Assessing the Benefits and Costs of the Hydrogen Cyanide Antiherbivore Defense in Trifolium repens
Abstract
1. Introduction
2. Results
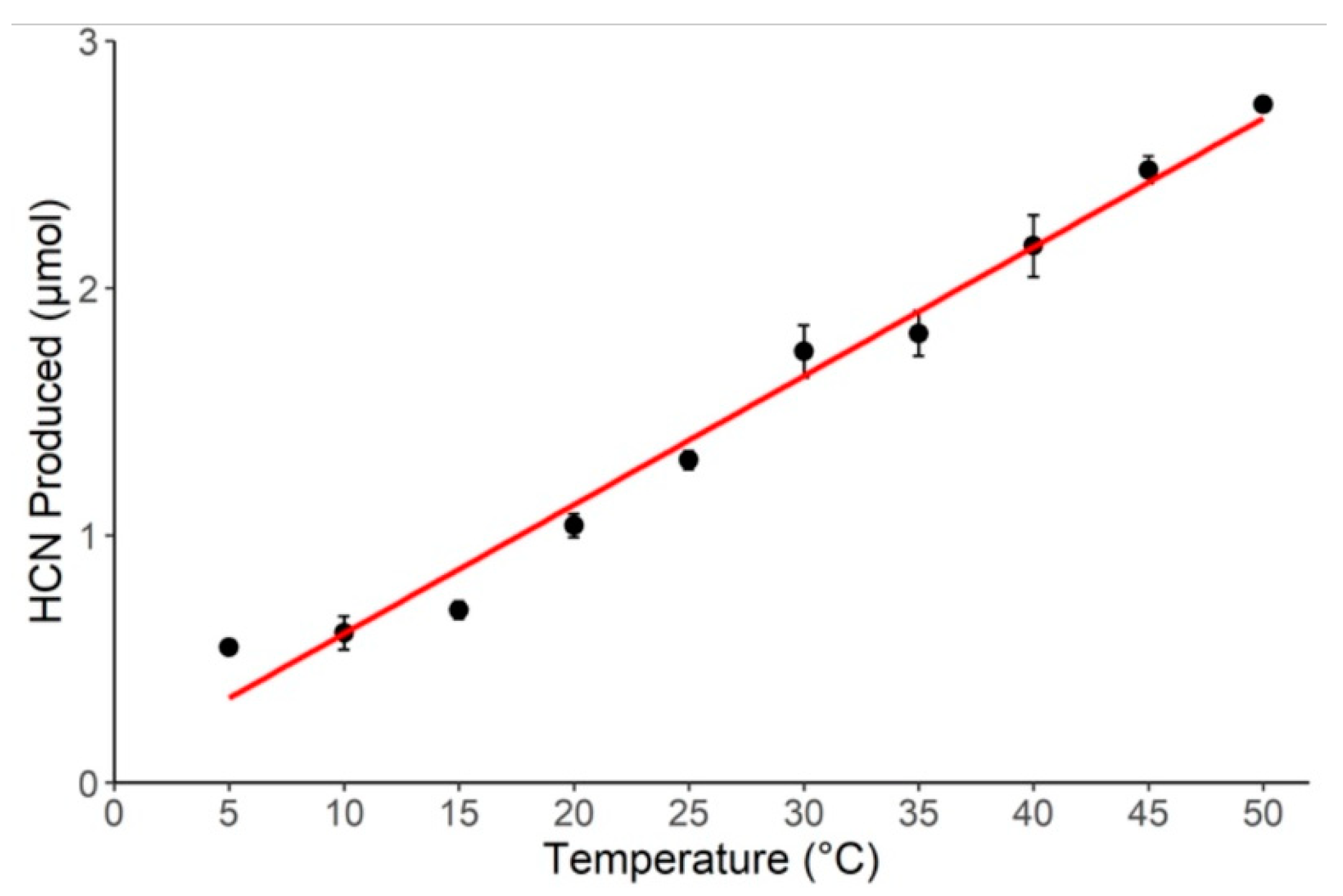
2.1. Effect of Temperature on HCN Production
2.2. Effect of Temperature on Efficacy of HCN Defense against Herbivores
2.2.1. No-Choice Assay
2.2.2. Choice Assay
2.3. Freezing and Autotoxicity
2.3.1. HCN Quantification
2.3.2. Analysis of Photosynthetic Activity Fv/Fm
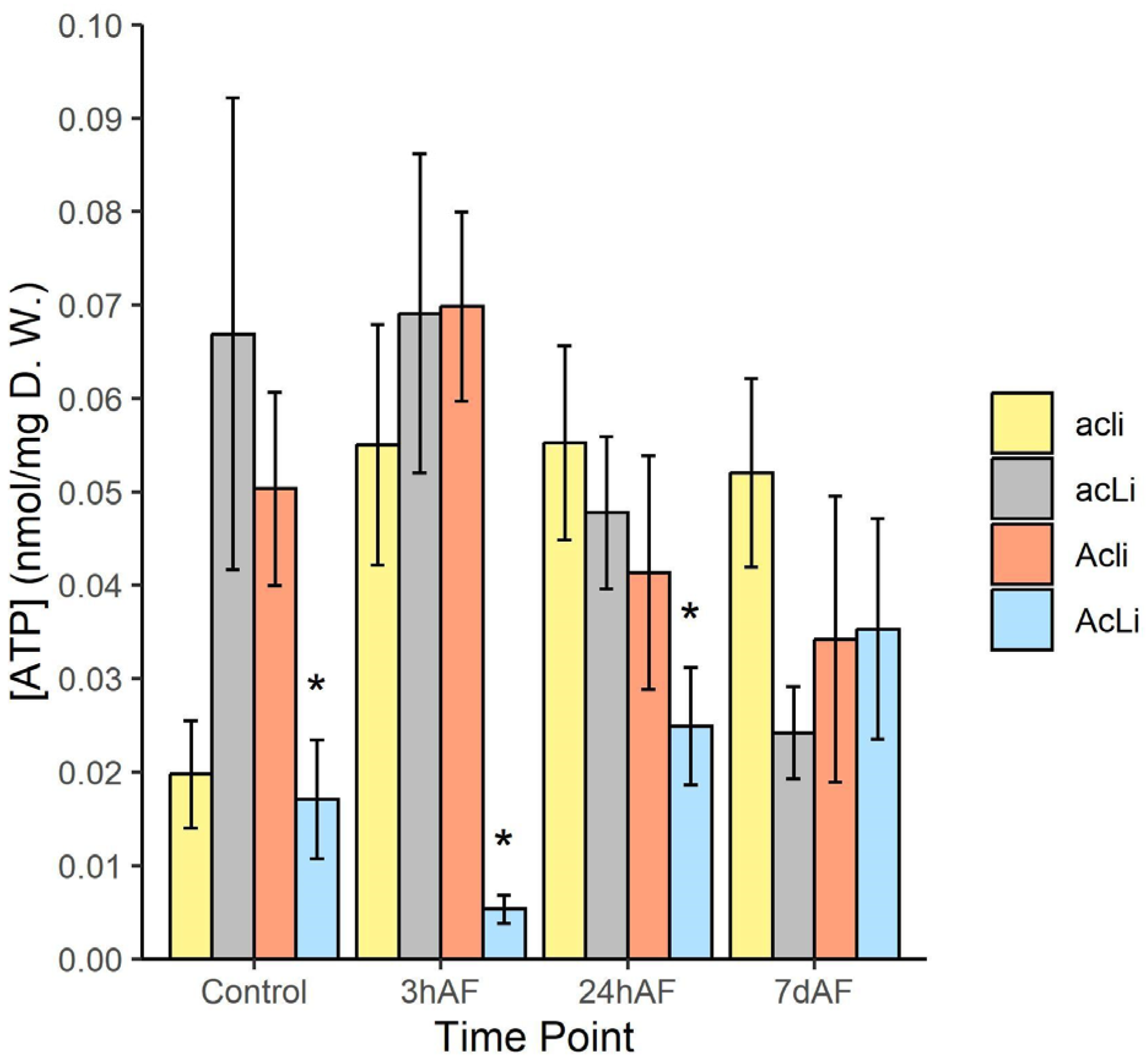
2.3.3. ATP Production
3. Discussion
3.1. Effect of Different Temperatures on HCN Production and Efficacy against Herbivores
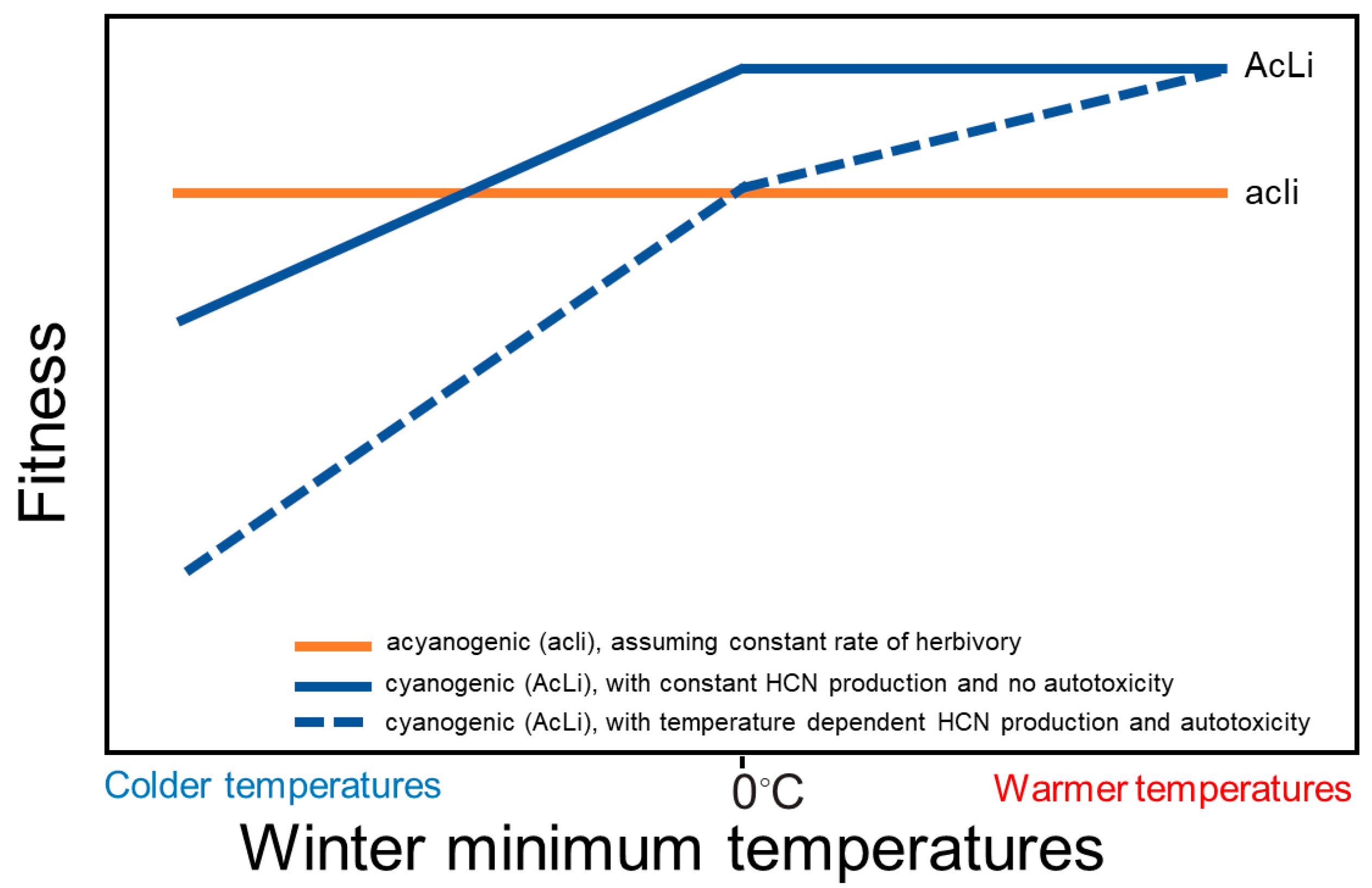
3.2. Freezing and Autotoxicity
4. Conclusions
5. Materials and Methods
5.1. Study System
Plant Materials
5.2. Influence of Temperature on the In-Vitro HCN Production
Colorimetric HCN Quantification
5.3. Effect of Temperature on T. repens Chemical Defense against Herbivores
5.3.1. No-Choice Assay
5.3.2. Choice Assay
5.4. Freezing Experiment
5.4.1. Colorimetric HCN Quantification
5.4.2. Measuring the Maximum Photochemical Yield of Photosystem II (Fv/Fm)
5.4.3. ATP Analysis
Metabolite Extraction
Metabolite Quantification
5.5. Statistical Analysis
5.5.1. Effect of Temperature on the Production of HCN
5.5.2. Effect of Temperature on Efficacy of HCN Defense against Herbivores
5.5.3. Freezing and Autotoxicity
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neilson, E.H.; Goodger, J.Q.D.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Stamp, N. Out of the quagmire of plant defense hypotheses. Q. Rev. Biol. 2003, 78, 23–55. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.G.; Zangerl, A.R.; DeLucia, E.H.; Berenbaum, M.R. The carbon-nutrient balance hypothe-sis: Its rise and fall. Ecol. Lett. 2001, 4, 86–95. [Google Scholar] [CrossRef]
- Sampedro, L.; Moreira, X.; Zas, R. Costs of constitutive and herbivore-induced chemical defences in pine trees emerge only under low nutrient availability. J. Ecol. 2011, 99, 818–827. Available online: https://www.jstor.org/stable/23028867 (accessed on 20 January 2023). [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- McKey, D. Adaptive patterns in alkaloid physiology. Am. Nat. 1974, 108, 305–320. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Rudgers, J.A.; Lau, J.A.; Irwin, R.E. Direct and ecological costs of resistance to herbivory. Trends Ecol. Evol. 2002, 17, 278–285. [Google Scholar] [CrossRef]
- Kakes, P. An analysis of the costs and benefits of the cyanogenic system in Trifolium repens L. Theor. Appl. Genet. 1989, 77, 111–118. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Extrinsic factors influencing production of secondary metabolites in plants. In Insect-Plant Interactions; CRC Press: Boca Raton, FL, USA, 2019; pp. 107–134. [Google Scholar] [CrossRef]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S. Resource availability and plant antiherbivore defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef]
- Fine, P.V.A.; Mesones, I.; Coley, P.D. Herbivores promote habitat specialization by trees in Amazonian forests. Science 2004, 305, 663–665. [Google Scholar] [CrossRef]
- Hahn, P.G.; Agrawal, A.A.; Sussman, K.I.; Maron, J.L. Population variation, environmental gradients, and the evolutionary ecology of plant defense against herbivory. Am. Nat. 2019, 193, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Anstett, D.N.; Nunes, K.A.; Baskett, C.; Kotanen, P.M. Sources of controversy surrounding latitudinal patterns in herbivory and defense. Trends Ecol. Evol. 2016, 31, 789–802. [Google Scholar] [CrossRef]
- Poulton, E.J. Cyanogenesis in Plants. Plant Physiol. 1990, 94, 401–405. [Google Scholar] [CrossRef]
- Hughes, M.A. The cyanogenic polymorphism in Trifolium repens L. (White Clover). Heredity 1991, 66, 105–115. [Google Scholar] [CrossRef]
- Yeats, T.H. Setting and diffusing the cyanide bomb in plant defense. Plant Physiol. 2018, 178, 956–957. [Google Scholar] [CrossRef]
- Daday, H. Gene frequencies in wild populations of Trifolium repens L. IV. Mechanism of natural selection. Heredity 1965, 20, 355–365. [Google Scholar] [CrossRef]
- Hayden, K.J.; Parker, I.M. Plasticity in cyanogenesis of Trifolium repens L.: Inducibility, fitness costs, and variable expression. Evol. Ecol. Res. 2002, 4, 155–168. [Google Scholar]
- Kooyers, N.J.; Hartman Bakken, B.; Ungerer, M.C.; Olsen, K.M. Freeze-induced cyanide toxicity does not maintain the cyanogenesis polymorphism in white clover (Trifolium repens). Am. J. Bot. 2018, 105, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Corkill, L. Cyanogenesis in white clover (Trifolium repens L.). I. Cyanogenesis in single plants. N. Z. J. Sci. Technol. 1940, 22, 65b–67b. [Google Scholar]
- Corkill, L. Cyanogenesis in white clover (Trifolium repens L.). V. The inheritance of cyanogenesis. N. Z. J. Sci. Technol. 1942, 23, 178–193. [Google Scholar]
- Olsen, K.M.; Sutherland, B.L.; Small, L.L. Molecular evolution of the Li/li chemical defence polymorphism in white clover (Trifolium repens L.). Mol. Ecol. 2007, 16, 4180–4193. [Google Scholar] [CrossRef]
- Olsen, K.M.; Hsu, S.-C.; Small, L.L. Evidence on the molecular basis of the Ac/ac adaptive cyanogenesis polymorphism in white clover (Trifolium repens L.). Genetics 2008, 179, 517–526. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Møller, B.L. Cyanogenic glycosides: Synthesis, physiology, and phenotypic plasticity. Annu. Rev. Plant Biol. 2014, 65, 155–185. [Google Scholar] [CrossRef]
- Olsen, K.M.; Small, L.L. Micro- and macroevolutionary adaptation through repeated loss of a complete metabolic pathway. New Phytol. 2018, 219, 757–766. [Google Scholar] [CrossRef]
- Olsen, K.M.; Kooyers, N.J.; Small, L.L. Recurrent gene deletions and the evolution of adaptive cyanogenesis polymorphisms in white clover (Trifolium repens L.). Mol. Ecol. 2013, 22, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, J.S.; Ness, R.W.; Cohan, B.; Fitzpatrick, C.R.; Innes, S.G.; Koch, S.; Miles, L.S.; Munim, S.; Peres-Neto, P.R.; Prashad, C.; et al. Global urban environmental change drives adaptation in white clover. Science 2022, 375, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Daday, H. Gene frequencies in wild populations of Trifolium repens. I. Distribution by latitude. Heredity 1954, 8, 61–78. [Google Scholar] [CrossRef]
- Daday, H. Gene frequencies in wild populations of Trifolium repens. II. Distribution by altitude. Heredity 1954, 8, 377–384. [Google Scholar] [CrossRef]
- Daday, H. Gene frequencies in wild populations of Trifolium repens L. III. World distribution. Heredity 1958, 12, 169–184. [Google Scholar] [CrossRef]
- Dirzo, R.; Harper, J.L. Experimental studies on slug-plant interactions: III. Differences in the acceptability of individual plants of Trifolium repens to slugs and snails. J. Ecol. 1982, 70, 101–117. [Google Scholar] [CrossRef]
- Burgess, R.S.L.; Ennos, R.A. Selective grazing of acyanogenic white clover: Variation in behaviour among populations of the slug Deroceras reticulatum. Oecologia 1987, 73, 432–435. Available online: http://www.jstor.org/stable/4218386 (accessed on 20 January 2023). [CrossRef]
- Kooyers, N.J.; Gage, L.R.; Al-Lozi, A.; Olsen, K.M. Aridity shapes cyanogenesis cline evolution in white clover (Trifolium repens L.). Mol. Ecol. 2014, 23, 1053–1070. [Google Scholar] [CrossRef]
- Kooyers, N.J.; Olsen, K.M. Searching for the bull’s eye: Agents and targets of selection vary among geographically disparate cyanogenesis clines in white clover (Trifolium repens L.). Heredity 2013, 111, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Foulds, W.; Grime, J.P. The response of cyanogenic and acyanogenic phenotypes of Trifolium repens to soil moisture supply. Heredity 1972, 28, 181–187. [Google Scholar] [CrossRef]
- Albano, L.; Johnson, M.T.J. Interactions between environmental factors drive selection on cyanogenesis in Trifolium repens. OIKOS 2023, e09629. [Google Scholar] [CrossRef]
- Innes, G.S.; Santangelo, S.J.; Kooyers, J.N.; Olsen, K.M.; Johnson, M.T.J. Evolution in response to climate in the native and introduced ranges of a globally distributed plant. Evolution 2022, 76, 1495–1511. [Google Scholar] [CrossRef] [PubMed]
- Ganders, F.R. Altitudinal clines for cyanogenesis in introduced populations of white clover near Vancouver, Canada. Heredity 1990, 64, 387–390. [Google Scholar] [CrossRef]
- Olsen, K.M.; Ungerer, M.C. Freezing tolerance and cyanogenesis in white clover (Trifolium repens L. Fabaceae). Int. J. Plant Sci. 2008, 169, 1141–1147. [Google Scholar] [CrossRef]
- Foulds, W.; Young, L. Effect of frosting, moisture stress and potassium cyanide on the metabolism of cyanogenic and acyanogenic phenotypes of Lotus corniculatus L. and Trifolium repens L. Heredity 1977, 38, 19–24. [Google Scholar] [CrossRef]
- Brighton, F.; Horne, M.T. Influence of temperature on cyanogenic polymorphisms. Nature 1977, 265, 437–438. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Lieberei, R.; Ganzhorn, J.U. Plant cyanogenesis of Phaseolus lunatus and its relevance for herbivore–plant interaction: The importance of quantitative data. J. Chem. Ecol. 2005, 31, 1445–1473. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Kautz, S.; Lieberei, S. How generalist and specialist herbivores respond to various cyanogenic plant features. Entomol. Exp. Appl. 2010, 134, 245–259. [Google Scholar] [CrossRef]
- Armstrong, H.E.; Armstrong, E.F.; Horton, E. Herbage studies. II. Variation in Lotus corniculatus and Trifolium repens (cyanophoric plants). Proc. Biol. Sci. 1913, 86, 262–269. [Google Scholar]
- Coop, I. Cyanogenesis in white clover (Trifolium repens L.). III. A study of linamarase, the enzyme which hydrolyses lotaustralin. N. Z. J. Sci. Technol. 1940, 22, 71B–83B. [Google Scholar]
- Collinge, D.B.; Hughes, M.A. Developmental and physiological studies on the cyanogenic glucosides of white clover. J. Exp. Bot. 1982, 33, 154–161. [Google Scholar] [CrossRef]
- Ikya, J.K.-H.; Ariahu, C.C.; Ayatse, J.O. Arrhenius and absolute reaction rate models for thermodynamic characterization of linamarase (B-glucosidase) applying linamarin substrate. Agro. Sci. 2013, 11, 16–20. [Google Scholar] [CrossRef]
- van Ohlen, M.; Herfurth, A.-M.; Kerbstadt, H.; Wittstock, U. Cyanide detoxification in an insect herbivore: Molecular identification of β-cyanoalanine synthases from Pieris rapae. Insect Biochem. Mol. Biol. 2016, 70, 99–110. [Google Scholar] [CrossRef]
- Angseesing, J.P.A.; Angseesing, W.S. Field observations on the cyanogenesis polymorphism in Trifolium repens. Heredity 1973, 31, 276–282. [Google Scholar] [CrossRef]
- Angseesing, J.P.A. Selective eating of the acyanogenic form of Trifolium repens. Heredity 1974, 32, 73–83. [Google Scholar] [CrossRef]
- Root, R.B. Organization of a plant-arthropod association in simple and diverse habitats: The fauna of collards (Brassica oleracea). Ecol. Monogr. 1973, 43, 95–120. [Google Scholar] [CrossRef]
- Barbosa, P.; Hines, J.; Kaplan, I.; Martinson, H.; Szczepaniec, A.; Szendrei, Z. Associational resistance and associational susceptibility: Having right or wrong neighbors. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 1–20. [Google Scholar] [CrossRef]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 2008, 40, 533–539. [Google Scholar] [CrossRef]
- Machingura, M.; Salomon, E.; Jez, J.M.; Ebbs, S.D. The β-cyanoalanine synthase pathway: Beyond cyanide detoxification. Plant Cell Environ. 2016, 39, 2329–2341. [Google Scholar] [CrossRef] [PubMed]
- Vyse, K.; Penzlin, J.; Sergeant, K.; Hincha, D.K.; Arora, R.; Zuther, E. Repair of sub-lethal freezing damage in leaves of Arabidopsis thaliana. BMC Plant Biol. 2020, 20, 35. [Google Scholar] [CrossRef]
- Bajerski, F.; Stock, J.; Hanf, B.; Darienko, T.; Heine-Dobbernack, E.; Lorenz, M.; Naujox, L.; Keller, E.R.J.; Schumacher, H.M.; Friedl, T.; et al. ATP content and cell viability as indicators for cryostress across the diversity of life. Front. Physiol. 2018, 17, 921. [Google Scholar] [CrossRef]
- Sobczyk, E.A.; Marszalek, A.; Kacperska, A. ATP involvement in plant tissue responses to low temperature. Physiol. Plant 1985, 63, 399–405. [Google Scholar] [CrossRef]
- Zhen, Y.; Dhakal, P.; Ungerer, M.C. Fitness benefits and costs of cold acclimation in Arabidopsis thaliana. Am. Nat. 2011, 178, 44–52. [Google Scholar] [CrossRef]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Johnson, M.T.J.; Rasmann, S. The latitudinal herbivory-defence hypothesis takes a detour on the map. New Phytol. 2011, 191, 592–596. [Google Scholar] [CrossRef]
- Burdon, J. Trifolium repens L. J. Ecol. 1983, 71, 307–330. [Google Scholar] [CrossRef]
- Feigl, F.; Anger, V. Replacement of benzidine by copper ethylacetoacetate and tetra base as spot-test reagent for hydrogen cyanide and cyanogen. Anal. 1966, 91, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Gleadow, R.; Bjarnholt, N.; Jørgensen, K.; Fox, J.; Miller, R. Research methods in plant science. In Soil Allelochemicals; Narwal, S., Szajdak, L., Sampietro, D.A., Eds.; Studium Press: Houston, TX, USA, 2011; Volume 1, pp. 283–310. [Google Scholar]
- Lambert, J.L.; Ramasamy, J.; Paukstelis, J.V. Stable reagents for the colorimetric determination of cyanide by modified König reactions. Anal. Chem. 1975, 47, 916–918. [Google Scholar] [CrossRef]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated Digital Image Analysis for Rapid and Accurate Measurement of Leaf Area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef] [PubMed]
- González-Cabanelas, D.; Hammerbacher, A.; Raguschke, B.J.; Gershenzon, J.; Wright, L.P. Quantifying the metabolites of the methylerythritol 4-phosphate (MEP) pathway in plants and bacteria. Meth. Enzymol. 2016, 576, 225–249. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 20 January 2023).
- Fox, J.; Weisberg, S. CAR—An R Companion to Applied Regression; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R package Version 1.4.2. 2019. Available online: https://CRAN.Rproject.org/package=emmeans (accessed on 20 January 2023).


| No-Choice | Choice | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | ||||||||
| Effect | d.f. | F | p | d.f. | F | p | d.f. | F | p |
| Cyanotype | 3 | 2.386 | 0.076 | 3 | 0.774 | 0.513 | 3 | 1.538 | 0.212 |
| Temperature | 1 | 0.090 | 0.765 | 1 | 1.578 | 0.213 | 1 | 0.014 | 0.906 |
| Cyanotype × Temperature | 3 | 2.514 | 0.065 | 3 | 1.416 | 0.245 | 3 | 0.534 | 0.661 |
| Effect | d.f. | F Value | p |
|---|---|---|---|
| Cyanotype | 3 | 2.359 | 0.075 |
| Time point | 3 | 2.782 | 0.044 |
| Cyanotype × Time point | 9 | 4.171 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emad Fadoul, H.; Albano, L.J.; Bergman, M.E.; Phillips, M.A.; Johnson, M.T.J. Assessing the Benefits and Costs of the Hydrogen Cyanide Antiherbivore Defense in Trifolium repens. Plants 2023, 12, 1213. https://doi.org/10.3390/plants12061213
Emad Fadoul H, Albano LJ, Bergman ME, Phillips MA, Johnson MTJ. Assessing the Benefits and Costs of the Hydrogen Cyanide Antiherbivore Defense in Trifolium repens. Plants. 2023; 12(6):1213. https://doi.org/10.3390/plants12061213
Chicago/Turabian StyleEmad Fadoul, Hind, Lucas J. Albano, Matthew E. Bergman, Michael A. Phillips, and Marc T. J. Johnson. 2023. "Assessing the Benefits and Costs of the Hydrogen Cyanide Antiherbivore Defense in Trifolium repens" Plants 12, no. 6: 1213. https://doi.org/10.3390/plants12061213
APA StyleEmad Fadoul, H., Albano, L. J., Bergman, M. E., Phillips, M. A., & Johnson, M. T. J. (2023). Assessing the Benefits and Costs of the Hydrogen Cyanide Antiherbivore Defense in Trifolium repens. Plants, 12(6), 1213. https://doi.org/10.3390/plants12061213






