Hyptis obtusiflora C. Presl ex Benth Methanolic Extract Exhibits Anti-Inflammatory and Anti-Gastritis Activities via Suppressing AKT/NF-κB Pathway
Abstract
1. Introduction
2. Results
2.1. Ho-ME Suppresses Nitric Oxide Production in TLR-Activated Macrophages
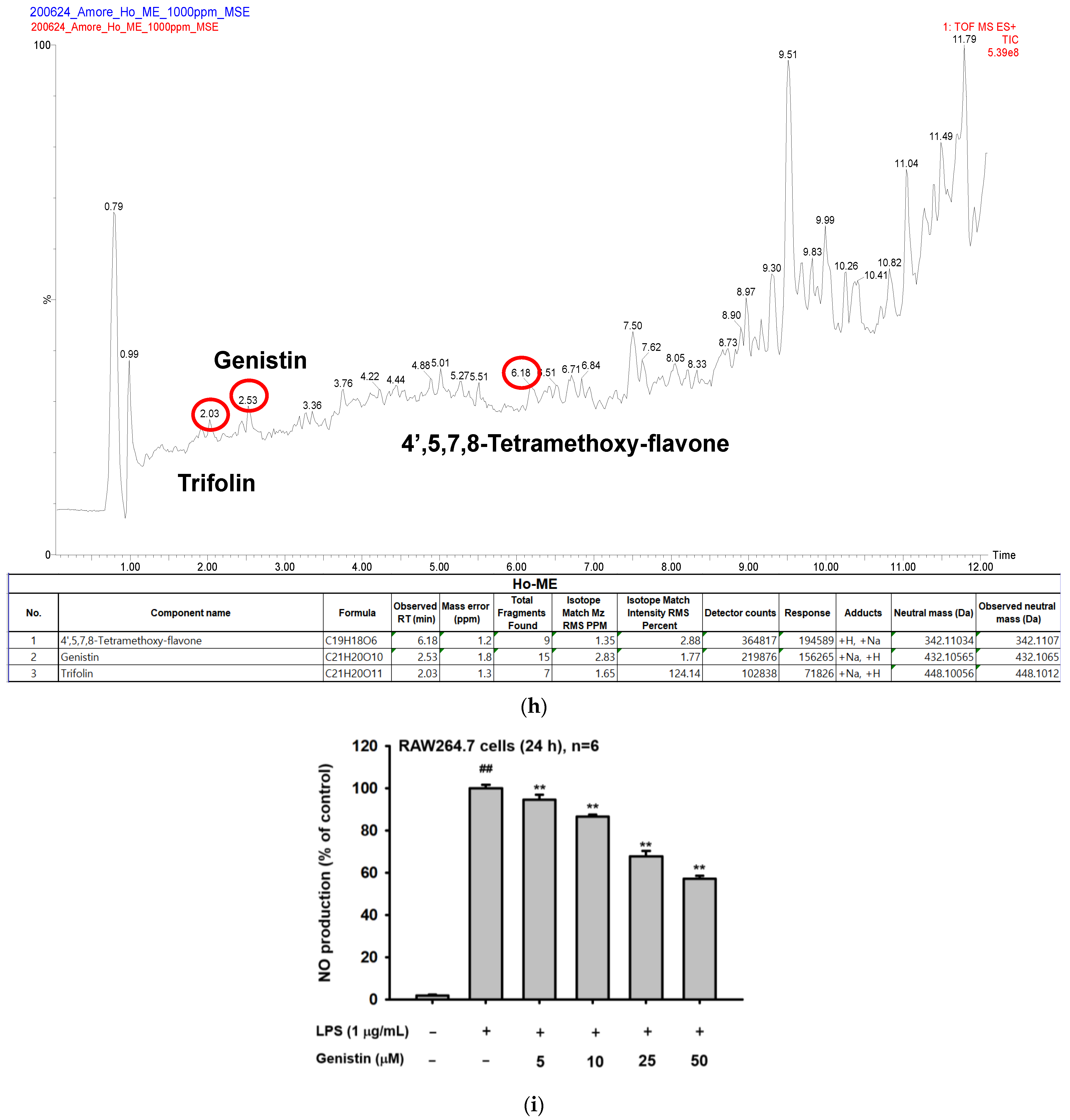
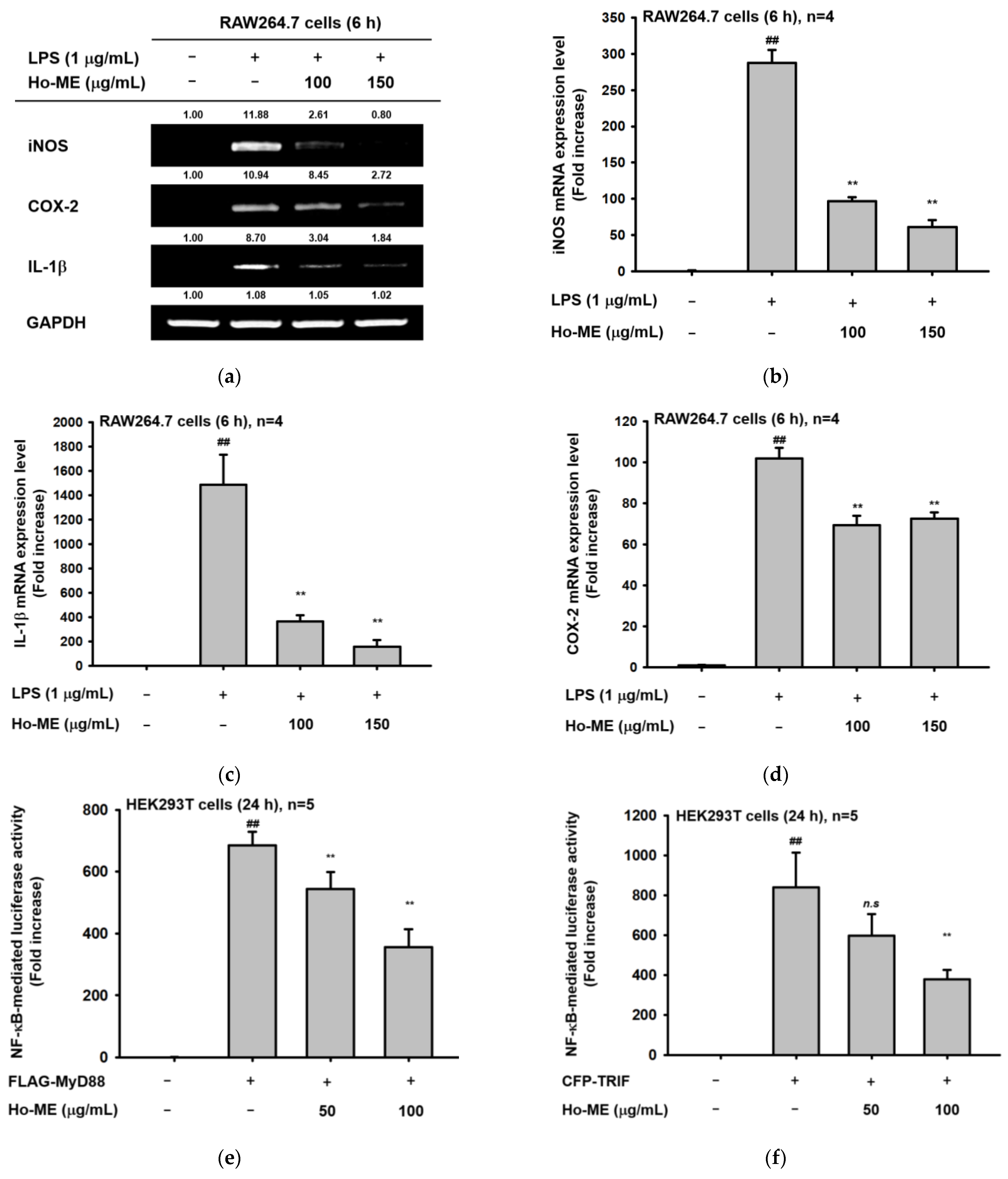
2.2. Ho-ME Altered Activities of Inflammation-Related Transcription Factors and mRNA Expression of Inflammatory Genes
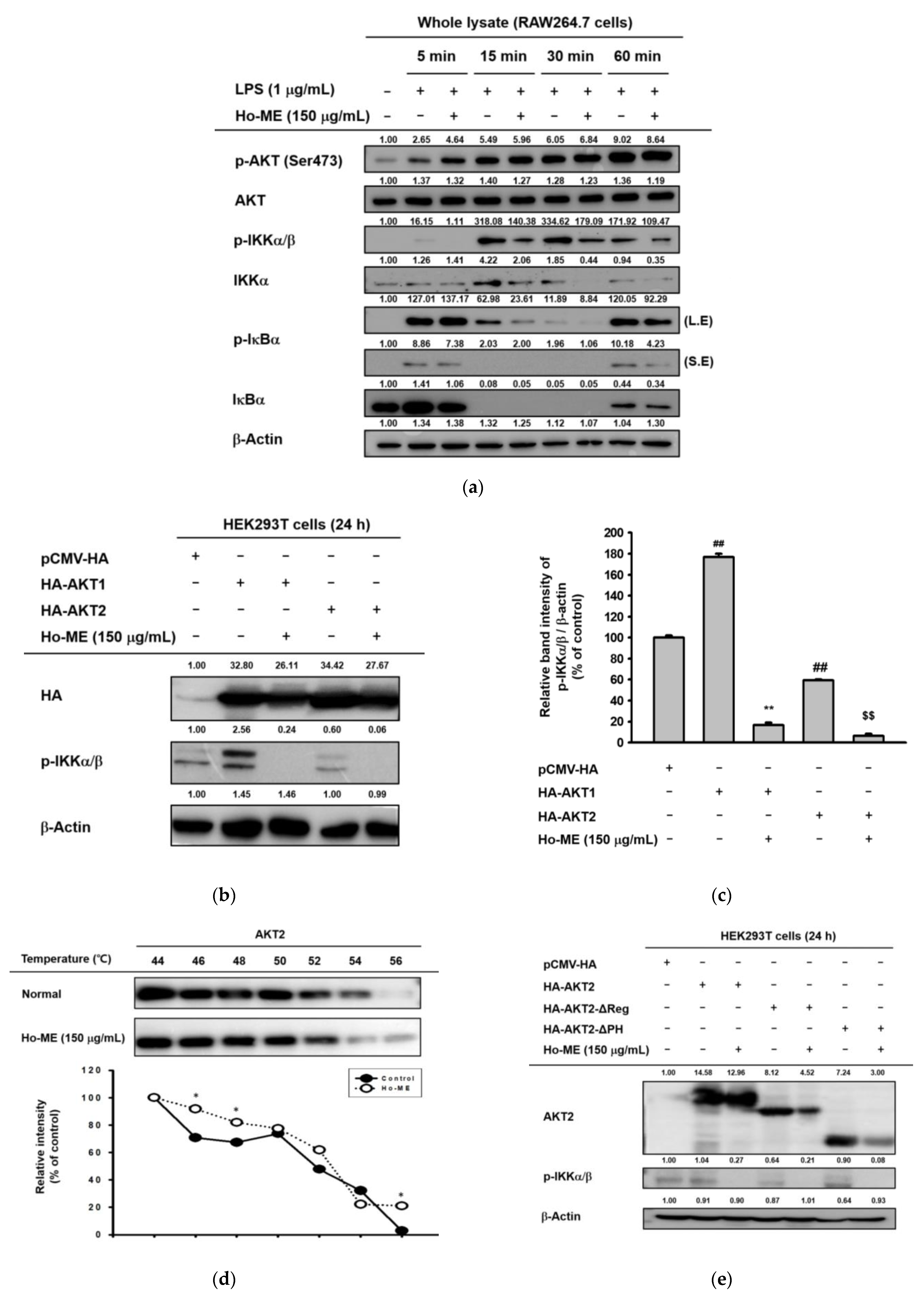
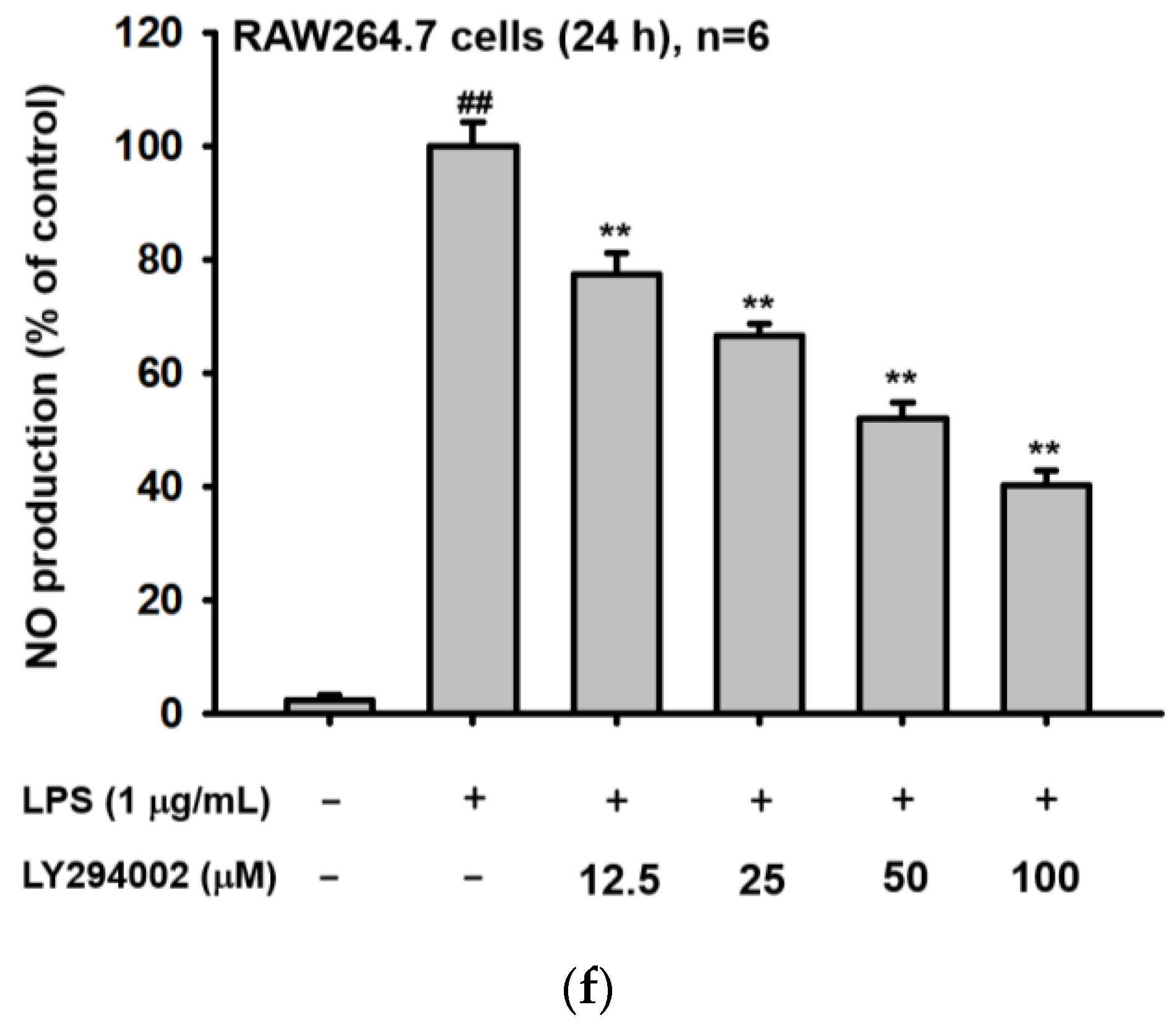
2.3. Ho-ME Interrupts AKT Phosphorylation during an Intracellular Signaling Cascade
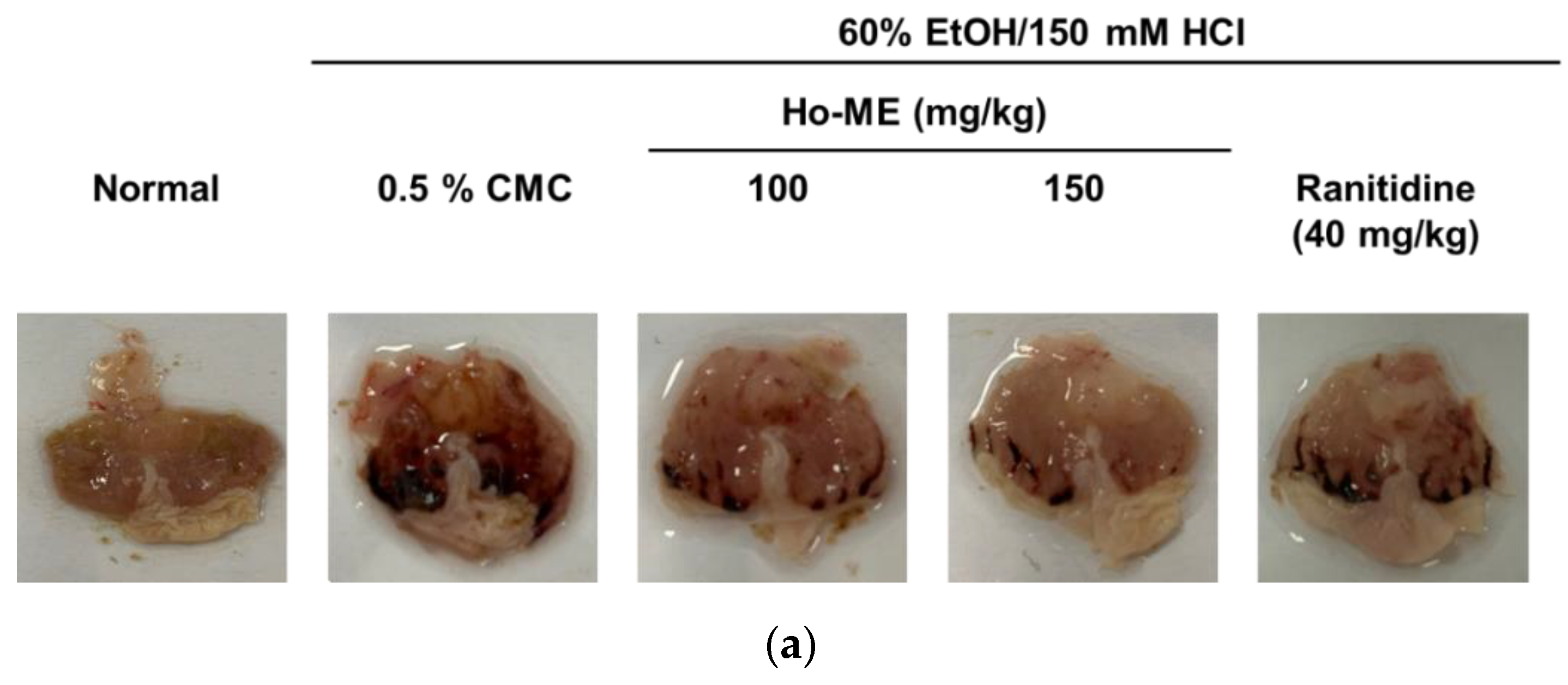
2.4. Ho-ME Alleviated DAMP-Induced Acute Gastritis
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Plant Extract Processing
4.3. Cell Culture
4.4. NO and MTT Assays
4.5. RNA Extraction and Polymerase Chain Reaction
4.6. Luciferase Assay
4.7. Western Blot
4.8. Overexpression
4.9. Cellular Thermal Shift Assay
4.10. Mice
4.11. Acute Gastritis Mouse Model Generated with HCl/EtOH
4.12. Quadrupole Time-of-Flight LC/MS
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, C.-H. Glycobiology in Innate Immunology; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Jambrina, M.; Eder, J.; Helgers, L.C.; Hertoghs, N.; Nijmeijer, B.M.; Stunnenberg, M.; Geijtenbeek, T.B.H. C-Type Lectin Receptors in Antiviral Immunity and Viral Escape. Front. Immunol. 2018, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7, 255–266. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Shin, K.K.; Kim, H.; Hong, Y.H.; Choi, W.; Kwak, Y.S.; Han, C.K.; Hyun, S.H.; Cho, J.Y. Korean Red Ginseng exerts anti-inflammatory and autophagy-promoting activities in aged mice. J. Ginseng Res. 2021, 45, 717–725. [Google Scholar] [CrossRef]
- You, L.; Cha, S.; Kim, M.Y.; Cho, J.Y. Ginsenosides are active ingredients in Panax ginseng with immunomodulatory properties from cellular to organismal levels. J. Ginseng Res. 2022, 46, 711–721. [Google Scholar] [CrossRef]
- Mitra, A.; Rahmawati, L.; Lee, H.P.; Kim, S.A.; Han, C.K.; Hyun, S.H.; Cho, J.Y. Korean Red Ginseng water extract inhibits cadmium-induced lung injury via suppressing MAPK/ERK1/2/AP-1 pathway. J. Ginseng Res. 2022, 46, 690–699. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Niu, Z.; Shen, T.; Zhang, J.; Wang, X.; Hu, W.; Cho, J.Y. A review of the immunomodulatory activities of polysaccharides isolated from Panax species. J. Ginseng Res. 2022, 46, 23–32. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Murakami, M.; Hirano, T. The molecular mechanisms of chronic inflammation development. Front. Immunol. 2012, 3, 323. [Google Scholar] [CrossRef]
- Harley, R.M.; Atkins, S.; Budantsev, A.L.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; Kok, R.D.; Krestovskaja, T.D.; Morales, R. Labiatae. In Flowering Plants Dicotyledons; Springer: Berlin/Heidelberg, Germany, 2004; pp. 167–275. [Google Scholar]
- Nieto, G. Biological activities of three essential oils of the Lamiaceae family. Medicines 2017, 4, 63. [Google Scholar] [CrossRef] [PubMed]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Roumy, V.; Mahieux, S.; Biabiany, M.; Standaert-Vitse, A.; Rivière, C.; Sahpaz, S.; Bailleul, F.; Neut, C.; Hennebelle, T. Rosmarinic acid and its methyl ester as antimicrobial components of the hydromethanolic extract of Hyptis atrorubens Poit.(Lamiaceae). Evid.-Based Complement. Altern. Med. 2013, 2013, 604536. [Google Scholar] [CrossRef] [PubMed]
- Almtorp, G.T.; Hazell, A.C.; Torssell, K.B. A lignan and pyrone and other constituents from Hyptis capitata. Phytochemistry 1991, 30, 2753–2756. [Google Scholar] [CrossRef]
- Costa, V.C.d.O.; Tavares, J.F.; Silva, A.B.; Duarte, M.C.; Agra, M.d.F.; Barbosa-Filho, J.M.; de Souza, I.L.; da Silva, B.A.; Silva, M.S. Hyptenolide, a new α-pyrone with spasmolytic activity from Hyptis macrostachys. Phytochem. Lett. 2014, 8, 32–37. [Google Scholar] [CrossRef]
- Machado, F.D.F.; de Oliveira Formiga, R.; de Morais Lima, G.R.; de Jesus, N.Z.T.; Júnior, E.B.A.; Marinho, A.F.; Tavares, J.F.; Santos, F.A.; Viana, A.F.S.C.; Araújo, A.A. Hyptis suaveolens (L.) Poit protects colon from TNBS-induced inflammation via immunomodulatory, antioxidant and anti-proliferative mechanisms. J. Ethnopharmacol. 2021, 265, 113153. [Google Scholar] [CrossRef]
- Barbosa, A.G.; Tintino, C.D.; Pessoa, R.T.; de Lacerda Neto, L.J.; Martins, A.O.; de Oliveira, M.R.; Coutinho, H.D.; Cruz-Martins, N.; Junior, L.J.Q.; Wilairatana, P. Anti-inflammatory and antinociceptive effect of Hyptis martiusii BENTH leaves essential oil. Biotechnol. Rep. 2022, 35, e00756. [Google Scholar] [CrossRef]
- McNeil, M.; Facey, P.; Porter, R. Essential Oils from the Hyptis genus—A Review (1909–2009). Nat. Prod. Commun. 2011, 6, 1934578X1100601149. [Google Scholar] [CrossRef]
- Luzuriaga-Quichimbo, C.X.; Blanco-Salas, J.; Cerón-Martínez, C.E.; Stanković, M.S.; Ruiz-Téllez, T. On the Possible Chemical Justification of the Ethnobotanical Use of Hyptis obtusiflora in Amazonian Ecuador. Plants 2018, 7, 104. [Google Scholar] [CrossRef]
- De la Torre, L.; Navarrete, H.; Muriel, P.; Macía, M.J.; Balslev, H. Enciclopedia de las Plantas Útiles del Ecuador (Con Wxtracto de Datos); Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia: Quito, Ecuador, 2008. [Google Scholar]
- Bryan, N.S.; Ahmed, S.; Lefer, D.J.; Hord, N.; von Schwarz, E.R. Dietary nitrate biochemistry and physiology. An update on clinical benefits and mechanisms of action. Nitric Oxide 2023, 132, 1–7. [Google Scholar] [CrossRef]
- Song, C.; Lee, C.Y.; Lee, H.P.; Hossain, M.A.; Zhang, Z.; Kim, S.Y.; Song, M.; Kim, J.H.; Cho, J.Y. Protective Function of Malus baccata (L.) Borkh Methanol Extract against UVB/Hydrogen Peroxide-Induced Skin Aging via Inhibition of MAPK and NF-kappaB Signaling. Plants 2022, 11, 2368. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Choi, W.; Kwon, K.W.; You, L.; Rahmawati, L.; Luong, V.D.; Kim, W.; Lee, B.H.; Lee, S.; Kim, J.H.; et al. Inhibitory Effects of Grewia tomentosa Juss. on IgE-Mediated Allergic Reaction and DNCB-Induced Atopic Dermatitis. Plants 2022, 11, 2540. [Google Scholar] [CrossRef]
- Lee, D.; Kim, J.W.; Lee, C.Y.; Oh, J.; Hwang, S.H.; Jo, M.; Kim, S.A.; Choi, W.; Noh, J.K.; Yi, D.K.; et al. Guettarda crispiflora Vahl Methanol Extract Ameliorates Acute Lung Injury and Gastritis by Suppressing Src Phosphorylation. Plants 2022, 11, 3560. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Choi, E.; Hong, Y.H.; Kim, H.; Jang, Y.J.; Lee, J.S.; Choung, E.S.; Woo, B.Y.; Hong, Y.D.; Lee, S.; et al. Syk/NF-kappaB-targeted anti-inflammatory activity of Melicope accedens (Blume) T.G. Hartley methanol extract. J. Ethnopharmacol. 2021, 271, 113887. [Google Scholar] [CrossRef]
- Kim, H.; Yang, W.S.; Htwe, K.M.; Lee, M.N.; Kim, Y.D.; Yoon, K.D.; Lee, B.H.; Lee, S.; Cho, J.Y. Dipterocarpus tuberculatus Roxb. Ethanol Extract Has Anti-Inflammatory and Hepatoprotective Effects In Vitro and In Vivo by Targeting the IRAK1/AP-1 Pathway. Molecules 2021, 26, 2529. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.G.; Hong, Y.H.; Shin, K.K.; Kim, J.K.; Kim, Y.D.; Yoon, K.D.; Kim, K.H.; Yoo, B.C.; Sung, G.H.; et al. Sauropus brevipes ethanol extract negatively regulates inflammatory responses in vivo and in vitro by targeting Src, Syk and IRAK1. Pharm. Biol. 2021, 59, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kwon, K.W.; Kim, M.Y.; Cho, J.Y. Potentilla paradoxa Nutt. Ethanol Extract Exhibits Anti-Inflammatory Effects by Suppression of the Src/NF-kappaB Signaling Pathway. Plants 2022, 11, 1750. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Chen, H.; Kim, S.A.; Lee, J.S.; Choung, E.S.; Zhang, Z.; Kim, S.Y.; Kim, J.H.; Cho, J.Y. Anti-Inflammatory Functions of Methanol Extract from Malus baccata (L.) Borkh. Leaves and Shoots by Targeting the NF-kappaB Pathway. Plants 2022, 11, 646. [Google Scholar] [CrossRef]
- Song, C.; Lorz, L.R.; Lee, J.; Cho, J.Y. In Vitro Photoprotective, Anti-Inflammatory, Moisturizing, and Antimelanogenic Effects of a Methanolic Extract of Chrysophyllum lucentifolium Cronquist. Plants 2021, 11, 94. [Google Scholar] [CrossRef]
- Weako, J.; Jang, H.; Keskin, O.; Nussinov, R.; Gursoy, A. The structural basis of Akt PH domain interaction with calmodulin. Biophys. J. 2021, 120, 1994–2008. [Google Scholar] [CrossRef]
- Rittirsch, D.; Flierl, M.A.; Ward, P.A. Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 2008, 8, 776–787. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, M.; Triggianese, P.; Conigliaro, P.; Candi, E.; Melino, G.; Perricone, R. The interplay between inflammation and metabolism in rheumatoid arthritis. Cell Death Dis. 2015, 6, e1887. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Islam, M.S.; Uddin, M.N.; Hasan, M.M.I.; Akanda, M.R. The potential health benefits of the isoflavone glycoside genistin. Arch. Pharmacal Res. 2020, 43, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Phromnoi, K.; Yodkeeree, S.; Anuchapreeda, S.; Limtrakul, P. Inhibition of MMP-3 activity and invasion of the MDA-MB-231 human invasive breast carcinoma cell line by bioflavonoids. Acta Pharmacol. Sin. 2009, 30, 1169–1176. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, Y.; Shi, Z.; Everaert, N.; Ren, G. Synergistic Effect of Bioactive Anticarcinogens from Soybean on Anti-Proliferative Activity in MDA-MB-231 and MCF-7 Human Breast Cancer Cells In Vitro. Molecules 2018, 23, 1557. [Google Scholar] [CrossRef]
- Hamdy, S.M.; Latif, A.K.; Drees, E.A.; Soliman, S.M. Prevention of rat breast cancer by genistin and selenium. Toxicol. Ind. Health 2012, 28, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zheng, A.B.; Jin, J.; Cui, Y.; Zhang, N.; Che, Z.P.; Wang, Y.; Zhan, J.; Tu, W.J. Cardioprotective Effects of Genistin in Rat Myocardial Ischemia-Reperfusion Injury Studies by Regulation of P2X7/NF-kappaB Pathway. Evid. Based Complement. Altern. Med. 2016, 2016, 5381290. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, J.G.; Lee, J.; Yang, W.S.; Park, G.W.; Kim, H.G.; Yi, Y.S.; Baek, K.S.; Sung, N.Y.; Hossen, M.J.; et al. The dietary flavonoid Kaempferol mediates anti-inflammatory responses via the Src, Syk, IRAK1, and IRAK4 molecular targets. Mediat. Inflamm 2015, 2015, 904142. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, J.G.; Sung, G.H.; Yang, S.; Yang, W.S.; Kim, E.; Kim, J.H.; Ha, V.T.; Kim, H.G.; Yi, Y.S.; et al. Kaempferol, a dietary flavonoid, ameliorates acute inflammatory and nociceptive symptoms in gastritis, pancreatitis, and abdominal pain. Mol. Nutr. Food Res. 2015, 59, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Z.; Cain, A.; Wang, B.; Long, M.; Taylor, J. Antifungal activity of camptothecin, trifolin, and hyperoside isolated from Camptotheca acuminata. J. Agric. Food Chem. 2005, 53, 32–37. [Google Scholar] [CrossRef]
- Kim, M.J.; Kwon, S.B.; Kim, M.S.; Jin, S.W.; Ryu, H.W.; Oh, S.R.; Yoon, D.Y. Trifolin induces apoptosis via extrinsic and intrinsic pathways in the NCI-H460 human non-small cell lung-cancer cell line. Phytomedicine 2016, 23, 998–1004. [Google Scholar] [CrossRef]
- Pandith, H.; Zhang, X.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W.; Baek, S.J. Effect of Siam weed extract and its bioactive component scutellarein tetramethyl ether on anti-inflammatory activity through NF-kappaB pathway. J. Ethnopharmacol. 2013, 147, 434–441. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- Srinivasula, S.M.; Poyet, J.L.; Razmara, M.; Datta, P.; Zhang, Z.; Alnemri, E.S. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem. 2002, 277, 21119–21122. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Aksentijevich, I.; Nowak, M.; Mallah, M.; Chae, J.J.; Watford, W.T.; Hofmann, S.R.; Stein, L.; Russo, R.; Goldsmith, D.; Dent, P.; et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): A new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002, 46, 3340–3348. [Google Scholar] [CrossRef]
- Feldmann, J.; Prieur, A.M.; Quartier, P.; Berquin, P.; Certain, S.; Cortis, E.; Teillac-Hamel, D.; Fischer, A.; de Saint Basile, G. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 2002, 71, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Gattorno, M.; Tassi, S.; Carta, S.; Delfino, L.; Ferlito, F.; Pelagatti, M.A.; D’Osualdo, A.; Buoncompagni, A.; Alpigiani, M.G.; Alessio, M.; et al. Pattern of interleukin-1beta secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 2007, 56, 3138–3148. [Google Scholar] [CrossRef]
- Hoffman, H.M.; Mueller, J.L.; Broide, D.H.; Wanderer, A.A.; Kolodner, R.D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 2001, 29, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.M.; Brydges, S.D. Genetic and molecular basis of inflammasome-mediated disease. J. Biol. Chem. 2011, 286, 10889–10896. [Google Scholar] [CrossRef]
- Goldbach-Mansky, R.; Dailey, N.J.; Canna, S.W.; Gelabert, A.; Jones, J.; Rubin, B.I.; Kim, H.J.; Brewer, C.; Zalewski, C.; Wiggs, E. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1β inhibition. N. Engl. J. Med. 2006, 355, 581–592. [Google Scholar] [CrossRef]
- Boni-Schnetzler, M.; Boller, S.; Debray, S.; Bouzakri, K.; Meier, D.T.; Prazak, R.; Kerr-Conte, J.; Pattou, F.; Ehses, J.A.; Schuit, F.C.; et al. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology 2009, 150, 5218–5229. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.W.; Hung, J.; Powell, B.L.; Wiltshire, S.; Foo, B.T.; Leow, Y.C.; McQuillan, B.M.; Jennens, M.; McCaskie, P.A.; Thompson, P.L.; et al. Association of Interleukin-1 gene polymorphisms with central obesity and metabolic syndrome in a coronary heart disease population. Hum. Genet. 2008, 124, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Prantner, D.; Darville, T.; Sikes, J.D.; Andrews, C.W., Jr.; Brade, H.; Rank, R.G.; Nagarajan, U.M. Critical role for interleukin-1beta (IL-1beta) during Chlamydia muridarum genital infection and bacterial replication-independent secretion of IL-1beta in mouse macrophages. Infect. Immun. 2009, 77, 5334–5346. [Google Scholar] [CrossRef]
- Schenk, M.; Fabri, M.; Krutzik, S.R.; Lee, D.J.; Vu, D.M.; Sieling, P.A.; Montoya, D.; Liu, P.T.; Modlin, R.L. Interleukin-1β triggers the differentiation of macrophages with enhanced capacity to present mycobacterial antigen to T cells. Immunology 2014, 141, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Mori, H.; Ohishi, M.; Aki, D.; Hanada, T. Negative regulation of cytokine signaling influences inflammation. Curr. Opin. Immunol. 2003, 15, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, P.E.; Thompson, P.A.; Rosenwasser, L.J.; Dinarello, C.A. The role of interleukin 1 in human B cell activation: Inhibition of B cell proliferation and the generation of immunoglobulin-secreting cells by an antibody against human leukocytic pyrogen. J. Immunol. 1983, 130, 2708–2714. [Google Scholar] [CrossRef]
- Chen, W.S.; Xu, P.Z.; Gottlob, K.; Chen, M.L.; Sokol, K.; Shiyanova, T.; Roninson, I.; Weng, W.; Suzuki, R.; Tobe, K.; et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001, 15, 2203–2208. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Wojtaszewski, J.F.P. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl. Physiol. Nutr. Metab. 2007, 32, 557–566. [Google Scholar] [CrossRef]
- Dummler, B.; Hemmings, B.A. Physiological roles of PKB/Akt isoforms in development and disease. Biochem. Soc. Trans. 2007, 35, 231–235. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, Y.; Guo, H.; Sun, B.; Niu, R.; Ying, G.; Zhang, N. Akt2 is required for macrophage chemotaxis. Eur. J. Immunol. 2009, 39, 894–901. [Google Scholar] [CrossRef]
- Don, M.J.; Liao, J.F.; Lin, L.Y.; Chiou, W.F. Cryptotanshinone inhibits chemotactic migration in macrophages through negative regulation of the PI3K signaling pathway. Br. J. Pharmacol. 2007, 151, 638–646. [Google Scholar] [CrossRef]
- Mosheimer, B.A.; Kaneider, N.C.; Feistritzer, C.; Djanani, A.M.; Sturn, D.H.; Patsch, J.R.; Wiedermann, C.J. Syndecan-1 is involved in osteoprotegerin-induced chemotaxis in human peripheral blood monocytes. J. Clin. Endocrinol. Metab. 2005, 90, 2964–2971. [Google Scholar] [CrossRef]
- Minhajuddin, M.; Bijli, K.M.; Fazal, F.; Sassano, A.; Nakayama, K.I.; Hay, N.; Platanias, L.C.; Rahman, A. Protein kinase C-delta and phosphatidylinositol 3-kinase/Akt activate mammalian target of rapamycin to modulate NF-kappaB activation and intercellular adhesion molecule-1 (ICAM-1) expression in endothelial cells. J. Biol. Chem. 2009, 284, 4052–4061. [Google Scholar] [CrossRef]
- Chen, Q.; Powell, D.W.; Rane, M.J.; Singh, S.; Butt, W.; Klein, J.B.; McLeish, K.R. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J. Immunol. 2003, 170, 5302–5308. [Google Scholar] [CrossRef]
- Ravetch, J.V.; Bolland, S. IgG Fc Receptors. Annu. Rev. Immunol. 2001, 19, 275–290. [Google Scholar] [CrossRef]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Lee, C.Y.; Mitra, A.; Kim, H.; Woo, B.Y.; Hong, Y.D.; Noh, J.K.; Yi, D.-K.; Kim, H.G.; Cho, J.Y. Anti-Inflammatory Effects of Huberia peruviana Cogn. Methanol Extract by Inhibiting Src Activity in the NF-κB Pathway. Plants 2021, 10, 2335. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Zhu, H.; Li, H.; Li, Y.; Zhao, B.; Jin, Y.-H. Ginsenoside compound K inhibits nuclear factor-kappa B by targeting Annexin A2. J. Ginseng Res. 2019, 43, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Kim, M.-Y.; Cho, J.Y. Olea europaea Suppresses Inflammation by Targeting TAK1-Mediated MAP Kinase Activation. Molecules 2021, 26, 1540. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, B.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Yi, S.A.; Han, J.W.; Kim, S.; Kim, J.K.; Kim, J.C.; et al. Identification of anti-adipogenic withanolides from the roots of Indian ginseng (Withania somnifera). J. Ginseng Res. 2022, 46, 357–366. [Google Scholar] [CrossRef]




| PCR | Organism | Target | Direction | Sequence (5′ to 3′) |
|---|---|---|---|---|
| Semi-quantitative | Mouse | IL-1β | Forward | CAGGATGAGGACATGAGCACC |
| Semi-quantitative | Mouse | IL-1β | Reverse | CTCTGCAGACTCAAACTCCAC |
| Semi-quantitative | Mouse | iNOS | Forward | TGCCAGGGTCACAACTTTACA |
| Semi-quantitative | Mouse | iNOS | Reverse | ACCCCAAGCAAGACTTGGAC |
| Semi-quantitative | Mouse | COX-2 | Forward | TCACGTGGAGTCCGCTTTAC |
| Semi-quantitative | Mouse | COX-2 | Reverse | CTTCGCAGGAAGGGGATGTT |
| Semi-quantitative | Mouse | GAPDH | Forward | CACTCACGGCAAATTCAACGGCA |
| Semi-quantitative | Mouse | GAPDH | Reverse | GACTCCACGACATACTCAGCAC |
| Realtime | Mouse | IL-1β | Forward | GTGAAATGCCACCTTTTACAGTG |
| Realtime | Mouse | IL-1β | Reverse | CCTGCCTGAAGCTCTTGTTG |
| Realtime | Mouse | iNOS | Forward | GCCACCAACAATGGCAACAT |
| Realtime | Mouse | iNOS | Reverse | TCGATGCACAACTGGGTGAA |
| Realtime | Mouse | COX-2 | Forward | TTGGAGGCGAAGTGGGTTTT |
| Realtime | Mouse | COX-2 | Reverse | TGGCTGTTTTGGTAGGCTGT |
| Realtime | Mouse | GAPDH | Forward | TGTGAACGGATTTGGCCGTA |
| Realtime | Mouse | GAPDH | Reverse | ACTGTGCCGTTGAATTTGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Cho, J.Y.; Kim, D. Hyptis obtusiflora C. Presl ex Benth Methanolic Extract Exhibits Anti-Inflammatory and Anti-Gastritis Activities via Suppressing AKT/NF-κB Pathway. Plants 2023, 12, 1146. https://doi.org/10.3390/plants12051146
Oh J, Cho JY, Kim D. Hyptis obtusiflora C. Presl ex Benth Methanolic Extract Exhibits Anti-Inflammatory and Anti-Gastritis Activities via Suppressing AKT/NF-κB Pathway. Plants. 2023; 12(5):1146. https://doi.org/10.3390/plants12051146
Chicago/Turabian StyleOh, Jieun, Jae Youl Cho, and Daewon Kim. 2023. "Hyptis obtusiflora C. Presl ex Benth Methanolic Extract Exhibits Anti-Inflammatory and Anti-Gastritis Activities via Suppressing AKT/NF-κB Pathway" Plants 12, no. 5: 1146. https://doi.org/10.3390/plants12051146
APA StyleOh, J., Cho, J. Y., & Kim, D. (2023). Hyptis obtusiflora C. Presl ex Benth Methanolic Extract Exhibits Anti-Inflammatory and Anti-Gastritis Activities via Suppressing AKT/NF-κB Pathway. Plants, 12(5), 1146. https://doi.org/10.3390/plants12051146







