Interactive Effect of Arbuscular Mycorrhizal Fungi (AMF) and Olive Solid Waste on Wheat under Arsenite Toxicity
Abstract
1. Introduction
2. Results
2.1. AMF Colonization
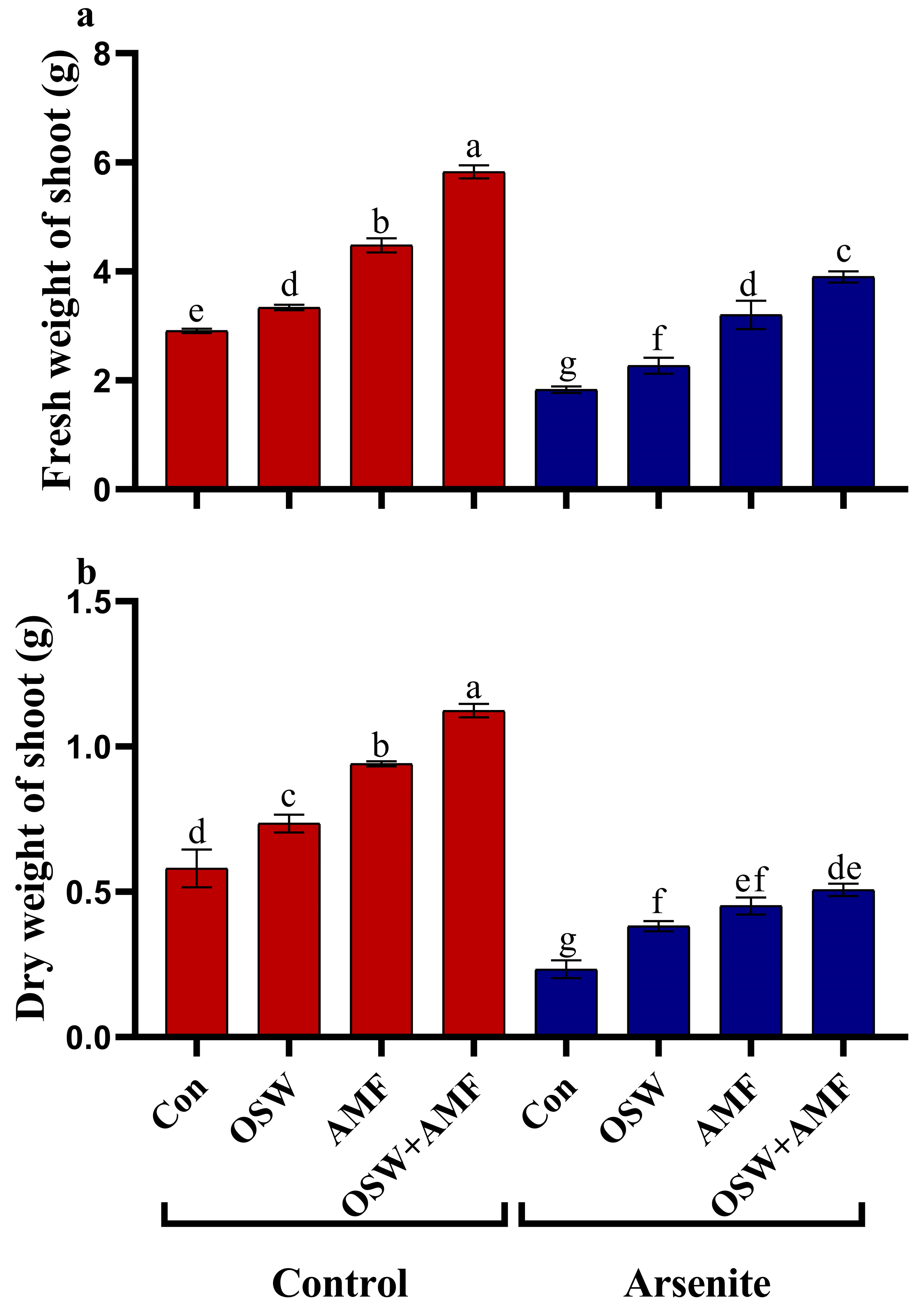
2.2. Growth Analysis
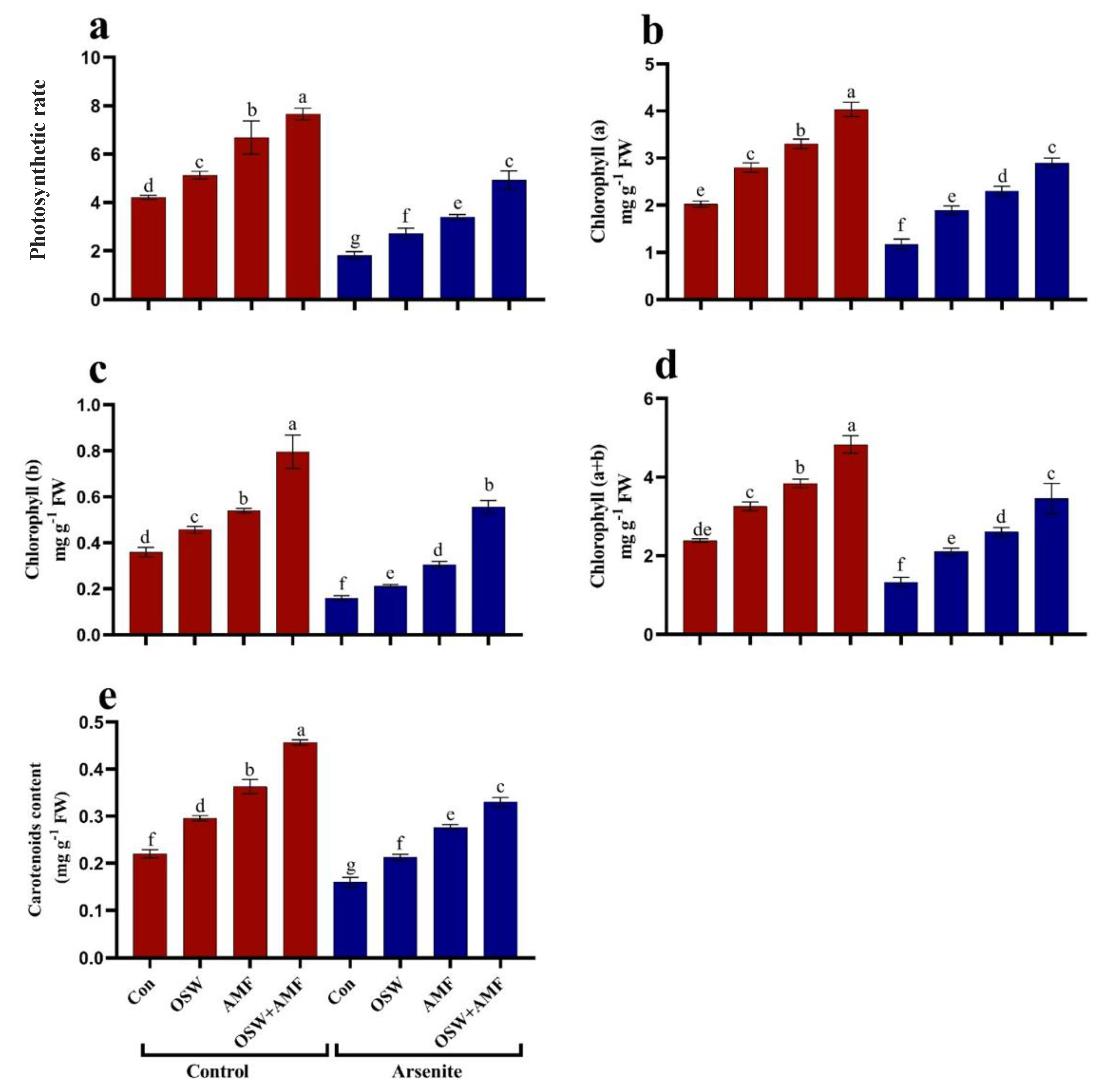
2.3. Photosynthetic Rate and Pigments Contents
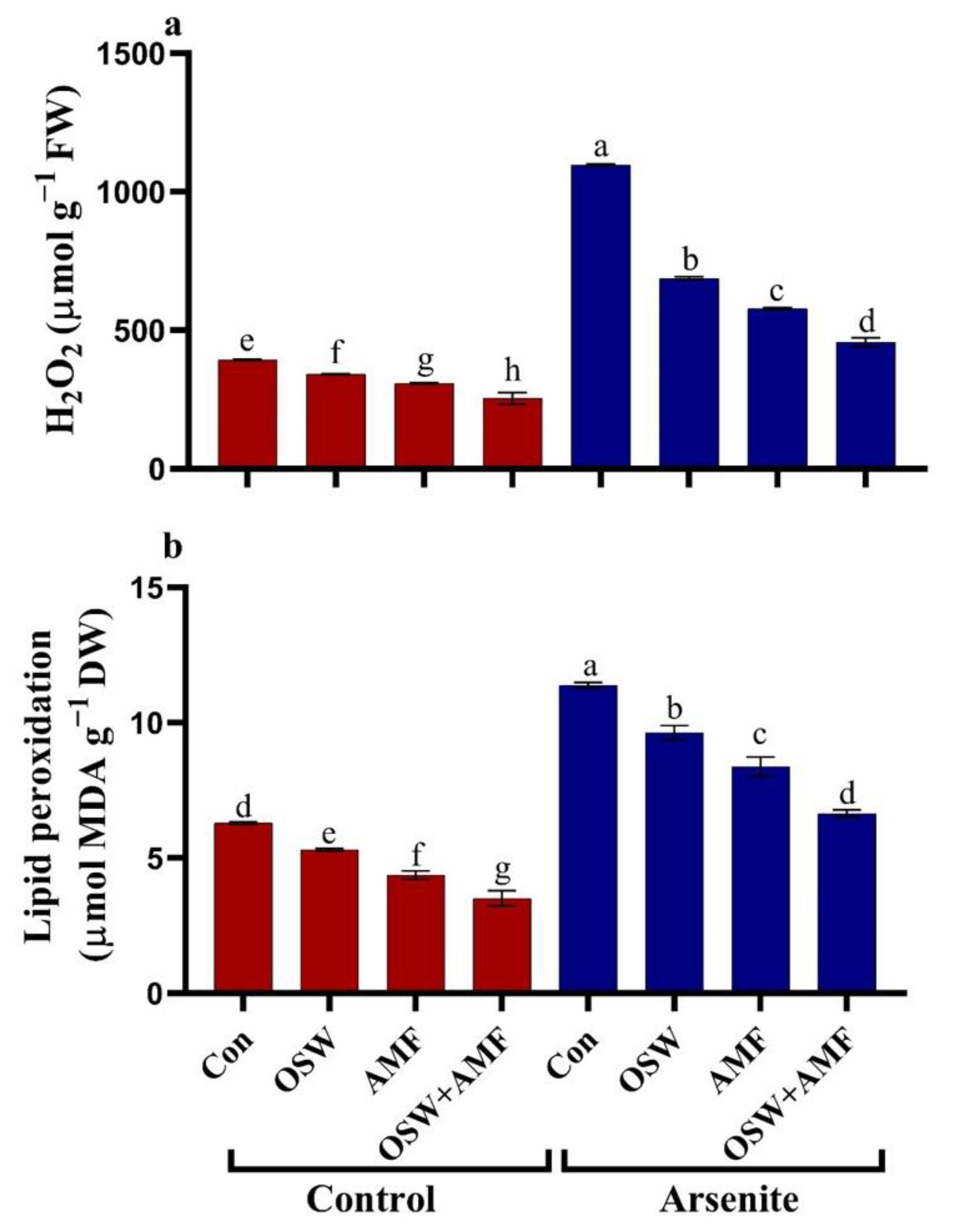
2.4. Oxidative Damage in Wheat Plants under AsIII Stress
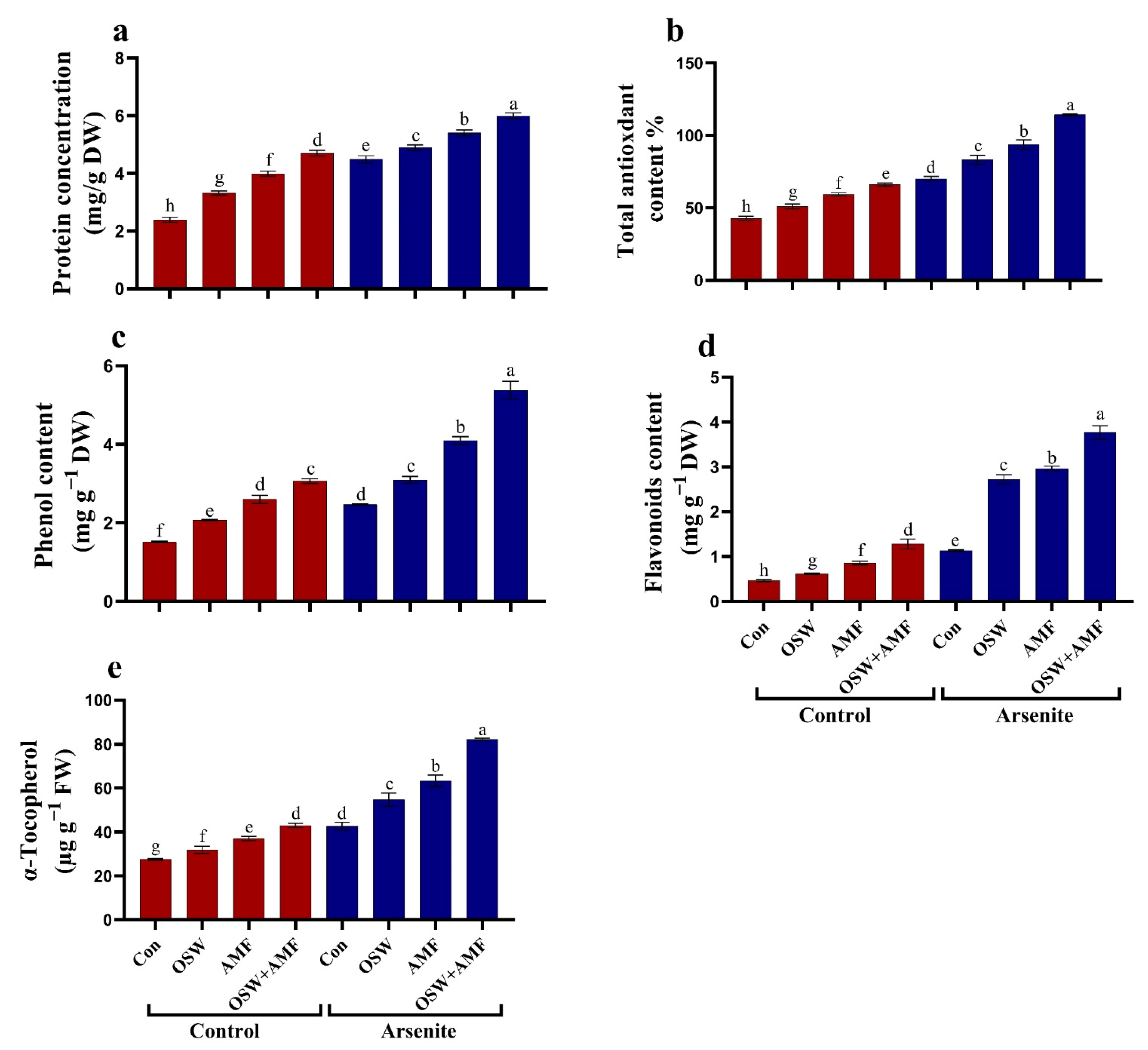
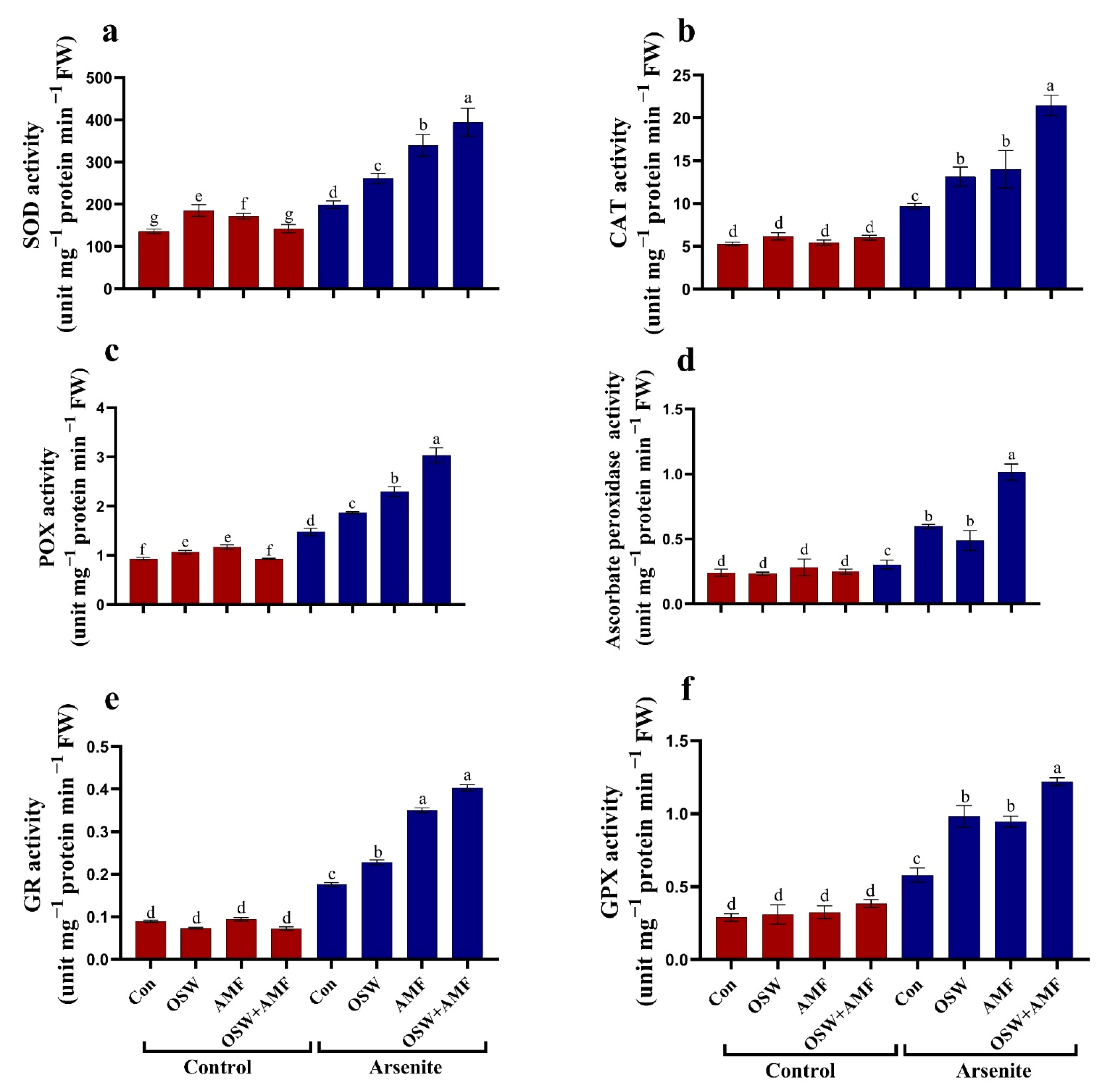
2.5. Antioxidant Defense System
Antioxidant Metabolites
2.6. Effects of OSW, AMF, and Combination (OSW+AMF) Application on Antioxidant Stressed Plants Were Enzymes in Wheat Plants under AsIII Stress
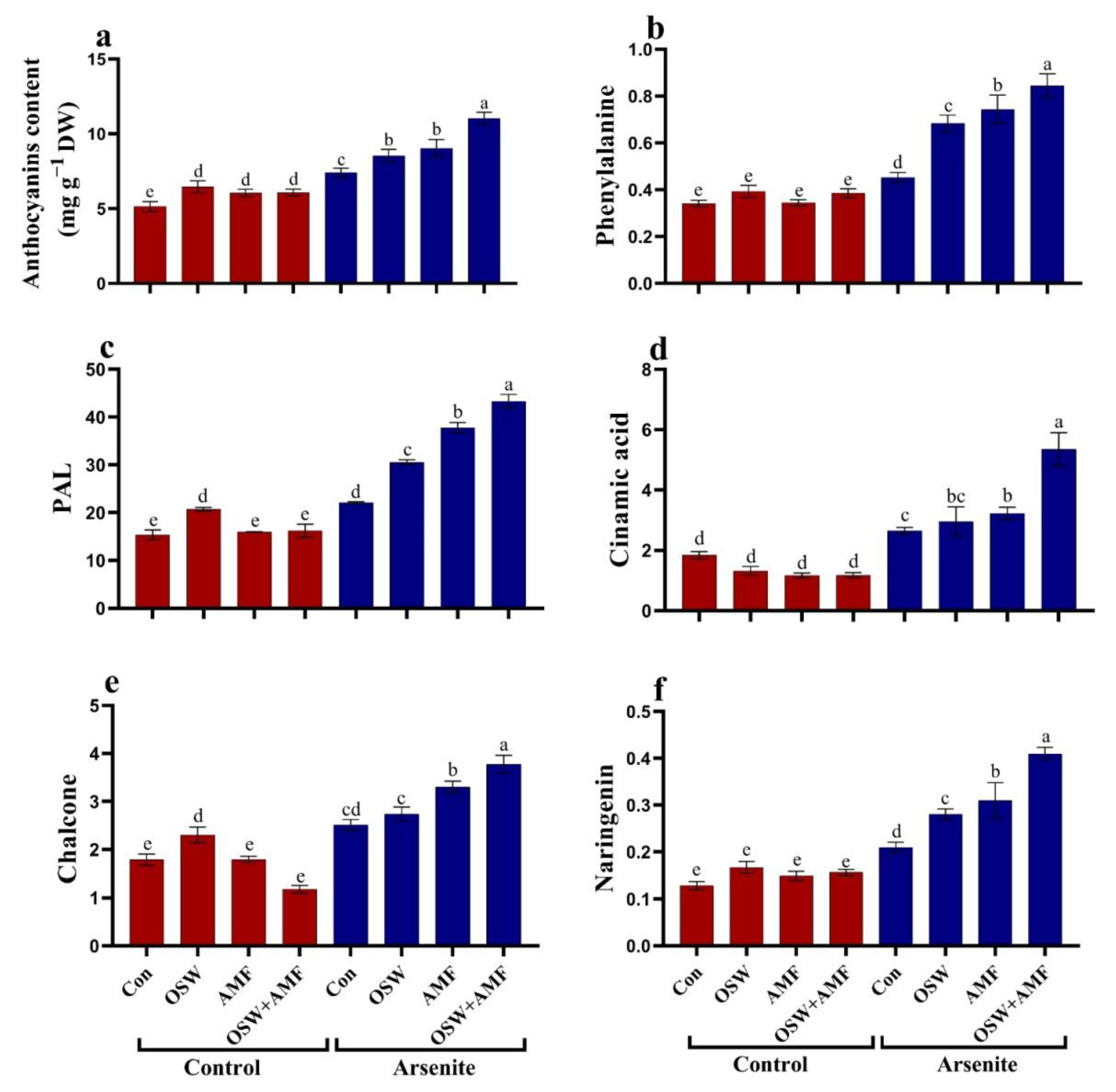
2.7. Anthocyanin Metabolism in Wheat Plants under AsIII Stress
3. Discussion
4. Material and Methods
4.1. Experimental Set up
4.2. Soil Analysis
4.3. Photosynthesis Related Parameters
4.4. Stress Markers
4.5. Antioxidant Metabolites
4.6. Antioxidant Enzyme Activities
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhtar, N.; Ishak, M.I.S.; Bhawani, S.A.; Umar, K. Various natural and anthropogenic factors responsible for water quality degradation: A review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs)(arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Kayode, O.T.; Aizebeokhai, A.P.; Odukoya, A.M. Arsenic in agricultural soils and implications for sustainable agriculture. In IOP Conference Series: A. M. Arsenic in agricultural soils and implications for sustainable agriculture. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; p. 012081. [Google Scholar]
- Vahter, M.; Concha, G.; Nermell, B.; Nilsson, R.; Dulout, F.; Natarajan, A.T. A unique metabolism of inorganic arsenic in native Andean women. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 1995, 293, 455–462. [Google Scholar] [CrossRef]
- Wu, J.; Liang, J.; Björn, L.O.; Li, J.; Shu, W.; Wang, Y. Phosphorus-arsenic interaction in the ‘soil-plant-microbe’system and its influence on arsenic pollution. Sci. Total Environ. 2022, 802, 149796. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, R.; Khan, M.H.; Choudhury, S. Arsenic in rice: An overview on stress implications, tolerance and mitigation strategies. In Plants under Metal and Metalloid Stress: Responses, Tolerance and Remediation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 401–415. [Google Scholar]
- Mondal, S.; Pramanik, K.; Ghosh, S.K.; Pal, P.; Ghosh, P.K.; Ghosh, A.; Maiti, T.K. Molecular insight into arsenic uptake, transport, phytotoxicity, and defense responses in plants: A critical review. Planta 2022, 255, 87. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yang, Y. Anthocyanin-mediated arsenic tolerance in plants. Environ. Pollut. 2022, 292, 118475. [Google Scholar] [CrossRef]
- Spagnoletti, F.; Carmona, M.; Gómez, N.E.T.; Chiocchio, V.; Lavado, R.S. Arbuscular mycorrhiza reduces the negative effects of M. phaseolina on soybean plants in arsenic-contaminated soils. Appl. Soil Ecol. 2017, 121, 41–47. [Google Scholar]
- Finnegan, P.M.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Abuelsoud, W.; Hassan, Y.M.; Alkhalifah, D.H.M.; Hozzein, W.N.; Zrieq, R.; Beemster, G.T.; Schoenaers, S. An actinomycete strain of Nocardiopsis lucentensis reduces arsenic toxicity in barley and maize. J. Hazard. Mater. 2021, 417, 126055. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A.; Hartley-Whitaker, J. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol. 2002, 154, 29–43. [Google Scholar] [CrossRef]
- Vejvodová, K.; Száková, J.; García-Sánchez, M.; Praus, L.; Romera, I.G.; Tlustoš, P. Effect of Dry Olive Residue–Based Biochar and Arbuscular Mycorrhizal Fungi Inoculation on the Nutrient Status and Trace Element Contents in Wheat Grown in the AsIII, Cd-, Pb-, and Zn-Contaminated Soils. J. Soil Sci. Plant Nutr. 2020, 20, 1067–1079. [Google Scholar] [CrossRef]
- Maksoud, M.A.; Bekhit, M.; El-Sherif, D.M.; Sofy, A.R.; Sofy, M.R. Gamma radiation-induced synthesis of a novel chitosan/silver/Mn-Mg ferrite nanocomposite and its impact on cadmium accumulation and translocation in brassica plant growth. Int. J. Biol. Macromol. 2022, 194, 306–316. [Google Scholar] [CrossRef]
- Badawy, I.H.; Hmed, A.A.; Sofy, M.R.; Al-Mokadem, A.Z. Alleviation of Cadmium and Nickel Toxicity and Phyto-Stimulation of Tomato plant L. by Endophytic Micrococcus luteus and Enterobacter cloacae. Plants 2022, 11, 2018. [Google Scholar] [CrossRef]
- Torres, M.; Pierantozzi, P.; Searles, P.; Rousseaux, M.C.; García-Inza, G.; Miserere, A.; Bodoira, R.; Contreras, C.; Maestri, D. Olive cultivation in the southern hemisphere: Flowering, water requirements and oil quality responses to new crop environments. Front. Plant Sci. 2017, 8, 1830. [Google Scholar] [CrossRef]
- Rusan, M.J.M.; Albalasmeh, A.A.; Malkawi, H.I. Treated Olive solid wasteeffects on soil properties and plant growth. Water Air Soil Pollut. 2016, 227, 135. [Google Scholar] [CrossRef]
- Marks, E.A.; Kinigopoulou, V.; Akrout, H.; Azzaz, A.A.; Doulgeris, C.; Jellali, S.; Rad, C.; Sánchez Zulueta, P.; Tziritis, E.; El-Bassi, L. Potential for production of biochar-based fertilizers from olive mill waste in Mediterranean Basin countries: An initial assessment for Spain, Tunisia, and Greece. Sustainability 2020, 12, 6081. [Google Scholar] [CrossRef]
- Zipori, I.; Erel, R.; Yermiyahu, U.; Ben-Gal, A.; Dag, A. Sustainable management of olive orchard nutrition: A review. Agriculture 2020, 10, 11. [Google Scholar] [CrossRef]
- Anli, M.; Fakhech, A.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laoaune, R.; Ait-Rahou, Y.; Meddich, A. Comparing the Response of Growth and Physiologic Variables of Onion to Olive solid wasteApplication and Arbuscular Mycorrhizal Fungi Inoculation. Gesunde Pflanz. 2022, 1–12. [Google Scholar] [CrossRef]
- Nanjundappa, A.; Bagyaraj, D.J.; Saxena, A.K.; Kumar, M.; Chakdar, H. Interaction between arbuscular mycorrhizal fungi and Bacillus spp. in soil enhancing growth of crop plants. Fungal Biol. Biotechnol. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Delta, A.K.; Kaushik, P. Effects of Funneliformis mosseae and potassium silicate on morphological and biochemical traits of onion cultivated under water stress. Horticulturae 2022, 8, 663. [Google Scholar] [CrossRef]
- Praveen, A.; Mehrotra, S.; Singh, N. Mixed plantation of wheat and accumulators in arsenic contaminated plots: A novel way to reduce the uptake of arsenic in wheat and load on antioxidative defence of plant. Ecotoxicol. Environ. Saf. 2019, 182, 109462. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Quraishi, U.M.; Malik, R.N. Arsenic uptake and toxicity in wheat (Triticum aestivum L.): A review of multi-omics approaches to identify tolerance mechanisms. Food Chem. 2021, 355, 129607. [Google Scholar] [CrossRef]
- Fatoki, J.O.; Badmus, J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022, 5, 100052. [Google Scholar] [CrossRef]
- Banerjee, M.; Banerjee, N.; Bhattacharjee, P.; Mondal, D.; Lythgoe, P.R.; Martínez, M.; Pan, J.; Polya, D.A.; Giri, A.K. High arsenic in rice is associated with elevated genotoxic effects in humans. Sci. Rep. 2013, 3, 2195. [Google Scholar] [CrossRef]
- Chandrakar, V.; Pandey, N.; Keshavkant, S. Plant responses to arsenic toxicity: Morphology and physiology. In Mechanisms of Arsenic Toxicity and Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 27–48. [Google Scholar]
- Zaheer, M.S.; Ali, H.H.; Erinle, K.O.; Wani, S.H.; Okon, O.G.; Nadeem, M.A.; Nawaz, M.; Bodlah, M.A.; Waqas, M.M.; Iqbal, J.; et al. Inoculation of Azospirillum brasilense and exogenous application of trans-zeatin riboside alleviates arsenic induced physiological damages in wheat (Triticum aestivum). Environ. Sci. Pollut. Res. 2022, 29, 33909–33919. [Google Scholar] [CrossRef]
- de Andrade, S.A.L.; Domingues Jr, A.P.; Mazzafera, P. Photosynthesis is induced in rice plants that associate with arbuscular mycorrhizal fungi and are grown under arsenite and arsenite stress. Chemosphere 2015, 134, 141–149. [Google Scholar] [CrossRef]
- Langer, I.; Syafruddin, S.; Steinkellner, S.; Puschenreiter, M.; Wenzel, W.W. Plant growth and root morphology of Phaseolus vulgaris L. grown in a split-root system is affected by heterogeneity of crude oil pollution and mycorrhizal colonization. Plant Soil 2010, 332, 339–355. [Google Scholar] [CrossRef]
- Curaqueo, G.; Schoebitz, M.; Borie, F.; Caravaca, F.; Roldán, A. Inoculation with arbuscular mycorrhizal fungi and addition of composted olive-mill waste enhance plant establishment and soil properties in the regeneration of a heavy metal-polluted environment. Environ. Sci. Pollut. Res. 2014, 21, 7403–7412. [Google Scholar] [CrossRef]
- Gupta, S.; Thokchom, S.D.; Kapoor, R. Arbuscular mycorrhiza improves photosynthesis and restores alteration in sugar metabolism in Triticum aestivum L. grown in arsenic contaminated soil. Front. Plant Sci. 2021, 12, 640379. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Anand, G.; Singh, N.; Kapoor, R. Arbuscular mycorrhiza augments arsenic tolerance in wheat (Triticum aestivum L.) by strengthening antioxidant defense system and thiol metabolism. Front. Plant Sci. 2017, 8, 906. [Google Scholar] [CrossRef]
- Hristozkova, M.; Geneva, M.; Stancheva, I.; Boychinova, M.; Djonova, E. Contribution of arbuscular mycorrhizal fungi in attenuation of heavy metal impact on Calendula officinalis development. Appl. Soil Ecol. 2016, 101, 57–63. [Google Scholar] [CrossRef]
- Alam, M.Z.; McGee, R.; Hoque, M.A.; Ahammed, G.J.; Carpenter-Boggs, L. Effect of arbuscular mycorrhizal fungi, selenium and biochar on photosynthetic pigments and antioxidant enzyme activity under arsenic stress in mung bean (Vigna radiata). Front. Physiol. 2019, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Garg, N.; Cheema, A. Relative roles of Arbuscular Mycorrhizae in establishing a correlation between soil properties, carbohydrate utilization and yield in Cicer arietinum L. under As stress. Ecotoxicol. Environ. Saf. 2021, 207, 111196. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.T.; Manias, D.M.; Kyritsis, D.C.; Goussis, D.A. NO formation and autoignition dynamics during combustion of H2O-diluted NH3/H2O2 mixtures with air. Energies 2020, 14, 84. [Google Scholar] [CrossRef]
- Harkousse, O.; Slimani, A.; Jadrane, I.; Aitboulahsen, M.; Mazri, M.A.; Zouahri, A.; Ouahmane, L.; Koussa, T.; Al Feddy, M.N. Role of local biofertilizer in enhancing the oxidative stress defence systems of date palm seedling (Phoenix dactylifera) against abiotic stress. Appl. Environ. Soil Sci. 2021, 2021, 6628544. [Google Scholar] [CrossRef]
- Langeroodi, A.R.S.; Campiglia, E.; Mancinelli, R.; Radicetti, E. Can biochar improve pumpkin productivity and its physiological characteristics under reduced irrigation regimes? Sci. Hortic. 2019, 247, 195–204. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Ahmed, M.; Mohammed, A.E.; Alotaibi, M.O.; Yehia, R.S.; Selim, S.; Saleh, A.M.; Beemster, G.T.; Sheteiwy, M.S. Increasing atmospheric CO2 differentially supports arsenite stress mitigating impact of arbuscular mycorrhizal fungi in wheat and soybean plants. Chemosphere 2022, 296, 134044. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Zhang, X.N.; Zhang, L.; Yan, P.; Zhang, L.P.; Fu, J.Y.; Han, W.Y. Melatonin-mediated regulation of anthocyanin biosynthesis and antioxidant defense confer tolerance to arsenic stress in Camellia sinensis L. J. Hazard. Mater. 2021, 403, 123922. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.N.; Tahjib-Ul-Arif, M.; Hannan, A.; Sultana, N.; Akhter, S.; Hasanuzzaman, M.; Akter, F.; Hossain, M.S.; Sayed, M.A.; Hasan, M.T.; et al. Melatonin modulates plant tolerance to heavy metal stress: Morphological responses to molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Noceto, P.A.; Bettenfeld, P.; Boussageon, R.; Hériché, M.; Sportes, A.; van Tuinen, D.; Courty, P.E.; Wipf, D. Arbuscular mycorrhizal fungi, a key symbiosis in the development of quality traits in crop production, alone or combined with plant growth-promoting bacteria. Mycorrhiza 2021, 31, 655–669. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Tennant, D. A test of a modified line intersect method of estimating root length. J. Ecol. 1975, 73, 995–1001. [Google Scholar] [CrossRef]
- Rillig, M.C.; Allen, M.F. What is the role of arbuscular mycorrhizal fungi in plant-to-ecosystem responses to elevated atmospheric CO2? Mycorrhiza 1999, 9, 1–8. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Brown, M.E.; Burlingham, S.K.; Jackson, R.M. Studies on Azotobacter species in soil, II. Populations of Azotobacter in the rhizosphere and effects of artificial inoculation. Plant Soil 1962, 17, 320–332. [Google Scholar] [CrossRef]
- De Sousa, A.; AbdElgawad, H.; Han, A.; Teixeira, J.; Matos, M.; Fidalgo, F. Oxidative metabolism of rye Secale cereale L. after short term exposure to aluminum, uncovering the glutathione–ascorbate redox network. Front. Plant Sci. 2016, 7, 685. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.H.; Westjohn, D.B.; Helsel, D.R.; Wanty, R.B. Arsenic in ground water of the United States: Occurrence and geochemistry. Groundwater 2000, 38, 589–604. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Farfan-Vignolo, E.R.; De Vos, D.; Asard, H. Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci. 2015, 231, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hodges, E.V.; Boivin, M.; Vitaro, F.; Bukowski, W.M. The power of friendship, protection against an escalating cycle of peer victimization. Dev. Psychol. 1999, 35, 94. [Google Scholar] [CrossRef]
- Levine, R.L.; Williams, J.A.; Stadtman, E.P.; Shacter, E. [37] Carbonyl assays for determination of oxidatively modified proteins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1994; Volume 233, pp. 346–357. [Google Scholar]
- Jiang, Z.Y.; Woollard, A.C.; Wolff, S.P. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids 1991, 26, 853–856. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.L.; Reid, D.M. Leaf senescence and lipid peroxidation, Effects of some phytohormones, and scavengers of free radicals and singlet oxygen. Physiol. Plant. 1982, 56, 453–457. [Google Scholar] [CrossRef]
- Kumar, K.B.; Khan, P.A. Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv.PR 202) leaves during senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- Murshed, S.S.; Tan, S.H.; Nguyen, N.T. Temperature dependence of interfacial properties and viscosity of nanofluids for droplet-based microfluidics. J. Phys. D Appl. Phys. 2008, 41, 085502. [Google Scholar] [CrossRef]
- Drotar, A.; Phelps, P.; Fall, R. Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci. 1985, 42, 35–40. [Google Scholar] [CrossRef]

| Root Colonization (%) | Hyphal Length (cm g−1 Soil) | Arbuscules Numbers (cm−1 Root) | |
|---|---|---|---|
| AMF | 56.98 ± 6.45 a | 24.17 ± 3.76 b | 6.04 ± 0.37 a |
| AMF+OSW | 61.8 ± 2.3 a | 26.17 ± 3.0 a | 5.95 ± 0.4 a |
| AsIII + AMF | 30.8 ± 1.18 c | 12.26 ± 0.48 c | 3.6 ± 0.68 c |
| AsIII + OSW + AMF | 34.01 ± 7.39 b | 13.63 ± 1.46 c | 4.2 ± 0.23 b |
| Treatment Code | Treatments |
|---|---|
| T1 | Control soil |
| T2 | Soil treated with olive solid waste (OSW, 4% w/w) |
| T3 | AMF-inoculated soil |
| T4 | AMF and OSW treated soil |
| T5 | AsIII-treated soil (as NaAsO2 at 100 mg/kg soil) |
| T6 | Soil treated with AsIII and OSW |
| T7 | Soil treated with AsIII + AMF |
| T8 | Soil treated with AsIII, OSW and AMF |
| Physico-Chemical Characterization | |
|---|---|
| Water content (%) | 85.46 ± 5.93 |
| Dry matter (%) | 13.36 ± 1.47 |
| pH | 5.96 ± 0.42 |
| Electric conductivity (mS cm−1 ) | 14.46 ± 1.03 |
| Chemical oxygen demand (g L−1 ) | 101.49 ± 11.2 |
| Biochemical oxygen demand (gL−1) | 52.11 ± 5.75 |
| Organic matter (g L−1) | 64.65 ± 4.6 |
| Salinity (g L−1 ) | 7.21 ± 0.51 |
| Minerals | |
| Nitrogen (N) (g L−1) | 1.96 ± 0.14 |
| Phosphorus (P) (g L−1) | 1.72 ± 0.19 |
| Potassium (K) (g L−1) | 9.71 ± 1.07 |
| Calcium (Ca) (g L−1) | 0.93 ± 0.07 |
| Magnesium (Mg) (g L−1) | 0.5 ± 0.04 |
| Sodium (Na) (g L−1) | 1.78 ± 0.13 |
| Chloride (Cl) (g L−1) | 1.25 ± 0.09 |
| Iron (Fe) (g L−1) | 0.71 ± 0.05 |
| Zinc (Zn) (g L−1) | 0.18 ± 0.01 |
| Antioxidant | |
| Antioxidant Activity (FRAP) | 53.66 ± 4.98 |
| Antioxidant Activity DPPH (%) | 65.34 ± 9.71 |
| Phenols | |
| Total phenols (g L−1) | 3.96 ± 0.97 |
| Flavonoids (g L−1) | 1 ± 0.24 |
| Caffeic acid | 0.03 ± 0 |
| Ferulic acid | 3.23 ± 0.16 |
| Protocatechuic acid | 0.32 ± 0.03 |
| Catechin | 0.97 ± 0.05 |
| Galic acid | 20.88 ± 2.48 |
| p-Coumaric acid | 4.57 ± 0.5 |
| Resorcinol | 0.04 ± 0.01 |
| Chlorogenic acid | 0.26 ± 0.04 |
| Syringic acid | 1.43 ± 0.08 |
| Quercetin | 0.06 ± 0 |
| Quercetrin | 0.1 ± 0.01 |
| Luteolin | 0.07 ± 0.03 |
| Apigenin | 0.4 ± 0.24 |
| Isoquercetrin | 0.45 ± 0.04 |
| Rutin | 0.05 ± 0 |
| Ellagic acid | 0.02 ± 0 |
| Velutin | 0.02 ± 0 |
| Naringenin | 0.07 ± 0.01 |
| Genistein | 0.11 ± 0.01 |
| Daidzein | 0.16 ± 0.01 |
| Fisetin | 0.21 ± 0.02 |
| O-hydroxydaidzein | 0.02 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albqmi, M.; Selim, S.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; Jaouni, S.K.A.; Hussein, S.; Warrad, M.; Sofy, M.R.; AbdElgawad, H. Interactive Effect of Arbuscular Mycorrhizal Fungi (AMF) and Olive Solid Waste on Wheat under Arsenite Toxicity. Plants 2023, 12, 1100. https://doi.org/10.3390/plants12051100
Albqmi M, Selim S, Al-Sanea MM, Alnusaire TS, Almuhayawi MS, Jaouni SKA, Hussein S, Warrad M, Sofy MR, AbdElgawad H. Interactive Effect of Arbuscular Mycorrhizal Fungi (AMF) and Olive Solid Waste on Wheat under Arsenite Toxicity. Plants. 2023; 12(5):1100. https://doi.org/10.3390/plants12051100
Chicago/Turabian StyleAlbqmi, Mha, Samy Selim, Mohammad M. Al-Sanea, Taghreed S. Alnusaire, Mohammed S. Almuhayawi, Soad K. Al Jaouni, Shaimaa Hussein, Mona Warrad, Mahmoud R. Sofy, and Hamada AbdElgawad. 2023. "Interactive Effect of Arbuscular Mycorrhizal Fungi (AMF) and Olive Solid Waste on Wheat under Arsenite Toxicity" Plants 12, no. 5: 1100. https://doi.org/10.3390/plants12051100
APA StyleAlbqmi, M., Selim, S., Al-Sanea, M. M., Alnusaire, T. S., Almuhayawi, M. S., Jaouni, S. K. A., Hussein, S., Warrad, M., Sofy, M. R., & AbdElgawad, H. (2023). Interactive Effect of Arbuscular Mycorrhizal Fungi (AMF) and Olive Solid Waste on Wheat under Arsenite Toxicity. Plants, 12(5), 1100. https://doi.org/10.3390/plants12051100










