Glyphosate-Induced Abscisic Acid Accumulation Causes Male Sterility in Sea Island Cotton
Abstract
1. Introduction
2. Results
2.1. Glyphosate Causes Yield Loss by Inducing Male Sterility in Sea Island Cotton
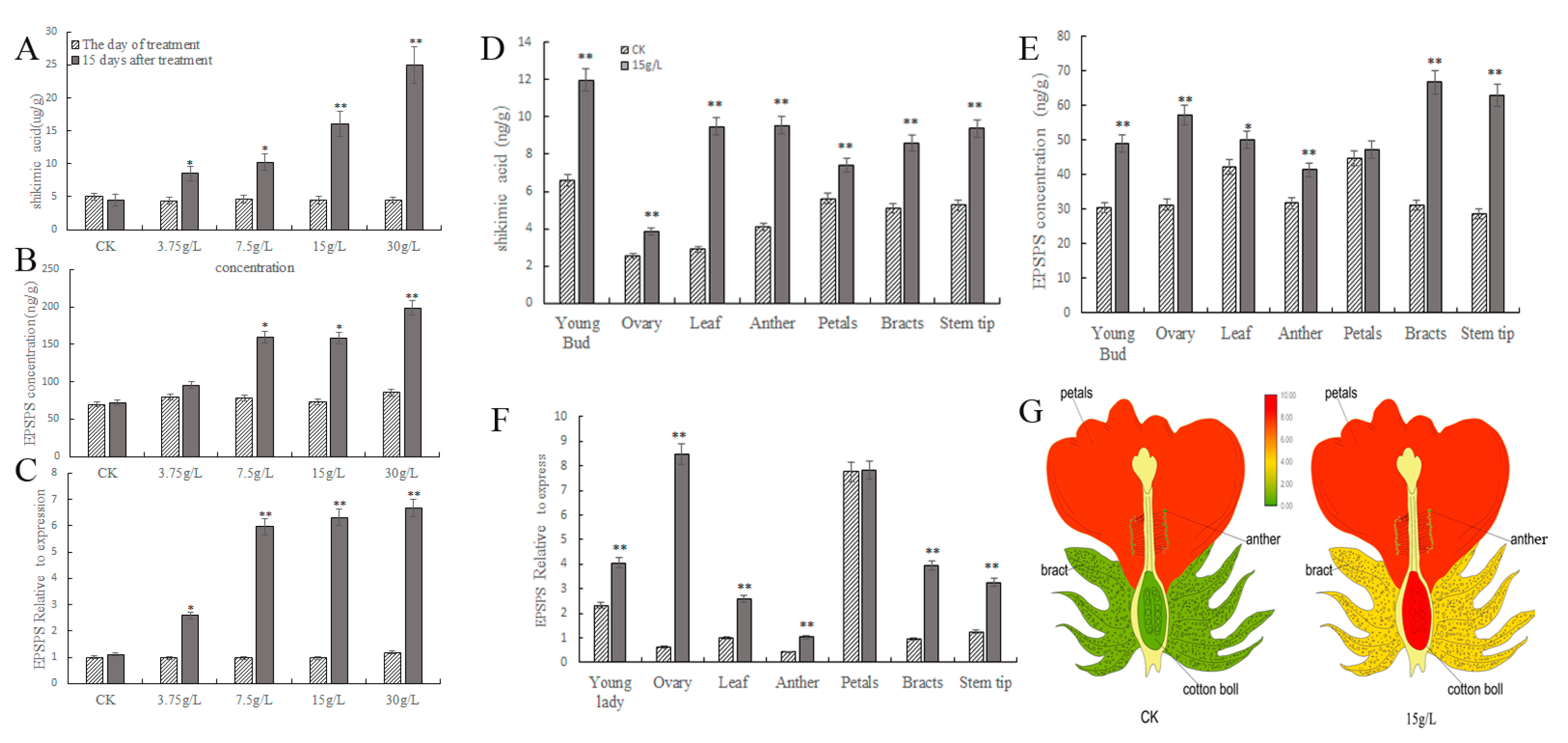
2.2. Glyphosate Attracts Male Sterility by Affecting the Shikimic Acid Pathway in Sea Island Cotton
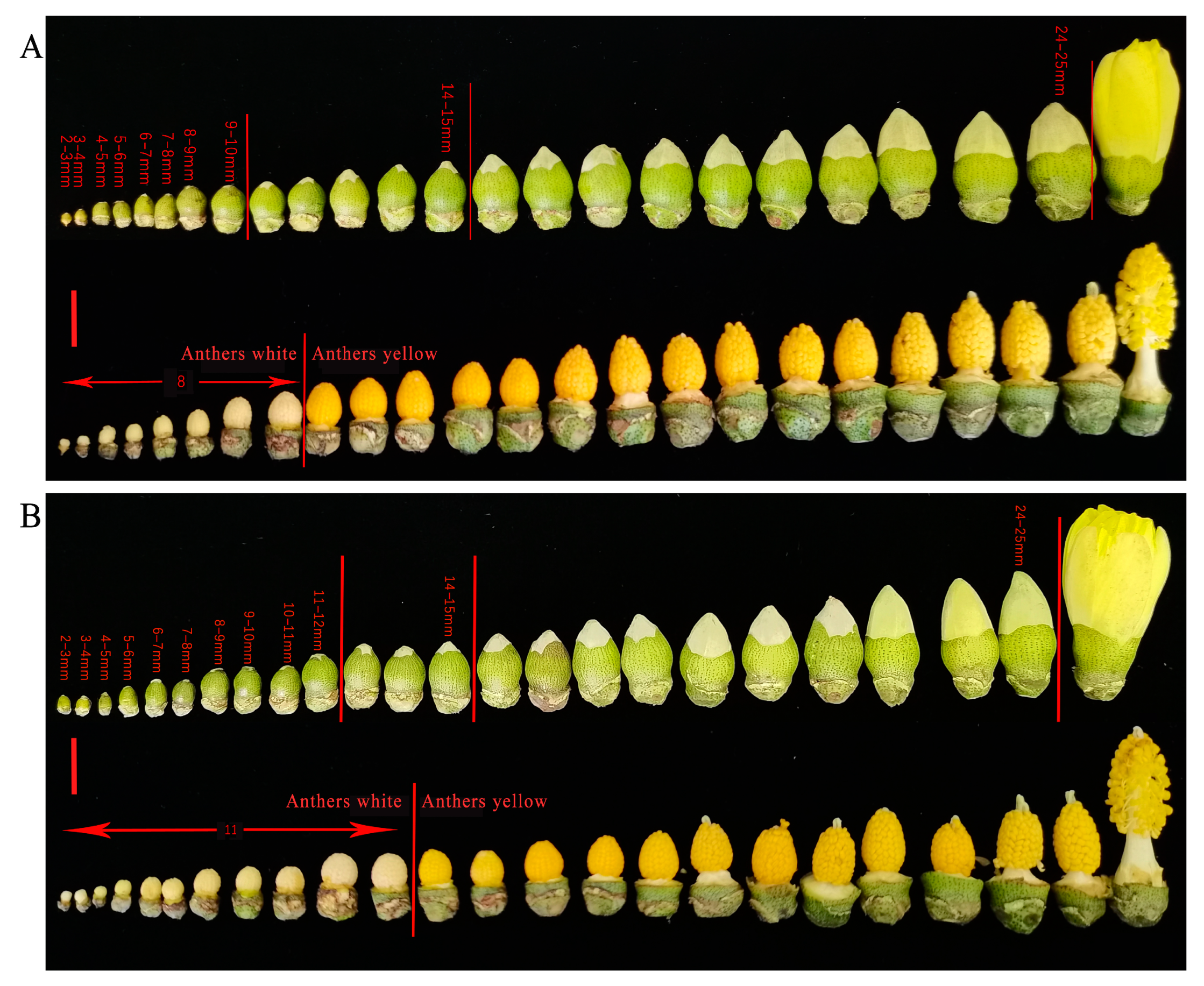
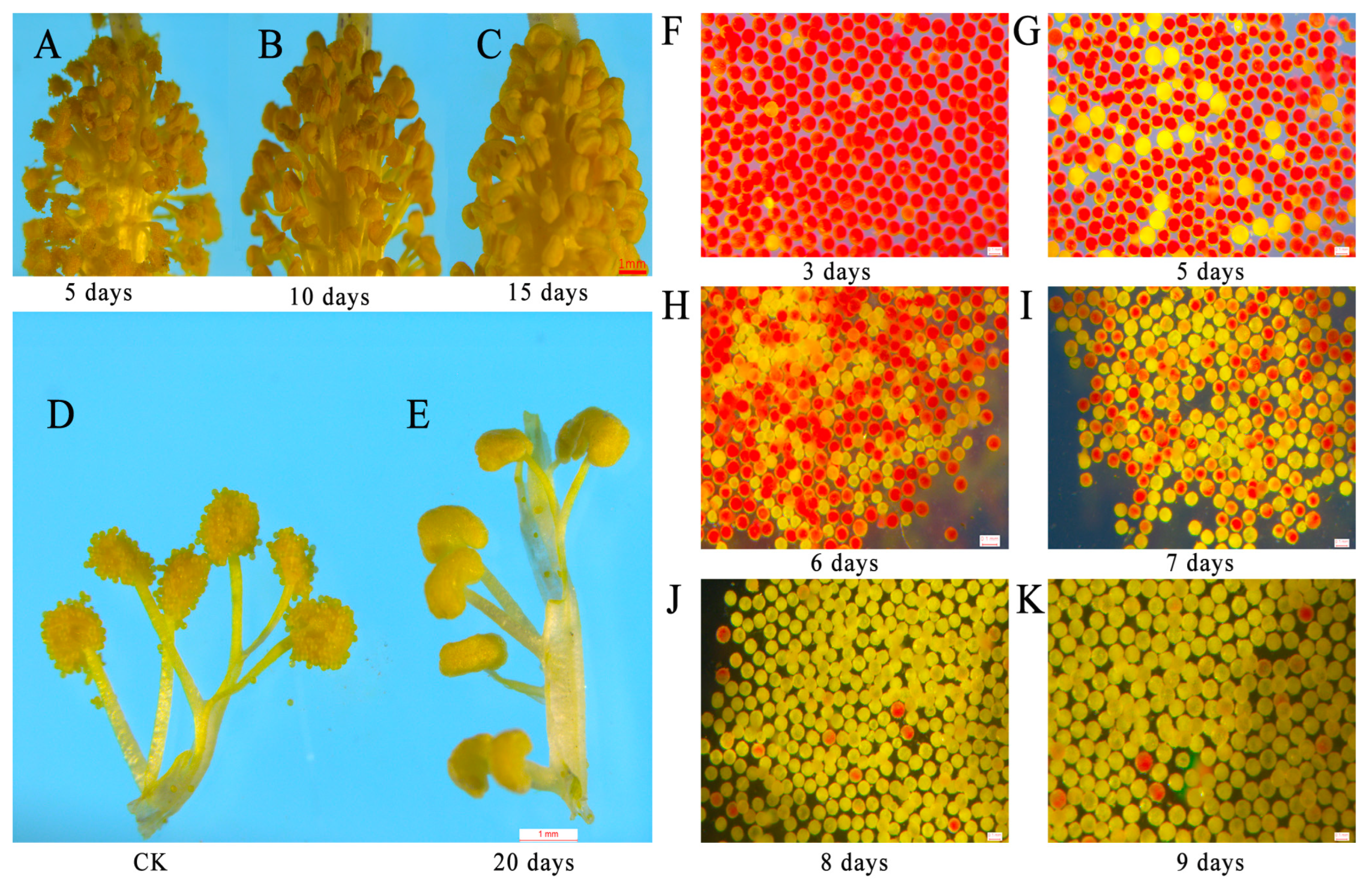
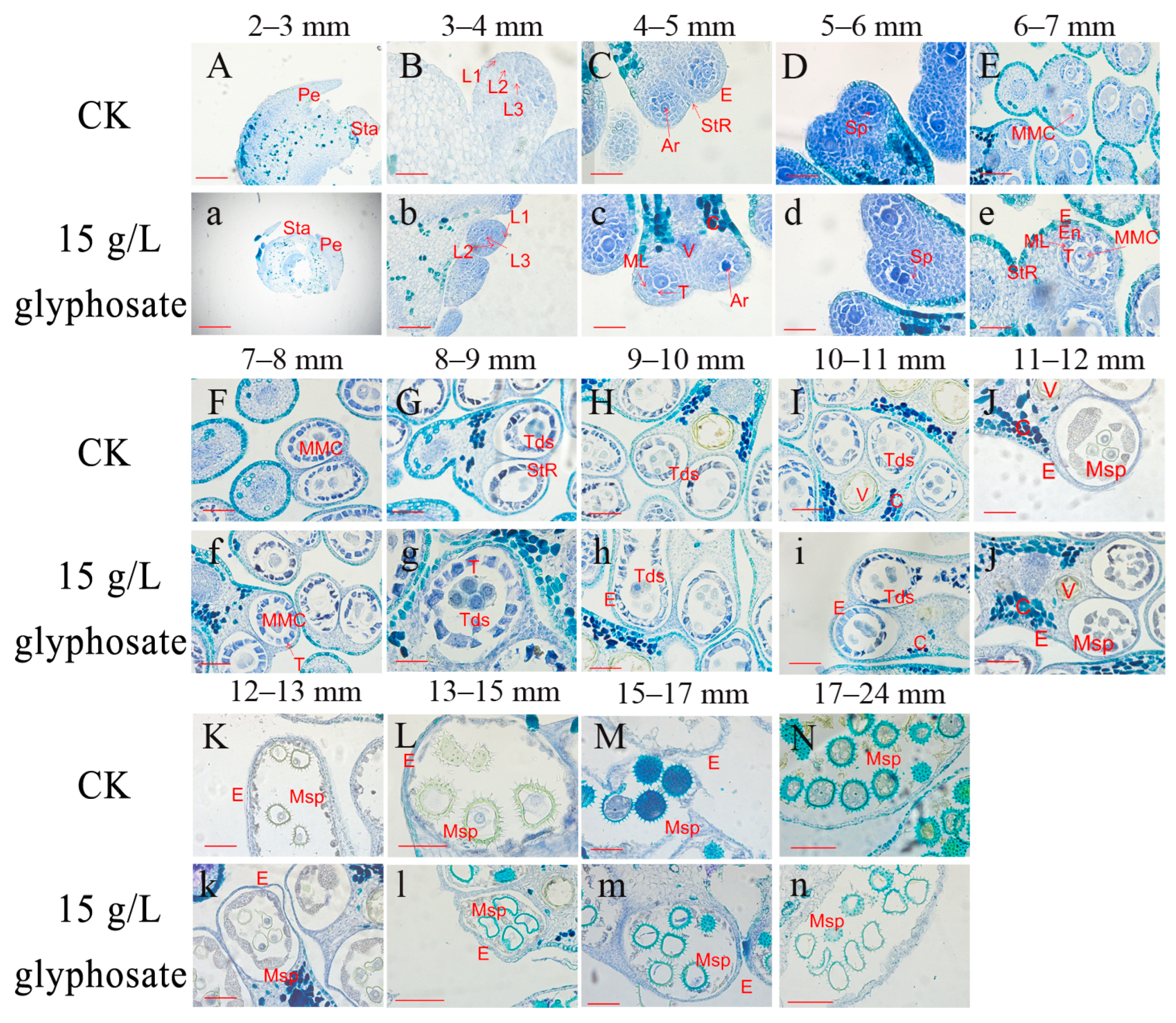
2.3. Tetrads Stage Is the Key Period during Which 15 g/L Glyphosate Affects Male Sterility in Sea Island Cotton
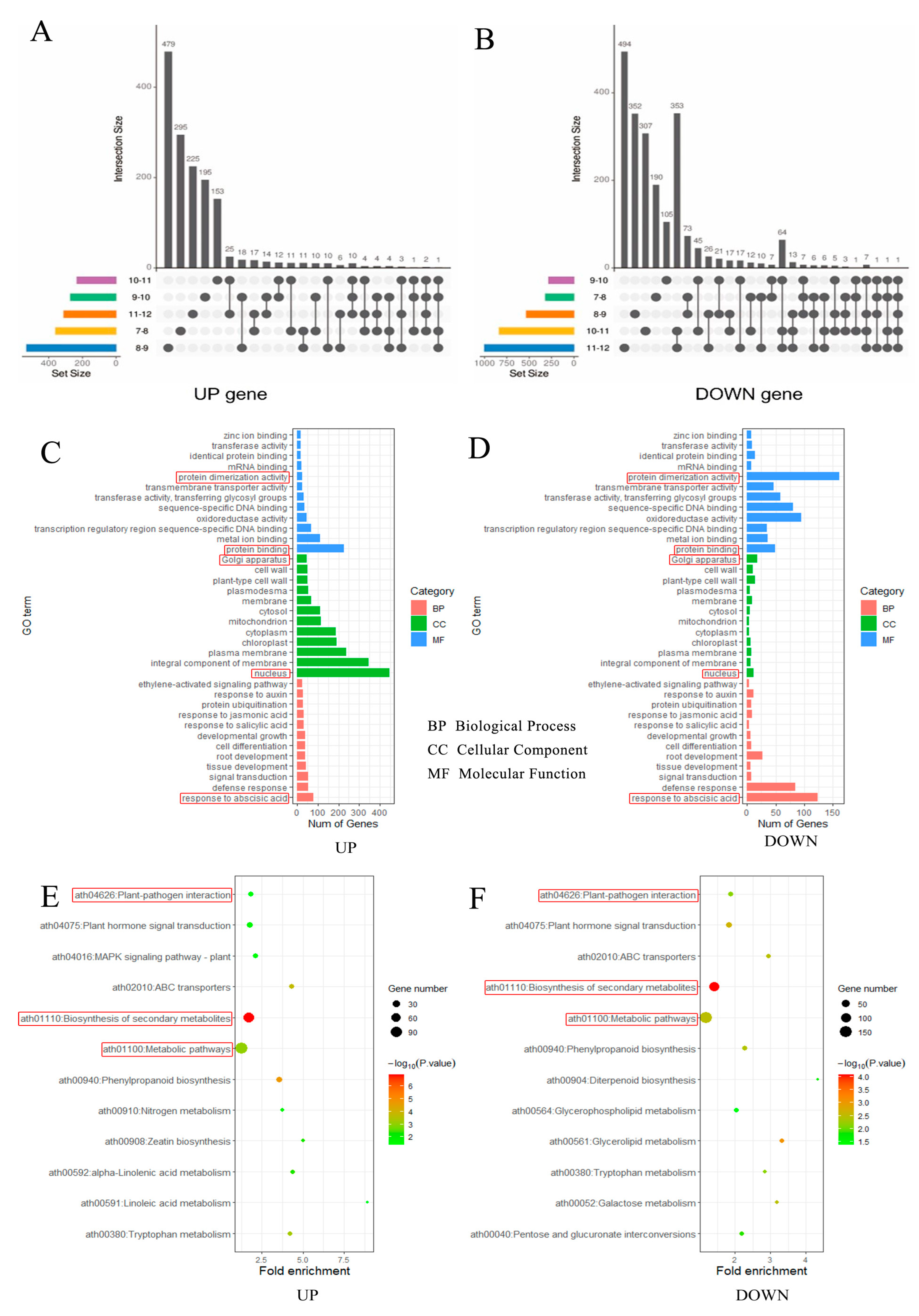
2.4. Transcriptome Reveals Genes Related to Male Sterility Evoked by Glyphosate in Sea Island Cotton
3. Discussion
3.1. Glyphosate Triggered Male Sterility in Cotton
3.2. The Critical Periods for the Emergence of Male Sterility
3.3. Abnormal Abscisic Acid Pathway Is Responsible for Male Sterility
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Treatment
4.3. Sampling Methods
4.4. Detection of EPSPS Gene Expression Levels
4.5. Examination of Shikimic Acid and EPSPS Synthase Contents
4.6. Histological Section Observation
4.7. Transcriptome Data Analysis
4.8. qRT-PCR Validation
4.9. Phytohormone Measurement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Y.; Li, W.; Li, A.; Ge, R.; Zhang, B.; Li, J.; Liu, G.; Li, J.; Liu, A.; Shang, H.; et al. Constructing a high-density linkage map for Gossypium hirsutum × Gossypium barbadenseand identifying QTLs for lint percentage. J. Integr. Plant Biol. 2014, 57, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhu, G.; Song, X.; Xu, H.; Li, W.; Ning, X.; Chen, Q.; Guo, W. Genome-wide association analysis reveals loci and candidate genes involved in fiber quality traits in sea island cotton (Gossypium barbadense). BMC Plant Biol. 2020, 20, 289. [Google Scholar] [CrossRef]
- Roberts, J.; Florentine, S.; Fernando, W.G.D.; Tennakoon, K.U. Achievements, Developments and Future Challenges in the Field of Bioherbicides for Weed Control: A Global Review. Plants 2022, 11, 2242. [Google Scholar] [CrossRef] [PubMed]
- Viator, R.P.; Jost, P.H.; Senseman, S.A.; Cothren, J.T. Effect of glyphosate application timings and methods on glyphosate-resistant cotton. Weed Sci. 2004, 52, 147–151. [Google Scholar] [CrossRef]
- Jones, M.A.; Snipes, C.E. Tolerance of transgenic cotton to topical applications of glyphosate. J. Cotton Sci. 1999, 3, 19–26. [Google Scholar] [CrossRef]
- Viator, R.P.; Senseman, S.A.; Cothren, J.T. Boll Abscission Responses of Glyphosate-Resistant Cotton (Gossypium hirsutum) to Glyphosate. Weed Technol. 2003, 17, 571–575. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hubmeier, C. Histochemical analyses of male reproductive development in glyphosate-tolerant cotton (Gossypium hirsutum). In Proceedings of the 18th Asian-Pacific Weed Science Society Conference, Beijing, China, 26–30 November 2001; pp. 437–441. [Google Scholar]
- Yasuor, H.; Riov, J.; Rubin, B. Glyphosate-induced male sterility in glyphosate-resistant cotton (Gossypium hirsutum L.) is associated with inhibition of anther dehiscence and reduced pollen viability. Crop Prot. 2007, 26, 363–369. [Google Scholar] [CrossRef]
- Villanueva, M.J.C.; Muñiz, B.F.; Tames, R.S. Effects of Glyphosate on Growth and the Chlorophyll and Carotenoid Levels of Yellow Nutsedge (Cyperus esculentus). Weed Sci. 1985, 33, 751–754. [Google Scholar] [CrossRef]
- Kim, D.; Koo, S. Concise and Practical Total Synthesis of (+)-Abscisic Acid. ACS Omega 2020, 5, 13296–13302. [Google Scholar] [CrossRef]
- Rajasekaran, K.; Hein, M.B.; Vasil, I.K. Endogenous Abscisic Acid and Indole-3-Acetic Acid and Somatic Embryogenesis in Cultured Leaf Explants of Pennisetum purpureum Schum. Plant Physiol. 1987, 84, 47–51. [Google Scholar] [CrossRef]
- Mei, L.; Feng, C.; Zhao, T.; Li, C.; Yan, S.; Li, C.; Feng, J.; Zhang, F.; Zhang, Y.; Xiao, Q.; et al. Characterizations of male sterility in a glyphosate-tolerant upland cotton (Gossypium hirsutum L.) induced by glyphosate and its assessments on safety utilization. Ind. Crops Prod. 2019, 134, 318–327. [Google Scholar] [CrossRef]
- Nie, T.; Jiang, Z.; Sun, L.; Chen, Y.; Li, J.; Yang, A.; Yin, Z. Analysis on the biological basis of stamen abortion during the second flowering of Magnolia × soulangeana ‘Changchun’. Trees 2022, 36, 1515–1528. [Google Scholar] [CrossRef]
- Zhang, J.-K.; Zong, X.-F.; Yu, G.-D.; Li, J.-N.; Zhang, W. Relationship Between Phytohormones and Male Sterility in Thermo-Photo-Sensitive Genic Male Sterile (TGMS) Wheat. Euphytica 2006, 150, 241–248. [Google Scholar] [CrossRef]
- Kasembe, J.N.R. Phenotypic Restoration of Fertility in a Male-sterile Mutant by Treatment with Gibberellic Acid. Nature 1967, 215, 668. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, G.; Li, Y.; Mo, N.; Zhang, J.; Liang, Y. Transcriptomic analysis implies that GA regulates sex expression via ethylene-dependent and ethylene-independent pathways in cucumber (Cucumis sativus L.). Front. Plant Sci. 2017, 8, 10. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Q.; Fu, H.; Chen, K.; Zhao, S.; Zhang, C.; Cai, T.; Wang, L.; Lu, W.; Dang, H.; et al. Identification of Key Gene Networks and Deciphering Transcriptional Regulators Associated with Peanut Embryo Abortion Mediated by Calcium Deficiency. Front. Plant Sci. 2022, 13, 814015. [Google Scholar] [CrossRef]
- Gan, Z.; Feng, Y.; Wu, T.; Wang, Y.; Xu, X.; Zhang, X.; Han, Z. Downregulation of the auxin transporter gene SlPIN8 results in pollen abortion in tomato. Plant Mol. Biol. 2019, 99, 561–573. [Google Scholar] [CrossRef]
- Sekhar, K.C.; Sawhney, V. Role of ABA in stamen and pistil development in the normal and solanifolia mutant of tomato (Lycopersicon esculentum). Sex. Plant Reprod. 1991, 4, 279–283. [Google Scholar] [CrossRef]
- Shi, T.; Iqbal, S.; Ayaz, A.; Bai, Y.; Pan, Z.; Ni, X.; Hayat, F.; Bilal, M.S.; Razzaq, M.K.; Gao, Z. Analyzing Differentially Expressed Genes and Pathways Associated with Pistil Abortion in Japanese Apricot via RNA-Seq. Genes 2020, 11, 1079. [Google Scholar] [CrossRef]
- Nag, A.; King, S.; Jack, T. miR319a targeting of TCP4 is critical for petal growth and development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 22534–22539. [Google Scholar] [CrossRef]
- Howarth, D.G.; Donoghue, M.J. Duplications inCYC-like Genes from Dipsacales Correlate with Floral Form. Int. J. Plant Sci. 2005, 166, 357–370. [Google Scholar] [CrossRef]
- Chai, W.; Jiang, P.; Huang, G.; Jiang, H.; Li, X. Identification and expression profiling analysis of TCP family genes involved in growth and development in maize. Physiol. Mol. Biol. Plants 2017, 23, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Lennon, K.; Lord, E. Pollination in Arabidopsis thaliana. Microsc. Microanal. 1998, 4, 1180–1181. [Google Scholar] [CrossRef]
- Yasuor, H.; Abu-Abied, M.; Belausov, E.; Madmony, A.; Sadot, E.; Riov, J.; Rubin, B. Glyphosate-Induced Anther Indehiscence in Cotton Is Partially Temperature Dependent and Involves Cytoskeleton and Secondary Wall Modifications and Auxin Accumulation. Plant Physiol. 2006, 141, 1306–1315. [Google Scholar] [CrossRef]
- Pline, W.A.; Viator, R.P.; Wilcut, J.W.; Edmisten, K.L.; Thomas, J.F.; Wells, R. Reproductive abnormalities in glyphosate-resistant cotton caused by lower CP4-EPSPS levels in the male reproductive tissue. Weed Sci. 2002, 50, 438–447. [Google Scholar] [CrossRef]
- Ren, M. Stamen fusion in plants: Diversity, adaptive significance, and taxonomic implications. J. Syst. Evol. 2008, 46, 452–466. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, X.; Li, S.; Zhang, L.; Song, X. Oxidative Stress and Aberrant Programmed Cell Death Are Associated with Pollen Abortion in Isonuclear Alloplasmic Male-Sterile Wheat. Front. Plant Sci. 2018, 9, 595. [Google Scholar] [CrossRef]
- Sheng, Z.; Tang, L.; Shao, G.; Xie, L.; Jiao, G.; Tang, S.; Hu, P. The rice thermo-sensitive genic male sterility gene tms9: Pollen abortion and gene isolation. Euphytica 2014, 203, 145–152. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Wang, L.; Wu, M.; Zhang, D.; Fang, W.; Chen, F.; Teng, N. Cytological and Molecular Characteristics of Pollen Abortion in Lily with Dysplastic Tapetum. Hortic. Plant J. 2019, 5, 281–294. [Google Scholar] [CrossRef]
- Kempken, F.; Pring, D. Plant Breeding: Male Sterility in Higher Plants—Fundamentals and Applications. Prog. Bot. 1999, 60, 139–166. [Google Scholar] [CrossRef]
- Sanders, P.M.; Bui, A.Q.; Weterings, K.; McIntire, K.N.; Hsu, Y.-C.; Lee, P.Y.; Truong, M.T.; Beals, T.P.; Goldberg, R.B. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 1999, 11, 297–322. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Liu, J.; Xia, M.; Wang, W.; Shen, F. Transcriptome Analysis of Early Anther Development of Cotton Revealed Male Sterility Genes for Major Metabolic Pathways. J. Plant Growth Regul. 2014, 34, 223–232. [Google Scholar] [CrossRef]
- Wu, Y.; Min, L.; Wu, Z.; Yang, L.; Zhu, L.; Yang, X.; Yuan, D.; Guo, X.; Zhang, X. Defective pollen wall contributes to male sterility in the male sterile line 1355A of cotton. Sci. Rep. 2015, 5, 9608. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hoekstra, S.; Van Bergen, S.; Lamers, G.E.M.; Oppedijk, B.J.; Van Der Heijden, M.W.; De Priester, W.; Schilperoort, R.A. Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. Plant Mol. Biol. 1999, 39, 489–501. [Google Scholar] [CrossRef]
- Brocard-Gifford, I.; Lynch, T.J.; Garcia, M.E.; Malhotra, B.; Finkelstein, R.R. The Arabidopsis thaliana ABSCISIC ACID-INSENSITIVE8 locus encodes a novel protein mediating abscisic acid and sugar responses essential for growth. Plant Cell. 2004, 16, 406–421. [Google Scholar] [CrossRef]
- Yang, C.; Vizcay-Barrena, G.; Conner, K.; Wilson, Z.A. MALE STERILITY1 is required for tapetal development and pollen wall biosynthesis. Plant Cell. 2007, 19, 3530–3548. [Google Scholar] [CrossRef]
- Zhu, Y.; Dun, X.; Zhou, Z.; Xia, S.; Yi, B.; Wen, J.; Shen, J.; Ma, C.; Tu, J.; Fu, T. A separation defect of tapetum cells and microspore mother cells results in male sterility in Brassica napus: The role of abscisic acid in early anther development. Plant Mol Biol. 2010, 72, 111–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Sun, Q.; Lu, S.; Chai, L.; Ye, J.; Deng, X. Citrus transcription factor CsHB5 regulates abscisic acid biosynthetic genes and promotes senescence. Plant J. 2021, 108, 151–168. [Google Scholar] [CrossRef]
- Xu, P.; Chen, H.; Cai, W. Transcription factor CDF4 promotes leaf senescence and floral organ abscission by regulating abscisic acid and reactive oxygen species pathways in Arabidopsis. EMBO Rep. 2020, 21, e48967. [Google Scholar] [CrossRef]
- Joseph, M.P.; Papdi, C.; Kozma-Bognár, L.; Nagy, I.; López-Carbonell, M.; Rigó, G.; Koncz, C.; Szabados, L. The Arabidopsis ZINC FINGER PROTEIN3 Interferes with Abscisic Acid and Light Signaling in Seed Germination and Plant Development. Plant Physiol. 2014, 165, 1203–1220. [Google Scholar] [CrossRef]
- Swarbreck, D.; Wilks, C.; Lamesch, P.; Berardini, T.Z.; Garcia-Hernandez, M.; Foerster, H.; Li, D.; Meyer, T.; Muller, R.; Ploetz, L.; et al. The Arabidopsis Information Resource (TAIR): Gene structure and function annotation. Nucleic Acids Res. 2007, 36, D1009–D1014. [Google Scholar] [CrossRef] [PubMed]
- Pagant, S.; Bichet, A.; Sugimoto, K.; Lerouxel, O.; Desprez, T.; McCann, M.; Lerouge, P.; Vernhettes, S.; Höfte, H. KOBITO1 Encodes a Novel Plasma Membrane Protein Necessary for Normal Synthesis of Cellulose during Cell Expansion in Arabidopsis. Plant Cell 2002, 14, 2001–2013. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Brown, M.S.; Kohel, R.J. Evaluation of seven tetrazolium salts as vital pollen stains in cotton Gossypium hirsutum L. Crop Sci. 1964, 4, 508–510. [Google Scholar] [CrossRef]
- Min, L.; Li, Y.; Hu, Q.; Zhu, L.; Gao, W.; Wu, Y.; Ding, Y.; Liu, S.; Yang, X.; Zhang, X. Sugar and Auxin Signaling Pathways Respond to High-Temperature Stress during Anther Development as Revealed by Transcript Profiling Analysis in Cotton. Plant Physiol. 2014, 164, 1293–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ding, Q.; Zeng, J.; Wang, F.-R.; Zhang, J.; Fan, S.-J.; He, X.-Q. An Improved CTAB-Ammonium Acetate Method for Total RNA Isolation from Cotton. Phytochem. Anal. 2012, 23, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Yang, Y.; Yang, X.; He, S.; Zhu, Z. Light-weight reference-based compression of FASTQ data. BMC Bioinform. 2015, 16, 188. [Google Scholar] [CrossRef]
- Yu, J.; Jung, S.; Cheng, C.-H.; Lee, T.; Zheng, P.; Buble, K.; Crabb, J.; Humann, J.; Hough, H.; Jones, D.; et al. CottonGen: The Community Database for Cotton Genomics, Genetics, and Breeding Research. Plants 2021, 10, 2805. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Harris, M.A.; Clark, J.; Ireland, A.; Lomax, J.; Ashburner, M.; Foulger, R.; Eilbeck, K.; Lewis, S.; Marshall, B.; Mungall, C.; et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004, 32, D258–D261. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Kanehisa, M. Using the KEGG Database Resource. Curr. Protoc. Bioinform. 2012, 38, 1.12.1–1.12.43. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cioć, M.; Dziurka, M.; Pawłowska, B. Changes in Endogenous Phytohormones of Gerbera jamesonii Axillary Shoots Multiplied under Different Light Emitting Diodes Light Quality. Molecules 2022, 27, 1804. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, G.; Zhao, N.; Wang, W.; Wang, M.; Zhu, J.; Yang, J.; Lin, F.; Huang, X.; Zhang, Y.; Min, L.; et al. Glyphosate-Induced Abscisic Acid Accumulation Causes Male Sterility in Sea Island Cotton. Plants 2023, 12, 1058. https://doi.org/10.3390/plants12051058
Qin G, Zhao N, Wang W, Wang M, Zhu J, Yang J, Lin F, Huang X, Zhang Y, Min L, et al. Glyphosate-Induced Abscisic Acid Accumulation Causes Male Sterility in Sea Island Cotton. Plants. 2023; 12(5):1058. https://doi.org/10.3390/plants12051058
Chicago/Turabian StyleQin, Guoli, Nan Zhao, Weiran Wang, Meng Wang, Jiahui Zhu, Jing Yang, Feng Lin, Xinglei Huang, Yanhui Zhang, Ling Min, and et al. 2023. "Glyphosate-Induced Abscisic Acid Accumulation Causes Male Sterility in Sea Island Cotton" Plants 12, no. 5: 1058. https://doi.org/10.3390/plants12051058
APA StyleQin, G., Zhao, N., Wang, W., Wang, M., Zhu, J., Yang, J., Lin, F., Huang, X., Zhang, Y., Min, L., Chen, G., & Kong, J. (2023). Glyphosate-Induced Abscisic Acid Accumulation Causes Male Sterility in Sea Island Cotton. Plants, 12(5), 1058. https://doi.org/10.3390/plants12051058





