Dynamic Regulation of the Light-Harvesting System through State Transitions in Land Plants and Green Algae
Abstract
1. Introduction
2. Highly Conserved Core Subunits and Variable LHCIs Constitute the PSI–LHCI Complex in Land Plants and Green Algae
3. Similarities and Differences of PSI–LHCI–LHCII Supercomplexes between Land Plants and Green Algae
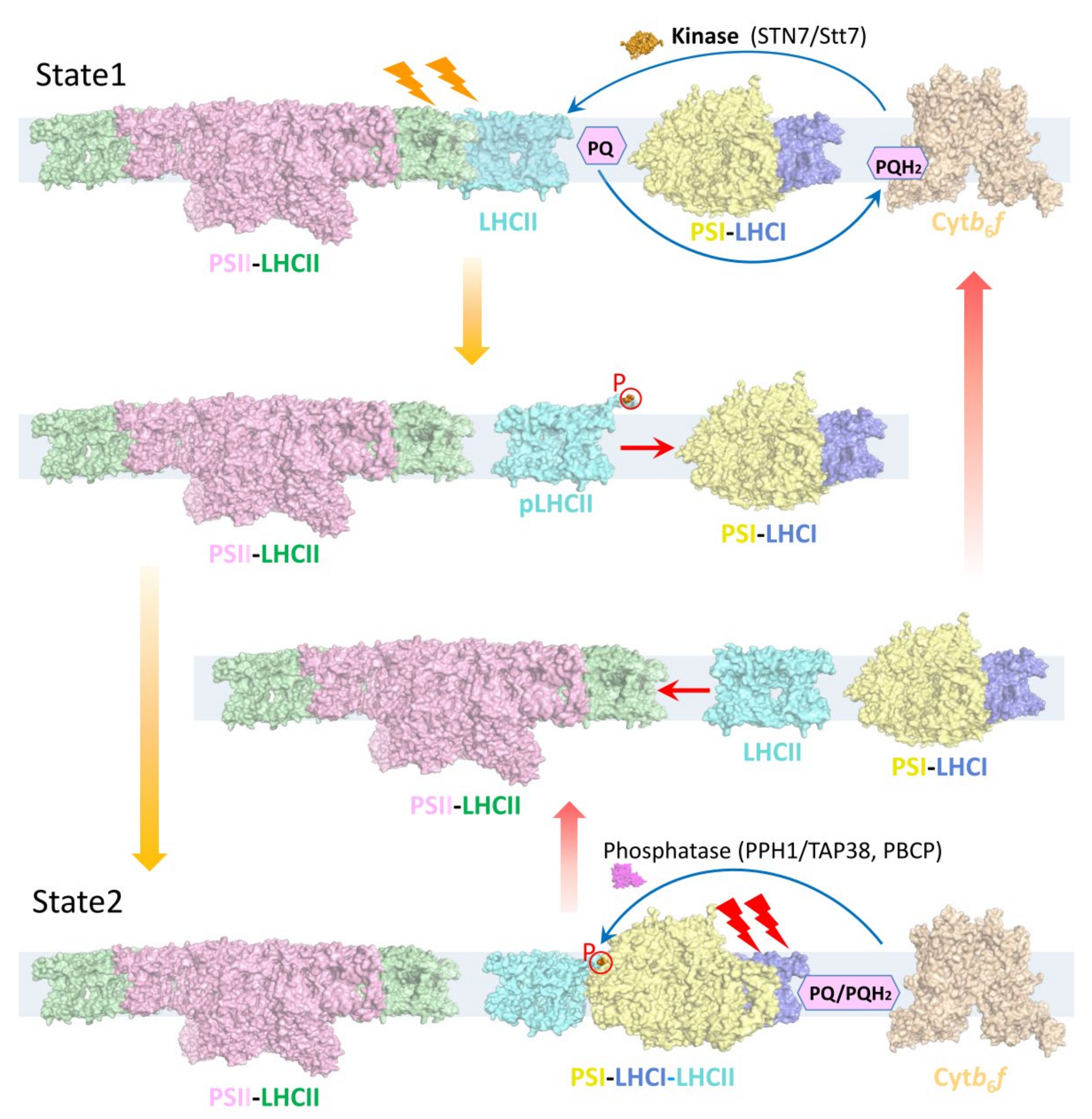
4. Phosphorylated LHCII Is Critical for the Stable PSI–LHCI–LHCII Assembly under State 2 Conditions
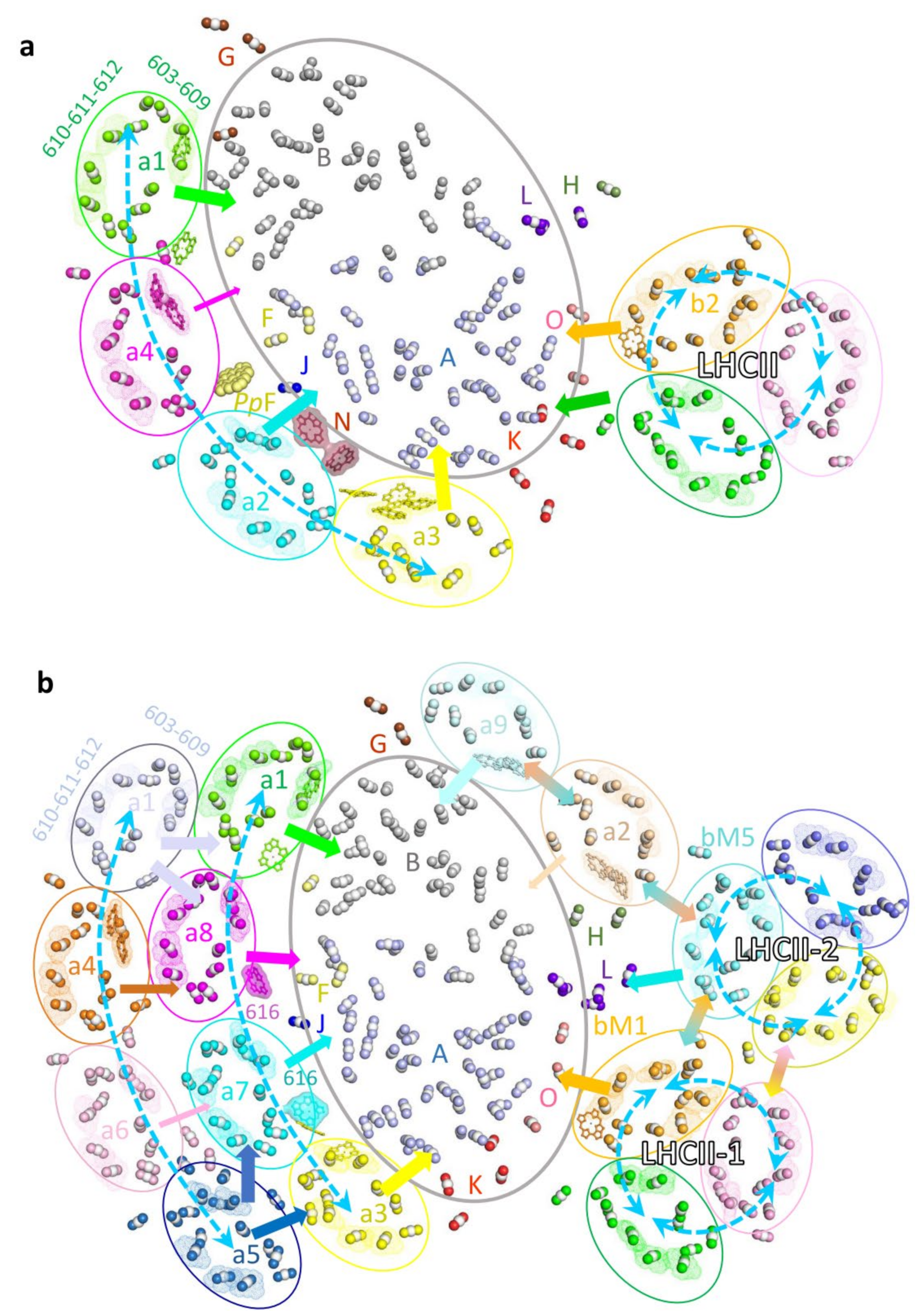
5. Well-Organized Pigment Molecules Constitute Potential Energy Transfer Pathways in PSI–LHCI–LHCII
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, N.; Yocum, C.F. Structure and function of photosystems I and II. Annu. Rev. Plant Biol. 2006, 57, 521–565. [Google Scholar] [CrossRef]
- Dekker, J.P.; Boekema, E.J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys Acta 2005, 1706, 12–39. [Google Scholar] [CrossRef]
- Jansson, S. A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 1999, 4, 236–240. [Google Scholar] [CrossRef]
- Pan, X.; Cao, P.; Su, X.; Liu, Z.; Li, M. Structural analysis and comparison of light-harvesting complexes I and II. Biochim. Biophys Acta Bioenerg. 2020, 1861, 148038. [Google Scholar] [CrossRef]
- Nelson, N.; Ben-Shem, A. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 2004, 5, 971–982. [Google Scholar] [CrossRef]
- Cao, P.; Pan, X.; Su, X.; Liu, Z.; Li, M. Assembly of eukaryotic photosystem II with diverse light-harvesting antennas. Curr. Opin. Struct. Biol. 2020, 63, 49–57. [Google Scholar] [CrossRef]
- Rochaix, J.D. Role of thylakoid protein kinases in photosynthetic acclimation. FEBS Lett. 2007, 581, 2768–2775. [Google Scholar] [CrossRef]
- Minagawa, J. State transitions—The molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim. Biophys. Acta 2011, 1807, 897–905. [Google Scholar] [CrossRef]
- Depege, N.; Bellafiore, S.; Rochaix, J.D. Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 2003, 299, 1572–1575. [Google Scholar] [CrossRef]
- Bellafiore, S.; Barneche, F.; Peltier, G.; Rochaix, J.D. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 2005, 433, 892–895. [Google Scholar] [CrossRef]
- Pribil, M.; Pesaresi, P.; Hertle, A.; Barbato, R.; Leister, D. Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol. 2010, 8, e1000288. [Google Scholar] [CrossRef]
- Cariti, F.; Chazaux, M.; Lefebvre-Legendre, L.; Longoni, P.; Ghysels, B.; Johnson, X.; Goldschmidt-Clermont, M. Regulation of Light Harvesting in Chlamydomonas reinhardtii Two Protein Phosphatases Are Involved in State Transitions. Plant Physiol. 2020, 183, 1749–1764. [Google Scholar] [CrossRef]
- Shapiguzov, A.; Ingelsson, B.; Samol, I.; Andres, C.; Kessler, F.; Rochaix, J.D.; Vener, A.V.; Goldschmidt-Clermont, M. The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 4782–4787. [Google Scholar] [CrossRef]
- Lemeille, S.; Rochaix, J.D. State transitions at the crossroad of thylakoid signalling pathways. Photosynth. Res. 2010, 106, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, W.J.; Santabarbara, S.; Mosebach, L.; Wollman, F.A.; Rappaport, F. State transitions redistribute rather than dissipate energy between the two photosystems in Chlamydomonas. Nat. Plants 2016, 2, 16031. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, G.; Rappaport, F.; Furia, A.; Fleischmann, M.; Rochaix, J.D.; Zito, F.; Forti, G. Involvement of state transitions in the switch between linear and cyclic electron flow in Chlamydomonas reinhardtii. Embo Rep. 2002, 3, 280–285. [Google Scholar] [CrossRef]
- Takahashi, H.; Clowez, S.; Wollman, F.A.; Vallon, O.; Rappaport, F. Cyclic electron flow is redox-controlled but independent of state transition. Nat. Commun. 2013, 4, 1954. [Google Scholar] [CrossRef]
- Chuartzman, S.G.; Nevo, R.; Shimoni, E.; Charuvi, D.; Kiss, V.; Ohad, I.; Brumfeld, V.; Reich, Z. Thylakoid membrane remodeling during state transitions in Arabidopsis. Plant Cell 2008, 20, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Unnep, R.; Zsiros, O.; Tokutsu, R.; Takizawa, K.; Porcar, L.; Moyet, L.; Petroutsos, D.; Garab, G.; Finazzi, G.; et al. Chloroplast remodeling during state transitions in Chlamydomonas reinhardtii as revealed by noninvasive techniques in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 5042–5047. [Google Scholar] [CrossRef]
- Unlu, C.; Drop, B.; Croce, R.; van Amerongen, H. State transitions in Chlamydomonas reinhardtii strongly modulate the functional size of photosystem II but not of photosystem I. Proc. Natl. Acad. Sci. USA 2014, 111, 3460–3465. [Google Scholar] [CrossRef]
- Ünlü, C.; Polukhina, I.; van Amerongen, H. Origin of pronounced differences in 77 K fluorescence of the green alga Chlamydomonas reinhardtii in state 1 and 2. Eur. Biophys. J. 2015, 45, 209–217. [Google Scholar] [CrossRef]
- Wu, G.; Ma, L.; Yuan, C.; Dai, J.; Luo, L.; Poudyal, R.S.; Sayre, R.T.; Lee, C.H. Formation of light-harvesting complex II aggregates from LHCII-PSI-LHCI complexes in rice plants under high light. J. Exp. Bot. 2021, 72, 4938–4948. [Google Scholar] [CrossRef] [PubMed]
- Kouril, R.; Zygadlo, A.; Arteni, A.A.; de Wit, C.D.; Dekker, J.P.; Jensen, P.E.; Scheller, H.V.; Boekema, E.J. Structural characterization of a complex of photosystem I and light-harvesting complex II of Arabidopsis thaliana. Biochemistry 2005, 44, 10935–10940. [Google Scholar] [CrossRef] [PubMed]
- Galka, P.; Santabarbara, S.; Khuong, T.T.; Degand, H.; Morsomme, P.; Jennings, R.C.; Boekema, E.J.; Caffarri, S. Functional analyses of the plant photosystem I-light-harvesting complex II supercomplex reveal that light-harvesting complex II loosely bound to photosystem II is a very efficient antenna for photosystem I in state II. Plant Cell 2012, 24, 2963–2978. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, E.; Albertsson, P.A. Heterogeneity in Photosystem-I—The Larger Antenna of Photosystem-I-Alpha Is Due to Functional Connection to a Special Pool of Lhcii. Biochim. Biophys. Acta 1993, 1141, 175–182. [Google Scholar] [CrossRef]
- Jansson, S.; Stefansson, H.; Nystrom, U.; Gustafsson, P.; Albertsson, P.A. Antenna protein composition of PS I and PS II in thylakoid sub-domains. Biochim. Biophys. Acta Bioenerg. 1997, 1320, 297–309. [Google Scholar] [CrossRef]
- Albertsson, P. A quantitative model of the domain structure of the photosynthetic membrane. Trends Plant Sci. 2001, 6, 349–358. [Google Scholar] [CrossRef]
- Benson, S.L.; Maheswaran, P.; Ware, M.A.; Hunter, C.N.; Horton, P.; Jansson, S.; Ruban, A.V.; Johnson, M.P. An intact light harvesting complex I antenna system is required for complete state transitions in Arabidopsis. Nat. Plants 2015, 1, 15176. [Google Scholar] [CrossRef]
- Bell, A.J.; Frankel, L.K.; Bricker, T.M. High Yield Non-detergent Isolation of Photosystem I-Light-harvesting Chlorophyll II Membranes from Spinach Thylakoids: Implications For The Organization Of The Ps I Antennae In Higher Plants. J. Biol. Chem. 2015, 290, 18429–18437. [Google Scholar] [CrossRef]
- Yadav, K.N.; Semchonok, D.A.; Nosek, L.; Kouril, R.; Fucile, G.; Boekema, E.J.; Eichacker, L.A. Supercomplexes of plant photosystem I with cytochrome b6f, light-harvesting complex II and NDH. Biochim. Biophys Acta Bioenerg. 2017, 1858, 12–20. [Google Scholar] [CrossRef]
- Guo, J.; Wei, X.; Li, M.; Pan, X.; Chang, W.; Liu, Z. Structure of the catalytic domain of a state transition kinase homolog from Micromonas algae. Protein Cell 2013, 4, 607–619. [Google Scholar] [CrossRef]
- Wei, X.; Guo, J.; Li, M.; Liu, Z. Structural Mechanism Underlying the Specific Recognition between the Arabidopsis State-Transition Phosphatase TAP38/PPH1 and Phosphorylated Light-Harvesting Complex Protein Lhcb1. Plant Cell 2015, 27, 1113–1127. [Google Scholar] [CrossRef]
- Pan, X.; Tokutsu, R.; Li, A.; Takizawa, K.; Song, C.; Murata, K.; Yamasaki, T.; Liu, Z.; Minagawa, J.; Li, M. Structural basis of LhcbM5-mediated state transitions in green algae. Nat. Plants 2021, 7, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shen, L.; Wang, W.; Mao, Z.; Yi, X.; Kuang, T.; Shen, J.R.; Zhang, X.; Han, G. Structure of photosystem I-LHCI-LHCII from the green alga Chlamydomonas reinhardtii in State 2. Nat. Commun. 2021, 12, 1100. [Google Scholar] [CrossRef]
- Pan, X.; Ma, J.; Su, X.; Cao, P.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Structure of the maize photosystem I supercomplex with light-harvesting complexes I and II. Science 2018, 360, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Suga, M.; Kuang, T.; Shen, J.R. Photosynthesis. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science 2015, 348, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Mazor, Y.; Borovikova, A.; Nelson, N. The structure of plant photosystem I super-complex at 2.8 A resolution. Elife 2015, 4, e07433. [Google Scholar] [CrossRef]
- Mazor, Y.; Borovikova, A.; Caspy, I.; Nelson, N. Structure of the plant photosystem I supercomplex at 2.6 A resolution. Nat. Plants 2017, 3, 17014. [Google Scholar] [CrossRef]
- Gorski, C.; Riddle, R.; Toporik, H.; Da, Z.; Dobson, Z.; Williams, D.; Mazor, Y. The structure of the Physcomitrium patens photosystem I reveals a unique Lhca2 paralogue replacing Lhca4. Nat. Plants 2022, 8, 307–316. [Google Scholar] [CrossRef]
- Yan, Q.; Zhao, L.; Wang, W.; Pi, X.; Han, G.; Wang, J.; Cheng, L.; He, Y.K.; Kuang, T.; Qin, X.; et al. Antenna arrangement and energy-transfer pathways of PSI-LHCI from the moss Physcomitrella patens. Cell Discov. 2021, 7, 10. [Google Scholar] [CrossRef]
- Suga, M.; Ozawa, S.I.; Yoshida-Motomura, K.; Akita, F.; Miyazaki, N.; Takahashi, Y. Structure of the green algal photosystem I supercomplex with a decameric light-harvesting complex I. Nat. Plants 2019, 5, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ma, J.; Pan, X.; Zhao, X.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Antenna arrangement and energy transfer pathways of a green algal photosystem-I-LHCI supercomplex. Nat. Plants 2019, 5, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Pi, X.; Wang, W.; Han, G.; Zhu, L.; Liu, M.; Cheng, L.; Shen, J.R.; Kuang, T.; Sui, S.F. Structure of a green algal photosystem I in complex with a large number of light-harvesting complex I subunits. Nat. Plants 2019, 5, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Perez-Boerema, A.; Klaiman, D.; Caspy, I.; Netzer-El, S.Y.; Amunts, A.; Nelson, N. Structure of a minimal photosystem I from the green alga Dunaliella salina. Nat. Plants 2020, 6, 321–327. [Google Scholar] [CrossRef]
- Caspy, I.; Malavath, T.; Klaiman, D.; Fadeeva, M.; Shkolnisky, Y.; Nelson, N. Structure and energy transfer pathways of the Dunaliella Salina photosystem I supercomplex. Biochim. Biophys Acta Bioenerg. 2020, 1861, 148253. [Google Scholar] [CrossRef]
- Klimmek, F.; Sjodin, A.; Noutsos, C.; Leister, D.; Jansson, S. Abundantly and rarely expressed Lhc protein genes exhibit distinct regulation patterns in plants. Plant Physiol. 2006, 140, 793–804. [Google Scholar] [CrossRef]
- Wientjes, E.; Croce, R. The light-harvesting complexes of higher-plant Photosystem I: Lhca1/4 and Lhca2/3 form two red-emitting heterodimers. Biochem. J. 2011, 433, 477–485. [Google Scholar] [CrossRef]
- Crepin, A.; Kucerova, Z.; Kosta, A.; Durand, E.; Caffarri, S. Isolation and characterization of a large photosystem I-light-harvesting complex II supercomplex with an additional Lhca1-a4 dimer in Arabidopsis. Plant J. 2020, 102, 398–409. [Google Scholar] [CrossRef]
- Peng, L.; Fukao, Y.; Fujiwara, M.; Takami, T.; Shikanai, T. Efficient operation of NAD(P)H dehydrogenase requires supercomplex formation with photosystem I via minor LHCI in Arabidopsis. Plant Cell 2009, 21, 3623–3640. [Google Scholar] [CrossRef]
- Su, X.; Cao, D.; Pan, X.; Shi, L.; Liu, Z.; Dall’Osto, L.; Bassi, R.; Zhang, X.; Li, M. Supramolecular assembly of chloroplast NADH dehydrogenase-like complex with photosystem I from Arabidopsis thaliana. Mol. Plant 2022, 15, 454–467. [Google Scholar] [CrossRef]
- Shen, L.; Tang, K.; Wang, W.; Wang, C.; Wu, H.; Mao, Z.; An, S.; Chang, S.; Kuang, T.; Shen, J.R.; et al. Architecture of the chloroplast PSI-NDH supercomplex in Hordeum vulgare. Nature 2022, 601, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Lucinski, R.; Schmid, V.H.; Jansson, S.; Klimmek, F. Lhca5 interaction with plant photosystem I. FEBS Lett. 2006, 580, 6485–6488. [Google Scholar] [CrossRef] [PubMed]
- Ganeteg, U.; Klimmek, F.; Jansson, S. Lhca5—An LHC-type protein associated with photosystem I. Plant Mol. Biol. 2004, 54, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, X.; Yang, G.; Song, J.; Zhao, X.; Zhu, L.; Qin, X. Assembly of LHCA5 into PSI blue shifts the far-red fluorescence emission in higher plants. Biochem. Biophys Res. Commun. 2022, 612, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, B.; Kumar, P.; Verma, V.; Irfan, M.; Sharma, R.; Bhargava, B. How plants conquered land: Evolution of terrestrial adaptation. J. Evol. Biol. 2023, 36, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Rensing, S.A.; Lang, D.; Zimmer, A.D.; Terry, A.; Salamov, A.; Shapiro, H.; Nishiyama, T.; Perroud, P.F.; Lindquist, E.A.; Kamisugi, Y.; et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 2008, 319, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Alboresi, A.; Caffarri, S.; Nogue, F.; Bassi, R.; Morosinotto, T. In silico and biochemical analysis of Physcomitrella patens photosynthetic antenna: Identification of subunits which evolved upon land adaptation. PLoS ONE 2008, 3, e2033. [Google Scholar] [CrossRef]
- Busch, A.; Petersen, J.; Webber-Birungi, M.T.; Powikrowska, M.; Lassen, L.M.; Naumann-Busch, B.; Nielsen, A.Z.; Ye, J.; Boekema, E.J.; Jensen, O.N.; et al. Composition and structure of photosystem I in the moss Physcomitrella patens. J. Exp. Bot. 2013, 64, 2689–2699. [Google Scholar] [CrossRef]
- Iwai, M.; Yokono, M. Light-harvesting antenna complexes in the moss Physcomitrella patens: Implications for the evolutionary transition from green algae to land plants. Curr. Opin. Plant Biol. 2017, 37, 94–101. [Google Scholar] [CrossRef]
- Nielsen, V.S.; Mant, A.; Knoetzel, J.; Moller, B.L.; Robinson, C. Import of barley photosystem I subunit N into the thylakoid lumen is mediated by a bipartite presequence lacking an intermediate processing site. Role of the delta pH in translocation across the thylakoid membrane. J. Biol. Chem. 1994, 269, 3762–3766. [Google Scholar] [CrossRef]
- He, W.Z.; Malkin, R. Specific release of a 9-kDa extrinsic polypeptide of photosystem I from spinach chloroplasts by salt washing. FEBS Lett. 1992, 308, 298–300. [Google Scholar] [CrossRef]
- Jensen, P.E.; Haldrup, A.; Zhang, S.; Scheller, H.V. The PSI-O subunit of plant photosystem I is involved in balancing the excitation pressure between the two photosystems. J. Biol. Chem. 2004, 279, 24212–24217. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Yokono, M.; Kono, M.; Noguchi, K.; Akimoto, S.; Nakano, A. Light-harvesting complex Lhcb9 confers a green alga-type photosystem I supercomplex to the moss Physcomitrella patens. Nat. Plants 2015, 1, 14008. [Google Scholar] [CrossRef]
- Pinnola, A.; Alboresi, A.; Nosek, L.; Semchonok, D.; Rameez, A.; Trotta, A.; Barozzi, F.; Kouril, R.; Dall’Osto, L.; Aro, E.M.; et al. A LHCB9-dependent photosystem I megacomplex induced under low light in Physcomitrella patens. Nat. Plants 2018, 4, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, P.; Ballottari, M.; Bonente, G.; Giuliano, G.; Bassi, R. LHCBM1 and LHCBM2/7 polypeptides, components of major LHCII complex, have distinct functional roles in photosynthetic antenna system of Chlamydomonas reinhardtii. J. Biol. Chem. 2012, 287, 16276–16288. [Google Scholar] [CrossRef]
- Lunde, C.; Jensen, P.E.; Haldrup, A.; Knoetzel, J.; Scheller, H.V. The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 2000, 408, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Nakane, T.; Kimanius, D.; Lindahl, E.; Scheres, S.H. Characterisation of molecular motions in cryo-EM single-particle data by multi-body refinement in RELION. Elife 2018, 7, e36861. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K.; Bjorkman, O.; Grossman, A.R. The roles of specific xanthophylls in photoprotection. Proc. Natl. Acad. Sci. USA 1997, 94, 14162–14167. [Google Scholar] [CrossRef]
- Standfuss, J.; Terwisscha van Scheltinga, A.C.; Lamborghini, M.; Kuhlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 A resolution. EMBO J. 2005, 24, 919–928. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, H.; Wang, K.; Kuang, T.; Zhang, J.; Gui, L.; An, X.; Chang, W. Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature 2004, 428, 287–292. [Google Scholar] [CrossRef]
- Croce, R.; Chojnicka, A.; Morosinotto, T.; Ihalainen, J.A.; van Mourik, F.; Dekker, J.P.; Bassi, R.; van Grondelle, R. The low-energy forms of photosystem I light-harvesting complexes: Spectroscopic properties and pigment-pigment interaction characteristics. Biophys. J. 2007, 93, 2418–2428. [Google Scholar] [CrossRef] [PubMed]
- Morosinotto, T.; Breton, J.; Bassi, R.; Croce, R. The nature of a chlorophyll ligand in Lhca proteins determines the far red fluorescence emission typical of photosystem I. J. Biol. Chem. 2003, 278, 49223–49229. [Google Scholar] [CrossRef] [PubMed]
- Rochaix, J.D. Regulation and dynamics of the light-harvesting system. Annu. Rev. Plant Biol. 2014, 65, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Ruban, A. Molecular design of the photosystem II light-harvesting antenna: Photosynthesis and photoprotection. J. Exp. Bot. 2005, 56, 365–373. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Truong, T.B. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr. Opin. Plant Biol. 2013, 16, 307–314. [Google Scholar] [CrossRef]
- Peers, G.; Truong, T.B.; Ostendorf, E.; Busch, A.; Elrad, D.; Grossman, A.R.; Hippler, M.; Niyogi, K.K. An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 2009, 462, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Li, M.; Liu, Z.; Cao, P.; Pan, X.; Zhang, H.; Zhao, X.; Zhang, J.; Chang, W. Crystal structures of the PsbS protein essential for photoprotection in plants. Nat. Struct. Mol. Biol. 2015, 22, 729–735. [Google Scholar] [CrossRef]
- Li, X.P.; Gilmore, A.M.; Caffarri, S.; Bassi, R.; Golan, T.; Kramer, D.; Niyogi, K.K. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 2004, 279, 22866–22874. [Google Scholar] [CrossRef]
- Goral, T.K.; Johnson, M.P.; Duffy, C.D.; Brain, A.P.; Ruban, A.V.; Mullineaux, C.W. Light-harvesting antenna composition controls the macrostructure and dynamics of thylakoid membranes in Arabidopsis. Plant J. 2012, 69, 289–301. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Cazzaniga, S.; Bressan, M.; Palecek, D.; Zidek, K.; Niyogi, K.K.; Fleming, G.R.; Zigmantas, D.; Bassi, R. Two mechanisms for dissipation of excess light in monomeric and trimeric light-harvesting complexes. Nat. Plants 2017, 3, 17033. [Google Scholar] [CrossRef]
- Bonente, G.; Ballottari, M.; Truong, T.B.; Morosinotto, T.; Ahn, T.K.; Fleming, G.R.; Niyogi, K.K.; Bassi, R. Analysis of LhcSR3, a protein essential for feedback de-excitation in the green alga Chlamydomonas reinhardtii. PLoS Biol. 2011, 9, e1000577. [Google Scholar] [CrossRef] [PubMed]
- Pinnola, A.; Cazzaniga, S.; Alboresi, A.; Nevo, R.; Levin-Zaidman, S.; Reich, Z.; Bassi, R. Light-Harvesting Complex Stress-Related Proteins Catalyze Excess Energy Dissipation in Both Photosystems of Physcomitrella patens. Plant Cell 2015, 27, 3213–3227. [Google Scholar] [CrossRef] [PubMed]
- Girolomoni, L.; Cazzaniga, S.; Pinnola, A.; Perozeni, F.; Ballottari, M.; Bassi, R. LHCSR3 is a nonphotochemical quencher of both photosystems in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2019, 116, 4212–4217. [Google Scholar] [CrossRef] [PubMed]
- Kromdijk, J.; Glowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, H.; Li, M.; Pan, X. Dynamic Regulation of the Light-Harvesting System through State Transitions in Land Plants and Green Algae. Plants 2023, 12, 1173. https://doi.org/10.3390/plants12051173
Shang H, Li M, Pan X. Dynamic Regulation of the Light-Harvesting System through State Transitions in Land Plants and Green Algae. Plants. 2023; 12(5):1173. https://doi.org/10.3390/plants12051173
Chicago/Turabian StyleShang, Hui, Mei Li, and Xiaowei Pan. 2023. "Dynamic Regulation of the Light-Harvesting System through State Transitions in Land Plants and Green Algae" Plants 12, no. 5: 1173. https://doi.org/10.3390/plants12051173
APA StyleShang, H., Li, M., & Pan, X. (2023). Dynamic Regulation of the Light-Harvesting System through State Transitions in Land Plants and Green Algae. Plants, 12(5), 1173. https://doi.org/10.3390/plants12051173





