Seed Bank Community under Different-Intensity Agrophytocenoses on Hilly Terrain in Lithuania
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Soil Description and Experimental Design
2.2. Trial Factors and Treatments
2.3. Methods of Analysis
2.4. Statistical Analysis
3. Results
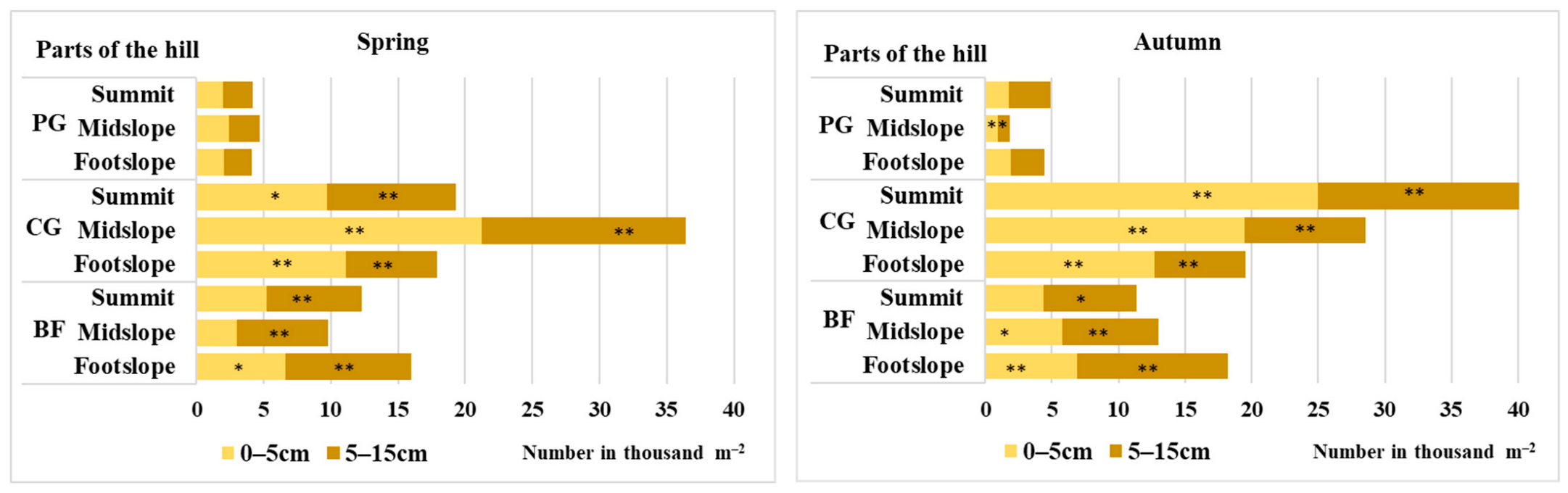
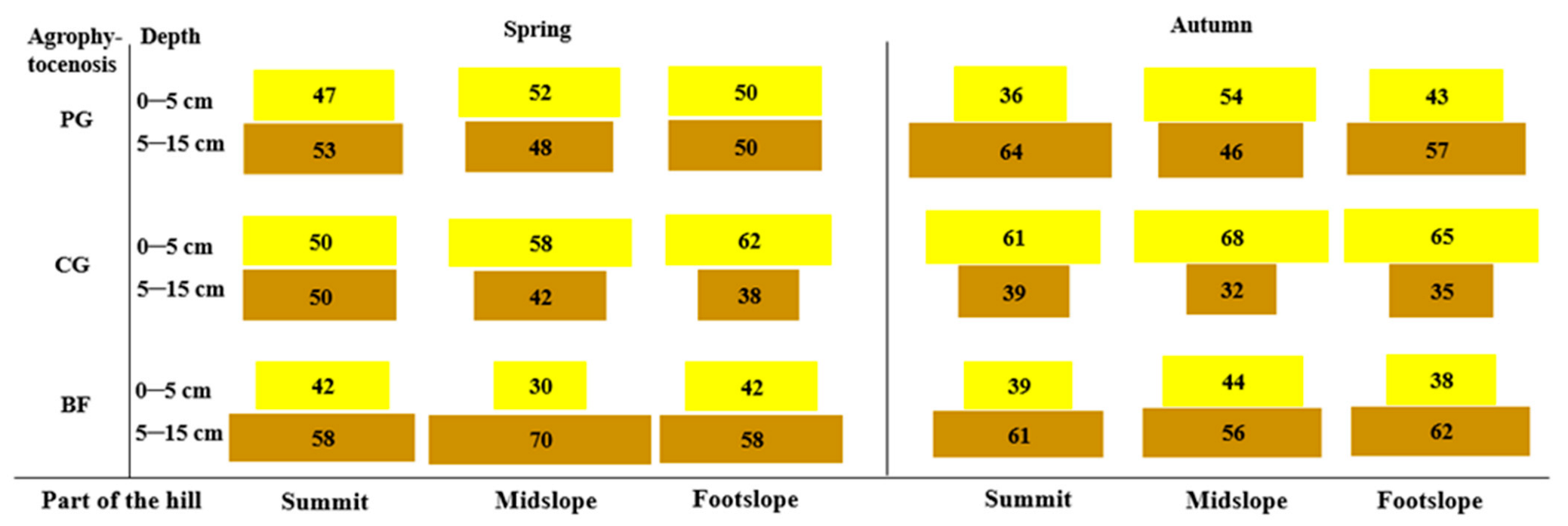
3.1. Seed Reserves in the Soil and the Vertical Distribution of the Soil Seed Bank
3.2. The Number of Seed Species
3.3. Seed Surface Morphological Traits of Soil Seed Bank
3.4. Correlation between the Seed Number and Soil Microbial Biomass Carbon
4. Discussion
4.1. Seed Reserves in the Soil and the Vertical Distribution of the Soil Seed Bank
4.2. The Number of Seed Species
4.3. Seed Surface Morphological Traits of Soil Seed Bank
4.4. Correlation between the Seed Number and Soil Microbial Biomass Carbon
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, S.; Pradhan, S.S.; Chauhan, J. Dynamics of weed seed bank and its management for sustainable crop production. Int. J. Chem. Stud. 2018, 6, 643–647. [Google Scholar]
- Hopfensperger, K.N. A Review of similarity between seed bank and standing vegetation across ecosystems. Oikos 2007, 116, 1438–1448. [Google Scholar] [CrossRef]
- Ghersa, C.M.; Martínez–Ghersa, M.A. Ecological correlates of weed seed size and persistence in the soil under different tilling systems: Implications for weed management. Field Crop. Res. 2000, 67, 141–148. [Google Scholar] [CrossRef]
- Bochet, E. The fate of seeds in the soil: A review of the influence of overland flow on seed removal and its consequences for the vegetation of arid and semiarid patchy ecosystems. Soil 2015, 1, 131–146. [Google Scholar] [CrossRef]
- Shiferaw, W.; Demissew, S.; Bekele, T. Ecology of soil seed banks: Implications for conservation and restoration of natural vegetation: A review. Int. J. Biodivers. Conserv. 2018, 10, 380–393. [Google Scholar]
- Bekker, R.M.; Bakker, J.P.; Grandin, U.; Kalamees, R.; Milberg, P.; Poschlod, P.; Thompson, K.; Willems, J.H. Seed size, shape and vertical distribution in the soil: Indicators of seed longevity. Funct. Ecol. 1998, 12, 834–842. [Google Scholar] [CrossRef]
- Tóth, Á.; Deák, B.; Tóth, K.; Kiss, R.; Lukács, K.; Rádai, Z.; Godó, L.; Borza, S.; Kelemen, A.; Miglécz, T.; et al. Vertical distribution of soil seed bank and the ecological importance of deeply buried seeds in alkaline grasslands. PeerJ 2022, 10, 13226. [Google Scholar] [CrossRef]
- Albrecht, H.; Auerswald, K. Sees traits in arable weed seed banks and their relationship to land-use changes. Basic. Appl. Ecol. 2009, 10, 516–524. [Google Scholar] [CrossRef]
- Gardarin, A.; Dürr, C.; Mannino, M.R.; Busset, H.; Colbach, N. Seed mortality in the soil is related to seed coat thickness. Seed Sci. Res. 2010, 20, 243–256. [Google Scholar] [CrossRef]
- Reine, R.; Chocarro, C.; Fillat, F. Soil seed bank and management regimes of semi-natural mountain meadow communities. Agric. Ecosyst Environ. 2004, 104, 567–575. [Google Scholar] [CrossRef]
- Santín-Montanyá, M.I.; Martín-Lammerding, D.; Zambrana, E.; Tenorio, J.L. Management of weed emergence and weed seed bank in response to different tillage, cropping systems and selected soil properties. Soil Tillage Res. 2016, 161, 38–46. [Google Scholar] [CrossRef]
- Auškalnienė, O.; Kadžienė, G.; Janušauskaitė, D.; Supronienė, S. Changes in weed seed bank and flora as affected by soil tillage systems. Zemdirbyste 2018, 105, 221–226. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Smagacz, J.; Kwiatkowski, C.A.; Harasim, E.; Wo’zniak, A. Weed Flora and Soil Seed Bank Composition as Affected by Tillage System in Three-Year Crop Rotation. Agriculture 2020, 10, 186. [Google Scholar] [CrossRef]
- Jankauskas, B. Soil Erosion: Case Study, Lithuania; Jakobsson, C., Ed.; Sustainable Agriculture; Ecosystem Health and Sustainable Agriculture: Uppsala, Sweden, 2012; pp. 231–238. [Google Scholar]
- Skuodienė, R.; Kinderienė, I.; Tomchuk, D.; Šlepetys, J.; Karčauskienė, D. Root development of temporary and permanent grasslands and their anti-erosion significance on a hilly terrain. Zemdirbyste 2020, 107, 209–216. [Google Scholar] [CrossRef]
- Burnside, O.C.; Wilson, R.G.; Weisberg, S.; Hubbard, K.G. Seed longevity of 41 weed species buried 17 years in eastern and western Nebraska. Weed Sci. 1996, 44, 74–86. [Google Scholar] [CrossRef]
- Benech-Arnold, R.L.; Sánchez, R.A.; Forcella, F.; Kruk, B.C.; Ghersa, C.M. Environmental control of dormancy in weed seed banks in soil. Field. Crops Res. 2000, 105–122. [Google Scholar] [CrossRef]
- Batlla, D.; Benech-Arnold, R.L. Predicting changes in dormancy level in natural seed soil banks. Plant Mol. Biol. 2010, 73, 3–13. [Google Scholar] [CrossRef]
- Swanton, C.J.; Shrestha, A.; Knezevic, S.Z.; Roy, R.C.; BallCoelho, B.R. Influence of tillage type on vertical weed seedbank distribution in a sandy soil. Can. J. Plant Sci. 2000, 80, 455–457. [Google Scholar] [CrossRef]
- Skuodienė, R.; Matyžiūtė, V. Soil Seed Bank in a Pre–Erosion Cereal–Grass Crop Rotation. Plants 2022, 11, 2636. [Google Scholar] [CrossRef]
- Rahman, A.; James, T.K.; Grbavac, N.; Mellsop, J. Evaluation of two methods for enumerating the soil weeds seedbank. In Proceedings of the 48th New Zealand Plant Protection Conference, Hastings, New Zealand, 8–10 August 1995; pp. 75–95. [Google Scholar]
- Grigas, A. Lietuvos Augalų Vaisiai ir Sėklos; Fruits and Seeds of Lithuanian Plants; Mokslas: Vilnius, Lithuania, 1986. (In Lithuanian) [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. Microbial biomass measurements in forest soils: Determination of KC values and tests of hypotheses to explain the failure of the chloroform fumigation-incubation method in acid soils. Soil Biol. Biochem. 1987, 19, 689–696. [Google Scholar] [CrossRef]
- Brookes, P.C. The use of microbial parameters in monitoring soil pollution by heavy metals. Biol. Fert. Soils. 1995, 19, 269–279. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil-I. Fumigation with chloroform. Soil Biol. Biochem. 1976, 8, 167–177. [Google Scholar] [CrossRef]
- Raudonius, S. Application of statistics in plant and crop research: Important issues. Zemdirbyste 2017, 104, 377–382. [Google Scholar] [CrossRef]
- Nei, M.; Li, W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef]
- Van de Peer, Y.; De Wachter, R. Treecon for Windows: A software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 1994, 10, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Cabin, R.J.; Mitchell, R.J.; Marshall, D.L. Do surface plant and soil seed bank populations differ genetically? A multipopulation study of the desert mustard Lesquerella fendleri (Brassicaceae). Am. J. Bot. 1998, 85, 1098–1109. [Google Scholar] [CrossRef]
- Plue, J.; Van Calster, H.; Auestad, I.; Basto, S.; Bekker, R.M.; Bruun, H.H.; Chevalier, R.; Decocq, G.; Grandin, U.; Hermy, M.; et al. Buffering effects of soil seed banks on plant community composition in response to land use and climate. Glob. Ecol. Biogeogr. 2020, 30, 128–139. [Google Scholar] [CrossRef]
- Wang, N.; He, X.; Zhao, F.; Wang, D.; Jiao, J. Soil seed bank in different vegetation types in the Loess Plateau region and its role in vegetation restoration. Restor. Ecol. 2020, 28, 5–12. [Google Scholar] [CrossRef]
- Wagner, M.; Poschlod, P.; Setchfield, R.P. Soil seed bank in managedand abandoned semi-natural meadows in Soomaa National Park, Estonia. Ann. Bot. Fenn. 2003, 40, 87–100. [Google Scholar]
- Skuodienė, R.; Matyžiūtė, V. Assessment of an abandoned grassland community and the soil seed bank of a hilly relief. Zemdirbyste 2022, 109, 3–10. [Google Scholar] [CrossRef]
- Lopez-Marino, A.; Luis-Calabuig, E.; Fillat, F.; Bermudez, F.F. Floristic composition of established vegetation and the soil seed bank in pasture communities under different traditional management regimes. Agric. Ecosyst. Environ. 2000, 78, 273–282. [Google Scholar] [CrossRef]
- Valkó, O.; Török, P.; Tóthmérész, B.; Matus, G. Restoration potential in seed banks of acidic fen and dry-mesophilous meadows: Can restoration be based on local seed banks? Restor. Ecol. 2011, 19, 9–15. [Google Scholar] [CrossRef]
- Janicka, M. The evaluation of soil seed bank in two arrhenatherion meadow habitats in Central Poland. Acta Sci. Pol. Agric. 2016, 15, 25–38. [Google Scholar]
- Streit, B.; Stamp, P.; Richner, W. Einfluss von unterschiedlicher Bodenbearbeitung Intersität auf Entwicklung von Unkraut Populationen in Ackerkulturen. Z. Für Pflanzenkrankh. Und Pflanzenschutz Sonderh. 2000, 17, 41–46. [Google Scholar]
- Skuodienė, R.; Karčauskienė, D.; Čiuberkis, S.; Repšienė, R.; Ambrazaitienė, D. The influence of primary soil tillage on soil weed seed bank and weed incidence in a cereal–grass crop rotation. Zemdirbyste 2013, 100, 25–32. [Google Scholar] [CrossRef]
- Čiuberkis, S. The influence of traditional and reduced soil tillage on crop weed infestation in crop rotation. Vagos 2008, 79, 37–42. [Google Scholar]
- Skuodienė, R.; Karčauskienė, D.; Repšienė, R.; Šiaudinis, G. Changes in the weed communities as affected by different primary soil tillage and deep loosening. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2018, 68, 643–648. [Google Scholar] [CrossRef]
- Albrecht, H. Development of arable weed seedbanks during the 6 years after the change from conventional to organic farming. Weed Res. 2005, 45, 339–350. [Google Scholar] [CrossRef]
- Stokes, A. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil. 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Trakhtenbrot, A.; Katul, G.G.; Nathan, R. Mechanistic modeling of seed dispersal by wind over hilly terrain. Ecol. Modell. 2014, 274, 29–40. [Google Scholar] [CrossRef]
- Lestari, D.A.; Pratiwi, A. Studi morfologi benih terpilih Annonaceae: Koleksi bank benih Kebun Raya Purwodadi. Pros. Sem. Nas. Masy. Biodiv. Indon. 2022, 8, 103–110. [Google Scholar]
- Kumar, A.; Choudhary, T.; Das, S.; Meena, K.S. Weed seed bank: Impacts and management for future crop production. Agron. Crop. 2019, 2, 207–223. [Google Scholar]
- Lewis, T.D.; Rowan, J.S.; Hawes, C.; McKenzie, B.M. Assessing the significance of soil erosion for arable weed seedbank diversity in agro-ecosystems. Prog. Phys. Geogr. 2013, 37, 622–641. [Google Scholar] [CrossRef]
- Schwartz–Lazaro, L.M.; Copes, J.T. A review of the soil seedbank from a weed scientists perspective. Agronomy 2019, 9, 369. [Google Scholar] [CrossRef]
- Nathan, R.; Schurr, F.M.; Spiegel, O.; Steinitz, O. Mechanisms of long–distance seed dispersal. Trends Ecol. Evol. 2008, 23, 638–647. [Google Scholar] [CrossRef]
- Diantina, S.; McGill, C.; Millner, J.; Nadarajan, J.; Pritchard, H.W.; McCormick, A.C. Comparative seed morphology of tropical and temperate orchid species with different growth habits. Plants 2020, 9, 161. [Google Scholar] [CrossRef]
- Gan, S.; Guo, J.; Zhang, Y.; Wang, X.; Huang, L. “Phoenix in flight”: An unique fruit morphology ensures wind dispersal of seeds of the phoenix tree (Firmiana simplex (L.) W. Wight). BMC Plant Biol. 2022, 22, 113–125. [Google Scholar] [CrossRef]
- Casper, H.A.; Leeuwen, V.; Sarneel, J.M.; Paassen, J.; Rip, W.J.; Bakker, E.S. Hydrology, shore morphology and species traits affect seed dispersal, germination and community assembly in shoreline plant communities. J. Ecol. 2014, 102, 998–1007. [Google Scholar]
- Csontos, P. Seed banks: Ecological definitions and sampling considerations. Community Ecol. 2007, 8, 75–85. [Google Scholar] [CrossRef]
- Chee–Sanford, J.; Fu, X. Investigating the role of microorganisms in soil seed bank management. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Méndez–Vilas, J., Ed.; Formatex Research Centre: Badajoz, Spain, 2010; Volume 1, pp. 257–266. [Google Scholar]
- Pollard, A. Seeds vs fungi: An enzymatic battle in the soil seedbank. Seed Sci. Res. 2018, 28, 197–214. [Google Scholar] [CrossRef]
- Bogužas, V.; Kairytė, A. Ploughless and no–tillage and residue impact on weed infestation in continuous barley. Vagos 2003, 57, 16–21. [Google Scholar]
- Dorado, J.; Monte, J.P.D.; López–Fando, C. Weed seedbank response to crop rotation and tillage in semiarid agroecosystems. Weed Sci. 1999, 47, 67–73. [Google Scholar] [CrossRef]
- Dick, W.A.; Daniel, T.C. Soil Chemical and Biological Properties as Affected by Conservation Tillage: Environmental Implications. In Effects Conservation Tillage on Ground Water Quality: Nitrates and Pesticides; Logan, T.J., Ed.; CRC Press: Chelsea, MI, USA, 1987; pp. 125–147. [Google Scholar]
- Poptrowska-Dlugosz, A.; Wilczewski, E. Changes in enzyme activities as affected by green manure catch crops and mineral nitrogen fertilization. Zemdirbyste 2014, 101, 139–146. [Google Scholar] [CrossRef]
- Nichols, V.; Verhulst, N.; Cox, R.; Govaerts, B. Weed dynamics and conservation agriculture principles: A review. Field Crop. Res. 2015, 183, 56–68. [Google Scholar] [CrossRef]
- Vigueira, O.C.; Vigueira, C.C.; Olsen, K.M.; Caicedo, A.L. The red queen in the corn: Agricultural weeds as models of rapid adaptive evolution. Heredity 2013, 110, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.E.; Burger, M.; Cavagnaro, T.R. Roots, nitrogen transformations, and ecosystem services. Annu. Rev. Plant Biol. 2008, 59, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, C.; Skovgaard, I.M. Crop and soil factors of importance for the distribution of plant species on arable fields in Denmark. Agric. Ecosyst. Environ. 2009, 133, 61–67. [Google Scholar] [CrossRef]
- Hawes, C.; Haughton, A.J.; Bohan, D.A.; Squire, G.R. Functional approaches for assessing plant and invertebrate abundance patterns in arable systems. Basic Appl. Ecol. 2009, 10, 34–42. [Google Scholar] [CrossRef]
- Kinderienė, I. The influence of catch crops on the composition, number and frequency on weeds growing in cereal crops on hilly soils. Zemdirbyste 2005, 91, 40–54. [Google Scholar]
- Jarašiūnas, G.; Kinderienė, I. Impact of agro–environmental systems on soil erosion processes and soil properties on hilly landscape in Western Lithuania. J. Environ. Eng. Landsc. Manag. 2016, 24, 60–69. [Google Scholar] [CrossRef]

| Soil Properties | Part of the Hill | |||||
|---|---|---|---|---|---|---|
| Summit | Midslope | Footslope | ||||
| 0–5 cm | 5–15 cm | 0–5 cm | 5–15 cm | 0–5 cm | 5–15 cm | |
| Permanent grassland | ||||||
| Soil acidity (pHKCl) | 5.5 | 5.8 | 6.4 | 6.0 | 5.1 | 4.9 |
| Mobile P2O5 (mg/kg) | 52.7 | 39.7 | 96.0 | 46.3 | 38.3 | 14.0 |
| Mobile K2O (mg/kg) | 253.7 | 138.3 | 250.7 | 132.3 | 329.7 | 154.0 |
| Total N (%) | 0.132 | 0.096 | 0.150 | 0.124 | 0.162 | 0.101 |
| Organic C (%) | 1.12 | 0.97 | 1.54 | 1.17 | 1.65 | 1.12 |
| Soil density Mg m−3 | 1.00 | 1.12 | 0.76 | 0.93 | 0.81 | 1.07 |
| Soil moisture 1 (%) | 21.4–25.5 | 14.5–18.0 | 22.3–42.7 | 22.3–23.4 | 23.0–30.7 | 19.5–20.7 |
| MBC µg/g C | 278.11 | 252.83 | 324.67 | 287.50 | 333.17 | 256.17 |
| Cereal–grass crop rotation | ||||||
| Soil acidity (pHKCl) | 5.6 | 5.4 | 5.3 | 5.1 | 5.1 | 5.1 |
| Mobile P2O5 (mg/kg) | 192 | 201 | 165 | 168 | 149 | 148 |
| Mobile K2O (mg/kg) | 209 | 112 | 198 | 98 | 223 | 107 |
| Total N (%) | 0.078 | 0.077 | 0.097 | 0.096 | 0.106 | 0.101 |
| Organic C (%) | 0.9 | 0.8 | 1.1 | 1.0 | 1.1 | 1.0 |
| Soil density Mg m−3 | 1.34 | 1.36 | 1.35 | 1.35 | 1.27 | 1.33 |
| Soil moisture 1 (%) | 12.1–16.1 | 11.9–14.7 | 15.4–21.2 | 14.1–19.4 | 17.8–21.8 | 16.9–20.6 |
| MBC µg/g C | 210.28 | 196.33 | 293.11 | 278.67 | 354.00 | 285.56 |
| Crop rotation with black fallow | ||||||
| Soil acidity (pHKCl) | 6.4 | 6.6 | 5.4 | 5.7 | 5.2 | 5.4 |
| Mobile P2O5 (mg/kg) | 211.7 | 213.7 | 174.3 | 174.0 | 163.7 | 140.0 |
| Mobile K2O (mg/kg) | 181.7 | 112.7 | 225.0 | 103.7 | 207.3 | 116.3 |
| Total N (%) | 0.072 | 0.057 | 0.084 | 0.082 | 0.091 | 0.085 |
| Organic C (%) | 0.65 | 0.68 | 0.82 | 0.82 | 0.94 | 0.82 |
| Soil density Mg m−3 | 1.32 | 1.38 | 1.30 | 1.30 | 1.38 | 1.44 |
| Soil moisture 1 (%) | 13.0–15.7 | 13.3–14.6 | 15.3–16.6 | 15.4–16.4 | 18.2–20.2 | 17.5–18.9 |
| MBC µg/g C | 192.28 | 220.00 | 335.22 | 285.17 | 354.83 | 325.61 |
| Treatment | Spring | Autumn | ||
|---|---|---|---|---|
| 0–5 cm | 5–15 cm | 0–5 cm | 5–15 cm | |
| Agrophytocenosis (factor A) | ||||
| Permanent grassland | 8.8 a | 4.3 b | 7.3 b | 3.8 b |
| Cereal–grass crop rotation | 8.4 a | 6.6 a | 8.6 a | 6.6 a |
| Crop rotation with black fallow | 7.2 b | 6.0 a | 7.6 b | 6.9 a |
| Part of the Hill (factor B) | ||||
| Summit | 7.2 b | 5.1 b | 6.4 c | 4.4 c |
| Midslope | 7.6 b | 5.8 a | 7.9 b | 6.0 b |
| Footslope | 9.6 a | 5.9 a | 9.2 a | 6.8 a |
| F (t-test) | ||||
| Factor A | ns | ** | * | ** |
| Factor B | ** | ns | ** | ** |
| Interaction of factors A × B | ns | ns | ns | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skuodienė, R.; Matyžiūtė, V.; Aleinikovienė, J.; Frercks, B.; Repšienė, R. Seed Bank Community under Different-Intensity Agrophytocenoses on Hilly Terrain in Lithuania. Plants 2023, 12, 1084. https://doi.org/10.3390/plants12051084
Skuodienė R, Matyžiūtė V, Aleinikovienė J, Frercks B, Repšienė R. Seed Bank Community under Different-Intensity Agrophytocenoses on Hilly Terrain in Lithuania. Plants. 2023; 12(5):1084. https://doi.org/10.3390/plants12051084
Chicago/Turabian StyleSkuodienė, Regina, Vilija Matyžiūtė, Jūratė Aleinikovienė, Birutė Frercks, and Regina Repšienė. 2023. "Seed Bank Community under Different-Intensity Agrophytocenoses on Hilly Terrain in Lithuania" Plants 12, no. 5: 1084. https://doi.org/10.3390/plants12051084
APA StyleSkuodienė, R., Matyžiūtė, V., Aleinikovienė, J., Frercks, B., & Repšienė, R. (2023). Seed Bank Community under Different-Intensity Agrophytocenoses on Hilly Terrain in Lithuania. Plants, 12(5), 1084. https://doi.org/10.3390/plants12051084









