Effect of Three Boron Concentrations in Soil on Growth and Physiology in Sweet Cherry Trees
Abstract
1. Introduction
2. Results and Discussion
2.1. Plant Growth Parameters
2.2. Physiological Parameters
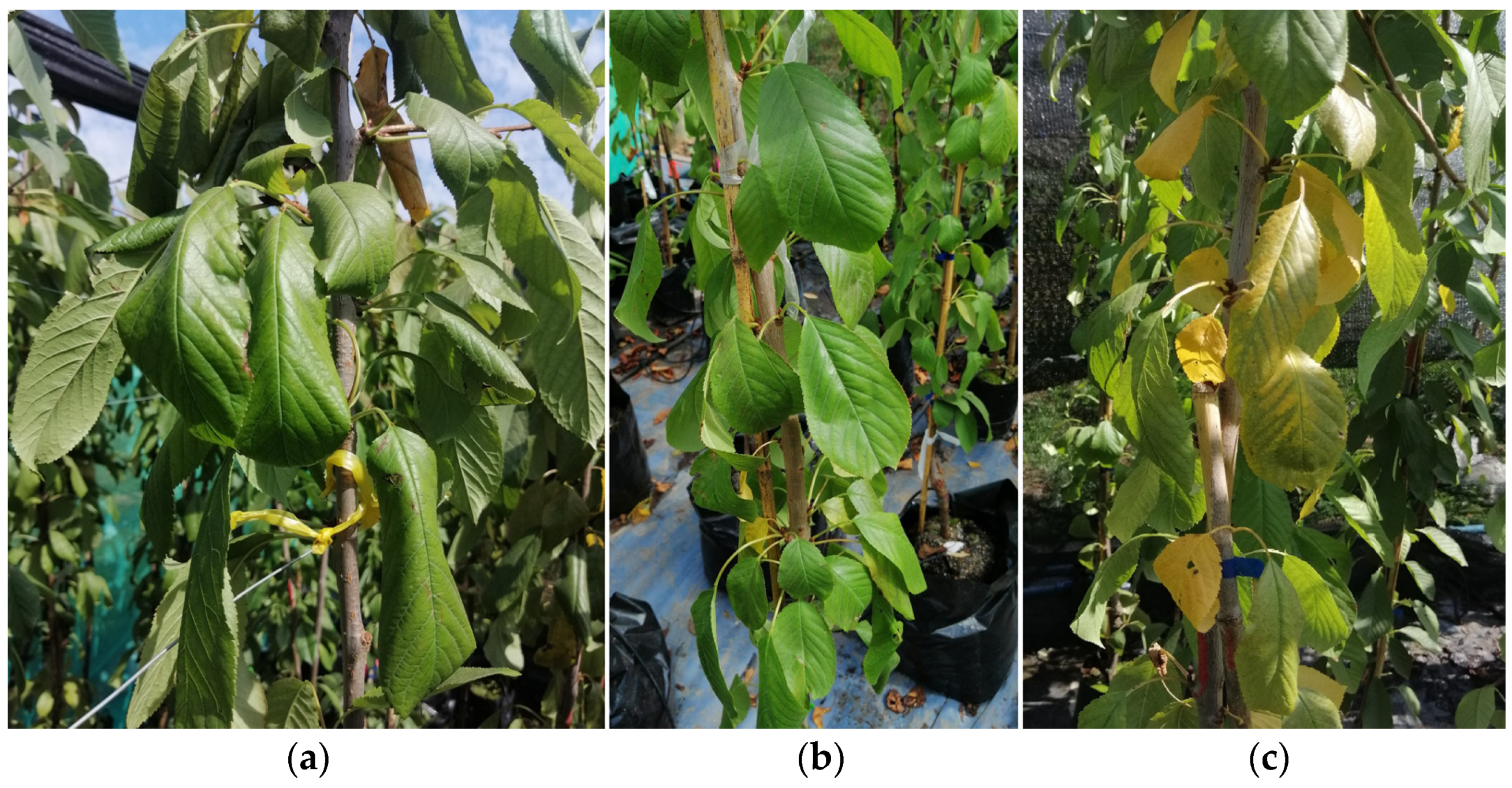
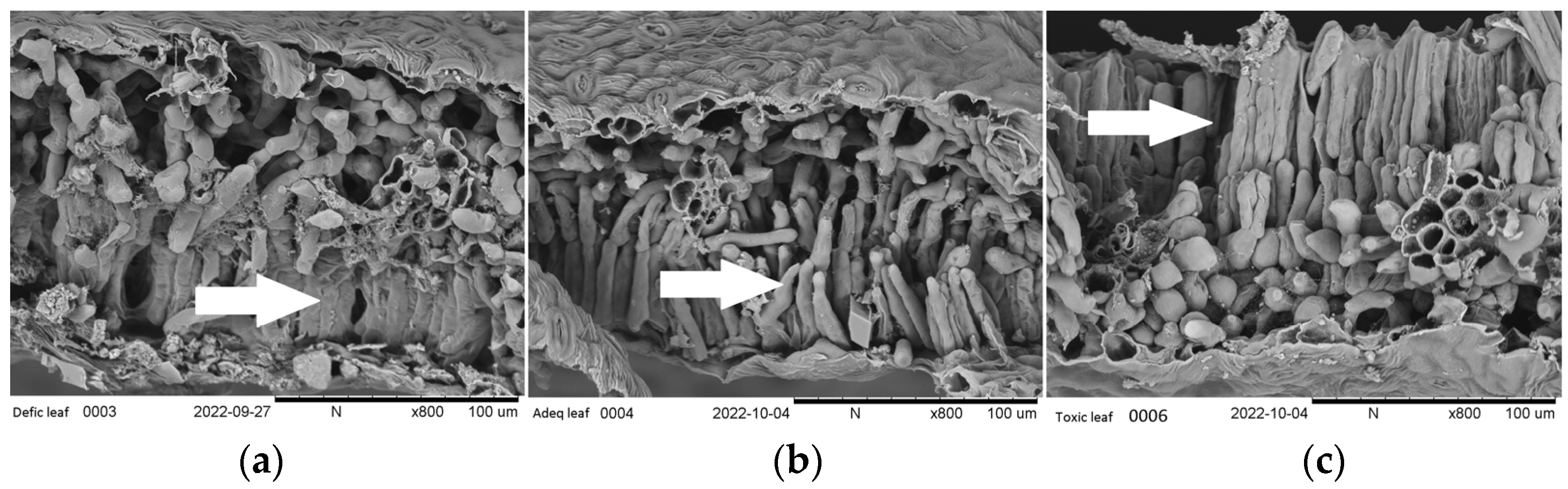
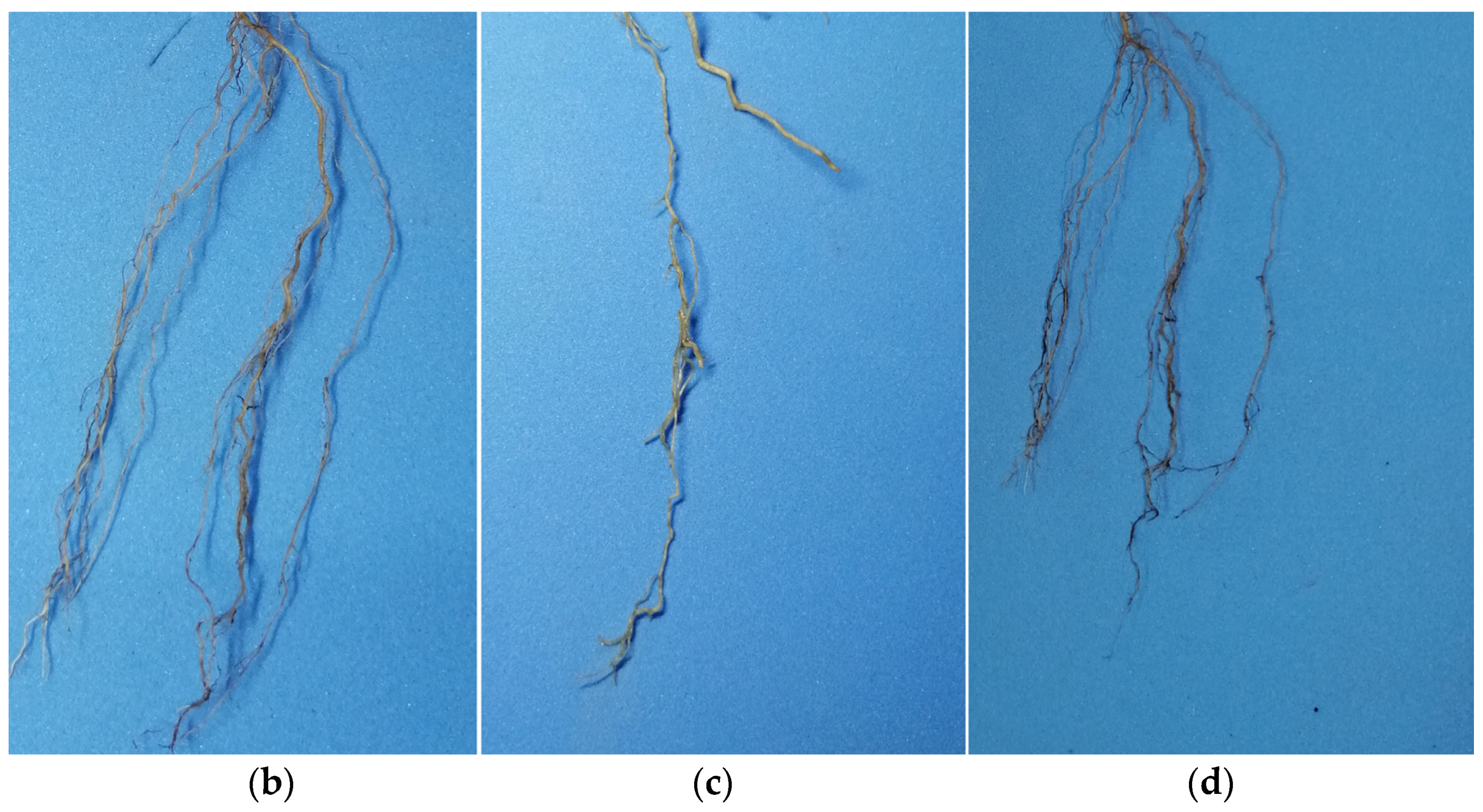
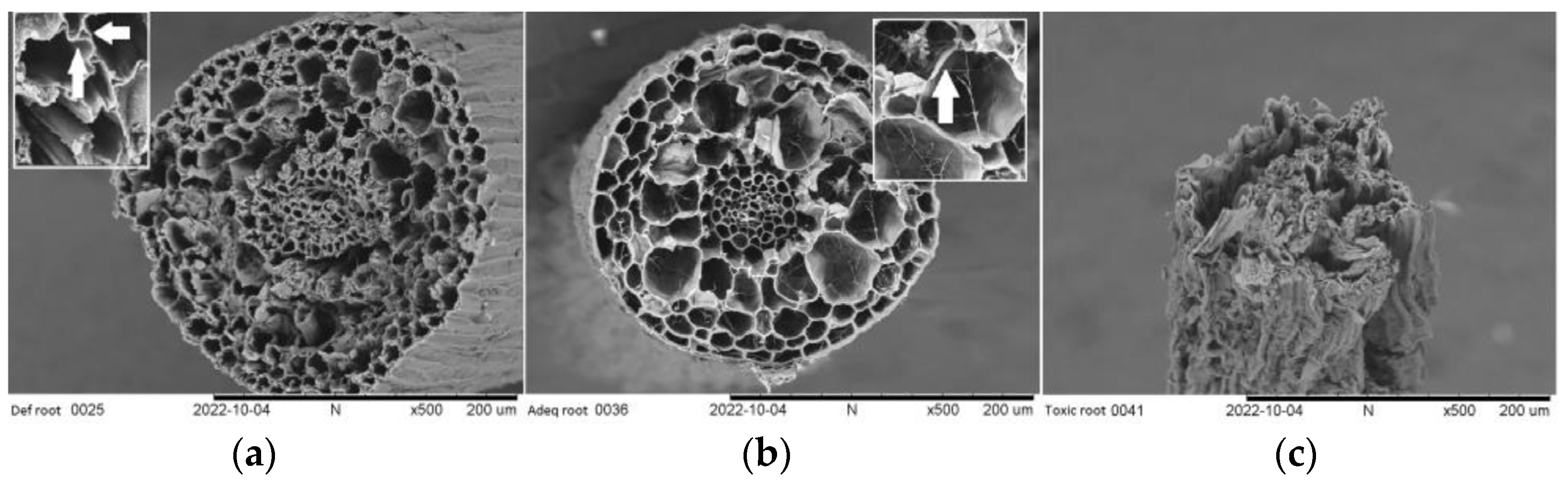
2.3. Symptoms and Morphological Characteristics
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Determination of the Experimental B Doses
3.3. Experimental Design
3.4. Soil Mineral Analysis
3.5. Biomass Components and Their Partitioning, DM, and Mineral Composition Measurements
3.6. Physiological Parameters
3.7. Morphological Study
3.8. Statistical Analysis and Data Presentation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nejad, S.A.; Etesami, H. The Importance of Boron in Plant Nutrition. In Metalloids in Plants: Advances and Future Prospects; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 433–449. [Google Scholar]
- Marschner, H. Mineral nutrition of higher plants. Ann. Bot. 1995, 78, 527–528. [Google Scholar]
- Aparicio-Durán, L.; Gmitter, F.G.; Arjona-López, J.M.; Grosser, J.W.; Calero-Velázquez, R.; Hervalejo, Á.; Arenas-Arenas, F.J. Evaluation of three new citrus rootstocks under boron toxicity conditions. Agronomy 2021, 11, 2490. [Google Scholar] [CrossRef]
- Wimmer, M.A.; Abreu, I.; Bell, R.W.; Bienert, M.D.; Brown, P.H.; Dell, B.; Fujiwara, T.; Goldbach, H.E.; Lehto, T.; Mock, H.P.; et al. Boron: An essential element for vascular plants: A comment on Lewis (2019) ‘Boron: The essential element for vascular plants that never was’. New Phytol. 2020, 226, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Peñaloza, P.; Toloza, P. Boron increases pollen quality, pollination, and fertlity of different genetic lines of pepper. J. Plant Nutr. 2018, 41, 969–979. [Google Scholar] [CrossRef]
- Brown, P.H.; Bellaloui, N.; Wimmer, M.A.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pfeffer, H.; Dannel, F. Boron in Plant Biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- González-Fontes, A.; Fujiwara, T. Advances in Plant Boron. Int. J. Mol. Sci. 2020, 21, 4107. [Google Scholar] [CrossRef]
- Niaz, A.; Hannan, A.; Waqas, M. Boron status of soils as affected by different soil characteristics–pH, CaCO3, organic matter and clay contents. Pak. J. Agric. Sci 2007, 44, 428–435. [Google Scholar]
- Rubio, F.; Vanzetti, G.; Buffa, E.V. Disponibilidad de Boro en un Suelo de Justiniano Posse y Respuesta a la Aplicación Foliar en Soja; Universidad Nacional de Cordoba: Córdoba, AR, USA, 2014; 11p. [Google Scholar]
- Kundu, D.; Khanam, R.; Saha, S.; Thingujam, U.; Hazra, G.C. Boron availability in relation to some important soil chemical properties in acid soils of Cooch Behar district, West Bengal. J. Appl. Nat. Sci. 2017, 9, 2400–2403. [Google Scholar] [CrossRef]
- Neenu, S.; Ramesh, K. Weather—Micronutrient Interactions in Soil and Plants—A Critical Review. Chem. Sci. Rev. Lett. 2020, 9, 205–219. [Google Scholar] [CrossRef]
- León Baque, E.E.; Vásquez Granda, V.D.; Valderrama Chávez, M.D. Cambios en patrones de precipitación y temperatura en el Ecuador: Regiones sierra y oriente. Dilemas Contemp. Educ. Política Valores 2021, 8, 1–22. [Google Scholar] [CrossRef]
- Landi, M.; Margaritopoulou, T.; Papadakis, I.E.; Araniti, F. Boron toxicity in higher plants: An update. Planta 2019, 250, 1011–1032. [Google Scholar] [CrossRef]
- Brdar-Jokanović, M. Boron toxicity and deficiency in agricultural plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef]
- Hua, T.; Zhang, R.; Sun, H.; Liu, C. Alleviation of boron toxicity in plants: Mechanisms and approaches. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2975–3015. [Google Scholar] [CrossRef]
- Shah, A.; Wu, X.; Ullah, A.; Fahad, S.; Muhammad, R.; Yan, L.; Jiang, C. Deficiency and toxicity of boron: Alterations in growth, oxidative damage and uptake by citrange orange plants. Ecotoxicol. Environ. Saf. 2017, 145, 575–582. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, F.; Simón-Grao, S.; Martínez-Nicolás, J.J.; Alfosea-Simón, M.; Liu, C.; Chatzissavvidis, C.; Pérez-Pérez, J.G.; Cámara-Zapata, J.M. Multiple stresses occurring with boron toxicity and deficiency in plants. J. Hazard. Mater. 2020, 397, 122713. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Brown, P.H.; Labavitch, J.M. Species variability in boron requirement is correlated with cell wall pectin. J. Exp. Bot. 1996, 47, 227–232. [Google Scholar] [CrossRef]
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef] [PubMed]
- Whiley, A.W.; Smith, T.E.; Wolstenholme, B.N.; Saranah, J.B. Boron Nutrition of Avocados. Talk. Avocados 1996, 7, 12–15. [Google Scholar]
- Figueroa, L.; Tapia, T.L.; Bastías, E.; Escobar, H.; Amador, A. Levels of boron in irrigation waters and soils cultivated with olives in Northern Chile. Idesia 1994, 13, 63–67. [Google Scholar]
- Larrañaga, E.P.; Osores, M.A. Catastro Frutícola Principales Resultados; Oficina de Estuduos y Políticas Agrárias (ODEPA); Ministerio de Agricultura: Santiago, Chile, 2019; pp. 1–48.
- Bernier, R.; Alfaro, M. Acidez de suelos y efectos del encalado. Inst. Investig. Agropecu. INIA 2006, 151, 1–46. [Google Scholar]
- Sade, N.; Del Mar Rubio-Wilhelmi, M.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2018, 69, 845–853. [Google Scholar] [CrossRef]
- Johnson, V.J.; Mirza, A. Role of Macro and Micronutrients in the Growth and Development of Plants. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 576–587. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; El-Esawi, M.A.; Hussain, S.; Wang, X. Boron-toxicity induced changes in cell wall components, boron forms, and antioxidant defense system in rice seedlings. Ecotoxicol. Environ. Saf. 2021, 216, 112192. [Google Scholar] [CrossRef]
- Eggert, K.; Von Wirén, N. Response of the plant hormone network to boron deficiency. New Phytol. 2017, 216, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Rutto, L.; Katuuramu, D. Melatonin acts synergistically with auxin to promote lateral root development through fine tuning auxin transport in Arabidopsis thaliana. PLoS ONE 2019, 14, e0221687. [Google Scholar] [CrossRef] [PubMed]
- Gökoğlan, B.; Nikpeyma, Y.; Çağlar, S. Effects of Late Fall Foliar Boron Sprays on the Fruit Set of Sweet Cherry Trees cv. 0900 Ziraat. Int. J. Sci. Res. 2017, 6, 2406–2408. [Google Scholar] [CrossRef]
- Wang, R.; Liu, G.; Liu, L.; Jiang, C. Relationship between leaf chlorosis and different boron forms in Trifoliate orange seedlings under excessive boron supply. Soil Sci. Plant Nutr. 2014, 60, 325–332. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Wojcik, P.; Wojcik, M.; Klamkowski, K. Response of apple trees to boron fertilization under conditions of low soil boron availability. Sci. Hortic. 2008, 116, 58–64. [Google Scholar] [CrossRef]
- Gotoh, E.; Suetsugu, N.; Higa, T.; Matsushita, T.; Tsukaya, H.; Wada, M. Palisade cell shape affects the light-induced chloroplast movements and leaf photosynthesis. Sci. Rep. 2018, 8, 1472. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M. Interaction of soil boron application with leaf B concentration, root length density, and canopy size of citrus affected by Huanglongbing. J. Plant Nutr. 2020, 43, 186–193. [Google Scholar] [CrossRef]
- Wang, N.; Yang, C.; Pan, Z.; Liu, Y.; Peng, S. Boron deficiency in woody plants: Various responses and tolerance mechanisms. Front. Plant Sci. 2015, 6, 916. [Google Scholar] [CrossRef]
- Koshiba, T.; Kobayashi, M.; Matoh, T. Boron deficiency: How does the defect in cell wall damage the cells? Plant Signal. Behav. 2009, 4, 557–558. [Google Scholar] [CrossRef] [PubMed]
- Bonomelli, C.; Mogollón, R.; de Freitas, S.T.; Zoffoli, J.P.; Contreras, C. Nutritional relationships in bitter pit-affected fruit and the feasibility of vis-nIR Models to Determine Calcium Concentration in “Fuji” Apples. Agronomy 2020, 10, 1476. [Google Scholar] [CrossRef]
- Casanoves, F.; Pla, L.; Di Rienzo, J.A.; Díaz, S. FDiversity: A software package for the integrated analysis of functional diversity. Methods Ecol. Evol. 2011, 2, 233–237. [Google Scholar] [CrossRef]

| Plant Tissues | Treatments (Boron in Soil) | p Value | ||
|---|---|---|---|---|
| Deficient | Adequate | Toxic | ||
| Leaves (n°) | 132.0 a 1 | 153.0 a | 128.0 a | 0.960 |
| Buds (n°) | 72.0 a | 74.0 a | 54.0 a | 0.346 |
| Spurs (n°) | 4.0 c | 6.0 b | 10.0 a | 0.040 |
| Stems (n°) | 6.0 a | 7.0 a | 2.0 a | 0.134 |
| Internode length (cm) | 4.03 a | 3.77 b | 3.27 c | 0.050 |
| Trunk length (cm) | 153.5 a | 150.5 a | 168.5 a | 0.282 |
| Fresh weight of (g) | ||||
| Leaves | 142.9 a | 155.3 a | 145.5 a | 0.962 |
| Buds | 3.15 a | 2.07 a | 1.27 a | 0.320 |
| Stems | 4.1 a | 4.1 a | 2.10 b | 0.050 |
| Trunk | 206.0 a | 202.5 a | 201.0 a | 0.990 |
| White roots | 50.5 a | 33.0 b | 22.0 c | 0.035 |
| Brown roots | 94.5 a | 94.0 a | 93.5 a | 0.868 |
| Dry Weight of Plant Tissues (%) | Treatments (Boron in Soil) | p Value | ||
|---|---|---|---|---|
| Deficient | Adequate | Toxic | ||
| Leaves | 12.97 a 1 | 14.96 a | 14.33 a | 0.960 |
| Buds | 0.75 a | 0.52 a | 0.34 a | 0.346 |
| Stems | 1.00 a | 1.06 a | 0.61 b | 0.048 |
| Trunk | 50.06 a | 51.10 a | 53.15 a | 0.134 |
| White roots | 12.20 a | 8.41 b | 7.16 c | 0.050 |
| Brown roots | 23.02 a | 23.95 a | 24.41 a | 0.282 |
| Physiological Parameters (%) | Treatments (Boron in Soil) | p Value | ||
|---|---|---|---|---|
| Deficient | Adequate | Toxic | ||
| Net Photosynthesis (Pn) umol CO2 m−2 s−1 | 7.98 c 1 | 14.85 a | 10.34 b | <0.001 |
| Stomatal conductance (Gs) mmol m−2 s−1 | 94.60 b | 146.20 a | 137.85 a | <0.001 |
| Transpiration rate (E) mmol m−2 s−1 | 2.38 b | 3.23 a | 2.66 b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arredondo, G.; Bonomelli, C. Effect of Three Boron Concentrations in Soil on Growth and Physiology in Sweet Cherry Trees. Plants 2023, 12, 1240. https://doi.org/10.3390/plants12061240
Arredondo G, Bonomelli C. Effect of Three Boron Concentrations in Soil on Growth and Physiology in Sweet Cherry Trees. Plants. 2023; 12(6):1240. https://doi.org/10.3390/plants12061240
Chicago/Turabian StyleArredondo, Gerardo, and Claudia Bonomelli. 2023. "Effect of Three Boron Concentrations in Soil on Growth and Physiology in Sweet Cherry Trees" Plants 12, no. 6: 1240. https://doi.org/10.3390/plants12061240
APA StyleArredondo, G., & Bonomelli, C. (2023). Effect of Three Boron Concentrations in Soil on Growth and Physiology in Sweet Cherry Trees. Plants, 12(6), 1240. https://doi.org/10.3390/plants12061240






