Comparison of the Allelopathic Potential of Non-Native and Native Species of Mediterranean Ecosystems
Abstract
1. Introduction
2. Results
2.1. Total Phenol and Flavonoid Amount
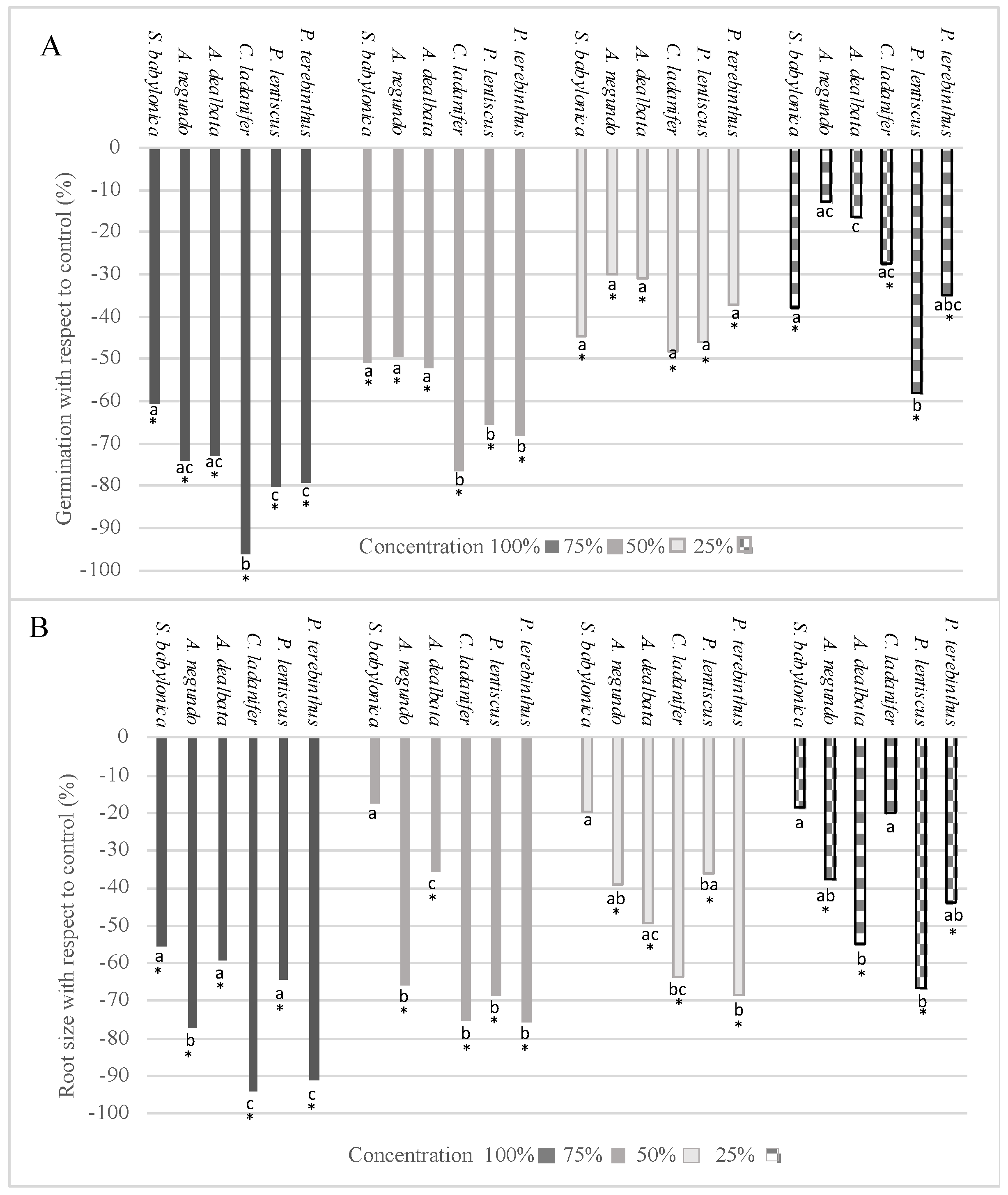
2.2. Bioactivity Test on Lactuca sativa
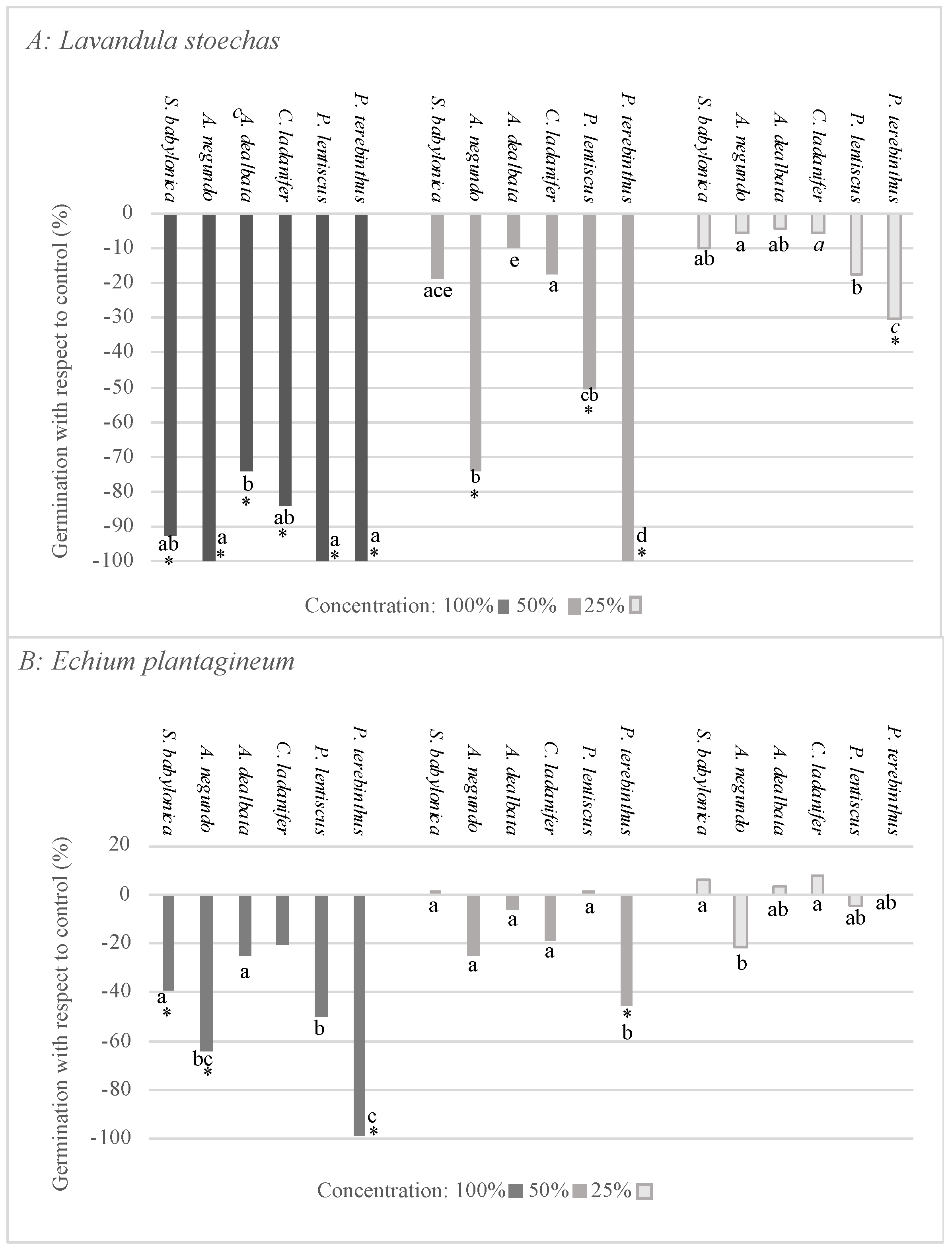
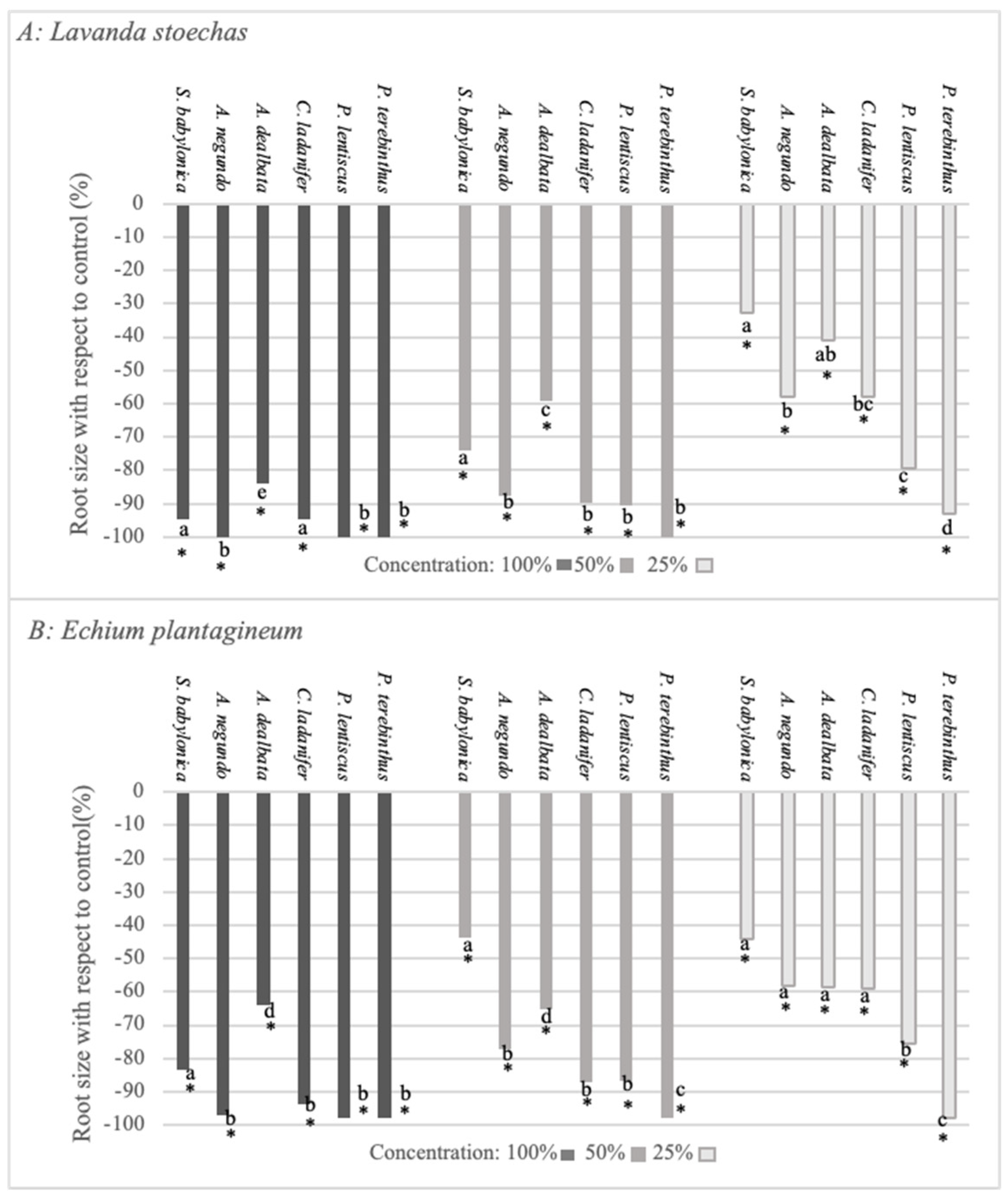
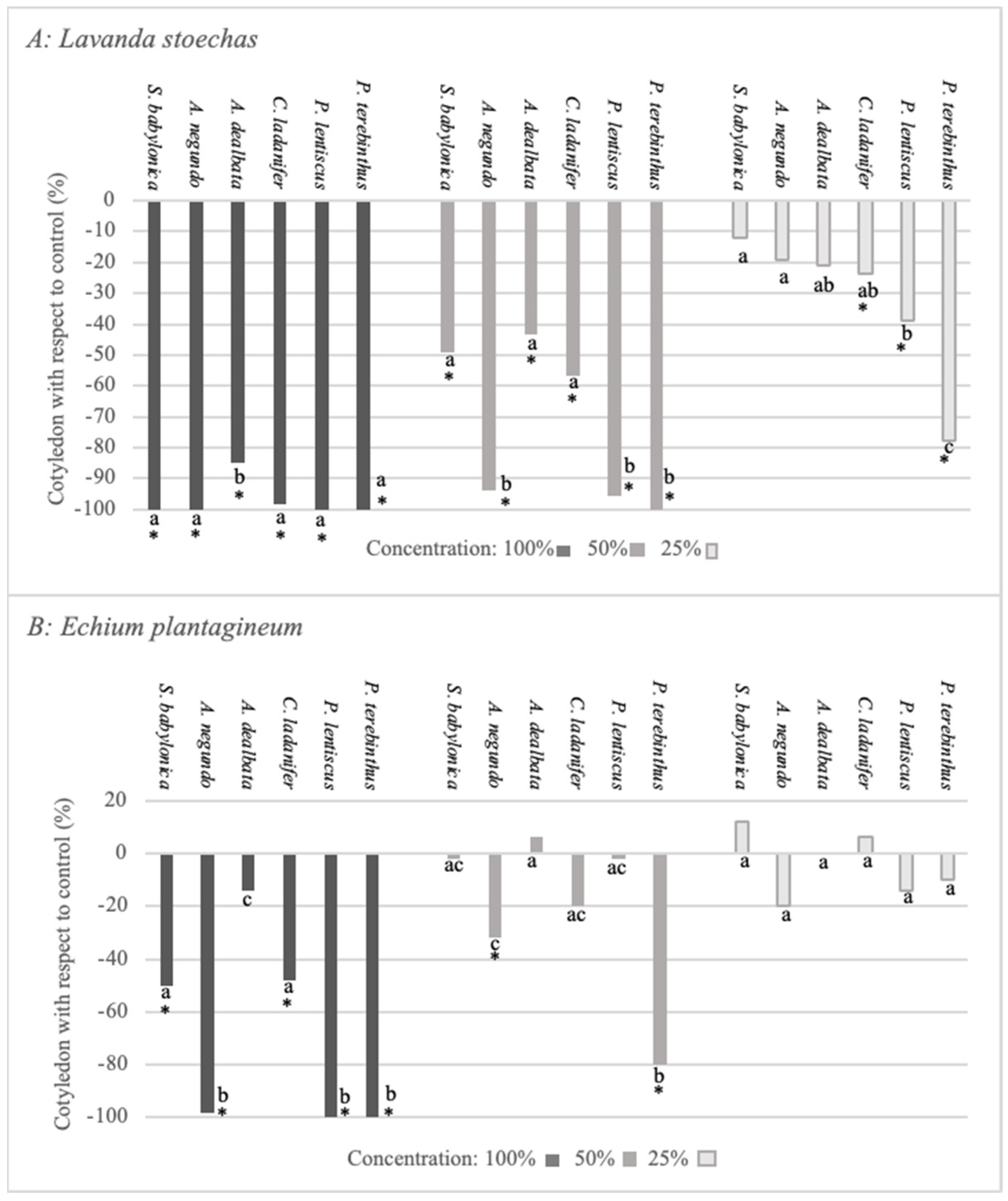
2.3. Bioactivity Test on Lavandula stoechas and Echium plantagineum
3. Discussion
4. Materials and Methods
4.1. Gathering of Materials and Sample Treatment
4.2. Preparation of the Aqueous Extracts
4.3. Extraction and Quantification of Total Phenols
4.4. Extraction and Quantification of Total Flavonoids
4.5. Bioassays
4.5.1. Bioassays on Lactuca sativa
4.5.2. Bioassays on Echium plantagineum and Lavandula stoechas
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kutiel, P. Fire and Ecosystem Heterogeneity: A mediterranean Case Study. Earth Surf. Process Landf. 1994, 19, 184–194. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Pedrol, N.; González, L. Allelopathy, a Physiological Process with Ecological Implications; Springer: Dordrecht, The Netherlands, 2006; ISBN 978-1-4020-4279-9. [Google Scholar]
- Gross, J.; Hilker, M. Chemoecological Studies of the Exocrine Glandular Larval Secretions of two Chrysomelid Species: Phaedon cochleariae and Chrysomela lapponica. Chemoecology 1995, 6, 185–189. [Google Scholar] [CrossRef]
- Ikonen, A.; Tahvanainen, J.; Roininen, H. Chlorogenic Acid as an Antiherbivore Defence of Willows Against Leaf Beetles. Entomol. Exp. Appl. 2001, 99, 47–54. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research. Research Signpost37/661 (2); Imperato, F., Ed.; Fort PO, Trivandrum-695 023: Kerala, India, 2006; pp. 23–67. [Google Scholar]
- Zobel, A.M.; Clarke, P.A.; Lynch, J.M. Production of phenolics in response to UV irradiation and heavy metals in seedlings of Acer species. In Recent Advances in Allelopathy; Narwal, S.S., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 1–12. [Google Scholar]
- Ryan, K.G.; Swinny, E.E.; Winefield, C.; Markham, K.R. Flavonoids and UV Photoprotection in Arabidopsis mutants. Zeitschrift fur Naturforschung—Section C. J. Biosci. 2001, 56, 745–754. [Google Scholar]
- Baas, W.J. Secondary plant compounds, their ecological significance and consequences for the carbon budget. Introduction of the carbon/nutrient cycle theory. In Causes and Consequences of Variation in Growth Rate and Productivity of Higher Plants; Lambers, H., Cambridge, M.L., Konings, H., Pons, T.L., Eds.; SPB Academic Publishing bv: The Hague, The Netherlands, 1989; pp. 313–340. [Google Scholar]
- Harborne, J.B. Biochemical plant ecology. In Plant Biochemistry; Dey, P.M., Harborne, J.B., Eds.; Academic Press: London, UK, 1997; pp. 503–516. [Google Scholar]
- Chaves, N.; Escudero, J.C. Allelopathic Effect of Cistus Ladanifer on Seed Germination. Funct. Ecol. 1997, 11, 432–440. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Escudero, J.C. Plant Growth Inhibiting Flavonoids in Exudate Of Cistus ladanifer and in Associated Soils. J. Chem. Ecol. 2001, 27, 623–631. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Alías, J.C.; Escudero, J.C. Identification and Effects of the Interaction of Phytotoxic Compounds from Exudate of Cistus ladanifer Leaves. J. Chem. Ecol. 2001, 27, 611–621. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy; Academic Press: New York, NY, USA, 1984. [Google Scholar]
- Prince, E.K.; Pohnert, G. Searching for Signal in the Noise: Metabolomics in Chemical Ecology. Anal. Bioanal. Chem. 2010, 396, 193–197. [Google Scholar] [CrossRef]
- Chaves, N.; Escudero, J.C.; Gutiérrez-Merino, C. Role of Ecological Variables in the Seasonal Variation of Flavonoid Content of Cistus ladanifer Exudate. J. Chem. Ecol. 1997, 23, 579–603. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Pickett, J.A. Plant Defence Signalling Induced By Biotic Attacks. Curr. Opin. Plant Biol. 2007, 10, 387–392. [Google Scholar] [CrossRef]
- Peñuelas, J.; Munné-Bosch, S. Isoprenoids: An Evolutionary Pool for Photoprotection. Trends Plant Sci. 2005, 10, 166–169. [Google Scholar] [CrossRef]
- Scognamiglio, M. Phytochemical Analysis of Mediterranean Plants: Metabolomic Approach to Study Allelopathic Interactions Among Coexisting Species. Ph.D. Thesis, University of Naples: Naples, Italy, 2011. [Google Scholar]
- Fernández, C.; Santonja, M.; Gros, R.; Monnier, Y.; Chomel, M.; Baldy, V.; Bousquet-Mélou, A. Allelochemicals of Pinus halepensis as Drivers of Biodiversity in Mediterranean Open Mosaic Habitats During the Colonization Stage of Secondary Succession. J. Chem. Ecol. 2013, 39, 298–311. [Google Scholar] [CrossRef]
- Fiorentino, A.; D’Abrosca, B.; Esposito, A.; Izzo, A.; Pascarella, M.T.; D’Angel, G.; Monaco, P. Potential Allelopathic Effect of Neo-Clerodane Diterpenes from Teucrium chamaedrys (L.) on Stenomediterranean and Weed Cosmopolitan Species. Biochem. Syst. Ecol. 2009, 37, 349–353. [Google Scholar] [CrossRef]
- Rolim de Almeida, L.F.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic Activities of Mediterranean Essential Oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef]
- Araniti, F.; Sorgona, A.; Lupini, A.; Abenavoli, M.R. Screening of Mediterranean Wild Plant Species for AlleloPathic Activity and Their Use as Bio-Herbicides. Allelopath. J. 2012, 29, 107–124. [Google Scholar]
- Scognamiglio, M.; Fiumano, V.; D’Abrosca, B.; Esposito, A.; Choi, Y.H.; Verpoorte, R.; Fiorentino, A. Chemical Interactions Between Plants in Mediterranean Vegetation: The Influence of Selected Plant Extracts on Aegilops geniculata Metabolome. Phytochemistry 2014, 106, 69–85. [Google Scholar] [CrossRef]
- Scognamiglio, M.; Graziani, V.; Tsafantakis, N.; Esposito, A.; Fiorentino, A.; D’Abrosca, B. NMR-Based Metabolomics and Bioassays to Study Phytotoxic Extracts and Putative Phytotoxins from Mediterranean Plant Species. Phytochem Anal. 2019, 30, 512–523. [Google Scholar] [CrossRef]
- Chaves, N.; Alías, J.C.; Sosa, T. Phytotoxicity of Cistus ladanifer L.: Role of Allelopathy. Allelopath. J. 2016, 38, 113–131. [Google Scholar]
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on Allelopathy of Exotic Invasive Plants. Procedia Eng. 2011, 18, 240–246. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. Allelopathy and Exotic Plant Invasion. Plant Soil. 2003, 256, 29–39. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Bossdorf, O.; Dawson, W. The Ecology and Evolution of Alien Plants. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 25–47. [Google Scholar] [CrossRef]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy Is Pervasive in Invasive Plants. Biol. Invasions 2021, 23, 367–371. [Google Scholar] [CrossRef]
- Catalán, P.; Vázquez-De-Aldana, B.R.; De Las Heras, P.; Fernández-Seral, A.; Pérez Corona, M.E. Comparing the Allelopathic Potential of Exotic and Native Plant Species on Understory Plants: Are Exotic Plants Better Armed? An. Biol. 2013, 35, 65–74. [Google Scholar] [CrossRef]
- Inderjit; Seastedt, T.R.; Callaway, R.M.; Pollock, J.L.; Kaur, J. Allelopathy and Plant Invasions: Traditional, Congeneric, and Bio-Geographical Approaches. Biol. Invasions 2008, 10, 875–890. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel Weapons: Invasive Success and the Evolution of Increased Competitive Ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Thorpe, A.S.; Thelen, G.C.; Diaconu, A.; Callaway, R.M. Root Exudate Is Allelopathic in Invaded Community But Not in Native Community: Field Evidence for the Novel Weapons Hypothesis. J. Ecol. 2009, 97, 641–645. [Google Scholar] [CrossRef]
- He, W.M.; Feng, Y.; Ridenour, W.M.; Thelen, G.C.; Pollock, J.L.; Diaconu, A.; Callaway, R.M. Novel Weapons and Invasion: Biogeographic Differences in the Competitive Effects of Centaurea maculosa and its Root Exudate (±)-Catechin. Oecologia 2009, 159, 803–815. [Google Scholar] [CrossRef]
- Ni, G.Y.; Schaffner, U.; Peng, S.L.; Callaway, R.M. Acroptilon repens, an Asian Invader, Has Stronger Competitive Effects on Species From America than Species From Its Native Range. Biol. Invasions 2010, 12, 3653–3663. [Google Scholar] [CrossRef]
- Thorpe, A.S.; Callaway, R.M. Biogeographic Differences in the Effects of Centaurea stoebe on the Soil Nitrogen Cycle: Novel Weapons and Soil Microbes. Biol. Invasions 2011, 13, 1435–1445. [Google Scholar] [CrossRef]
- Kim, Y.O.; Lee, E.J. Comparison of Phenolic Compounds and the Effects of Invasive and Native Species in East Asia: Support for the Novel Weapons Hypothesis. Ecol. Res. 2011, 26, 87–94. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Feng, Y.L.; Zhang, L.Q.; Callaway, R.M.; Valiente-Banuet, A.; Lei, Y.B.; Luo, D.Q.; Liao, Z.Y.; Barclay, G.F.; Silva-Pereyra, C. Integrating Novel Chemical Weapons and Evolutionarily Increased Competitive Ability in Success of a Tropical Invader. New Phytol. 2015, 205, 1350–1359. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, J.; Ma, K. Soil Biota Reduce Allelopathic Effects of the Invasive Eupatorium adenophorum. PLoS ONE 2011, 6, e25393. [Google Scholar] [CrossRef]
- Lind, E.M.; Parker, J.D. Novel Weapons Testing: Are Invasive Plants More Chemically Defended than Native Plants? PLoS ONE 2010, 5, e10429. [Google Scholar] [CrossRef]
- Medina-Villar, S.; Alonso, Á.; Castro-Díez, P.; Pérez-Corona, E. Allelopathic Potentials of Exotic Invasive and Native Trees Over Coexisting Understory Species: The Soil as Modulator. Plant Ecol. 2017, 218, 579–594. [Google Scholar] [CrossRef]
- Oduor, A.M.O.; van Kleunen, M.; Stift, M. Allelopathic Effects of Native And Invasive Brassica Nigra do not Support The Novel-Weapons Hypothesis. Am. J. Bot. 2020, 107, 1106–1113. [Google Scholar] [CrossRef]
- Castroviejo, S.; Acedo, C.; Cirujano, S.; Laínz, M.; Monserrat, P.; Morales, R.; Soriano, C. Flora Ibérica: Plantas Vasculares de la Península Ibérica e Islas Baleares (Vol. 3); Real Jardín Botánico, CSIC: Madrid, Spain, 2006. [Google Scholar]
- Dias, A.S.; Dias, L.S.; Pereira, I.P. Activity of water extracts of Cistus ladanifer and Lavandula stoechas in soil on germination and early growth of wheat and Phalaris minor. Allelopath. J. 2004, 14, 59–64. [Google Scholar]
- Castroviejo, S.; Acedo, C.; Cirujano, S.; Laínz, M.; Monserrat, P.; Morales, R.; Soriano, C. Flora Ibérica: Plantas Vasculares de la Península Ibérica e Islas Baleares (Vol. 9); Real Jardín Botánico, CSIC: Madrid, Spain, 2015. [Google Scholar]
- Demirbüker Kavak, D.; Altıok, E.; Bayraktar, O.; Ülkü, S. Pistacia terebinthus Extract: As A Potential Antioxidant, Antimicrobial and Possible Β-Glucuronidase Inhibitor. J. Mol. Catal. B Enzym. 2010, 64, 167–171. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Madani, K.; Falé, P.L.V.; Serralheiro, M.L.; Araújo, M.E.M. Antioxidant Capacity and Phenolic Contents of Some Mediterranean Medicinal Plants and Their Potential Role in the Inhibition of Cyclooxygenase-1 and Acetylcholinesterase Activities. Ind. Crops Prod. 2014, 53, 6–15. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional Uses, Phytochemistry and Pharmacology of Chios Mastic Gum (Pistacia lentiscus var. Chia, Anacardiaceae): A Review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef]
- Ismail, A.; Lamia, H.; Mohsen, H.; Bassen, J. Chemical Composition and Herbicidal Effects of Pistacea lentiscus L. Essential Oil Against Weeds. Int. J. Med. Arom. Plants 2012, 2, 558–565. [Google Scholar]
- Saludes-Zanfaño, M.I.; Vivar-Quintana, A.M.; Morales-Corts, M.R. Pistacia Root and Leaf Extracts as Potential Bioherbicides. Plants 2022, 11, 916. [Google Scholar] [CrossRef]
- Bulut, Y.; Atabeyoğlu, Ö.; Kordali, Ş. The Effects of Pistacia terebinthus Leaf Extracts and Giberellic Acid on Plant Height, Inflorescence Survival and Inflorescence Numbers of Pelargonium ‘Ringo Deep Scarlet’. Atatürk Üniversitesi Ziraat Fakültesi Derg. 2011, 39, 123–126. [Google Scholar]
- Blanco, J.; Vázquez, F.M.; García, D.; Márquez, F.; Palacios, M.J. Flora of the Spanish List and Catalog of Invasive Alien Species Present in the Extremadura Region. EEI Congr. Nac. Sobre Especies Exóticas Invasoras 2012, 80–82. [Google Scholar]
- Lorenzo, P.; González, L.; Reigosa, M.J. The Genus Acacia as Invader: The Characteristic Case of Acacia dealbata Link in Europe. Ann. For. Sci. 2010, 67, 101. [Google Scholar] [CrossRef]
- Rodríguez-Echeverría, S.; Afonso, C.; Correia, M.; Lorenzo, P.; Roiloa, S.R. The Effect of Soil Legacy on Competition and Invasion by Acacia dealbata Link. Plant Ecol. 2013, 214, 1139–1146. [Google Scholar] [CrossRef]
- Carballeira, A.; Reigosa, M.J. Effects of Natural Leachates of Acacia dealbata Link in Galicia (NW Spain). Bot. Bull. Acad. Sin. 1999, 40, 87–92. [Google Scholar]
- Lorenzo, P.; Pazos-Malvido, E.; González, L.; Reigosa, M.J. Allelopathic Interference of Invasive Acacia dealbata: Physiological Effects. Allelopath. J. 2008, 22, 452–462. [Google Scholar]
- Lorenzo, P.; Palomera-Pérez, A.; Reigosa, M.J.; Gónzalez, L. Allelopathic Interference of Invasive Acacia dealbata Link on the Physiological Parameters of Native Understory Species. Plant Ecol. 2011, 212, 403–412. [Google Scholar] [CrossRef]
- Aguilera, N.; Becerra, J.; Villaseñor-Parada, C.; Lorenzo, P.; González, L.; Hernández, V. Effects and Identification of Chemical Compounds Released from the Invasive Acacia dealbata Link. Chem. Ecol. 2015, 31, 479–493. [Google Scholar] [CrossRef]
- Dollar, K.E.; Pallardy, S.G.; Garrett, H.G. Composition and Environment of Floodplain Forests of Northern Missouri. Can. J. For. Res. 1992, 22, 1343–1350. [Google Scholar] [CrossRef]
- Yeryomenko, Y.A. Allelopathic Activity of Invasive Arboreal Species. Russ. J. Biol. Invasions 2014, 5, 146–150. [Google Scholar] [CrossRef]
- Nikolaeva, A.A.; Golosova, E.V.; Shelepova, O.V. Allelopathic Activity of Acer negundo L. Leaf Litter as a Vector of Invasion Species into Plant Communities. BIO Web Conf. 2021, 38, 00088. [Google Scholar] [CrossRef]
- Flora of China. Salix Babylonica Linnaeus FOC Vol, 4, 186. Available online: http://www.efloras.org (accessed on 21 November 2022).
- Baquero, R.; Falcón, S.; Nicola, G. Guía Didáctica de Educación Ambiental. In Especies Exóticas Invasoras; Universidad de Castillas la Mancha: Cuenca, Spain, 2022. [Google Scholar]
- Mutlu-Durak, H.; Yildiz Kutman, B. Seed Treatment with Biostimulants Extracted from Weeping Willow (Salix babylonica) Enhances Early Maize Growth. Plants 2021, 10, 1449. [Google Scholar] [CrossRef]
- Devesa, J.A.; Arroyo, J.; Herrera, J. Contribución al Conocimiento de la Biología Floral del Género Lavandula L. Anales Jard. Bot. Madrid 1985, 42, 165–186. [Google Scholar]
- Valdés, B.; Talavera, S.; Fernández-Galiano, E. Flora Vascular de Andalucía Occidental; Ketres Ed.: Barcelona, Spain, 1987; Volume 2. [Google Scholar]
- Luís, A.; Gil, N.; Amaral, M.E.; Duarte, A.P. Antioxidant Activities of Extracts from Acacia melanoxylon, Acacia dealbata and Olea europaea and Alkaloids Estimation. Int. J. Pharm. Pharm. Sci. 2012, 4, 225–231. [Google Scholar]
- Mcewan, R.W.; Arthur-Paratley, L.G.; Rieske, L.K.; Arthur, M.A. A Multi-Assay Comparison of Seed Germination Inhibition by Lonicera Maackii and Co-Occurring Native Shrubs. Flora 2010, 205, 475–483. [Google Scholar] [CrossRef]
- Cipollini, K.; Titus, K.; Wagner, C. Allelopathic Effects of Invasive Species (Alliaria petiolata, Lonicera maackii and Ranunculus ficaria) in the Midwestern United States. Allelopathy J. 2012, 29, 63–76. [Google Scholar]
- Cipollini, K.; Greenawalt, M. Comparison of Allelopathic Effects of Five Invasive Species on two Native Species. J. Torrey Bot. Society 2016, 143, 427–436. [Google Scholar] [CrossRef]
- Chon, S.U.; Nelson, C.J. Allelopathy in Compositae Plants. A Review. Agron. Sustain. Dev. 2010, 30, 349–358. [Google Scholar] [CrossRef]
- Del Fabbro, C.; Güsewell, S.; Prati, D. Allelopathic Effects of Three Plant Invaders on Germination of Native Species: A Field Study. Biol. Invasions 2014, 16, 1035–1042. [Google Scholar] [CrossRef]
- De Las Heras, P.; Medina-Villar, S.; Pérez-Corona, M.E.; Vázquez-De- Aldana, B. Leaf Litter Age Regulates the Effect of Native and Exotic Tree Species on Understory Herbaceous Vegetation of Riparian Forests. Basic Appl. Ecol. 2020, 48, 11–25. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana Camara as an Invasive Plant. Plants 2021, 10, 1028. [Google Scholar] [CrossRef]
- Arroyo, A.I.; Pueyo, Y.; Saiz, H.; Alados, C.L. Plant-Plant Interactions as a Mechanism Structuring Plant Diversity in a Mediterranean Semi-Arid Ecosystem. Ecol. Evol. 2015, 5, 5305–5317. [Google Scholar] [CrossRef]
- Hashoum, H.; Santonja, M.; Gauquelin, T.; Saatkamp, A.; Ganivet, J.; Greff, S.; Lecareux, C.; Fernández, C.; Bousquet-Mélou, A. Biotic Interactions in a Mediterranean Oak Forest: Role of Allelopathy Along Phenological Development of Woody Species. Eur. J. Forest Res. 2017, 136, 699–710. [Google Scholar] [CrossRef]
- Fernández, C.; Monnier, Y.; Santoja, M.; Gallet, C.; Weston, L.A.; Prévosto, B.; Saunier, A.; Baldy, V.; Bousquet-Mélou, A. The Impact of Competition and Allelopathy on the Trade-Off Between Plant Defense and Growth in two Contrasting Tree Species. Front. Plant Sci. 2016, 7, 594. [Google Scholar] [CrossRef]
- Chen, B.; Liao, H.; Chen, W.; Wei, H.; Peng, S. Role of Allelopathy in Plant Invasion and Control of Invasive Plants. Allelopathy J. 2017, 41, 155–166. [Google Scholar] [CrossRef]
- Christina, M.; Rouifed, S.; Puijalon, S.; Vallier, F.; Meiffren, G.; Bellvert, F.; Piola, F. Allelopathic Effect of a Native Species on A Major Plant Invader in Europe. Sci. Nat. 2015, 102, 12–19. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-phosphotungstic Acid Reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Chang, C.; Yang, M.; Wen, H.; Chern, J. Estimation of Total Flavonoid Content in Propolis by two Complementary Colorimetric Methods. J. Food Drug Analaysis 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Macías, F.A.; Simonet, A.M.; Pacheco, P.C.; Barrero, A.F.; Cabrera, E.; Jiménez-González, D. Natural and Synthetic Podolactones with Potential Use as Natural Herbicide Models. J. Agric. Food Chem. 2000, 48, 3003–3007. [Google Scholar] [CrossRef]
- Qasim, M.; Fujii, Y.; Zaheer, M.; Irfan Aziz, A.; Watanabe, K.N.; Ajmal Khan, M. Phytotoxic Analysis of Coastal Medicinal Plants and Quantification of Phenolic Compounds Using HPLC. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2019, 153, 767–774. [Google Scholar] [CrossRef]

| Phenol Total | Flavonoids | ||
|---|---|---|---|
| GAE (mg/mg dw) | EQ (mg/mg dw) | EQ (mg/mg dw) | |
| S. babylonica | 0.010 a | 0.008 a | 0.004 a |
| A. negundo | 0.012 a | 0.010 a | 0.008 b |
| A. dealbata | 0.025 b | 0.021 b | 0.016 c |
| C. ladanifer | 0.013 a | 0.012 a | 0.010 b |
| P. lentiscus | 0.024 b | 0.020 b | 0.012 c,b |
| P. terebinthus | 0.026 b | 0.022 b | 0.012 c,b |
| Lavandula stoechas | |||||
|---|---|---|---|---|---|
| Extract | Species | Germination | Root Size | Cotyledon | Cotyledon Size |
| 100% | Native | −94.68 a | −98.20 a | −99.50 a | −96.36 a |
| Non native | −88.88 a | −92.83 a | −95.02 a | −89.82 a | |
| 50% | Native | −56.03 a | −93.40 a | −84.07 a | −79.68 a |
| Non native | −34.29 a | −73.69 b | −62.18 b | −57.93 a | |
| 25% | Native | −17.87 a | −76.74 a | −46.76 a | −51.93 a |
| Non native | −6.76 a | −43.86 b | −17.41 b | −11.44 b | |
| Echium plantagineum | |||||
| Germination | Root size | Cotyledon | Cotyledon size | ||
| 100% | Native | −56.25 a | −96.54 a | −82.66 a | −83.57 a |
| Non native | −42.70 a | −81.47 b | −54.00 b | −40.14 b | |
| 50% | Native | −20.83 a | −90.51 a | −34 a | −43.42 a |
| Non native | −9.89 a | −62.04 b | −9.33 a | −20.69 a | |
| 25% | Native | 1.04 a | −77.47 a | −6.00 a | −17.69 a |
| Non native | −4.16 a | −53.9 b | −2.66 a | 3.91 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves Lobón, N.; González Félix, M.; Alías Gallego, J.C. Comparison of the Allelopathic Potential of Non-Native and Native Species of Mediterranean Ecosystems. Plants 2023, 12, 972. https://doi.org/10.3390/plants12040972
Chaves Lobón N, González Félix M, Alías Gallego JC. Comparison of the Allelopathic Potential of Non-Native and Native Species of Mediterranean Ecosystems. Plants. 2023; 12(4):972. https://doi.org/10.3390/plants12040972
Chicago/Turabian StyleChaves Lobón, Natividad, Marisa González Félix, and Juan Carlos Alías Gallego. 2023. "Comparison of the Allelopathic Potential of Non-Native and Native Species of Mediterranean Ecosystems" Plants 12, no. 4: 972. https://doi.org/10.3390/plants12040972
APA StyleChaves Lobón, N., González Félix, M., & Alías Gallego, J. C. (2023). Comparison of the Allelopathic Potential of Non-Native and Native Species of Mediterranean Ecosystems. Plants, 12(4), 972. https://doi.org/10.3390/plants12040972






