Agronomical Practices and Management for Commercial Cultivation of Portulaca oleracea as a Crop: A Review
Abstract
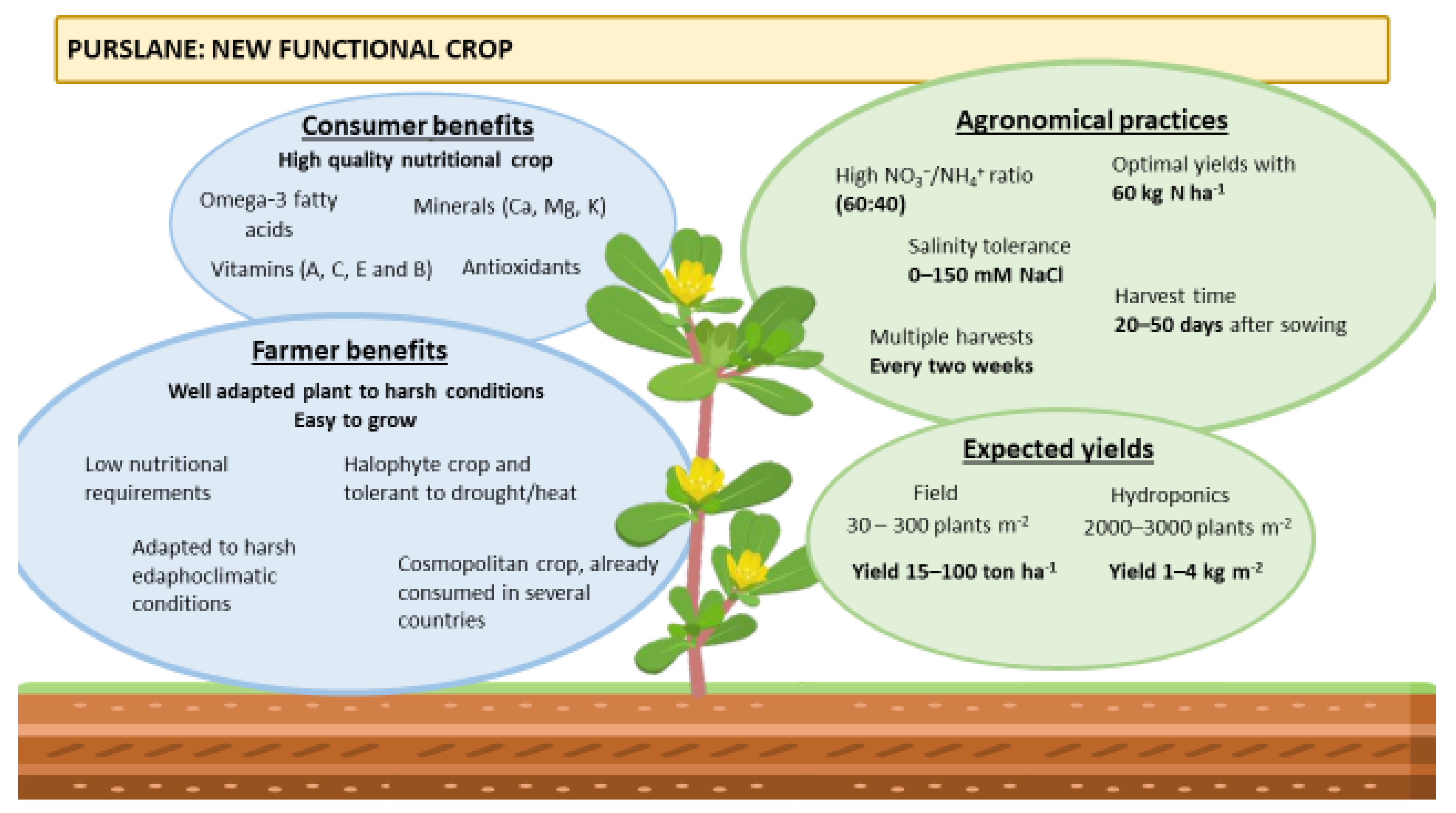
Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Cultivation Practices
3.1. Propagation and Growing Conditions
3.2. Irrigation
3.3. Mineral Fertilization
3.4. Planting and Harvesting Time
4. Sustainable Practices and Cropping Systems
5. Agronomic Recommendations
6. Future Prospects and Conclusionary Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations; International Fund for Agricultural Development; UNICEF; World Food Programme; World Health Organization. The State and Food Security and Nutrition in the World 2019. Safeguardin against Economic Slowdowns and Downturns; FAO: Rome, Italy; IFAD: Rome, Italy; UNICEF: New York, NY, USA; WFP: Rome, Italy; WHO: Geneva, Switzerland, 2019; ISBN 9789251315705. [Google Scholar]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Ray, D.K.; Ramankutty, N.; Mueller, N.D.; West, P.C.; Foley, J.A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 2012, 3, 1293. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Bini, C. Soil: A Precious Natural Resource; Nova Science Publishers: Hauppauge, NY, USA, 2009. [Google Scholar]
- European Union. Agriculture and Food Security in Climate Sensitive Areas in the Mediterranean; European Union: Strasbourg, France, 2020. [Google Scholar]
- Lal, R. Climate Change and Soil Degradation Mitigation by Sustainable Management of Soils and Other Natural Resources. Agric. Res. 2012, 1, 199–212. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Phytochemical composition and bioactive compounds of common purslane (Portulaca oleracea L.) as affected by crop management practices. Trends Food Sci. Technol. 2016, 55, 1–10. [Google Scholar] [CrossRef]
- Luczaj, L.; Pieroni, A.; Tardío, J.; Pardo-De-Santayana, M.; Sõukand, R.; Svanberg, I.; Kalle, R.; Łuczaj, Ł.; Pieroni, A.; Tardío, J.; et al. Wild food plant use in 21st century Europe: The disappearance of old traditions and the search for new cuisines involving wild edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Pardo-de-Santayana, M.; Tardío, J. Mediterranean non-cultivated vegetables as dietary sources of compounds with antioxidant and biological activity. LWT—Food Sci. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Cabrera Loera, R.D.; Morales, P.; Fernández-Ruiz, V.; Cámara, M.; Díez Marqués, C.; Pardo-de-Santayana, M.; Tardío, J. Wild vegetables of the Mediterranean area as valuable sources of bioactive compounds. Genet. Resour. Crop Evol. 2012, 59, 431–443. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean wild edible plants: Weeds or “new functional crops”? Molecules 2018, 23, 2299. [Google Scholar] [CrossRef] [PubMed]
- Gonz, M.R.; González-Tejero, M.R.; Casares-Porcel, M.; Sánchez-Rojas, C.P.; Ramiro-Gutiérrez, J.M.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.E.; Censorii, E.; de Pasquale, C.; et al. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef]
- García-Herrera, P.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Díez-Marqués, C.; Molina, M.; Tardío, J. Nutrient composition of six wild edible Mediterranean Asteraceae plants of dietary interest. J. Food Compos. Anal. 2014, 34, 163–170. [Google Scholar] [CrossRef]
- Tepper, B.J. Nutritional implications of genetic taste variation: The role of PROP sensitivity and other taste phenotypes. Annu. Rev. Nutr. 2008, 28, 367–388. [Google Scholar] [CrossRef]
- Heinrich, M.; Nebel, S.; Leonti, M.; Rivera, D.; Obón, C. “Local food-nutraceuticals”: Bridging the gap between local knowledge and global needs. Forum Nutr. 2006, 59, 1–17. [Google Scholar] [CrossRef]
- Akbar, S. Portulaca oleracea L. (Portulacaceae). In Handbook of 200 Medicinal Plants; Akbar, S., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1491–1504. ISBN 978-3-030-16807-0. [Google Scholar]
- Miyanishi, K.; Cavers, P.B. The biology of Canadian weeds. 40. Portulaca oleracea L. Can. J. Plant Sci. 1980, 60, 953–963. [Google Scholar] [CrossRef]
- Saheri, F.; Barzin, G.; Pishkar, L.; Boojar, M.M.A.; Babaeekhou, L. Foliar spray of salicylic acid induces physiological and biochemical changes in purslane (Portulaca oleracea L.) under drought stress. Biologia 2020, 75, 2189–2200. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Ren, S.; Weeda, S.; Akande, O.; Guo, Y.; Rutto, L.; Mebrahtu, T. Drought tolerance and AFLP-based genetic diversity in purslane (Portulaca oleracea L.). J. Biotech Res. 2011, 3, 51–61. [Google Scholar]
- Jin, R.; Shi, H.; Han, C.; Zhong, B.; Wang, Q.; Chan, Z. Physiological changes of purslane (Portulaca oleracea L.) after progressive drought stress and rehydration. Sci. Hortic. 2015, 194, 215–221. [Google Scholar] [CrossRef]
- Jin, R.; Wang, Y.; Liu, R.; Gou, J.; Chan, Z. Physiological and metabolic changes of purslane (Portulaca oleracea L.) in response to drought, heat, and combined stresses. Front. Plant Sci. 2016, 6, 1123. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, G.Q.; Tian, X.S.; Yang, H.M.; Yue, M.F.; Yang, C.H. The hotter the weather, the greater the infestation of Portulaca oleracea: Opportunistic life-history traits in a serious weed. Weed Res. 2015, 55, 396–405. [Google Scholar] [CrossRef]
- Egea-Gilabert, C.; Ruiz-Hernández, M.V.; Parra, M.Á.; Fernández, J.A. Characterization of purslane (Portulaca oleracea L.) accessions: Suitability as ready-to-eat product. Sci. Hortic. 2014, 172, 73–81. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Vasilakoglou, I.B.; Petrotos, K.; Barros, L.; Ferreira, I.C.F.R. Nutritional value, chemical composition and cytotoxic properties of common purslane (Portulaca oleracea L.) in relation to harvesting stage and plant part. Antioxidants 2019, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Tarkergari, S.; Waghray, K.; Gulla, S. Acceptability studies of value added products with Purslane (Portulaca oleracea). Pakistan J. Nutr. 2013, 12, 93–96. [Google Scholar] [CrossRef]
- Uddin, K.; Juraimi, A.S.; Hossain, S.; Altaf, M.; Nahar, U.; Ali, E.; Rahman, M.M.; Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; et al. Purslane weed (Portulaca oleracea): A prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci. World J. 2014, 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Montoya-García, C.O.; García-Mateos, R.; Becerra-Martínez, E.; Toledo-Aguilar, R.; Volke-Haller, V.H.; Jesús Magdaleno-Villar, J. Bioactive compounds of purslane (Portulaca oleracea L.) according to the production system: A review. Sci. Hortic. 2023, 308, 111584. [Google Scholar] [CrossRef]
- Kumar, A.; Sreedharan, S.; Kashyap, A.K.; Singh, P.; Ramchiary, N. A review on bioactive phytochemicals and ethnopharmacological potential of purslane (Portulaca oleracea L.). Heliyon 2022, 8, e08669. [Google Scholar] [CrossRef]
- Stroescu, M.; Stoica-Guzun, A.; Ghergu, S.; Chira, N.; Jipa, I. Optimization of fatty acids extraction from Portulaca oleracea seed using response surface methodology. Ind. Crops Prod. 2013, 43, 405–411. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Norman, H.A.; Gillaspy, J.E. Purslane in human nutrition and its potential for world agriculture. World Rev. Nutr. Diet. 1995, 77, 47–74. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.H.; Ghalavand, A.; Mashhadi-Akbar-Boojar, M.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A. Increased Medicinal Contents of Purslane by Nitrogen and Arbuscular Mycorrhiza under Drought Stress. Commun. Soil Sci. Plant Anal. 2020, 51, 118–135. [Google Scholar] [CrossRef]
- Santos, R.V.; Machado, R.M.A.; Alves-Pereira, I.; Ferreira, R.M.A. The influence of nitrogen fertilization on growth, yield, nitrate and oxalic acid concentration in purslane (Portulaca oleracea). Acta Hortic. 2016, 1142, 299–304. [Google Scholar] [CrossRef]
- Kaymak, H.C. Effect of nitrogen forms on growth, yield and nitrate accumulation of cultivated purslane (Portulaca oleracea L.). Bulg. J. Agric. Sci. 2013, 19, 444–449. [Google Scholar]
- Petropoulos, S.; Karkanis, A.; Fernandes, Â.; Barros, L.; Ferreira, I.C.F.R.; Ntatsi, G.; Petrotos, K.; Lykas, C.; Khah, E. Chemical composition and yield of six genotypes of common purslane (Portulaca oleracea L.): An alternative source of omega-3 fatty acids. Plant Foods Hum. Nutr. 2015, 70, 420–426. [Google Scholar] [CrossRef]
- Montoya-García, C.O.; Volke-Haller, V.; Trinidad-Santos, A.; Villanueva-Verduzco, C.; Sánchez-Escudero, J. Purslane (Portulaca oleracea L.) response to NPK fertilization. Rev. Fitotec. Mex. 2017, 40, 325–332. [Google Scholar] [CrossRef]
- Fontana, E.; Hoeberechts, J.; Nicola, S.; Cros, V.; Palmegiano, G.B.; Peiretti, P.G. Nitrogen concentration and nitrate ammonium ratio affect yield and change the oxalic acid concentration and fatty acid profile of purslane (Portulaca oleracea L.) grown in a soilless culture system. J. Sci. Food Agric. 2006, 86, 2417–2424. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Tawaha, A.; Al-Tawaha, A.R.; Gammoh, S.; Ereifej, K.I.; Al-Karaki, G.; Hamasha, H.R.; Tranchant, C.C.; et al. Herbal yield, nutritive composition, phenolic contents and antioxidant activity of purslane (Portulaca oleracea L.) grown in different soilless media in a closed system. Ind. Crops Prod. 2019, 141, 111746. [Google Scholar] [CrossRef]
- Kumar, A.; Sreedharan, S.; Singh, P.; Achigan-Dako, E.G.; Ramchiary, N. Improvement of a Traditional Orphan Food Crop, Portulaca oleracea L. (Purslane) Using Genomics for Sustainable Food Security and Climate-Resilient Agriculture. Front. Sustain. Food Syst. 2021, 5, 1–16. [Google Scholar] [CrossRef]
- Simpson, C.R.; Franco, J.G.; King, S.R.; Volder, A. Intercropping halophytes to mitigate salinity stress in watermelon. Sustainability 2018, 10, 681. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental impact of different agricultural management practices: Conventional vs. Organic agriculture. CRC. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Abiven, S.; Menasseri, S.; Chenu, C. The effects of organic inputs over time on soil aggregate stability—A literature analysis. Soil Biol. Biochem. 2009, 41, 1–12. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.S.; Horwath, W.R.; Shennan, C.; Scow, K.M. Changes in soil chemical properties resulting from organic and low-input farming practices. Agron. J. 1998, 90, 662–671. [Google Scholar] [CrossRef]
- Lou, X.; Zhao, J.; Lou, X.; Xia, X.; Feng, Y.; Li, H. The Biodegradation of Soil Organic Matter in Soil-Dwelling Humivorous Fauna. Front. Bioeng. Biotechnol. 2022, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: Mechanisms and thresholds. Biol. Fertil. Soils 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Ali, F.; Rehman, S.U.; Tareen, N.M.; Ullah, K.; Ullah, A.; Bibi, T.; Laghari, S. Effect of waste water treatment on the growth of selected leafy vegetable plants. Appl. Ecol. Environ. Res. 2019, 17, 1585–1597. [Google Scholar] [CrossRef]
- Yadegari, M. Performance of purslane (Portulaca oleracea) in nickel and cadmium contaminated soil as a heavy metals-removing crop. Iran. J. Plant Physiol. 2018, 8, 2447–2455. [Google Scholar] [CrossRef]
- Elshamy, M.M.; Heikal, Y.M.; Bonanomi, G. Phytoremediation efficiency of Portulaca oleracea L. naturally growing in some industrial sites, Dakahlia District, Egypt. Chemosphere 2019, 225, 678–687. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A Review of the Use of Organic Amendments and the Risk to Human Health; Elsevier: Amsterdam, The Netherlands, 2013; Volume 120, ISBN 9780124076860. [Google Scholar]
- Raleigh, C.; Urdal, H. Climate change, environmental degradation and armed conflict. Polit. Geogr. 2007, 26, 674–694. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Seed germination ecology of Portulaca oleracea L.: An important weed of rice and upland crops. Ann. Appl. Biol. 2009, 155, 61–69. [Google Scholar] [CrossRef]
- Mitich, L.W. Common purslane (Portulaca oleracea). Weed Technol. 1997, 11, 394–397. [Google Scholar] [CrossRef]
- Dahlquist, R.M.; Prather, T.S.; Stapleton, J.J. Time and Temperature Requirements for Weed Seed Thermal Death. Weed Sci. 2007, 55, 619–625. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Role of temperature in regulating the timing of germination in Portulaca oleracea. Can. J. Bot. 1987, 66, 563–567. [Google Scholar] [CrossRef]
- Benvenuti, S.; Macchia, M.; Miele, S. Quantitative analysis of emergence of seedlings from buried weed seeds with increasing soil depth. Weed Sci. 2001, 49, 528–535. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Liu, Q.; Ben, C.; Todd, C.D.; Shi, J.; Yang, Y.; Hu, X. Comparative proteomic analysis of the thermotolerant plant Portulaca oleracea acclimation to combined high temperature and humidity stress. J. Proteome Res. 2012, 11, 3605–3623. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Ganjeali, A.; Farhoosh, R.; Cheniany, M.; Lari, Z. Interactive effects of chilling and wounding stresses on antioxidant compounds and fatty acid profile of purslane. Acta Physiol. Plant. 2022, 44, 1–12. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate impacts on agriculture: Implications for crop production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Jensen, C.R.; Liu, F. Improving crop production in the arid Mediterranean climate. F. Crop. Res. 2012, 128, 34–47. [Google Scholar] [CrossRef]
- Tramblay, Y.; Koutroulis, A.; Samaniego, L.; Vicente-Serrano, S.M.; Volaire, F.; Boone, A.; Le Page, M.; Llasat, M.C.; Albergel, C.; Burak, S.; et al. Challenges for drought assessment in the Mediterranean region under future climate scenarios. Earth-Science Rev. 2020, 210, 103348. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global Warming, Climate Change, and Environmental Pollution: Recipe for a Multifactorial Stress Combination Disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- D’Andrea, R.M.; Andreo, C.S.; Lara, M.V.; Andrea, R.M.D.; Andreo, C.S.; Lara, M.V. Deciphering the mechanisms involved in Portulaca oleracea (C4) response to drought: Metabolic changes including crassulacean acid-like metabolism induction and reversal upon re-watering. Physiol. Plant. 2014, 152, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.C.; Bittencourt, P.P.; Rodrigues, M.A.; Moreno-Villena, J.J.; Alves, F.R.R.; Gastaldi, V.D.; Boxall, S.F.; Dever, L.V.; Demarco, D.; Andrade, S.C.S.; et al. C4 and crassulacean acid metabolism within a single leaf: Deciphering key components behind a rare photosynthetic adaptation. New Phytol. 2020, 225, 1699–1714. [Google Scholar] [CrossRef]
- Ferrari, R.C.; Cruz, B.C.; Gastaldi, V.D.; Storl, T.; Ferrari, E.C.; Boxall, S.F.; Hartwell, J.; Freschi, L. Exploring C4–CAM plasticity within the Portulaca oleracea complex. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- D’Andrea, R.M.; Triassi, A.; Casas, M.I.; Andreo, C.S.; Lara, M.V. Identification of genes involved in the drought adaptation and recovery in Portulaca oleracea by differential display. Plant Physiol. Biochem. 2015, 90, 38–49. [Google Scholar] [CrossRef]
- Rahdari, P.; Hoseini, S.M. Effect of Different Levels of Drought Stress (PEG 6000 Concentrations) On Seed Germination and Inorganic Elements Content in Purslane (Portulaca oleraceae L.) Leaves. J. Stress Physiol. Biochem. 2012, 8, 51–61. [Google Scholar]
- Rahdari, P.; Tavakoli, S.; Hosseini, S.M. Studying of salinity stress effect on germination, proline, sugar, protein, lipid and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Sress Physiol. Biochem. 2012, 8, 182–193. [Google Scholar]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Uddin, M.K.; Sam, S.G.; Awang, A.; Juraimi, A.S.; Jalloh, M.B.; Madon, S.; Shamsuzzaman, S.M. Effect of water regimes on growth, total flavonoid and phenolic content of purslane (Portulaca oleracea L.). Bangladesh J. Bot. 2017, 46, 255–262. [Google Scholar]
- Duarte, B.; Feijão, E.; Pinto, M.V.; Matos, A.R.; Silva, A.; Figueiredo, A.; Fonseca, V.F.; Reis-Santos, P.; Caçador, I. Nutritional valuation and food safety of endemic mediterranean halophytes species cultivated in abandoned salt pans under a natural irrigation scheme. Estuar. Coast. Shelf Sci. 2022, 265, 107733. [Google Scholar] [CrossRef]
- Karkanis, A.C.; Petropoulos, S.A. Physiological and growth responses of several genotypes of common purslane (Portulaca oleracea L.) under Mediterranean semi-arid conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 569–575. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Teixeira, M.; Carvalho, I.S. Effects of salt stress on purslane (Portulaca oleracea) nutrition. Ann. Appl. Biol. 2009, 154, 77–86. [Google Scholar] [CrossRef]
- Bekmirzaev, G.; Ouddane, B.; Beltrao, J.; Khamidov, M.; Fujii, Y.; Sugiyama, A. Effects of salinity on the macro-and micronutrient contents of a halophytic plant species (Portulaca oleracea L.). Land 2021, 10, 481. [Google Scholar] [CrossRef]
- Franco, J.A.; Cros, V.; Vicente, M.J.; Martínez-Sánchez, J.J. Effects of salinity on the germination, growth, and nitrate contents of purslane (Portulaca oleracea L.) cultivated under different climatic conditions. J. Hortic. Sci. Biotechnol. 2011, 86, 1–6. [Google Scholar] [CrossRef]
- Alam, M.A.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Aslani, F.; Alam, M.Z. Effects of salinity and salinity-induced augmented bioactive compounds in purslane (Portulaca oleracea L.) for possible economical use. Food Chem. 2015, 169, 439–447. [Google Scholar] [CrossRef]
- Anastaćio, A.; Carvalho, I.S. Accumulation of fatty acids in purslane grown in hydroponic salt stress conditions. Int. J. Food Sci. Nutr. 2013, 64, 235–242. [Google Scholar] [CrossRef]
- Yazici, I.; Türkan, I.; Sekmen, A.H.; Demiral, T. Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ. Exp. Bot. 2007, 61, 49–57. [Google Scholar] [CrossRef]
- Giménez, A.; Martínez-Ballesta, M.D.C.; Egea-Gilabert, C.; Gómez, P.A.; Artés-Hernández, F.; Pennisi, G.; Orsini, F.; Crepaldi, A.; Fernández, J.A. Combined effect of salinity and led lights on the yield and quality of purslane (Portulaca oleracea L.) microgreens. Horticulturae 2021, 7, 180. [Google Scholar] [CrossRef]
- Xing, J.-C.; Dong, J.; Wang, M.-W.; Liu, C.; Zhao, B.-Q.; Wen, Z.-G.; Zhu, X.-M.; Ding, H.-R.; Zhao, X.-H.; Hong, L.-Z. Effects of NaCl stress on growth of Portulaca oleracea and underlying mechanisms. Brazilian J. Bot. 2019, 42, 217–226. [Google Scholar] [CrossRef]
- Borsai, O.; Al Hassan, M.; Negrușier, C.; Raigón, M.D.; Boscaiu, M.; Sestraș, R.E.; Vicente, O. Responses to salt stress in portulaca: Insight into its tolerance mechanisms. Plants 2020, 9, 1660. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeny, S.E.; El-Saadany, S.S.; Youssef, A.A.; El-Massry, R.A.; El-Newary, S.A. Response of Portulaca Oleracea L. plants to various fertilizers ratios on growth, yield and chemical composition under Egyption conditions. World J. Pharm. Sci. 2015, 3, 2297–2307. [Google Scholar]
- Mortley, D.G.; Oh, J.H.; Johnson, D.S.; Bonsi, C.K.; Hill, W.A. Influence of harvest intervals on growth responses and fatty acid content of purslane (Portulaca oleracea). HortScience 2012, 47, 437–439. [Google Scholar] [CrossRef]
- Montoya-García, C.O.; Volke-Haller, V.H.; Trinidad-Santos, A.; Villanueva-Verduzco, C. Change in the contents of fatty acids and antioxidant capacity of purslane in relation to fertilization. Sci. Hortic. 2018, 234, 152–159. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Soteriou, G.A.; Colla, G.; Rouphael, Y. The occurrence of nitrate and nitrite in Mediterranean fresh salad vegetables and its modulation by preharvest practices and postharvest conditions. Food Chem. 2019, 285, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Kaşkar, Ç.; Fernándeza, J.A.; Ochoa, J.; Niñirola, D.; Conesa, E.; Tüzel, Y. Agronomic behaviour and oxalate and nitrate content of different purslane cultivars (Portulaca oleracea) grown in a hydroponic floating system. Acta Hortic. 2009, 807, 521–526. [Google Scholar] [CrossRef]
- Szalai, G.; Dai, N.; Danin, A.; Dudai, N.; Barazani, O. Effect of nitrogen source in the fertilizing solution on nutritional quality of three members of the Portulaca oleracea aggregate. J. Sci. Food Agric. 2010, 90, 2039–2045. [Google Scholar] [CrossRef]
- Camalle, M.; Standing, D.; Jitan, M.; Muhaisen, R.; Bader, N.; Bsoul, M.; Ventura, Y.; Soltabayeva, A.; Sagi, M. Effect of salinity and nitrogen sources on the leaf quality, biomass, and metabolic responses of two ecotypes of Portulaca oleracea. Agronomy 2020, 10, 656. [Google Scholar] [CrossRef]
- Ghamari, H.; Shafagh Kolvanagh, J.; Sabaghpour, S.H.; Dabbagh Mohammadi Nassab, A. The effect of intercropping and nitroxin biofertilizer on yield components and relative yield total of purslane (Portulaca oleracea L.) and dragon’s head (Lallemantia iberica Fisch. & C.A. Mey). Not. Sci. Biol. 2016, 8, 472–476. [Google Scholar] [CrossRef]
- Rayan, A.M.; Swailam, H.M.; Hamed, Y.S. Composition, Structure, and Techno-Functional Characteristics of the Flour, Protein Concentrate, and Protein Isolate from Purslane (Portulaca oleracea L.) Seeds. Plant Foods Hum. Nutr. 2022, 78, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Brennan, E.B. The slide hammer seeder: A novel tool for planting small seeds. Horttechnology 2018, 28, 764–775. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Ganjeali, A.; Farhoosh, R.; Cheniany, M. Variation in phenolic compounds, α-linolenic acid and linoleic acid contents and antioxidant activity of purslane (Portulaca oleracea L.) during phenological growth stages. Physiol. Mol. Biol. Plants 2020, 26, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K.; Juraimi, A.S.; Ali, M.E.; Ismail, M.R. Evaluation of antioxidant properties and mineral composition of purslane (Portulaca oleracea L.) at different growth stages. Int. J. Mol. Sci. 2012, 13, 10257–10267. [Google Scholar] [CrossRef]
- Nastou, E.; Thalassinos, G.; Polyzos, N.; Antoniadis, V.; Petropoulos, S.A. The effect of nitrogen fertilization rate on growth and physiological parameters of three purslane genotypes grown in a soilless cultivation system. Acta Hortic. 2021, 1321, 125–132. [Google Scholar] [CrossRef]
- Fallah, S.; Omrani, B. Substitution of inorganic fertilizers with organic manure reduces nitrate accumulation and improves quality of purslane. Iran. J. Plant Physiol. 2019, 9, 2651–2660. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.H.; Ghalavand, A.; Boojar, M.M.A.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A. Application of manure and biofertilizer to improve soil properties and increase grain yield, essential oil and ω3 of purslane (Portulaca oleracea L.) under drought stress. Soil Tillage Res. 2021, 205, 104633. [Google Scholar] [CrossRef]
- Yang, Y.; Syed, S.; Mao, S.; Li, Q.; Ge, F.; Lian, B.; Lu, C. Bioorganic–Mineral Fertilizer Can Remediate Chemical Fertilizer-Oversupplied Soil: Purslane Planting as an Example. J. Soil Sci. Plant Nutr. 2020, 20, 892–900. [Google Scholar] [CrossRef]
- D’Imperio, M.; Parente, A.; Montesano, F.F.; Renna, M.; Logrieco, A.F.; Serio, F. Boron biofortification of Portulaca oleracea L. through soilless cultivation for a new tailored crop. Agronomy 2020, 10, 999. [Google Scholar] [CrossRef]
- D’Imperio, M.; Durante, M.; Gonnella, M.; Renna, M.; Montesano, F.F.; Parente, A.; Mita, G.; Serio, F. Enhancing the nutritional value of Portulaca oleracea L. by using soilless agronomic biofortification with zinc. Food Res. Int. 2022, 155, 111057. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Pintimalli, L.; Malorgio, F. Selenium biofortification of three wild species, Rumex acetosa L., Plantago coronopus L., and Portulaca oleracea L., grown as microgreens. Agronomy 2021, 11, 1155. [Google Scholar] [CrossRef]
- Rahbarian, R.; Azizi, E.; Behdad, A.; Mirblook, A. Effects of Chromium on Enzymatic/Nonenzymatic Antioxidants and Oxidant Levels of Portulaca oleracea L. J. Med. Plants By-Prod. 2019, 1, 21–31. [Google Scholar]
- Alyazouri, A.; Jewsbury, R.; Tayim, H.; Humphreys, P.; Al-Sayah, M.H. Uptake of Chromium by Portulaca oleracea from Soil: Effects of Organic Content, pH, and Sulphate Concentration. Appl. Environ. Soil Sci. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Thalassinos, G.; Nastou, E.; Petropoulos, S.A.; Antoniadis, V. Soil dynamics of Cr(VI) and responses of Portulaca oleracea L. grown in a Cr(VI)-spiked soil under different nitrogen fertilization regimes. Environ. Sci. Pollut. Res. 2022, 29, 14469–14478. [Google Scholar] [CrossRef]
- Yang, H.; Deng, Y.; Wang, M.W.; Zhu, M.; Liu, C.; Ge, Z.J.; Xing, J.C. Risk of heavy metal contamination in three seawater-cultivated vegetables. Polish J. Environ. Stud. 2020, 29, 961–967. [Google Scholar] [CrossRef]
- Alyazouri, A.; Jewsbury, R.; Tayim, H.; Humphreys, P.; Al-Sayah, M.H. Applicability of Heavy-Metal Phytoextraction in United Arab Emirates: An Investigation of Candidate Species. Soil Sediment Contam. 2014, 23, 557–570. [Google Scholar] [CrossRef]
- Subpiramaniyam, S. Portulaca oleracea L. for phytoremediation and biomonitoring in metal-contaminated environments. Chemosphere 2021, 280, 130784. [Google Scholar] [CrossRef]
- Abdallah, S.M.; Farahat, E.A.; Shaltout, K.H.; Eid, E.M. Assessing macro-nutrient removal potential of nine native plant species grown at a sewage sludge dump site. Appl. Ecol. Environ. Res. 2020, 18, 1799–1817. [Google Scholar] [CrossRef]
- Yadav, S.; Pandey, V.C.; Kumar, M.; Singh, L. Plant diversity and ecological potential of naturally colonizing vegetation for ecorestoration of fly ash disposal area. Ecol. Eng. 2022, 176, 106533. [Google Scholar] [CrossRef]
- Kiliç, C.C.; Kukul, Y.S.; Anaç, D. Performance of purslane (Portulaca oleracea L.) as a salt-removing crop. Agric. Water Manag. 2008, 95, 854–858. [Google Scholar] [CrossRef]
- Luo, J.; Xu, M.; Liu, C.; Wei, S.; Tang, H. Effects comparation of different mulching methods on soil in pitaya orchards. Int. Agrophysics 2021, 35, 269–278. [Google Scholar] [CrossRef]
- Cros, V.; Martinez-Sanchez, J.; Franco, J.A. Good Yields of Common Purslane with a High Fatty Acid Content Can Be Obtained in a Peat-based Floating System. Horttechnology 2007, 17, 14–20. [Google Scholar] [CrossRef]
- He, J.; You, X.; Qin, L. High Salinity Reduces Plant Growth and Photosynthetic Performance but Enhances Certain Nutritional Quality of C4 Halophyte Portulaca oleracea L. Grown Hydroponically Under LED Lighting. Front. Plant Sci. 2021, 12, 1–16. [Google Scholar] [CrossRef]
- Palaniswamy, U.R.; McAvoy, R.J.; Bible, B. Oxalic acid concentrations in purslane (Portulaca oleraceae L.) is altered by the stage of harvest and the nitrate to ammonium ratios in hydroponics. Sci. Hortic. 2004, 629, 299–305. [Google Scholar] [CrossRef]
- Lazcano-Escobar, J.F.; Quiñones-Islas, N.S.; Trejo-Estrada, S.R.; Ramírez-López, C. Optimization of the Biomass Production From Sprouts of Neo-Tropical Vegetable Species. Agrociencia 2022, 56, 704–726. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Vincentini, O.; Cantatore, V.; Cavoski, I.; Gobbetti, M. Fermented portulaca oleracea L. Juice: A novel functional beverage with potential ameliorating effects on the intestinal inflammation and epithelial injury. Nutrients 2019, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Apostol, L.C.; Ropciuc, S.; Prisacaru, A.E.; Albu, E. Characterization of tomato sauce enriched with purslane (Portulaca oleracea) leaves. J. Hyg. Eng. Des. 2020, 31, 127–132. [Google Scholar]
- Pratiwi, I.; Susilowati, A.; Pangastuti, A. Incorporation of purslane extract (Portulaca oleracea) to chitosan edible film as a packaging material to prevent damage of mozzarella cheese during storage. IOP Conf. Ser. Earth Environ. Sci. 2021, 828, 012026. [Google Scholar] [CrossRef]
- Delvarianzadeh, M.; Nouri, L.; Mohammadi Nafchi, A.; Ebrahimi, H. Physicochemical, rheological, and sensory evaluation of voluminous breads enriched by purslane (Portulaca oleracea L.). Ital. J. Food Sci. 2020, 32, 815–830. [Google Scholar] [CrossRef]
- Melilli, M.G.; Pagliaro, A.; Scandurra, S.; Gentile, C.; Di Stefano, V. Omega-3 rich foods: Durum wheat spaghetti fortified with Portulaca oleracea. Food Biosci. 2020, 37, 100730. [Google Scholar] [CrossRef]
- Melilli, M.G.; Di Stefano, V.; Sciacca, F.; Pagliaro, A.; Bognanni, R.; Scandurra, S.; Virzì, N.; Gentile, C.; Palumbo, M. Improvement of fatty acid profile in durum wheat breads supplemented with Portulaca oleracea L. quality traits of purslane-fortified bread. Foods 2020, 9, 764. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Saenz, Y.O.; López-Palestina, C.U.; Gutiérrez-Tlahque, J.; Monroy-Torres, R.; Pinedo-Espinoza, J.M.; Hernández-Fuentes, A.D. Nutritional and functional evaluation of three powder mixtures based on mexican quelites: Alternative ingredients to formulate food supplements. Food Sci. Technol. 2020, 40, 1029–1037. [Google Scholar] [CrossRef]
- Guo, S.; Li, H.; Zhang, M.; Lan, X.; Yang, Y.; Ying, X. Two new alkaloids with carboxylic acid moieties from Portulaca oleracea and their anti-inflammatory effects. Phytochem. Lett. 2022, 52, 67–71. [Google Scholar] [CrossRef]
- Liu, P.; Lan, X.; Tao, X.; Tian, J.; Ying, X.; Stien, D. A new alkaloid and two organic acids from Portulaca oleracea L. and their bioactivities. Nat. Prod. Res. 2022, 1–8. [Google Scholar] [CrossRef]
- Cui, X.; Ying, Z.; Ying, X.; Jia, L.; Yang, G. Three new alkaloids from Portulaca oleracea L. and their bioactivities. Fitoterapia 2021, 154, 1–8. [Google Scholar] [CrossRef]
- Lan, X.; Guo, S.; Song, M.; Liu, P.; Tian, J.; Zhang, W.; Ying, X. A Novel Amide Alkaloid from Portulaca oleracea. Chem. Nat. Compd. 2022, 58, 1089–1092. [Google Scholar] [CrossRef]
- Fu, J.; Wang, H.; Dong, C.; Xi, C.; Xie, J.; Lai, S.; Chen, R.; Kang, J. Water-soluble alkaloids isolated from Portulaca oleracea L. Bioorg. Chem. 2021, 113, 105023. [Google Scholar] [CrossRef]
- Wang, C.; Guo, S.; Tian, J.; Liu, P.; Song, M.; Zhang, W.; Ying, X. Two new lignans with their biological activities in Portulaca oleracea L. Phytochem. Lett. 2022, 50, 95–99. [Google Scholar] [CrossRef]
- Hoseini, S.S.; Najafi, G.; Sadeghi, A. Chemical characterization of oil and biodiesel from Common Purslane (Portulaca) seed as novel weed plant feedstock. Ind. Crops Prod. 2019, 140, 111582. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Arampatzis, D.A.; Tsiropoulos, N.G.; Petrović, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Seed oil and seed oil byproducts of common purslane (Portulaca oleracea L.): A new insight to plant-based sources rich in omega-3 fatty acids. LWT—Food Sci. Technol. 2020, 123, 109099. [Google Scholar] [CrossRef]
| Cultivation Conditions | Water Stress | Duration (Days) | Plant Density | Fresh Yield | Country | References | |
|---|---|---|---|---|---|---|---|
| Field experiment (June to July, 2016); sandy clay loam soil, with pH 7.4 and 1.3% of organic matter; mean daily temperatures of 28 °C; precipitations of 12 mm during the experiment; plants harvested at 45 DAS. | Mediterranean arid conditions | 45 | 240 plants m−2 | 25 ton ha−1 | 10.42 g plant−1 | Greece | [77] |
| Pots experiment (July to August, 2015); plants grown in 20 cm × 30 cm (d/h) pots with soil pH 5.23; EC 1.15 dS m−1 and organic matter content of 22.02%; three plants per pot; plants harvested at 30 DAE. | Field capacity | 30 no stress | - | 3.01 kg m−2 ** | 91.26 g plant−1 | Malaysia | [75] |
| Continuous saturated | 30 stressed | - | 2.28 kg m−2 ** | 68.97 g plant−1 | |||
| Continuous flooded | 30 stressed | - | 1.41 kg m−2 ** | 42.67 g plant−1 | |||
| 10 days flooded and saturated next | 10 flooded/20 saturated | - | 1.57 kg m−2 ** | 47.67 g plant−1 | |||
| 10 days saturated and field capacity next | 10 saturated/no stress | - | 2.74 kg m−2 ** | 82.93 g plant−1 | |||
| Greenhouse experiment; plants grown in 10 cm × 15 cm (d/h) pots with hummus, clay and sand (2:1:3); average day/night temperatures (25/18 °C); relative humidity (60/70%) and photoperiod (16/8 h); one plant per pot. Plants harvested at 45 DAE. | Control (90% field capacity) | 45 no stress | - | 0.45 kg m−2 ** | 13.70 g plant−1 | Iran | [21] |
| Mild drought (60% field capacity) | 30 no stress/15 stressed | - | 0.32 kg m−2 ** | 9.83 g plant−1 | |||
| Severe drought (30% field capacity) | 30 no stress/15 stressed | - | 0.24 kg m−2 ** | 7.23 g plant−1 | |||
| Cultivation Conditions | Salinity Stress | Duration (Days) | Plant Density | Fresh Yield | Country | References | |
|---|---|---|---|---|---|---|---|
| Glasshouse experiment (July to October 2013); 10-day-old seedlings were transplanted to 10 L pots with rice field soil; pH 4.8; 2.64% organic carbon; 1.25 g cc1bulk density and CEC of 7.06 me 100 g−1 soil; one plant per pot; saline treatment applied with NaCl; plants harvested at 60 DAT. | 0 dS m−1 | 60 no stress | - | 42.75 ton ha−1 ** | 129.48 g plant−1 | Malaysia | [82] |
| 8 dS m−1 | 29 no stress/30 stressed | - | 37.29 ton ha−1 ** | 112.94 g plant−1 | |||
| 16 dS m−1 | 29 no stress/30 stressed | - | 34.71 ton ha−1 ** | 105.14 g plant−1 | |||
| 24 dS m−1 | 29 no stress/30 stressed | - | 30.83 ton ha−1 ** | 93.40 g plant−1 | |||
| Greenhouse experiment (January to March, 2016); plants grown in 3 L pots with 1 kg of soil; pH 5; average day/night temperatures (22.2/17.9 °C); relative humidity from 12.9% to 88.3%; one plant per pot; saline treatment applied with NaCl; plants harvested at 50 DAS. | 0 | 50 no stress | - | 16.5 ton ha−1 ** | 50 g plant−1 | France | [80] |
| 5 dS m−1 | 4 no stress/46 stressed | - | 14.85 ton ha−1 ** | 45 g plant−1 | |||
| 9.8 dS m−1 | 4 no stress/46 stressed | - | 12.54 ton ha−1 ** | 38 g plant−1 | |||
| 20 dS m−1 | 4 no stress/46 stressed | - | 5.94 ton ha−1 ** | 18 g plant−1 | |||
| Greenhouse experiment (April to May 2017); plants grown in 26 mL capacity cells in floating system with nutritive solution; pH 7.7; day/night temperatures (38.1/13.6 °C) and relative humidity from 10% to 85%. Saline treatment applied with NaCl; plants harvested at 50 DAS. | 2.5 dS m−1 | 22 no stress/28 stressed | 3500 plants m−2 | 3.84 kg m−2 | 1.097 g plant−1 ** | Spain | [81] |
| 5 dS m−1 | 22 no stress/28 stressed | 3500 plants m−2 | 3.58 kg m−2 | 1.02 g plant−1 ** | |||
| 7.5 dS m−1 | 22 no stress/28 stressed | 3500 plants m−2 | 3.25 kg m−2 | 0.927 g plant−1 ** | |||
| 10 dS m−1 | 22 no stress/28 stressed | 3500 plants m−2 | 3.24 kg m−2 | 0.926 g plant−1 ** | |||
| 15 dS m−1 | 22 no stress/28 stressed | 3500 plants m−2 | 2.88 kg m−2 | 0.822 g plant−1 ** | |||
| Greenhouse experiment (2018); plants grown in artificial soil with Hoagland´s solution; day/night temperatures (28.6/19.8 °C) and relative humidity (76.8%/82.4%); one plant per pot; saline treatment applied with NaCl when plant height reached 15 cm; harvest took place 14 days after. | 0 mM | 14 no stress | - | 0.43 kg m−2 ** | 13.3 g plant−1 | China | [86] |
| 50 mM | 14 stress | - | 0.46 kg m−2 ** | 14.0 g plant−1 | |||
| 100 mM | 14 stress | - | 0.42 kg m−2 ** | 12.6 g plant−1 | |||
| 150 mM | 14 stress | - | 0.29 kg m−2 ** | 8.7 g plant−1 | |||
| 200 mM | 14 stress | - | 0.25 kg m−2 ** | 7.6 g plant−1 | |||
| Greenhouse experiment (2020); plants grown in 0.5 L pots with peat:vermiculite:perlite (50:25:25) and Hoagland´s nutrient solution; day/night temperatures (23/17 °C); photoperiod (16/8 h) and relative humidity ranged between 50 and 80%; one plant per pot; saline treatment applied with NaCl; plants harvested at 77 DAS. | 0 mM | 77 days | - | 2.064 kg m−2 ** | 62.55 g plant−1 | Spain | [87] |
| 100 mM | 42 no stress/35 stressed | - | 1.86 kg m−2 ** | 56.3 g plant−1 | |||
| 200 mM | 42 no stress/35 stressed | - | 1.28 kg m−2 ** | 38.78 g plant−1 | |||
| 400 mM | 42 no stress/35 stressed | - | 0.62 kg m−2 ** | 18.77 g plant−1 | |||
| Cultivation Conditions | Nitrogen Form | Nitrogen Dose | Plant Density | Fresh Yield | Country | References | |
|---|---|---|---|---|---|---|---|
| Field experiment (June to July, 2016); sandy clay loam soil, with pH 7.4 and 1.3% of organic matter; mean daily temperatures of 28 °C; precipitations of 12 mm during the experiment; plants harvested at 45 DAS. | None | None | 240 plants m−2 | 25 ton ha−1 | 10.42 g plant−1 | Greece | [77] |
| Field experiment (May to July, 2014); loam soil with pH 7.4 and 1.3% organic matter content; mean daily temperatures of 25 °C; plants harvested at 65 DAS. | None | None | 33 plants m−2 | 24 ton ha−1 | 72 g plant−1 | Greece | [38] |
| Field experiment (June to July, 2009 and 2010); sandy loam soil, pH 7.23, organic matter content of 1.16% and total nitrogen of 0.06%; plants harvested at 35 DAS; the obtained yield was the mean of both years. | None | None | - | 5.46 ton ha−1 | 16.54 g plant−1 ** | Turkey | [37] |
| NH4NO3 | 150 kg N ha−1 | - | 12.71 ton ha−1 | 38.51 g plant−1 ** | |||
| Urea | 150 kg N ha−1 | - | 11.54 ton ha−1 | 34.96 g plant−1 ** | |||
| (NH4)2SO4 | 150 kg N ha−1 | - | 10.8 ton ha−1 | 32.72 g plant−1 ** | |||
| (Ca(NH4NO3)2) | 150 kg N ha−1 | - | 10.92 ton ha−1 | 33.09 g plant−1 ** | |||
| Field experiment (June 2007 and 2008); clay soil with average pH 7.70, electical conductivity of 2.5 dS m−1, 0.50% organic matter content and 0.05% of total N; plants harvested at 61 DAS. | (NH4)2SO4 | 49 kg N ha−1 | 16 plants m−2 | 115.40 ton ha−1 | 721.30 g plant−1 | Egypt | [88] |
| (NH4)2SO4 | 73 kg N ha−1 | 16 plants m−2 | 140.90 ton ha−1 | 880.80 g plant−1 | |||
| (NH4)2SO4 | 98 kg N ha−1 | 16 plants m−2 | 159.77 ton ha−1 | 998.60 g plant−1 | |||
| Field experiment (July to August, 2014); sandy clay soil with pH 8.2; maximum day tempertures ranged from 19 °C to 30 °C and minimum temperatures ranged from 6 °C to 11 °C; plants harvested at 42 DAS. | None | None | 1750 plants m−2 | 110 ton ha−1 | 6.28 g plant−1 | Mexico | [39] |
| (NH4)2SO4 | 100 kg kg N ha−1 | 1750 plants m−2 | 120 ton ha−1 | 6.86 g plant−1 | |||
| NH4H2PO4 | 200 kg N ha−1 | 1750 plants m−2 | 126 ton ha−1 | 7.20 g plant−1 | |||
| Cultivation Conditions | Nitrogen Form | Nitrogen Dose | Plant Density | Fresh Yield | Country | References | |
|---|---|---|---|---|---|---|---|
| Hydroponic experiment conducted in greenhouse conditions (July 2004) in trays with peat floating on the nutrient solution; day/night temperatures (35/15 °C); plants harvested at 20 DAS. | None | None | 3105 plants m−2 | 0.27 kg m−2 | 0.09 g plant−1 | Italy | [40] |
| NO3−/NH4+ (40:60) | 12 mmol L−1 | 3105 plants m−2 | 1.39 kg m−2 | 0.45 g plant−1 | |||
| NO3−/NH4+ (40:60) | 24 mmol L−1 | 3105 plants m−2 | 1.50 kg m−2 | 0.48 g plant−1 | |||
| NO3−/NH4+ (40:60) | 36 mmol L−1 | 3105 plants m−2 | 1.81 kg m−2 | 0.58 g plant−1 | |||
| Hydroponic experiment conducted in greenhouse conditions (July 2004) in trays with peat floating on the nutrient solution; day/night temperatures (35/15 °C); plants harvested at 20 DAS. | NO3−/NH4+ (60:40) | 12 mmol L−1 | 3105 plants m−2 | 1.38 kg m−2 | 0.40 g plant−1 | ||
| NO3−/NH4+ (40:60) | 12 mmol L−1 | 3105 plants m−2 | 1.48 kg m−2 | 0.48 g plant−1 | |||
| NO3−/NH4+ (0:100) | 12 mmol L−1 | 3105 plants m−2 | 0.71 kg m−2 | 0.23 g plant−1 | |||
| Hydroponic experiment conducted in greenhouse (September 2007) in trays with vermiculite floating on the nutrient solution, pH 7.7, EC 0.85 dS m−1; plants were harvested at 13 DAS. | NO3−/NH4+ (60:40) | 80 mmol L−1 | 3200 plants m−2 | 1.38 kg m−2 | 0.43 g plant−1 | Spain | [92] |
| Hydroponic experiment conducted in greenhouse conditions from February to July in trays with tuff:peatmoss (2:1); maximun temperature 28 °C; total yield of plants harvested five times during 60 days. | Clark´s nutrient solution | 22 mM NO3− and 2.78 mM NH4+ | - | 26.92 kg m−2 | 8.97 g plant−1 ** | Jordan | [41] |
| Greenhouse experiment; plants cultivated in 8 L styrofoam boxes with organic substrate (forest residue, compost and white peat), pH 6, EC 2 mS cm−1, total nitrogen 300 mg L−1; day/night temperature (33.7/16.7 °C) and relative humidity (49.7%/12.2%); plants harvested at 31 DAS. | None | None | 2200 plants m−2 | 2.38 kg m−2 | 1.08 g plant−1 | Portugal | [36] |
| NH4NO3 | 30 kg N ha−1 | 2200 plants m−2 | 4.01 kg m−2 | 1.82 g plant−1 | |||
| NH4NO3 | 60 kg N ha−1 | 2200 plants m−2 | 5.10 kg m−2 | 2.32 g plant−1 | |||
| NH4NO3 | 90 kg N ha−1 | 2200 plants m−2 | 5.30 kg m−2 | 2.41 g plant−1 | |||
| Plants grown on October in 2 L pots with peat and perlite (2:1 v/v), pH 6, EC 0.35 dS m−1, total nitrogen 0.14%; plants harvested at 37 DAS. | NO3−/NH4+/Urea | None | - | 1.10 kg m−2 ** | 33 g plant−1 | Greece | [100] |
| NO3−/NH4+/Urea | 200 mg N L−1 | - | 2.50 kg m−2 ** | 75.7 g plant−1 | |||
| NO3−/NH4+/Urea | 400 mg N L−1 | - | 4.19 kg m−2 ** | 126.9 g plant−1 | |||
| NO3−/NH4+/Urea | 600 mg N L−1 | - | 7.24 kg m−2 ** | 219.4 g plant−1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrascosa, A.; Pascual, J.A.; Ros, M.; Petropoulos, S.A.; Alguacil, M.d.M. Agronomical Practices and Management for Commercial Cultivation of Portulaca oleracea as a Crop: A Review. Plants 2023, 12, 1246. https://doi.org/10.3390/plants12061246
Carrascosa A, Pascual JA, Ros M, Petropoulos SA, Alguacil MdM. Agronomical Practices and Management for Commercial Cultivation of Portulaca oleracea as a Crop: A Review. Plants. 2023; 12(6):1246. https://doi.org/10.3390/plants12061246
Chicago/Turabian StyleCarrascosa, Angel, Jose Antonio Pascual, Margarita Ros, Spyridon A. Petropoulos, and Maria del Mar Alguacil. 2023. "Agronomical Practices and Management for Commercial Cultivation of Portulaca oleracea as a Crop: A Review" Plants 12, no. 6: 1246. https://doi.org/10.3390/plants12061246
APA StyleCarrascosa, A., Pascual, J. A., Ros, M., Petropoulos, S. A., & Alguacil, M. d. M. (2023). Agronomical Practices and Management for Commercial Cultivation of Portulaca oleracea as a Crop: A Review. Plants, 12(6), 1246. https://doi.org/10.3390/plants12061246






