Boosting Immunity and Management against Wheat Fusarium Diseases by a Sustainable, Circular Nanostructured Delivery Platform
Abstract
1. Introduction
2. Results
2.1. CNC Synthesis, HAS Extraction, and Characterization from Cadenza SBEIIa Wheat Bran
2.2. Synthesis and Characterization of the Nanostructured Particle Formulation (NPF)
2.3. The NPF Displayed Several In Vitro Antifungal Modes of Action
2.4. The NPF Displayed Biocompatibility on Bread Wheat Plants
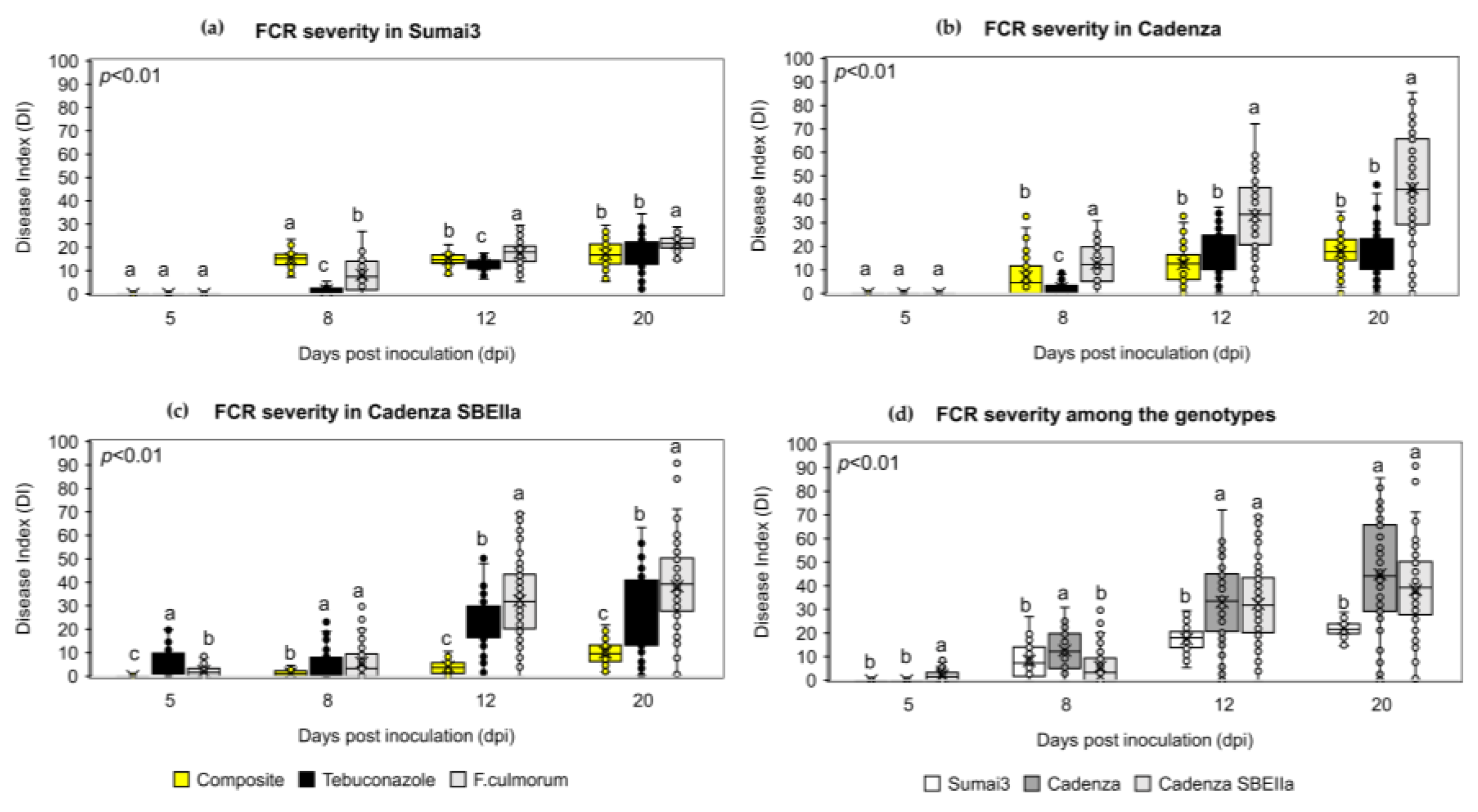
2.5. The NPF Reduced FHB and FCR Symptoms Progression
2.6. The NPF Acts as an Elicitor-like Molecule by Greatly Boosting Innate Immunity in Cadenza SBEIIa
2.7. The NPF Increased Grain Yield in the Two Cadenza Genotypes while Decreasing the Fusarium graminearum Biomass Spread into the Spikes
2.8. The Interaction among Data Revealed That the Preventive Application of the NPF Contributed to Control In Vivo FHB and FCR
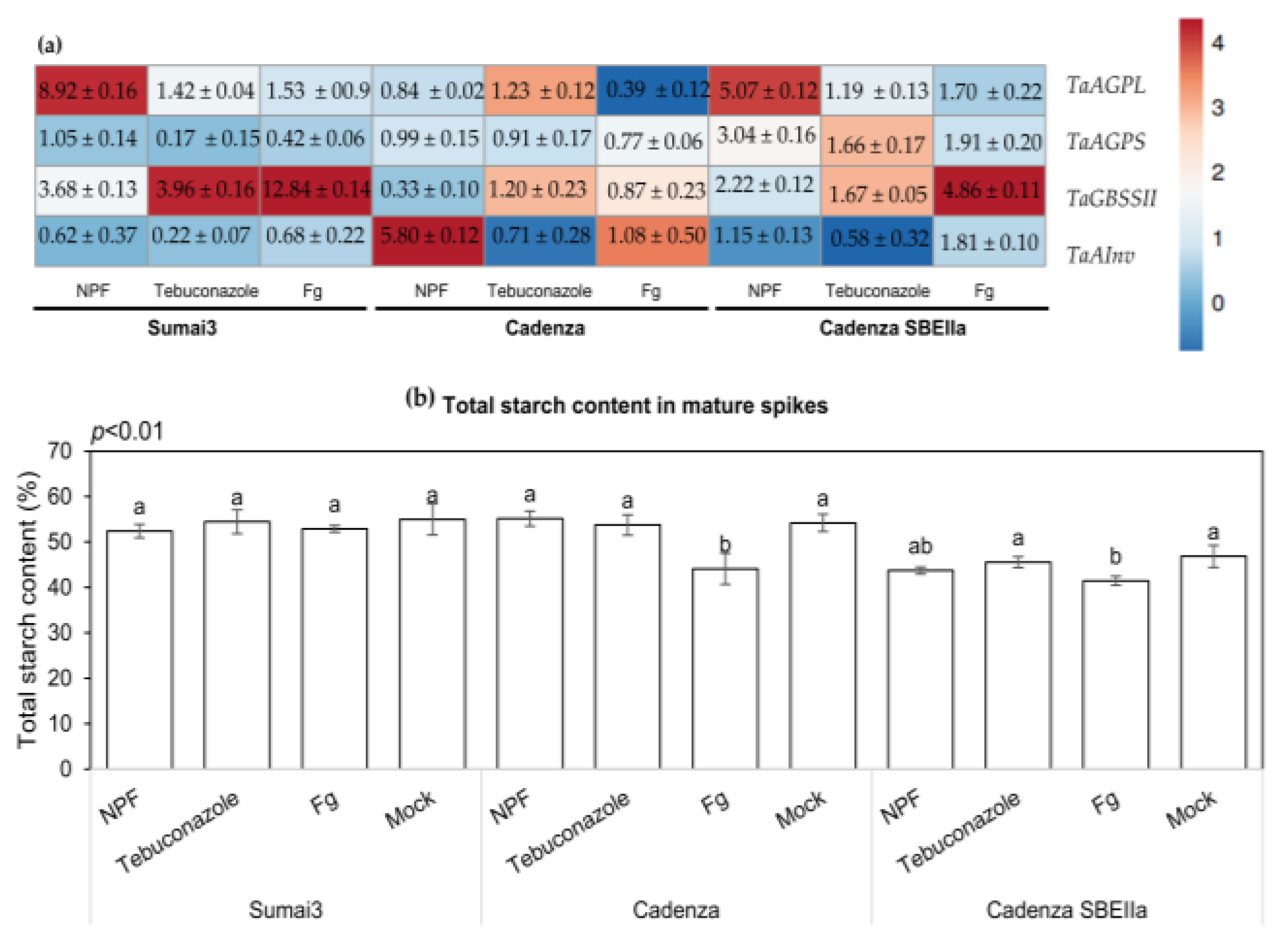
2.9. The NPF Application Affects the Expression Levels of Starch Biosynthetic Genes and Total Starch Content in Grains
3. Discussion
4. Materials and Methods
4.1. Fungal, Chemical, and Plant Materials
4.2. Cellulose Extraction and Synthesis of CNC from Bread Wheat Bran
4.3. CNC Characterization
4.4. Synthesis of Aminated CNC (dCNC-NH2)
4.5. Synthesis and Characterization of the NPF
4.6. In Vitro Antifungal Assays
4.6.1. The 96-Microtiter-Plates Assay
4.6.2. Incorporated Medium Assay
4.6.3. Cellular Aggregation Assay
4.6.4. Cellular Adhesion Assay
4.7. In Vivo Biocompatibility and Antifungal Assays
4.7.1. In Vivo Biocompatibility
4.7.2. In Vivo Antifungal Assay
4.8. Evaluation of Resistance Induction by Real-Time qPCR
4.9. Quantification of Fungal Biomass and Impact on Yield
4.10. Quantification of Total Starch from Mature Treated and Infected Kernels
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Hu, C.; Chen, P.; Zhou, X.; Li, Y.; Ma, K.; Li, S.; Liu, H.; Li, L. Arms race between the host and pathogen associated with Fusarium head blight of wheat. Cells 2022, 11, 2275. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Powell, J.; Gao, S.; Percy, C.; Kelly, A.; Macdonald, B.; Zhou, M.; Davies, P.; Liu, C. Investigation of two QTL conferring seedling resistance to Fusarium crown rot in barley on reducing grain yield loss under field environments. Agronomy 2022, 12, 1282. [Google Scholar] [CrossRef]
- Schöneberg, T.; Kibler, K.; Wettstein, F.E.; Bucheli, T.D.; Forrer, H.R.; Musa, T.; Mascher, F.; Bertossa, M.; Keller, B.; Vogelgsang, S. Influence of temperature, humidity duration and growth stage on the infection and mycotoxin production by Fusarium langsethiae and Fusarium poae in oats. Plant Pathol. 2019, 68, 173–184. [Google Scholar] [CrossRef]
- Bolanos-Carriel, C.; Wegulo, S.N.; Baenziger, P.S.; Funnell-Harris, D.; Hallen-Adams, H.E.; Eskridge, K.M. Effects of fungicide chemical class, fungicide application timing, and environment on Fusarium head blight in winter wheat. Eur. J. Plant Pathol. 2020, 158, 667–679. [Google Scholar] [CrossRef]
- Spolti, P.; Pathology, P.; Biology, P.; Ny, I. Triazole sensitivity in a contemporary population of Fusarium graminearum from New York wheat and competitiveness of a tebuconazole-resistant isolate. Plant Dis. 2014, 98, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, M.G. Characterization of Fusarium graminearum isolates resistant to both carbendazim and a new fungicide JS399-19. Phytopathology 2009, 99, 441–446. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Baudy, P.; Schulz, R.; Bundschuh, M. Effects of current-use fungicides and their mixtures on the feeding and survival of the key shredder Gammarus fossarum. Aquat. Toxicol. 2014, 150, 133–143. [Google Scholar] [CrossRef]
- de Souza, R.M.; Seibert, D.; Quesada, H.B.; de Jesus Bassetti, F.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- An, C.; Sun, C.; Li, N.; Huang, B.; Jiang, J.; Shen, Y.; Wang, C.; Zhao, X.; Cui, B.; Wang, C.; et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: Strategies towards sustainable agriculture. J. Nanobiotechnol. 2022, 20, 11. [Google Scholar] [CrossRef]
- Chaka, K.T. Extraction of cellulose nanocrystals from agricultural by-products: A review. Green Chem. Lett. Rev. 2022, 15, 582–597. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Starch-based nanocarriers as cutting-edge natural cargos for nutraceutical delivery. Trends Food Sci. Technol. 2019, 88, 397–415. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, G.; Gu, Z. Recent advances of starch-based excipients used in extended-release tablets: A review. Drug Deliv. 2016, 23, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Botticella, E.; Sestili, F.; Sparla, F.; Moscatello, S.; Marri, L.; Cuesta-Seijo, J.A.; Falini, G.; Battistelli, A.; Trost, P.; Lafiandra, D. Combining mutations at genes encoding key enzymes involved in starch synthesis affects the amylose content, carbohydrate allocation and hardness in the wheat grain. Plant Biotechnol. J. 2018, 16, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Sharif, R.; Mujtaba, M.; Rahman, M.U.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The multifunctional role of chitosan in horticultural crops; a review. Molecules 2018, 23, 872. [Google Scholar] [CrossRef]
- Farag, M.A.; Al-Mahdy, D.A.; Salaheldine, R.; Fahmy, S.; Yassin, A.; Porzel, A.; Brandt, W. Structure-activity relationships of antimicrobial gallic acid derivatives from pomegranate and acacia fruit extracts against potato bacterial wilt pathogen. Chem. Biodivers. 2015, 12, 955–962. [Google Scholar] [CrossRef]
- Schiavi, D.; Balbi, R.; Giovagnoli, S.; Camaioni, E.; Botticella, E.; Sestili, F.; Balestra, G.M. A green nanostructured pesticide to control tomato bacterial speck disease. Nanomaterials 2021, 11, 1852. [Google Scholar] [CrossRef]
- Francesconi, S.; Steiner, B.; Buerstmayr, H.; Lemmens, M.; Sulyok, M.; Balestra, G.M. Chitosan hydrochloride decreases Fusarium graminearum growth and virulence and boosts growth, development and systemic acquired resistance in two durum wheat genotypes. Molecules 2020, 25, 4752. [Google Scholar] [CrossRef]
- Francesconi, S.; Harfouche, A.; Maesano, M.; Balestra, G.M. UAV-based thermal, RGB imaging and gene expression analysis allowed detection of Fusarium head blight and gave new insights into the physiological responses to the disease in durum wheat. Front. Plant Sci. 2021, 12, 628575. [Google Scholar] [CrossRef]
- Francesconi, S.; Balestra, G.M. The modulation of stomatal conductance and photosynthetic parameters is involved in Fusarium head blight resistance in wheat. PLoS ONE 2020, 15, e0235482. [Google Scholar] [CrossRef] [PubMed]
- Francesconi, S.; Mazzaglia, A.; Balestra, G.M. Different inoculation methods affect components of Fusarium head blight resistance in wheat. Phytopathol. Mediterr. 2019, 58, 679–691. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Dufresne, A.; Elkasabgy, N.A. Nanocellulose: From an agricultural waste to a valuable pharmaceutical ingredient. Int. J. Biol. Macromol. 2020, 163, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Rescignano, N.; Botticella, E.; La Fiandra, D.; Renzi, M.; Mazzaglia, A.; Torre, L.; Kenny, J.M.; Balestra, G.M. Effect of poly(DL-lactide-co-glycolide) nanoparticles or cellulose nanocrystals-based formulations on Pseudomonas syringae pv tomato (Pst) and tomato plant development. J. Plant Dis. Prot. 2016, 123, 301–310. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Giovanale, G.; Mazzaglia, A.; Torre, L.; Balestra, G.M. Cellulose nanocrystals from Actinidia deliciosa pruning residues combined with carvacrol in PVA_CH films with antioxidant/antimicrobial properties for packaging applications. Int. J. Biol. Macromol. 2017, 104, 43–55. [Google Scholar] [CrossRef]
- Schiavi, D.; Francesconi, S.; Taddei, A.R.; Fortunati, E.; Balestra, G.M. Exploring cellulose nanocrystals obtained from olive tree wastes as sustainable crop protection tool against bacterial diseases. Sci. Rep. 2022, 12, 6149. [Google Scholar] [CrossRef]
- Sun, C.; Li, X.; Huang, B.; Li, N.; Wang, A.; An, C.; Jiang, J.; Shen, Y.; Wang, C.; Zhan, S.; et al. Construction and characterization of ethyl cellulose-based nano-delivery system for phenamacril. Int. J. Biol. Macromol. 2022, 221, 1251–1258. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Soppimath, K.S.; Aminabhavi, T.M.; Dave, A.M.; Mehta, M.H. Urea-formaldehyde crosslinked starch and guar gum matrices for encapsulation of natural liquid pesticide [Azadirachta Indica a. Juss. (neem) seed oil]: Swelling and release kinetics. J. Appl. Polym. Sci. 1999, 73, 2437–2446. [Google Scholar] [CrossRef]
- Kheiri, A.; Moosawi Jorf, S.A.; Mallihipour, A.; Saremi, H.; Nikkhah, M. Application of chitosan and chitosan nanoparticles for the control of Fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int. J. Biol. Macromol. 2016, 93, 1261–1272. [Google Scholar] [CrossRef]
- Kheiri, A.; Moosawi Jorf, S.A.; Malihipour, A.; Saremi, H.; Nikkhah, M. Synthesis and characterization of chitosan nanoparticles and their effect on Fusarium head blight and oxidative activity in wheat. Int. J. Biol. Macromol. 2017, 102, 526–538. [Google Scholar] [CrossRef]
- Gunupuru, L.R.; Patel, J.S.; Sumarah, M.W.; Renaud, J.B.; Mantin, E.G.; Prithiviraj, B. A plant biostimulant made from the marine brown algae Ascophyllum nodosum and chitosan reduce Fusarium head blight and mycotoxin contamination in wheat. PLoS ONE 2019, 14, e0220562. [Google Scholar] [CrossRef] [PubMed]
- Zachetti, V.G.L.; Cendoya, E.; Nichea, M.J.; Chulze, S.N.; Ramirez, M.L. Preliminary study on the use of chitosan as an eco-friendly alternative to control Fusarium growth and mycotoxin production on maize and wheat. Pathogens 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Buzón-Durán, L.; Martín-Gil, J.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Pérez-Lebeña, E.; Martín-Ramos, P. Antifungal activity of chitosan oligomers–amino acid aonjugate complexes against Fusarium culmorum in spelt (Triticum spelta L.). Agronomy 2020, 10, 1427. [Google Scholar] [CrossRef]
- Attjioui, M.; Gillet, D.; El Gueddari, N.E.; Moerschbacher, B.M. Synergistic antimicrobial effect of chitosan polymers and oligomers. Mol. Plant-Microbe Interact. 2021, 34, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Deshaies, M.; Lamari, N.; Ng, C.K.Y.; Ward, P.; Doohan, F.M. The impact of chitosan on the early metabolomic response of wheat to infection by Fusarium graminearum. BMC Plant Biol. 2022, 22, 73. [Google Scholar] [CrossRef]
- Hadjout, S.; Zouidi, M.; Merdas, S. Comparative evaluation of free and bound phenolic acid contents in early grains of durum wheat line for its resistance to Fusarium head blight with some other sensitive varieties in Algeria. J. Plant Prot. Res. 2022, 62, 287–294. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef]
- Boutigny, A.L.; Atanasova-Pénichon, V.; Benet, M.; Barreau, C.; Richard-Forget, F. Natural phenolic acids from wheat bran inhibit Fusarium culmorum trichothecene biosynthesis in vitro by repressing Tri gene expression. Eur. J. Plant Pathol. 2010, 127, 275–286. [Google Scholar] [CrossRef]
- Pagnussatt, F.A.; Del Ponte, E.M.; Garda-Buffon, J.; Badiale-Furlong, E. Inhibition of Fusarium graminearum growth and mycotoxin production by phenolic extract from Spirulina sp. Pestic. Biochem. Physiol. 2014, 108, 21–26. [Google Scholar] [CrossRef]
- Forrer, H.R.; Musa, T.; Schwab, F.; Jenny, E.; Bucheli, T.D.; Wettstein, F.E.; Vogelgsang, S. Fusarium head blight control and prevention of mycotoxin contamination in wheat with botanicals and tannic acid. Toxins 2014, 6, 830–849. [Google Scholar] [CrossRef]
- Matei, P.M.; Iacomi, B.M.; Martín-Gil, J.; Pérez-Lebena, E.; Ramos-Sánchez, M.C.; Barrio-Arredondo, M.T.; Martín-Ramos, P. In vitro antifungal activity of composites of AgNPs and polyphenol inclusion compounds against Fusarium culmorum in different dispersion media. Agronomy 2018, 8, 239. [Google Scholar] [CrossRef]
- Drakopoulos, D.; Luz, C.; Torrijos, R.; Meca, G.; Weber, P.; Bänziger, I.; Voegele, R.T.; Six, J.; Vogelgsang, S. Use of botanicals to suppress different stages of the life cycle of Fusarium graminearum. Phytopathology 2019, 109, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Lipke, P.N. What we do not know about fungal cell adhesion molecules. J. Fungi 2018, 4, 59. [Google Scholar] [CrossRef]
- Epstein, L.; Nicholson, R. Adhesion and adhesives of fungi and oomycetes. In Biological Adhesives, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–378. ISBN 9783319460826. [Google Scholar]
- Sun, X.; Lu, Q.; Boluk, Y.; Liu, Y. The Impact of Cellulose Nanocrystals on the Aggregation and Initial Adhesion of Pseudomonas Fluorescens Bacteria. Soft Matter 2014, 10, 8923–8931. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shao, Y.; Boluk, Y.; Liu, Y. The impact of cellulose nanocrystals on the aggregation and initial adhesion to a solid surface of Escherichia coli K12: Role of solution chemistry. Colloids Surf. B Biointerfaces 2015, 136, 570–576. [Google Scholar] [CrossRef]
- D’Orazio, G.; Munizza, L.; Zampolli, J.; Forcella, M.; Zoia, L.; Fusi, P.; Di Gennaro, P.; La Ferla, B. Cellulose nanocrystals are effective in inhibiting host cell bacterial adhesion. J. Mater. Chem. B 2017, 5, 7018–7020. [Google Scholar] [CrossRef] [PubMed]
- Boubakeur, B.; Drabo, M.S.; Khadem, H.; Segda, R.; Shah, M.A.; Savadogo, A. Antimicrobial, antibiofilm, and probiofilm effects of gallic acid on exopolysaccharide-dependent and -independent biofilm of model strains Streptococcus thermophilus Cnrz 447 and Staphylococcus aureus Atcc 43300. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e1781. [Google Scholar] [CrossRef]
- Lee, S.A.; Wallis, C.M.; Rogers, E.E.; Burbank, L.P. Grapevine phenolic compounds influence cell surface adhesion of Xylella fastidiosa and bind to lipopolysaccharide. PLoS ONE 2020, 15, e0240101. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Zainol Hilmi, N.H.; Daim, L.D.J. Phytotoxicity of chitosan-based agronanofungicides in the vegetative growth of oil palm seedling. PLoS ONE 2020, 15, e0231315. [Google Scholar] [CrossRef]
- Hegab, M.M.; Erdei, L.; AbdElgawad, H. Isolation and phytotoxicity of an active fraction and its pure compound (gallic acid) from sun spurge (Euphorbia helioscopia L.) against harmful weeds. Acta Biol. Szeged. 2016, 60, 17–25. [Google Scholar]
- Bravetti, M.M.D.M.; Carpinella, M.C.; Palacios, S.M. Phytotoxicity of Cortaderia speciosa extract, active principles, degradation in soil and effectiveness in field tests. Chemoecology 2020, 30, 15–24. [Google Scholar] [CrossRef]
- Kang, Z.; Buchenauer, H. Ultrastructural and immunocytochemical investigation of pathogen development and host responses in resistant and susceptible wheat spikes infected by Fusarium culmorum. Physiol. Mol. Plant Pathol. 2000, 57, 255–268. [Google Scholar] [CrossRef]
- Kheiri, A.; Moosawi Jorf, S.A.; Malihipour, A. Infection process and wheat response to Fusarium head blight caused by Fusarium graminearum. Eur. J. Plant Pathol. 2019, 153, 489–502. [Google Scholar] [CrossRef]
- Zwart, R.S.; Muylle, H.; Van Bockstaele, E.; Roldán-Ruiz, I. Evaluation of genetic diversity of Fusarium head blight resistance in European winter wheat. Theor. Appl. Genet. 2008, 117, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Funnell-Harris, D.L.; Graybosch, R.A.; O’Neill, P.M.; Duray, Z.T.; Wegulo, S.N. Amylose-free (“waxy”) wheat colonization by Fusarium spp. and response to Fusarium head blight. Plant Dis. 2019, 103, 972–983. [Google Scholar] [CrossRef]
- Liatukas, Ž.; Ruzgas, V.; Gorash, A.; Cecevičienė, J.; Armonienė, R.; Statkevičiūtė, G.; Jaškūnė, K.; Brazauskas, G. Development of the new waxy winter wheat cultivars Eldija and Sarta. Czech J. Genet. Plant Breed. 2021, 57, 149–157. [Google Scholar] [CrossRef]
- Kazan, K.; Gardiner, D.M. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: Recent progress and future prospects. Mol. Plant Pathol. 2018, 19, 1547–1562. [Google Scholar] [CrossRef]
- Su, J.; Zhao, J.; Zhao, S.; Li, M.; Pang, S.; Kang, Z.; Zhen, W.; Chen, S.; Chen, F.; Wang, X. Genetics of resistance to common root rot (spot blotch), Fusarium crown rot, and sharp eyespot in wheat. Front. Genet. 2021, 12, 699342. [Google Scholar] [CrossRef]
- Han, Q.M.; Kang, Z.S.; Buchenauer, H.; Huang, L.L.; Zhao, J. Cytological and immunocytochemical studies on the effects of the fungicide tebuconazole on the interaction of wheat with stripe rust. J. Plant Pathol. 2006, 88, 263–271. [Google Scholar]
- Chetouhi, C.; Bonhomme, L.; Lecomte, P.; Cambon, F.; Merlino, M.; Biron, D.G.; Langin, T. A proteomics survey on wheat susceptibility to Fusarium head blight during grain development. Eur. J. Plant Pathol. 2015, 141, 407–418. [Google Scholar] [CrossRef]
- Chetouhi, C.; Bonhomme, L.; Lasserre-Zuber, P.; Cambon, F.; Pelletier, S.; Renou, J.P.; Langin, T. Transcriptome dynamics of a susceptible wheat upon Fusarium head blight reveals that molecular responses to Fusarium graminearum infection fit over the grain development processes. Funct. Integr. Genom. 2016, 16, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Perlikowski, D.; Wisniewska, H.; Góral, T.; Kwiatek, M.; Majka, M.; Kosmala, A. Identification of kernel proteins associated with the resistance to Fusarium head blight in winter wheat (Triticum aestivum L). PLoS ONE 2014, 9, e110822. [Google Scholar] [CrossRef]
- Kumar, J.; Rai, K.M.; Pirseyedi, S.; Elias, E.M.; Xu, S.; Dill-Macky, R.; Kianian, S.F. Epigenetic regulation of gene expression improves Fusarium head blight resistance in durum wheat. Sci. Rep. 2020, 10, 17610. [Google Scholar] [CrossRef]
- Fortunati, E.; Benincasa, P.; Balestra, G.M.; Luzi, F.; Mazzaglia, A.; Del Buono, D.; Puglia, D.; Torre, L. Revalorization of barley straw and husk as precursors for cellulose nanocrystals extraction and their effect on PVA CH nanocomposites. Ind. Crop. Prod. 2016, 92, 201–217. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Wang, X.; Li, M.; Lei, H.; Xu, H. Cellulose nanocrystals prepared from wheat bran: Characterization and cytotoxicity assessment. Int. J. Biol. Macromol. 2019, 140, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Yazdanbakhsh, M.F.; Rashidi, A. The effect of ultrasonic waves on alpha-cellulose extraction from wheat bran to prepare alpha-cellulose nanofibers. J. Text. Inst. 2020, 111, 1518–1529. [Google Scholar] [CrossRef]
- Garvey, C.J.; Parker, I.H.; Simon, G.P. On the interpretation of X-ray diffraction powder patterns in terms of the nanostructure of cellulose I fibres. Macromol. Chem. Phys. 2005, 206, 1568–1575. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal structure and hydrogen bonding system in cellulose Ib from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef]
- Langan, P.; Nishiyama, Y.; Chanzy, H. X-ray structure of mercerized cellulose II at 1 Å resolution. Biomacromolecules 2001, 2, 410–416. [Google Scholar] [CrossRef]
- Dong, S.; Cho, H.J.; Lee, Y.W.; Roman, M. Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting. Biomacromolecules 2014, 15, 1560–1567. [Google Scholar] [CrossRef]
- Wang, G.; Song, H.; Li, R.; Li, Z.; Chen, J. Olefin oligomerization via new and efficient Brönsted acidic ionic liquid catalyst systems. Chin. J. Catal. 2018, 39, 1110–1120. [Google Scholar] [CrossRef]
- Mojumdar, A.; Upadhyay, A.K.; Raina, V.; Ray, L. A simple and rapid colorimetric method for the estimation of chitosan produced by microbial degradation of chitin waste. J. Microbiol. Methods 2019, 158, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Daniels, S.; Mott, E.; Hammond-Kosack, K. Arabidopsis is susceptible to the cereal ear blight fungal pathogens Fusarium graminearum and Fusarium culmorum. Plant J. 2002, 32, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Yang, Y.R.; Shen, X.C.; Zhang, Z.L.; Shen, P.; Xie, Z.X. Role of DNA in bacterial aggregation. Curr. Microbiol. 2008, 57, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Noronha, V.T.; Camargos, C.H.M.; Jackson, J.C.; Souza Filho, A.G.; Paula, A.J.; Rezende, C.A.; Faria, A.F. Physical membrane-stress-mediated antimicrobial properties of cellulose nanocrystals. ACS Sustain. Chem. Eng. 2021, 9, 3203–3212. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Tenea, G.N.; Peres Bota, A.; Cordeiro Raposo, F.; Maquet, A. Reference genes for gene expression studies in wheat flag leaves grown under different farming conditions. BMC Res. Notes 2011, 4, 373. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Horevaj, P.; Milus, E.A.; Bluhm, B.H. A real-time qPCR assay to quantify Fusarium graminearum biomass in wheat kernels. J. Appl. Microbiol. 2011, 111, 396–406. [Google Scholar] [CrossRef]
- Beccari, G.; Covarelli, L.; Nicholson, P. Infection processes and soft wheat response to root rot and crown rot caused by Fusarium culmorum. Plant Pathol. 2011, 60, 671–684. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, 566–570. [Google Scholar] [CrossRef]
- Kovács, V.; Gondor, O.K.; Szalai, G.; Darkó, É.; Majláth, I.; Janda, T.; Pál, M. Synthesis and role of salicylic acid in wheat varieties with different levels of cadmium tolerance. J. Hazard. Mater. 2014, 280, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Bolton, M.D.; Kolmer, J.A.; Xu, W.W.; Garvin, D.F. Lr34-mediated leaf rust resistance in wheat: Transcript profiling reveals a high energetic demand supported by transient recruitment of multiple metabolic pathways. Mol. Plant-Microbe Interact. 2008, 21, 1515–1527. [Google Scholar] [CrossRef]
- Lu, Z.X.; Gaudet, D.; Puchalski, B.; Despins, T.; Frick, M.; Laroche, A. Inducers of resistance reduce common bunt infection in wheat seedlings while differentially regulating defence-gene expression. Physiol. Mol. Plant Pathol. 2006, 67, 138–148. [Google Scholar] [CrossRef]
- Desmond, O.J.; Manners, J.M.; Stephens, A.E.; Maclean, D.J.; Schenk, P.M.; Gardiner, D.M.; Munn, A.L.; Kazan, K. The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol. Plant Pathol. 2008, 9, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Desmond, O.J.; Edgar, C.I.; Manners, J.M.; Maclean, D.J.; Schenk, P.M.; Kazan, K. Methyl jasmonate induced gene expression in wheat delays symptom development by the crown rot pathogen Fusarium pseudograminearum. Physiol. Mol. Plant Pathol. 2006, 67, 171–179. [Google Scholar] [CrossRef]
- Gaudet, D.A.; Wang, Y.; Frick, M.; Puchalski, B.; Penniket, C.; Ouellet, T.; Robert, L.; Singh, J.; Laroche, A. Low temperature induced defence gene expression in winter wheat in relation to resistance to snow moulds and other wheat diseases. Plant Sci. 2011, 180, 99–110. [Google Scholar] [CrossRef]
- Li, N.; Yin, N.; Niu, Z.; Hui, W.; Song, J.; Huang, C.; Wang, H.; Kong, L.; Feng, D. Isolation and characterization of three TaYUC10 genes from wheat. Gene 2014, 546, 187–194. [Google Scholar] [CrossRef]
- Xiang, Y.; Song, M.; Wei, Z.; Tong, J.; Zhang, L.; Xiao, L.; Ma, Z.; Wang, Y. A jacalin-related lectin-like gene in wheat is a component of the plant defence system. J. Exp. Bot. 2011, 62, 5471–5483. [Google Scholar] [CrossRef]
- Yang, F.; Dong, F.S.; Hu, F.H.; Liu, Y.W.; Chai, J.F.; Zhao, H.; Lv, M.Y.; Zhou, S. Genome-wide identification and expression analysis of the calmodulin-binding transcription activator (CAMTA) gene family in wheat (Triticum aestivum L.). BMC Genet. 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Usadel, B.; Kostadinova, S.; Morcuende, R. Quantitative RT-PCR platform to measure transcriptlLevels of C and N metabolism-related genes in durum wheat: Transcript profiles in elevated [CO2] and high temperature at different levels of N supply. Plant Cell Physiol. 2015, 56, 1556–1573. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Qi, J.; Shi, P.; Yin, Y. Starch accumulation, activities of key enzyme and gene expression in starch synthesis of wheat endosperm with different starch contents. J. Food Sci. Technol. 2014, 51, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Tundo, S.; Janni, M.; Moscetti, I.; Mandalá, G.; Savatin, D.; Blechl, A.; Favaron, F.; D’Ovidio, R. PvPGIP2 accumulation in specific floral tissues but not in the endosperm limits Fusarium graminearum infection in wheat. Mol. Plant-Microbe Interact. 2016, 29, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Mudge, A.M.; Dill-Macky, R.; Dong, Y.; Gardiner, D.M.; White, R.G.; Manners, J.M. A role for the mycotoxin deoxynivalenol in stem colonisation during crown rot disease of wheat caused by Fusarium graminearum and Fusarium pseudograminearum. Physiol. Mol. Plant Pathol. 2006, 69, 73–85. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francesconi, S.; Ronchetti, R.; Camaioni, E.; Giovagnoli, S.; Sestili, F.; Palombieri, S.; Balestra, G.M. Boosting Immunity and Management against Wheat Fusarium Diseases by a Sustainable, Circular Nanostructured Delivery Platform. Plants 2023, 12, 1223. https://doi.org/10.3390/plants12061223
Francesconi S, Ronchetti R, Camaioni E, Giovagnoli S, Sestili F, Palombieri S, Balestra GM. Boosting Immunity and Management against Wheat Fusarium Diseases by a Sustainable, Circular Nanostructured Delivery Platform. Plants. 2023; 12(6):1223. https://doi.org/10.3390/plants12061223
Chicago/Turabian StyleFrancesconi, Sara, Riccardo Ronchetti, Emidio Camaioni, Stefano Giovagnoli, Francesco Sestili, Samuela Palombieri, and Giorgio Mariano Balestra. 2023. "Boosting Immunity and Management against Wheat Fusarium Diseases by a Sustainable, Circular Nanostructured Delivery Platform" Plants 12, no. 6: 1223. https://doi.org/10.3390/plants12061223
APA StyleFrancesconi, S., Ronchetti, R., Camaioni, E., Giovagnoli, S., Sestili, F., Palombieri, S., & Balestra, G. M. (2023). Boosting Immunity and Management against Wheat Fusarium Diseases by a Sustainable, Circular Nanostructured Delivery Platform. Plants, 12(6), 1223. https://doi.org/10.3390/plants12061223









