populations in Silene paradoxa L. (Caryophyllaceae): A chloroplast microsatellite analysis. Mol. Ecol. 2001, 10, 1909–1916. [Google Scholar] [CrossRef]
- Mengoni, A.; Gonnelli, C.; Galardi, F.; Gabbrielli, R.; Bazzicalupo, M. Genetic diversity and heavy metal tolerance in populations of Silene paradoxa L. (Caryophyllaceae): A random amplified polymorphic DNA analysis. Mol. Ecol. 2000, 9, 1319–1324. [Google Scholar] [CrossRef]
- Boquete, M.T.; Schmid, M.W.; Wagemaker, N.C.A.M.; Carey, S.B.; McDaniel, S.F.; Richards, C.; Alonso, C. Molecular basis of intraspecific differentiation for heavy metal tolerance in the copper moss Scopelophila cataractae. Environ. Exp. Bot. 2022, 201, 104970. [Google Scholar] [CrossRef]
- Boonmee, S.; Neeratanaphan, L.; Tanee, T.; Khamon, P. The genetic differentiation of Colocasia esculenta growing in gold mining areas with arsenic contamination. Environ. Monit. Assess. 2015, 187, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Xu, B.; Li, Z.-M.; Sun, H. The ‘Ward Line–Mekong–Salween Divide’ is an important floristic boundary between the eastern Himalaya and Hengduan mountains: Evidence from the phylogeographical structure of subnival herbs Marmoritis complanatum (Lamiaceae). Bot. J. Linn. Soc. 2017, 185, 482–496. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Landis, J.B.; Zhang, J.; Yang, L.; Lin, N.; Zhang, H.; Guo, R.; Li, L.; Zhang, Y.; et al. Genomic insights into adaptation to heterogeneous environments for the ancient relictual Circaeaster agrestis (Circaeasteraceae, Ranunculales). New Phytol. 2020, 228, 285–301. [Google Scholar] [CrossRef]
- Zhu, L.; Bu, Q.; Xu, X.; Paik, I.; Huang, X.; Hoecker, U.; Deng, X.W.; Huq, E. CUL4 forms an E3 ligase with COP1 and SPA to promote light-induced degradation of PIF1. Nat. Commun. 2015, 6, 7245. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, X.; Liu, S.; Su, T.; Huang, H.; Ren, H.; Gao, Z.; Wang, X.; Lin, D.; Wang, Q.; et al. Regulation of Arabidopsis photoreceptor CRY2 by two distinct E3 ubiquitin ligases. Nat. Commun. 2021, 12, 2155. [Google Scholar] [CrossRef] [PubMed]
- Manfield, I.W.; Devlin, P.F.; Jen, C.-H.; Westhead, D.R.; Gilmartin, P.M. Conservation, convergence, and divergence of light-responsive, circadian-regulated, and tissue-specific expression patterns during evolution of the Arabidopsis GATA gene family. Plant Physiol. 2007, 143, 941–958. [Google Scholar] [CrossRef]
- Jones, A.M.; Xuan, Y.; Xu, M.; Wang, R.-S.; Ho, C.-H.; Lalonde, S.; You, C.H.; Sardi, M.I.; Parsa, S.A.; Smith-Valle, E.; et al. Border control—a membrane-linked interactome of Arabidopsis. Science 2014, 344, 711–716. [Google Scholar] [CrossRef]
- Yaish, M.W.F.; Peng, M.; Rothstein, S.J. AtMBD9 modulates Arabidopsis development through the dual epigenetic pathways of DNA methylation and histone acetylation. Plant J. 2009, 59, 123–135. [Google Scholar] [CrossRef]
- Peng, M.; Cui, Y.; Bi, Y.-M.; Rothstein, S.J. AtMBD9: A protein with a methyl-CpG-binding domain regulates flowering time and shoot branching in Arabidopsis. Plant J. 2006, 46, 282–296. [Google Scholar] [CrossRef]
- Shimizu, N.; Ishida, T.; Yamada, M.; Shigenobu, S.; Tabata, R.; Kinoshita, A.; Yamaguchi, K.; Hasebe, M.; Mitsumasu, K.; Sawa, S. BAM 1 and RECEPTOR-LIKE PROTEIN KINASE 2 constitute a signaling pathway and modulate CLE peptide-triggered growth inhibition in Arabidopsis root. New Phytol. 2015, 208, 1104–1113. [Google Scholar] [CrossRef]
- Barth, C.; Gouzd, Z.A.; Steele, H.P.; Imperio, R.M. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. J. Exp. Bot. 2010, 61, 379–394. [Google Scholar] [CrossRef]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef]
- Joshi, V.; Jander, G. Arabidopsis methionine gamma-lyase is regulated according to isoleucine biosynthesis needs but plays a subordinate role to threonine deaminase. Plant Physiol. 2009, 151, 367–378. [Google Scholar] [CrossRef]
- Su, Y.; Li, M.; Guo, L.; Wang, X. Different effects of phospholipase Dζ2 and non-specific phospholipase C4 on lipid remodeling and root hair growth in Arabidopsis response to phosphate deficiency. Plant J. 2018, 94, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.; Singh, A. Emerging role of phospholipase C mediated lipid signaling in abiotic stress tolerance and development in plants. Plant Cell Rep. 2021, 40, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Wang, X.; Dong, J.; Hou, Z.; Zhao, W.; Gao, X.; Yang, M. Advances in the Study of Phospholipase C Response to Stress in Plants. Biotechnol. Bull. 2021, 37, 154. [Google Scholar] [CrossRef]
- Xia, X.; Yang, Z.; Cui, Y.; Li, Y.; Hou, Q.; Yu, T. Soil heavy metal concentrations and their typical input and output fluxes on the southern Song-nen Plain, Heilongjiang Province, China. J. Geochem. Explor. 2014, 139, 85–96. [Google Scholar] [CrossRef]
- Zhao, W.; Shan, Y.; Zhao, L.; Nie, L. Spatial distribution characteristics of five heavy metals elements in rice: A case study for different basins in Heilongjiang Province. In Advances in Applied Chemistry and Industrial Catalysis; CRC Press: Boca Raton, FL, USA, 2022; pp. 390–397. [Google Scholar]
- Krämer, U.; Clemens, S. Functions and homeostasis of zinc, copper, and nickel in plants. In Topics in Current Genetics; Springer Verlag: New York, NY, USA, 2005; pp. 216–271. [Google Scholar]
- Manara, A. Plant responses to heavy metal toxicity. In Plants and Heavy Metals; Furini, A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 27–53. [Google Scholar]
- Tommasini, R.; Vogt, E.; Fromenteau, M.; Hörtensteiner, S.; Matile, P.; Amrhein, N.; Martinoia, E. An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J. 1998, 13, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Bovet, L.; Maeshima, M.; Martinoia, E.; Lee, Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007, 50, 207–218. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Tian, W.; Qin, L.; Meng, L.; Wu, D.; Huang, Y.; Li, D.; Zhao, D.; He, T. Identification of novel heavy metal detoxification proteins in Solanum tuberosum: Insights to improve food security protection from metal ion stress. Sci. Total Environ. 2021, 779, 146197. [Google Scholar] [CrossRef]
- Tilley, D.M.; Evans, C.R.; Larson, T.M.; Edwards, K.A.; Friesen, J.A. Identification and characterization of the nuclear isoform of Drosophila melanogaster CTP: Phosphocholine cytidylyltransferase. Biochemistry 2008, 47, 11838–11846. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Hakimuddin, F.; Bakovic, M. Developmental and metabolic effects of disruption of the mouse CTP: Phosphoethanolamine cytidylyltransferase gene (Pcyt2). Mol. Cell. Biol. 2007, 27, 3327–3336. [Google Scholar] [CrossRef]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Morash, S.C.; McMaster, C.R.; Hjelmstad, R.H.; Bell, R.M. Studies employing Saccharomyces cerevisiae cpt1 and ept1 null mutants implicate the CPT1 gene in coordinate regulation of phospholipid biosynthesis. J. Biol. Chem. 1994, 269, 28769–28776. [Google Scholar] [CrossRef]
- Yap, W.S.; Shyu, P.; Gaspar, M.L.; Jesch, S.A.; Marvalim, C.; Prinz, W.A.; Henry, S.A.; Thibault, G. Yeast FIT2 homolog is necessary to maintain cellular proteostasis by regulating lipid homeostasis. J. Cell Sci. 2020, 133, jcs248526. [Google Scholar] [CrossRef] [PubMed]
- Wild, R.; Gerasimaite, R.; Jung, J.-Y.; Truffault, V.; Pavlovic, I.; Schmidt, A.; Saiardi, A.; Jessen, H.J.; Poirier, Y.; Hothorn, M.; et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 2016, 352, 986–990. [Google Scholar] [CrossRef]
- Wege, S.; Khan, G.A.; Jung, J.-Y.; Vogiatzaki, E.; Pradervand, S.; Aller, I.; Meyer, A.J.; Poirier, Y. The EXS domain of PHO1 participates in the response of shoots to phosphate deficiency via a root-to-shoot signal. Plant Physiol. 2016, 170, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Stracke, R.; De Vos, R.C.H.; Bartelniewoehner, L.; Ishihara, H.; Sagasser, M.; Martens, S.; Weisshaar, B. Metabolomic and genetic analyses of flavonol synthesis in Arabidopsis thaliana support the in vivo involvement of leucoanthocyanidin dioxygenase. Planta 2009, 229, 427–445. [Google Scholar] [CrossRef]
- Prescott, A.G.; Stamford, N.P.J.; Wheeler, G.; Firmin, J.L. In vitro properties of a recombinant flavonol synthase from Arabidopsis thaliana. Phytochemistry 2002, 60, 589–593. [Google Scholar] [CrossRef]
- Gutha, L.R.; Casassa, L.F.; Harbertson, J.F.; Naidu, R.A. Modulation of flavonoid biosynthetic pathway genes and anthocyanins due to virus infection in grapevine (Vitis vinifera L.) leaves. BMC Plant Biol. 2010, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Satishchandran, C.; Markham, G.D. Mechanistic studies of Escherichia coli adenosine-5′-phosphosulfate kinase. Arch. Biochem. Biophys. 2000, 378, 210–215. [Google Scholar] [CrossRef]
- Ganea, E.; Harding, J.J. Glutathione-related enzymes and the eye. Curr. Eye Res. 2006, 31, 1–11. [Google Scholar] [CrossRef]
- Peng, C.; Xia, Y.; Zhang, W.; Wu, Y.; Shi, J. Proteomic analysis unravels response and antioxidation defense mechanism of rice plants to copper oxide nanoparticles: Comparison with bulk particles and dissolved Cu ions. ACS Agric. Sci. Technol. 2022, 2, 671–683. [Google Scholar] [CrossRef]
- Nebert, D.W.; Vasiliou, V. Analysis of the glutathione S-transferase (GST) gene family. Hum. Genom. 2004, 1, 1–5. [Google Scholar] [CrossRef]
- Teng, Z.; Zheng, W.; Jiang, S.; Hong, S.-B.; Zhu, Z.; Zang, Y. Role of melatonin in promoting plant growth by regulating carbon assimilation and ATP accumulation. Plant Sci. 2022, 319, 111276. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Saba, H.; Jyoti, P.; Neha, S. Mycorrhizae and phytochelators as remedy in heavy metal contaminated land remediation. Int. Res. J. Environ. Sci. 2013, 2, 74–78. [Google Scholar]
- Liu, Q.; Zhang, Y.; Wang, Y.; Wang, W.; Gu, C.; Huang, S.; Yuan, H.; Dhankher, O.P. Quantitative proteomic analysis reveals complex regulatory and metabolic response of Iris lactea Pall. var. chinensis to cadmium toxicity. J. Hazard. Mater. 2020, 400, 123165. [Google Scholar] [CrossRef]
- Borges, K.L.R.; Salvato, F.; Alcântara, B.K.; Nalin, R.S.; Piotto, F.Â.; Azevedo, R.A. Temporal dynamic responses of roots in contrasting tomato genotypes to cadmium tolerance. Ecotoxicology 2018, 27, 245–258. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Zhang, F.; Tang, M.; Chen, Y.; Wang, J.; Karanja, B.K.; Luo, X.; Zhang, W.; Liu, L. Dissecting root proteome changes reveals new insight into cadmium stress response in radish (Raphanus sativus L.). Plant Cell Physiol. 2017, 58, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.-L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A.; Schikora, A.; Hirt, H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009, 12, 421–426. [Google Scholar] [CrossRef]
- Takahashi, F.; Mizoguchi, T.; Yoshida, R.; Ichimura, K.; Shinozaki, K. Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol. Cell 2011, 41, 649–660. [Google Scholar] [CrossRef]
- Sethi, V.; Raghuram, B.; Sinha, A.K.; Chattopadhyay, S. A mitogen-activated protein kinase cascade module, MKK3-MPK6 and MYC2, is involved in blue light-mediated seedling development in Arabidopsis. Plant Cell 2014, 26, 3343–3357. [Google Scholar] [CrossRef] [PubMed]
- Vitti, A.; Nuzzaci, M.; Scopa, A.; Tataranni, G.; Remans, T.; Vangronsveld, J.; Sofo, A. Auxin and cytokinin metabolism and root morphological modifications in Arabidopsis thaliana seedlings infected with Cucumber mosaic virus (CMV) or exposed to cadmium. Int. J. Mol. Sci. 2013, 14, 6889–6902. [Google Scholar] [CrossRef]
- Farinati, S.; DalCorso, G.; Varotto, S.; Furini, A. The Brassica juncea BjCdR15, an ortholog of Arabidopsis TGA3, is a regulator of cadmium uptake, transport and accumulation in shoots and confers cadmium tolerance in transgenic plants. New Phytol. 2010, 185, 964–978. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, C.; Liang, Y.; Wang, C.; Yang, C.; Liu, G. A novel bZIP gene from Tamarix hispida mediates physiological responses to salt stress in tobacco plants. J. Plant Physiol. 2010, 167, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Smeets, K.; Opdenakker, K.; Remans, T.; Forzani, C.; Hirt, H.; Vangronsveld, J.; Cuypers, A.N.N. The role of the kinase OXI1 in cadmium- and copper-induced molecular responses in Arabidopsis thaliana. Plant Cell Environ. 2013, 36, 1228–1238. [Google Scholar] [CrossRef]
- van De Mortel, J.E.; Schat, H.; Moerland, P.D.; Van Themaat, E.V.L.; Van Der Ent, S.; Blankestijn, H.; Ghandilyan, A.; Tsiatsiani, S.; Aarts, M.G.M. Expression differences for genes involved in lignin, glutathione and sulphate metabolism in response to cadmium in Arabidopsis thaliana and the related Zn/Cd-hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 2008, 31, 301–324. [Google Scholar] [CrossRef]
- Opdenakker, K.; Remans, T.; Vangronsveld, J.; Cuypers, A. Mitogen-Activated Protein (MAP) kinases in plant metal stress: Regulation and responses in comparison to other biotic and abiotic stresses. Int. J. Mol. Sci. 2012, 13, 7828–7853. [Google Scholar] [CrossRef]
- Ramos, J.; Clemente, M.R.; Naya, L.; Loscos, J.; Pérez-Rontomé, C.; Sato, S.; Tabata, S.; Becana, M. Phytochelatin synthases of the model legume Lotus japonicus. A small multigene family with differential response to cadmium and alternatively spliced variants. Plant Physiol. 2007, 143, 1110–1118. [Google Scholar] [CrossRef]
- Gracheva, E.; Chitale, S.; Wilhelm, T.; Rapp, A.; Byrne, J.; Stadler, J.; Medina, R.; Cardoso, M.C.; Richly, H. ZRF1 mediates remodeling of E3 ligases at DNA lesion sites during nucleotide excision repair. J. Cell Biol. 2016, 213, 185–200. [Google Scholar] [CrossRef]
- Bélanger, F.; Angers, J.-P.; Fortier, É.; Hammond-Martel, I.; Costantino, S.; Drobetsky, E.; Wurtele, H. Mutations in replicative stress response pathways are associated with s phase-specific defects in nucleotide excision repair. J. Biol. Chem. 2016, 291, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Lipkin, S.M.; Wang, V.; Jacoby, R.; Banerjee-Basu, S.; Baxevanis, A.D.; Lynch, H.T.; Elliott, R.M.; Collins, F.S. MLH3: A DNA mismatch repair gene associated with mammalian microsatellite instability. Nat. Genet. 2000, 24, 27–35. [Google Scholar] [CrossRef] [PubMed]
- de Barros, A.C.; Takeda, A.A.S.; Dreyer, T.R.; Velazquez-Campoy, A.; Kobe, B.; Fontes, M.R.M. DNA mismatch repair proteins MLH1 and PMS2 can be imported to the nucleus by a classical nuclear import pathway. Biochimie 2018, 146, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Hartung, F.; Suer, S.; Puchta, H. Two closely related RecQ helicases have antagonistic roles in homologous recombination and DNA repair in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 18836–18841. [Google Scholar] [CrossRef] [PubMed]
- Holloman, W.K. Unraveling the mechanism of BRCA2 in homologous recombination. Nat. Struct. Mol. Biol. 2011, 18, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Kwok, S.F.; von Arnim, A.G.; Lee, A.; McNellis, T.W.; Piekos, B.; Deng, X.W. Arabidopsis COP8, COP10, and COP11 genes are involved in repression of photomorphogenic development in darkness. Plant Cell 1994, 6, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Franciosini, A.; Moubayidin, L.; Du, K.; Matari, N.H.; Boccaccini, A.; Butera, S.; Vittorioso, P.; Sabatini, S.; Jenik, P.D.; Costantino, P.; et al. The COP9 SIGNALOSOME is required for postembryonic meristem maintenance in Arabidopsis thaliana. Mol. Plant. 2015, 8, 1623–1634. [Google Scholar] [CrossRef]
- Manara, A.; DalCorso, G.; Guzzo, F.; Furini, A. Loss of the Atypical Kinases ABC1K7 and ABC1K8 Changes the Lipid Composition of the Chloroplast Membrane. Plant. Cell. Physiol. 2015, 56, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Staneloni, R.J.; Rodriguez-Batiller, M.J.; Legisa, D.; Scarpin, M.R.; Agalou, A.; Cerdán, P.D.; Meijer, A.H.; Ouwerkerk, P.B.; Casal, J.J. Bell-like homeodomain selectively regulates the high-irradiance response of phytochrome A. Proc. Natl. Acad. Sci. USA 2009, 106, 13624–13629. [Google Scholar] [CrossRef] [PubMed]
- Perea-Resa, C.; Carrasco-López, C.; Catalá, R.; Turečková, V.; Novak, O.; Zhang, W.; Sieburth, L.; Jiménez-Gómez, J.M.; Salinas, J. The LSM1-7 Complex Differentially Regulates Arabidopsis Tolerance to Abiotic Stress Conditions by Promoting Selective mRNA Decapping. Plant Cell 2016, 28, 505–520. [Google Scholar] [CrossRef]
- Kanwar, P.; Sanyal, S.K.; Mahiwal, S.; Ravi, B.; Kaur, K.; Fernandes, J.L.; Yadav, A.K.; Tokas, I.; Srivastava, A.K.; Suprasanna, P.; et al. CIPK9 targets VDAC3 and modulates oxidative stress responses in Arabidopsis. Plant J. 2022, 109, 241–260. [Google Scholar] [CrossRef]
- Xu, T.; Lee, K.; Gu, L.; Kim, J.I.; Kang, H. Functional characterization of a plastid-specific ribosomal protein PSRP2 in Arabidopsis thaliana under abiotic stress conditions. Plant Physiol. Biochem. 2013, 73, 405–411. [Google Scholar] [CrossRef]
- Poland, J.A.; Brown, P.J.; Sorrells, M.E.; Jannink, J.-L. Development of high-density genetic maps for barley and wheat using a novel two-enzyme genotyping-by-sequencing approach. PLoS ONE 2012, 7, e32253. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.N.; Nei, M. Limitations of the evolutionary parsimony method of phylogenetic analysis. Mol. Biol. Evol. 1990, 7, 82–102. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]




| Population | GF | MKS | ML | HL | TQL | AH | WYL | LJ | ZH | π × 10−2 | Tajima’s D |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GF | 0 | 0.2181 | 0.3892 | ||||||||
| MKS | 0.1018 | 0 | 0.2285 | 0.1205 | |||||||
| ML | 0.0643 | 0.0878 | 0 | 0.2289 | 0.0526 | ||||||
| HL | 0.0628 | 0.0909 | 0.0399 | 0 | 0.2272 | 0.1307 | |||||
| TQL | 0.0599 | 0.0838 | 0.0294 | 0.0371 | 0 | 0.2319 | 0.0297 | ||||
| AH | 0.0529 | 0.0938 | 0.0500 | 0.0544 | 0.0462 | 0 | 0.2212 | 0.1657 | |||
| WYL | 0.1282 | 0.1569 | 0.1166 | 0.1182 | 0.1115 | 0.1191 | 0 | 0.1943 | 0.5110 | ||
| LJ | 0.0623 | 0.0873 | 0.0322 | 0.0399 | 0.0254 | 0.0488 | 0.1137 | 0 | 0.2351 | 0.0507 | |
| ZH | 0.0491 | 0.0842 | 0.0408 | 0.0416 | 0.0371 | 0.0450 | 0.1042 | 0.0404 | 0 | 0.2314 | 0.1875 |
| Population | NO. | Collection Site | Latitude | Longitude |
|---|---|---|---|---|
| GF | 10 | Mengke Mountain, Heilongjiang | 52.63 | 124.31 |
| MKS | 13 | Hailin, Heilongjiang | 44.42 | 128.54 |
| ML | 15 | Muling, Heilongjiang | 44.21 | 130.21 |
| HL | 15 | Linjiang, Jilin | 41.99 | 127.24 |
| TQL | 13 | Tianqiaoling, Jilin | 43.36 | 129.37 |
| AH | 14 | Wuyinling, Heilongjiang | 48.67 | 129.42 |
| WYL | 14 | Zhanhe, Heilongjiang | 48.33 | 128.07 |
| LJ | 11 | Aihui, Heilongjiang | 49.62 | 126.81 |
| ZH | 15 | Gaofeng, Heilongjiang | 49.43 | 125.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jiang, Z.; Qin, A.; Wang, F.; Chang, E.; Liu, Y.; Nie, W.; Tan, C.; Yuan, Y.; Dong, Y.; et al. Population Structure, Genetic Diversity and Candidate Genes for the Adaptation to Environmental Stress in Picea koraiensis. Plants 2023, 12, 1266. https://doi.org/10.3390/plants12061266
Wang Y, Jiang Z, Qin A, Wang F, Chang E, Liu Y, Nie W, Tan C, Yuan Y, Dong Y, et al. Population Structure, Genetic Diversity and Candidate Genes for the Adaptation to Environmental Stress in Picea koraiensis. Plants. 2023; 12(6):1266. https://doi.org/10.3390/plants12061266
Chicago/Turabian StyleWang, Ya, Zeping Jiang, Aili Qin, Fude Wang, Ermei Chang, Yifu Liu, Wen Nie, Cancan Tan, Yanchao Yuan, Yao Dong, and et al. 2023. "Population Structure, Genetic Diversity and Candidate Genes for the Adaptation to Environmental Stress in Picea koraiensis" Plants 12, no. 6: 1266. https://doi.org/10.3390/plants12061266
APA StyleWang, Y., Jiang, Z., Qin, A., Wang, F., Chang, E., Liu, Y., Nie, W., Tan, C., Yuan, Y., Dong, Y., Huang, R., Jia, Z., & Wang, J. (2023). Population Structure, Genetic Diversity and Candidate Genes for the Adaptation to Environmental Stress in Picea koraiensis. Plants, 12(6), 1266. https://doi.org/10.3390/plants12061266






