Molecular Fingerprinting and Phytochemical Investigation of Syzygium cumini L. from Different Agro-Ecological Zones of India
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals and Reagents
2.1.2. Plant Materials
2.2. Method
2.2.1. Genomic DNA Isolation from S. cumini
2.2.2. Molecular Analysis by Using ISSR Marker
2.2.3. Phytochemical Study of S. cumini
- Extraction and estimation of polyphenols
- 2.
- Separation of polyphenols using TLC
- 3.
- Separation of polyphenols using HPLC
- 4.
- Statistical Analysis
3. Results and Discussion
3.1. Molecular Analysis
3.1.1. DNA Extraction Analysis
3.1.2. Genetic Diversity Analysis
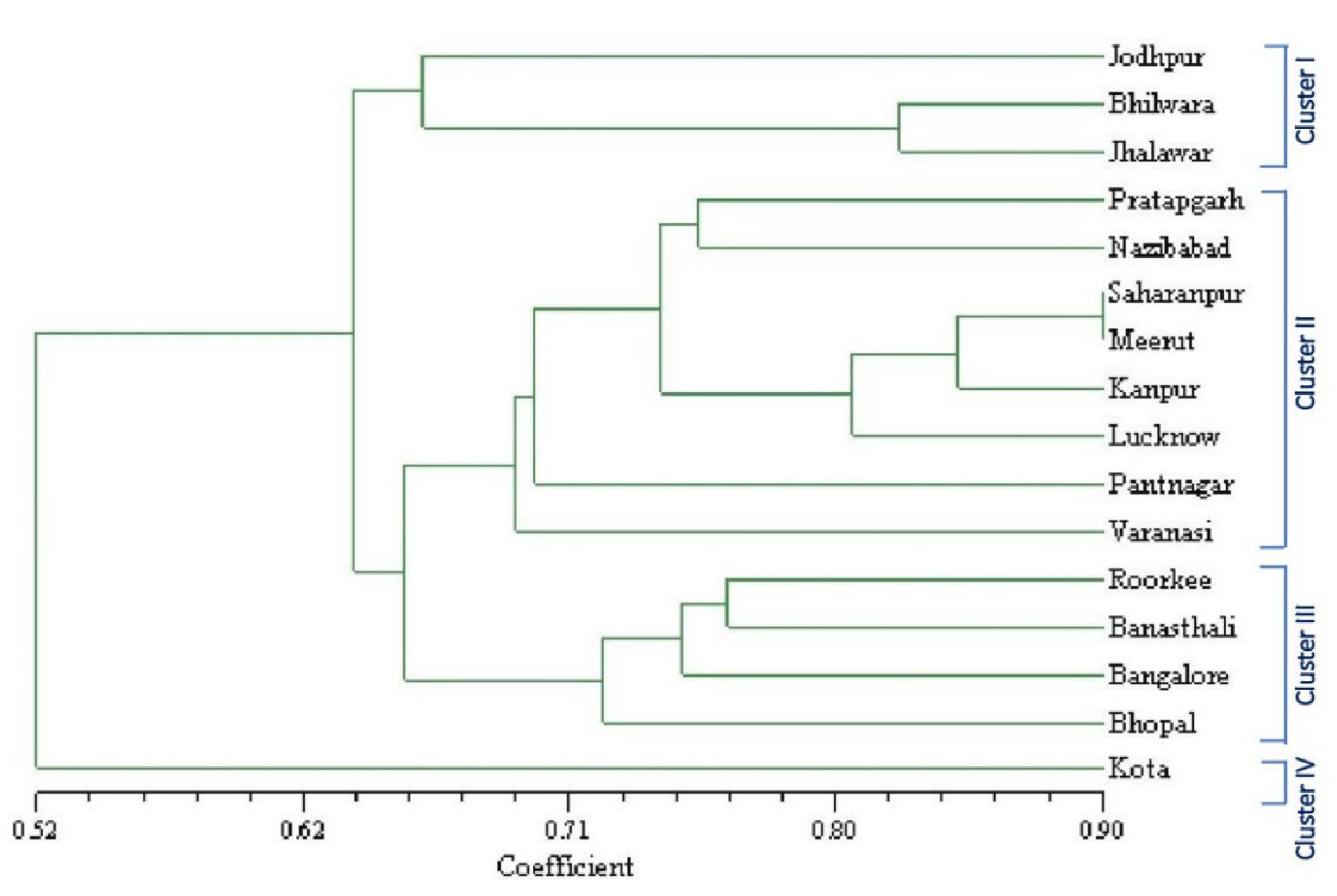
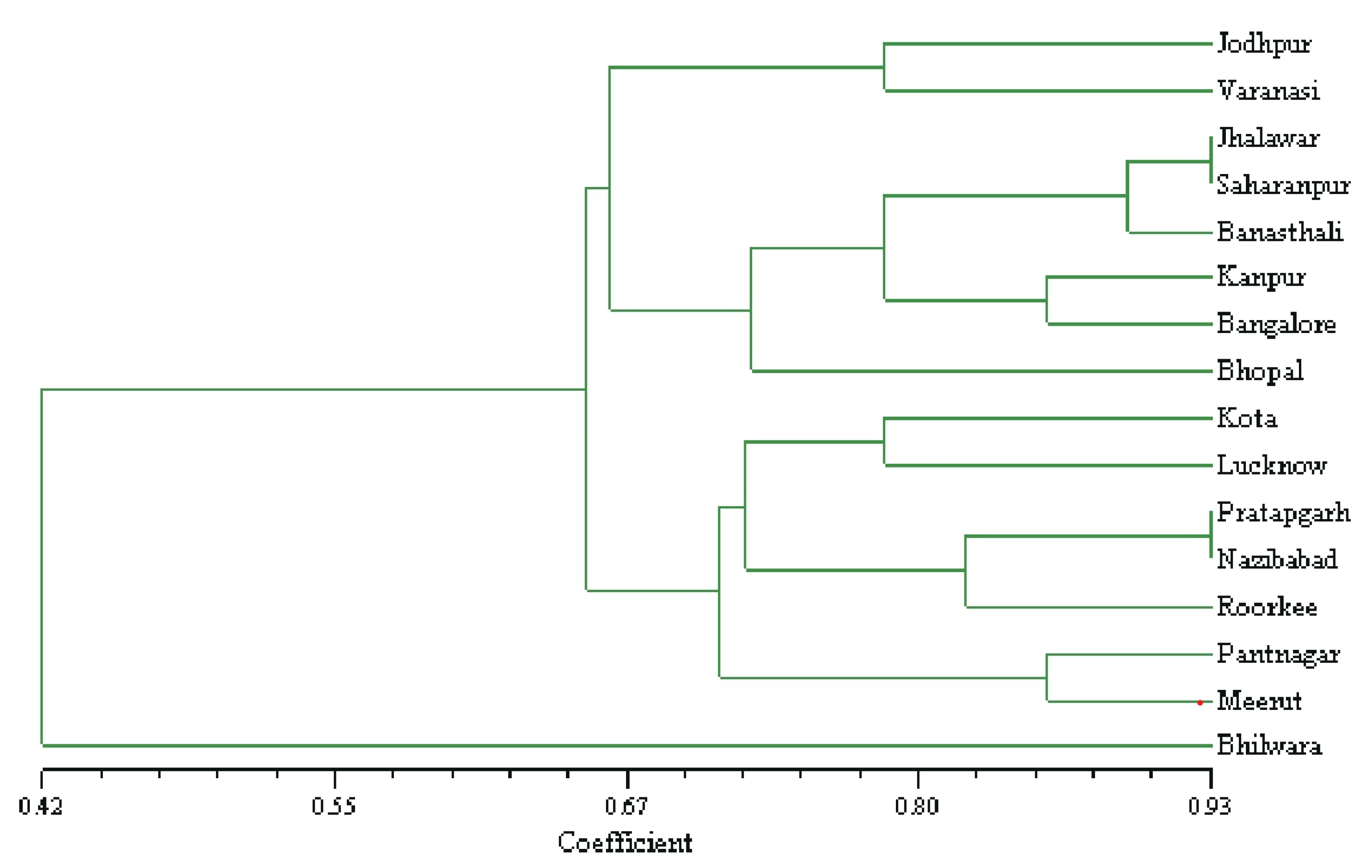
3.1.3. ISSR Marker-Based Genetic Similarity and Cluster Analysis
3.1.4. Shannon Diversity Index Based upon ISSR Markers
3.2. Phytochemical Analysis
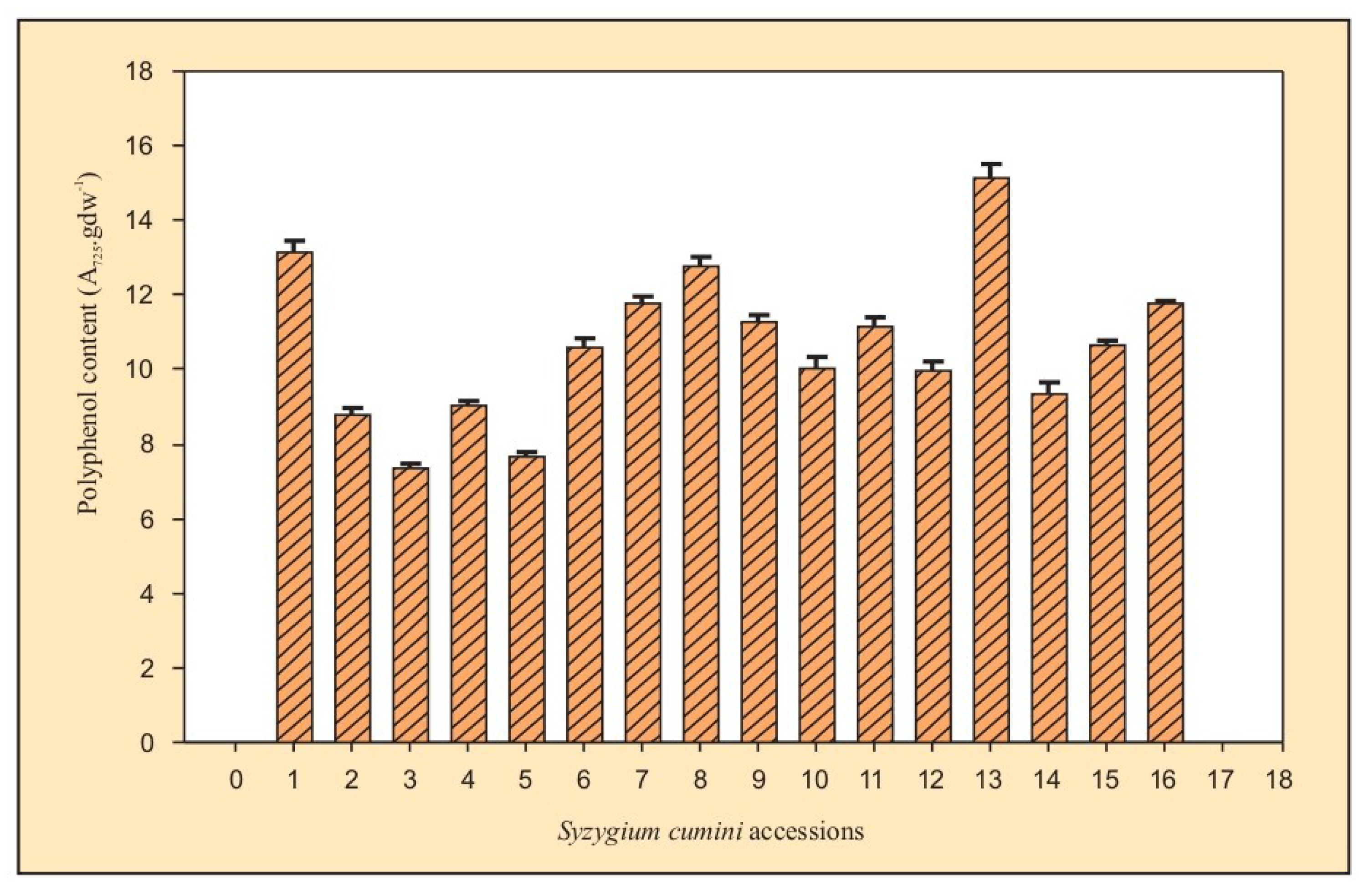
3.2.1. Total Phenol
3.2.2. Qualitative Analysis of Total Phenols Using TLC
3.2.3. Qualitative Analysis of Phenolic Acids Using HPLC
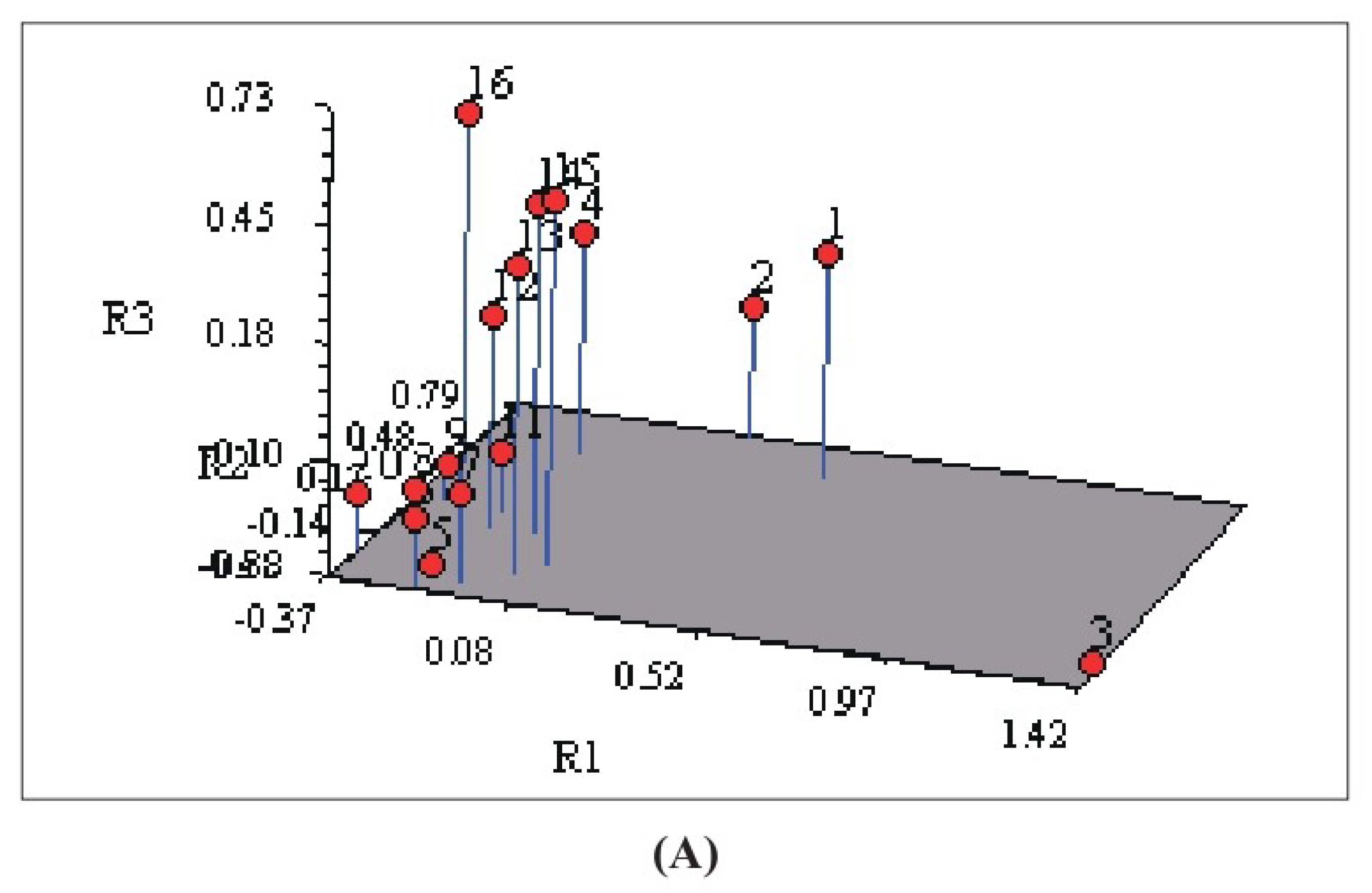
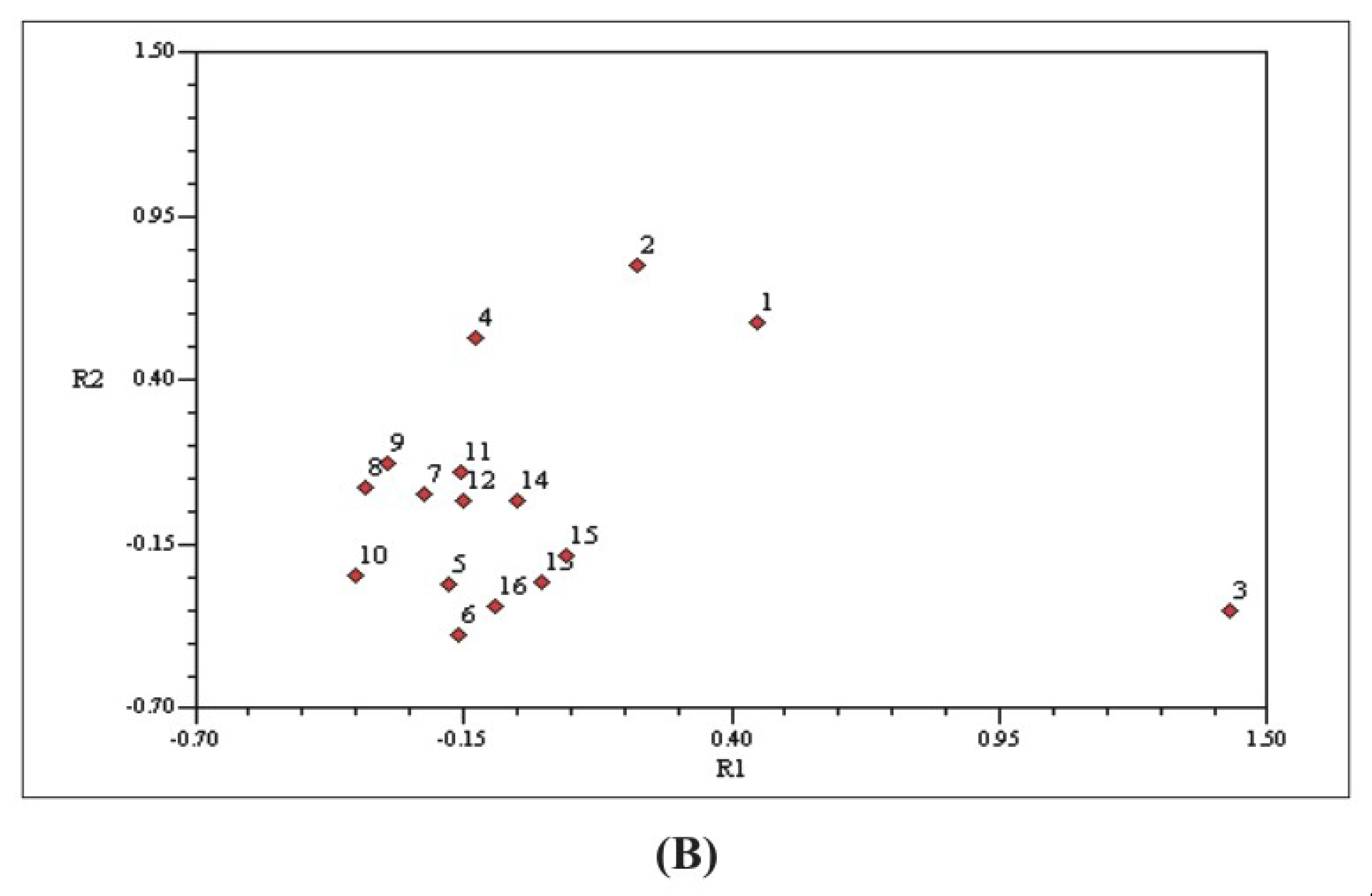
3.3. Correlation between Phytochemical and Molecular Profiling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brummitt, N.A.; Bachman, S.P.; Griffiths-Lee, J.; Lutz, M.; Moat, J.F.; Farjon, A.; Donaldson, J.S.; Hilton-Taylor, C.; Meagher, T.R.; Albuquerque, S.; et al. Green Plants in the Red: A Baseline Global Assessment for the IUCN Sampled Red List Index for Plants. PLoS ONE 2015, 10, e0135152. [Google Scholar] [CrossRef] [PubMed]
- Pironon, S.; Borrell, J.S.; Ondo, I.; Douglas, R.; Phillips, C.; Khoury, C.K.; Kantar, M.B.; Fumia, N.; Soto Gomez, M.; Viruel, J.; et al. Toward unifying global hotspots of wild and domesticated biodiversity. Plants 2020, 9, 1128. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Kang, D.; Ren, Y.; Qu, L.J.; Zhen, Y.; Gu, H. Genetic diversity of the natural populations of Arabidopsis thaliana in China. Heredity 2007, 99, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Madani, B.; Mirshekari, A.; Yahia, E.M.; Golding, J.B.; Hajivand, S.; Dastjerdy, A.M. Jamun (Syzygium cumini L. Skeels): A Promising Fruit for the Future. Hortic. Rev. 2021, 48, 275–306. [Google Scholar] [CrossRef]
- Singh, J.S.; Chaturvedi, R.K. Diversity of Ecosystem Types in India: A Review. Proc. Indian Natl. Sci. Acad. 2017, 83, 569–594. [Google Scholar] [CrossRef]
- Sardar, N.R.; Akbari, S.H.; Bhatt, H.G.; Tagalpallewar, G.P. Chemical and mineral composition of jamun fruit pulp (Syzygium cumini L.). Pharma Innov. 2022, 11, 1976–1979. [Google Scholar]
- De Carvalho, J.A.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Kano, R.; Bonifaz, A.; Toriello, C.; Negroni, R.; Rego, R.D.M.; Gremião, I.D.F.; et al. Trends in the molecular epidemiology and population genetics of emerging Sporothrix species. Stud. Mycol. 2021, 100, 100131. [Google Scholar] [CrossRef]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome Fingerprinting by Simple Sequence Repeat (SSR)-Anchored Polymerase Chain Reaction Amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef]
- Pianzzola, M.J.; Zarantonelli, L.; González, G.; Fraguas, L.F.; Vázquez, A. Genetic, phytochemical and biochemical analyses as tools for biodiversity evaluation of wild accessions of Solanum commersonii. Biochem. Syst. Ecol. 2004, 33, 67–78. [Google Scholar] [CrossRef]
- Jiang, H.; Xie, Z.; Koo, H.J.; McLaughlin, S.P.; Timmermann, B.N.; Gang, D.R. Metabolic profiling and phylogenetic analysis of medicinal Zingiber species: Tools for authentication of ginger (Zingiber officinale Rosc.). Phytochemistry 2006, 67, 1673–1685. [Google Scholar] [CrossRef]
- Grass, S.; Zidorn, C.; Blattner, F.R.; Stuppner, H. Comparative molecular and phytochemical investigation of Leontodon autumnalis (Asteraceae, Lactuceae) populations from Central Europe. Phytochemistry 2006, 67, 122–131. [Google Scholar] [CrossRef]
- Pandey, A.; Belwal, T.; Tamta, S.; Bhatt, I.D.; Rawal, R.S. Phenolic compounds, antioxidant capacity and antimutagenic activity in different growth stages of in vitro raised plants of Origanum vulgare L. Mol. Biol. Rep. 2019, 46, 2231–2241. [Google Scholar] [CrossRef]
- Khan, S.; Sharma, V. Genetic differentiation and diversity analysis of medicinal tree Syzygium cumini (Myrtaceae) from eco-logically different regions of India. Physiol. Mol. Biol. Plants 2010, 16, 149–158. [Google Scholar] [CrossRef]
- Jana, S.; Shekhawat, G.S. In vitro regeneration of Anethum graveolens, antioxidative enzymes during organogenesis and RAPD analysis for clonal fidelity. Biol. Plant 2012, 56, 9–14. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Yen, G.-C. Antioxidant Activity of Phenolic Compounds Isolated from Mesona procumbens Hemsl. J. Agric. Food Chem. 2002, 50, 2993–2997. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYS-Pc Numerical Taxonomy and Multivariate Analysis System, Version 2.1; Exeter Software: Setauket, NY, USA, 1993. [Google Scholar]
- Jaccard, P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 1908, 44, 223–270. [Google Scholar]
- Prevost, A.; Wilkinson, M.J. A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor. Appl. Genet. 1999, 98, 107–112. [Google Scholar] [CrossRef]
- Anderson, J.A.; Churchill, G.A.; Autrique, J.E.; Tanksley, S.D.; Sorrells, M.E. Optimizing parental selection for genetic linkage maps. Genome 1993, 36, 181–186. [Google Scholar] [CrossRef]
- Powell, W.; Morgante, M.; Andre, C. The comparision of RFLP, RAPD, AFLP and SSR (microsatellite) marker for germplasm analysis. Mol. Breed. 1996, 2, 225–238. [Google Scholar] [CrossRef]
- Singh, V.K.; Avtar, R.; Mahavir, N.K.; Manjeet, R.K.; Rathore, V. Assessment of genetic relationship among diverse Indian mustard (Brassica juncea L.) genotypes using XLSTAT. Electron. J. Plant Breed. 2020, 11, 674–680. [Google Scholar] [CrossRef]
- Kulkarni, M.; Borse, T.; Chaphalkar, S. Isolation and purification of genomic DNA from Black Plum (Eugenia jambolana Lam.) for analytical applications. Int. J. Biotechnol. Biochem. 2007, 3, 1–8. [Google Scholar]
- Morgante, M.; Oliveri, A.M. PCR-amplified microsatellites as markers in plant genetics. Plant J. 1993, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Amel, S.-H.; Khaled, C.; Messaoud, M.; Mohamed, M.; Mokhtar, T. Comparative Analysis of Genetic Diversity in Two Tunisian Collections of Fig Cultivars Based on Random Amplified Polymorphic DNA and Inter Simple Sequence Repeats Fingerprints. Genet. Resour. Crop. Evol. 2005, 52, 563–573. [Google Scholar] [CrossRef]
- Hamrick, J.L.; Godt, M.J.W. Allozyme diversity in plant species. In Plant Population Genetics, Breeding and Genetic Resources, Brown, A.H.D., Clegg, M.T., Kahler, A., Eds; Sinauer Associates Inc: Sunderland, UK, 1989; pp. 43–63. [Google Scholar]
- Qiu, Y.-X.; Hong, D.-Y.; Fu, C.-X.; Cameron, K.M. Genetic variation in the endangered and endemic species Changium smyrnioides (Apiaceae). Biochem. Syst. Ecol. 2003, 32, 583–596. [Google Scholar] [CrossRef]
- Bahulikar, R.A.; Lagu, M.D.; Kulkarmi, B.G.; Pandit, S.S.; Suresh, H.S.R.; Ranjekar, M.K.V.; Gupta, V.S. Genetic diversity among spatially isolated populations of Euriya nitida Korth (Theacea) based on inter-simple sequences repeats. Curr. Sci. 2004, 86, 824–831. [Google Scholar]
- Cruz, E.P.; Gepts, P.; GarciaMarín, P.C.; Villareal, D.Z. Spatial Distribution of Genetic Diversity in Wild Populations of Phaseolus vulgaris L. from Guanajuato and Michoacán, Méexico. Genet. Resour. Crop. Evol. 2005, 52, 589–599. [Google Scholar] [CrossRef]
- Li, J.-M.; Jin, Z.-X. Genetic structure of endangered Emmenopterys henryi Oliv. based on ISSR polymorphism and implications for its conservation. Genetica 2007, 133, 227–234. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Elam, D.R. Population genetic consequences of small population size. Implications for plant conservation. Annu. Rev. Ecol. Syst. 1993, 24, 217–242. [Google Scholar] [CrossRef]
- Sun, M.K.; Wong, C. Genetic structure of three orchid species with contrasting breeding systems using RAPD and allozyme markers. Am. J. Bot. 2001, 88, 2180–2188. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.; He, T. Ex situ genetic conservation of endangered Vatica guangxiensis (Dipterocarpaceae) in China. Biol. Conserv. 2002, 106, 151–156. [Google Scholar] [CrossRef]
- Vaishali; Khan, S.; Sharma, V. RAPD based assessment of genetic diversity of Butea monosperma from different agro-ecological regions of India. Indian J. Biotechnol. 2008, 7, 320–327. [Google Scholar]
- Pour Nikfardjam, M.S.; Márk, L.; Avar, P.; Figler, M.; Ohmacht, R. Polyphenols, anthocyanins, and trans-resveratrol in red wines from the Hungarian Villány region. Food Chem. 2006, 98, 453–462. [Google Scholar] [CrossRef]
- Gambelli, L.; Santaroni, G. Polyphenols content in some Italian red wines of different geographical origins. J. Food Compos. Anal. 2004, 17, 613–618. [Google Scholar] [CrossRef]
- Maksimović, Z.; Malenčić, D.; Kovačević, N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Contour-Ansel, D.; Bernhard-Reversat, F. High-performance liquid chromatography of water-soluble phenolics in leaf litter of three Eucalyptus hybrids (Congo). Plant Sci. 2002, 163, 217–222. [Google Scholar] [CrossRef]
- Tanrıöven, D.; Ekşi, A. Phenolic compounds in pear juice from different cultivars. Food Chem. 2005, 93, 89–93. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem. 2004, 87, 307–311. [Google Scholar] [CrossRef]
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavanoids of apple and pear by high performance liquid chromatography. J. Liq. Chromatogr. A 2001, 910, 265–273. [Google Scholar] [CrossRef]
- Palade, L.M.; Popa, M.E. Polyphenol Fingerprinting Approaches in Wine Traceability and Authenticity: Assessment and Implications of Red Wines. Beverages 2018, 4, 75. [Google Scholar] [CrossRef]
- McKeehen, J.D.; Busch, R.H.; Fulcher, R.G. Evaluation of wheat (Triticum aestivum L.) phenolic acids during grain development and their contribution to Fusarium resistance. J. Agri. Food Chem. 1999, 47, 1476–1482. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.; Robards, K.; Ryan, D. Sample preparation in the determination of phenolic compounds in fruits. Analyst 2000, 125, 989–1009. [Google Scholar] [CrossRef]
- Khanuja, S.P.; Shasany, A.K.; Pawar, A.; Lal, R.; Darokar, M.; Naqvi, A.; Rajkumar, S.; Sundaresan, V.; Lal, N.; Kumar, S. Essential oil constituents and RAPD markers to establish species relationship in Cymbopogon Spreng. (Poaceae). Biochem. Syst. Ecol. 2005, 33, 171–186. [Google Scholar] [CrossRef]
- Hu, Y.; Hou, T.T.; Xin, H.L.; Zhang, Q.Y.; Zheng, H.C.; Rahman, K.; Qin, L.P. Estrogen like activity of volatile components from Vitex royundifolia L. Indian J. Med. Res. 2007, 126, 68–72. [Google Scholar] [PubMed]
- Smelcerovic, A.; Verma, V.; Spiteller, M.; Ahmad, S.M.; Puri, S.C.; Qazi, G.N. Phytochemical analysis and genetic character-ization of six Hypericum species from Serbia. Phytochemistry 2006, 67, 171–177. [Google Scholar] [CrossRef]

| S. No. | Major Zones | Sub Zones | Place | Sample Code | State | Latitude | Longitude |
|---|---|---|---|---|---|---|---|
| 1. | Arid | E-1.2 | Jodhpur | JD1 | Rajasthan | N 26°23′ | E 73°8′ |
| 2. | Semi-arid | D-4.2 | Bhilwara | JD2 | Rajasthan | N 22°25′ | E 74°38′ |
| 3. | D-4.2 | Kota | JD3 | Rajasthan | N 24°14′ | E 75°49′ | |
| 4. | D-4.2 | Jhalawar | JD4 | Rajasthan | N 24°40′ | E 76°10′ | |
| 5. | D-4.2 | Pratapgarh | JD5 | Rajasthan | N 24°20′ | E 74°40′ | |
| 6. | D-4.2 | Bhopal | JD14 | MP | N 23°20′ | E 77°30′ | |
| 7. | D-4.3 | Meerut | JD9 | UP | N 29°10′ | E 77°42′ | |
| 8. | D-3.3 | Banasthali | JD15 | Rajasthan | N 26°60′ | E 75°54′ | |
| 9. | D-4.4 | Bangalore | JD16 | Karnataka | N 12°59′ | E 77°40′ | |
| 10. | Dry sub-humid | CD-5.4 | Saharanpur | JD7 | UP | N 29°58′ | E 77°33′ |
| 11. | CD-5.4 | Kanpur | JD8 | UP | N 26°28′ | E 80°20′ | |
| 12. | CD-5.4 | Lucknow | JD11 | UP | N 26°50′ | E 81°00′ | |
| 13. | CD-4.1 | Varanasi | JD12 | UP | N 25°22′ | E 83°00′ | |
| 14. | Moist sub-humid | CM-6.2 | Pantnagar | JD6 | Uttrakhand | N 29°31′ | E 79°30′ |
| 15. | CD-6.1 | Nazibabad | JD10 | Uttrakhand | N 29°36′ | E 78°18′ | |
| 16. | CD-6.1 | Roorkee | JD14 | Uttrakhand | N 29°52′ | E 77°59′ |
| S. No. | Primer Code | Oligomer Length (bp) | Tm | Primer Sequence (5’-3′) |
|---|---|---|---|---|
| 1. | 15BV17C1 | 17 | 52 | (AG)8 C |
| 2. | 16BV17A2 | 17 | 50 | (GA)8A |
| 3. | 17BV18T3 | 18 | 52 | (GA)8YT |
| 4. | 18BV18C4 | 18 | 54 | (GA)8YC |
| 5. | 19BV19C5 | 19 | 55 | (AC)8YAC |
| 6. | 20BV17A6 | 17 | 56 | (AGC)5CA |
| 7. | 21BV17G7 | 17 | 53 | (AGC)5GC |
| 8. | 22BV17T8 | 17 | 56 | (AGC)5GT |
| 9. | 23BV17C9 | 17 | 58 | (AGC)5GC |
| 10. | 24BV16T10 | 16 | 46 | (CA)7AT |
| 11. | 25BV16C11 | 16 | 48 | (CA)7AC |
| 12. | 26BV16T12 | 16 | 46 | (CA)7GT |
| 13. | 27BV16C13 | 16 | 50 | (CA)7GC |
| 14. | 28BV16A14 | 16 | 48 | (CA)7GA |
| 15. | 29BV16A15 | 16 | 46 | (CA)7AA |
| 16. | 30BV16A16 | 16 | 46 | (GT)7TA |
| 17. | 31BV16G17 | 16 | 48 | (GT)7TG |
| 18. | 32BV16A18 | 16 | 48 | (GT)7CA |
| 19. | 33BV16T19 | 16 | 48 | (GT)7CT |
| 20. | 34BV16T20 | 16 | 46 | (GT)7AT |
| 21. | 35BV16C21 | 16 | 48 | (GT)7AC |
| 22. | 36BV17A22 | 18 | 52 | GCTG(AG)6 A |
| 23. | 37BV17C23 | 17 | 52 | (GA)8C |
| 24. | 38BV17C24 | 17 | 52 | (AC)8C |
| 25. | 39BV19C25 | 19 | 58 | (AG)8 GCTC |
| 26. | 40BV17T26 | 17 | 50 | (GA)7CTT |
| S. No. | Places | Spectrophotometric Based | Gel Doc Based | ||||
|---|---|---|---|---|---|---|---|
| λ260\λ280 | Conc. μg.μL−1 | Yield μg.gdw−1 | MW (Kb) | Conc. μg.μL−1 | Yield μg.gdw−1 | ||
| 1. | Jodhpur | 1.81 | 3.0 | 300 | 20.4 | 1.07 | 214 |
| 2. | Bhilwara | 1.92 | 2.5 | 250 | 19.8 | 1.03 | 206 |
| 3. | Kota | 1.97 | 3.0 | 300 | 20.4 | 0.65 | 130 |
| 4. | Jhalawar | 2.00 | 3.3 | 330 | 20.4 | 0.94 | 188 |
| 5. | Pratapgarh | 1.82 | 2.6 | 260 | 19.5 | 0.96 | 192 |
| 6. | Pantnagar | 2.02 | 3.5 | 350 | 19.5 | 0.81 | 162 |
| 7. | Saharanpur | 1.86 | 3.2 | 320 | 19.5 | 0.85 | 170 |
| 8. | Kanpur | 1.91 | 3.8 | 380 | 19.5 | 0.97 | 194 |
| 9. | Meerut | 2.16 | 3.2 | 320 | 19.6 | 0.95 | 190 |
| 10. | Nazibabad | 1.74 | 2.6 | 260 | 20.4 | 0.82 | 164 |
| 11. | Lucknow | 1.83 | 3.8 | 380 | 20.4 | 0.75 | 150 |
| 12. | Varanasi | 2.00 | 2.1 | 210 | 20.4 | 1.16 | 232 |
| 13. | Roorkee | 1.80 | 4.1 | 410 | 20.4 | 1.13 | 226 |
| 14. | Bhopal | 2.06 | 1.9 | 190 | 19.5 | 0.92 | 184 |
| 15. | Banasthali | 1.93 | 2.3 | 230 | 20.4 | 0.77 | 154 |
| 16. | Bangalore | 2.04 | 3.2 | 320 | 19.5 | 0.95 | 190 |
| S. No. | Primer Code | Sequence (5′-3′) | Total no. of the Amplified Band (NAB) | Total no. of Polymorphic Fragments (NPB) | % Polymorphic Band (PPB) | Resolving Power | Gene Diversity (PIC) |
|---|---|---|---|---|---|---|---|
| 1. | 15BV17 C1 | (AG)8 C | 15 | 15 | 100.00 | 14.00 | 0.61 |
| 2. | 17BV18 T3 | (GA)8YT | 17 | 16 | 94.11 | 11.5 | 0.65 |
| 3. | 18BV18C4 | (GA)8YC | 11 | 10 | 90.90 | 8.00 | 0.74 |
| 4. | 20BV17 A6 | (AGC)5CA | 12 | 12 | 100.00 | 6.25 | 0.77 |
| 5. | 22BV17 T8 | (AGC)5GT | 7 | 7 | 100.00 | 6.50 | 0.75 |
| 6. | 26BV16 T12 | (CA)7GT | 11 | 11 | 100.00 | 11.00 | 0.68 |
| 7. | 27BV16 C13 | (CA)7GC | 11 | 10 | 90.90 | 9.12 | 0.73 |
| 8. | 40BV17 T26 | (GA)7CTT | 14 | 14 | 100.00 | 10.50 | 0.84 |
| Average | 12.25 | 11.87 | 96.25 | 10.08 | 0.74 |
| Primer Code | Sequence (5′-3’) | H Arid | H Semi-Arid | H Dry Sub-Humid | H Moist Sub-Humid | Hcol | Hcol/Hsp | Gst = (Hsp-Hcol)/Hsp | Hsp |
|---|---|---|---|---|---|---|---|---|---|
| 15BV17 C1 | (AG)8 C | - | 3.22 | 1.81 | 1.46 | 1.62 | 0.47 | 0.52 | 3.44 |
| 17BV18 T3 | (GA)8YT | - | 3.21 | 1.68 | 1.18 | 1.69 | 0.54 | 0.45 | 3.12 |
| 18BV18C4 | (GA)8YC | - | 1.17 | 0.69 | 2.28 | 1.03 | 0.41 | 0.58 | 2.48 |
| 20BV17A6 | (AGC)5CA | - | 2.27 | 1.73 | 2.56 | 1.22 | 0.46 | 0.53 | 2.65 |
| 22BV17T8 | (AGC)5GT | - | 2.38 | 2.16 | 1.18 | 1.43 | 0.62 | 0.37 | 2.30 |
| 26BV16T12 | (CA)7GT | - | 2.42 | 2.03 | 2.92 | 1.84 | 0.61 | 0.38 | 3.00 |
| 27BV16C13 | (CA)7GC | - | 2.45 | 1.94 | 2.28 | 1.67 | 0.55 | 0.44 | 3.02 |
| 40BV17T26 | (GA)7CTT | - | 4.09 | 1.38 | 2.46 | 1.86 | 0.61 | 0.38 | 3.98 |
| Average | - | 2.65 | 1.82 | 2.04 | 1.54 | 0.53 | 0.46 | 3.00 | |
| Nm | 0.30 |
| S. No. | Major Zones | Sub-Zones | Place | Sample Code | Yield (A725.gdw−1) * |
|---|---|---|---|---|---|
| 1. | Arid | E-1.2 | Jodhpur | JS1 | 13.16 ± 0.31 |
| 2. | Semi-arid | D-4.2 | Bhilwara | JS2 | 8.77 ± 0.22 |
| 3. | D-4.2 | Kota | JS3 | 7.38 ± 0.11 | |
| 4. | D-4.2 | Jhalawar | JS4 | 9.04 ± 0.12 | |
| 5. | D-4.2 | Pratapgarh | JS5 | 7.65 ± 0.16 | |
| 6. | D-4.2 | Bhopal | JS13 | 10.57 ± 0.29 | |
| 7. | D-4.3 | Meerut | JS9 | 11.74± 0.23 | |
| 8. | D-3.3 | Banasthali | JS15 | 12.75 ± 0.27 | |
| 9. | D-4.4 | Bangalore | JS16 | 11.25 ± 0.21 | |
| 10. | Dry sub-humid | CD-5.4 | Saharanpur | JS7 | 10.01 ± 0.34 |
| 11. | CD-5.4 | Kanpur | JS8 | 11.12 ± 0.29 | |
| 12. | CD-5.4 | Lucknow | JS11 | 9.96 ± 0.23 | |
| 13. | CD-4.1 | Varanasi | JS12 | 15.11 ± 0.37 | |
| 14. | Moist sub-humid | CM-6.2 | Pantnagar | JS6 | 9.36 ± 0.28 |
| 15. | CD-6.1 | Nazibabad | JS10 | 10.62 ± 0.15 | |
| 16. | CD-6.1 | Roorkee | JS14 | 11.77 ± 0.07 |
| S. No. | Phenolic Acid | Solvent 1 | Solvent 5 | Solvent 8 | Solvent 9 |
|---|---|---|---|---|---|
| 1. | Caffeic acid | 0.36 | 0.84 | 0.87 | 0.91 |
| 2. | p-Coumaric acid | 0.54 | 0.88 | 0.90 | 0.88 |
| 3. | o-Coumaric acid | 0.50 | 0.84 | 0.85 | 0.86 |
| 4. | Sinapic acid | 0.59 | 0.88 | 0.87 | 0.86 |
| 5. | Ferulic acid | 0.68 | 0.90 | 0.80 | 0.88 |
| S. No. | Standard Phenolic Acids | Retention Time (RT) | Area |
|---|---|---|---|
| 1. | Caffeic acid | 6.88 | 7,642,471 |
| 2. | Ferulic acid | 9.40 | 8,017,806 |
| 3. | Sinapic acid | 9.07 | 8,801,563 |
| 4. | p-Coumaric acid | 9.09 | 11,156,442 |
| 5. | o-Coumaric acid | 12.32 | 10,651,248 |
| Total Area | 46,269,530 |
| Arid | Semi-Arid | Dry Sub-Humid | Moist Sub-Humid | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak No. | Retention Time | Probable Identity | Jodhpur | Bhilwara | Kota | Jhalawar | Pratapgarh | Bhopal | Meerut | Banasthali | Bangalore | Saharanpur | Kanpur | Lucknow | Varanasi | Pantnagar | Nazibabad | Roorkee |
| 1. | 3.6 | - | 0.01 | 0.07 | 0.08 | 0.08 | - | 0.31 | 0.01 | - | - | 0.02 | 0.03 | 0.21 | 0.01 | 0.05 | - | - |
| 2. | 3.9 | - | 0.10 | 0.04 | - | 0.08 | 0.65 | - | 0.08 | 40.47 | 142.51 | 0.09 | 0.11 | - | 70.11 | 0.04 | 0.25 | 241.06 |
| 3. | 4.4 | - | 3.29 | 0.54 | 1.47 | 1.70 | 3.56 | 1.55 | 1.41 | 2.44 | 5.48 | 1.70 | 3.18 | 1.75 | 2.20 | 1.70 | 6.63 | 6.36 |
| 4. | 5.5 | - | 1.55 | 0.58 | 1.19 | 2.45 | 5.04 | 1.16 | 1.25 | 1.70 | 2.04 | 1.88 | 2.87 | 4.22 | 1.87 | 2.54 | 6.26 | 3.90 |
| 5. | 6.1 | - | 0.26 | - | 0.02 | 0.72 | 0.33 | - | 0.41 | 0.41 | 0.20 | 0.12 | - | 0.04 | 1.68 | 0.07 | 2.07 | - |
| 6. | 6.3 | - | 0.06 | - | 0.08 | - | 0.04 | - | - | - | - | - | - | 0.04 | 0.13 | 0.25 | - | 0.20 |
| 7. | 6.5 | - | 0.04 | 0.06 | - | 0.18 | - | 0.08 | - | 0.11 | 0.04 | 0.09 | 1.93 | - | 0.13 | - | - | - |
| 8. | 6.8 | Caffeic Acid | 1.67 | 0.56 | 3.63 | 5.00 | 1.42 | 1.60 | 5.14 | 3.58 | 1.69 | 2.04 | 3.94 | 7.14 | 5.65 | 2.47 | 1.87 | 1.60 |
| 9. | 7.3 | - | 0.13 | 0.06 | 0.08 | 0.24 | 0.45 | 0.30 | 0.20 | 0.06 | 0.28 | 0.30 | 0.85 | 0.12 | 0.22 | 0.35 | 0.67 | 1.26 |
| 10. | 8.1 | - | 0.05 | 0.06 | 1.67 | 0.07 | 0.06 | 0.30 | 0.08 | 0.06 | - | 0.03 | - | 0.02 | 0.01 | - | 0.12 | 0.08 |
| 11. | 8.4 | - | 0.01 | 0.05 | - | 0.10 | 0.41 | 0.01 | - | 0.01 | - | 0.06 | 0.01 | - | - | 0.01 | - | - |
| 12. | 8.6 | - | 0.01 | 0.02 | - | - | - | 0.17 | - | - | - | - | 0.01 | - | 0.04 | 0.01 | - | - |
| 13. | 9.1 | p-Coumaric | 0.26 | 0.02 | 0.20 | 0.02 | 0.04 | 0.05 | 0.06 | 0.03 | 0.06 | 0.03 | 0.02 | 0.12 | 0.03 | 0.10 | 0.03 | 0.07 |
| 14. | 9.4 | Ferulic Acid | 0.04 | 0.12 | - | 0.08. | - | 0.06 | - | - | - | - | - | 0.12 | 0.14 | - | - | 2.31 |
| S. No. | Sample | Caffeic Acid | Ferulic Acid | p-Coumaric Acid |
|---|---|---|---|---|
| 1. | Jodhpur | 167.71 | 4.65 | 26.79 |
| 2. | Bhilwara | 56.66 | 11.96 | 2.03 |
| 3. | Kota | 363.04 | nd | 20.27 |
| 4. | Jhalawar | 500.96 | 0.85 | 2.71 |
| 5. | Pratapgarh | 142.15 | nd | 4.42 |
| 6. | Pantnagar | 247.26 | nd | 9.08 |
| 7. | Saharanpur | 204.17 | nd | 3.59 |
| 8. | Kanpur | 39.47 | nd | 2.91 |
| 9. | Meerut | 514.06 | nd | 6.85 |
| 10. | Nazibabad | 187.67 | nd | 3.71 |
| 11. | Lucknow | 714.04 | 12.62 | 12.27 |
| 12. | Varanasi | 565.76 | 14.80 | 3.29 |
| 13. | Roorkee | 160.72 | 231.40 | 7.19 |
| 14. | Bhopal | 160.27 | 0.69 | 5.57 |
| 15. | Banasthali | 358.05 | nd | 3.66 |
| 16. | Banglore | 169.07 | nd | 6.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Agarwal, S.; Singh, K.; Chuturgoon, A.; Pareek, A. Molecular Fingerprinting and Phytochemical Investigation of Syzygium cumini L. from Different Agro-Ecological Zones of India. Plants 2023, 12, 931. https://doi.org/10.3390/plants12040931
Khan S, Agarwal S, Singh K, Chuturgoon A, Pareek A. Molecular Fingerprinting and Phytochemical Investigation of Syzygium cumini L. from Different Agro-Ecological Zones of India. Plants. 2023; 12(4):931. https://doi.org/10.3390/plants12040931
Chicago/Turabian StyleKhan, Suphiya, Swati Agarwal, Krati Singh, Anil Chuturgoon, and Ashutosh Pareek. 2023. "Molecular Fingerprinting and Phytochemical Investigation of Syzygium cumini L. from Different Agro-Ecological Zones of India" Plants 12, no. 4: 931. https://doi.org/10.3390/plants12040931
APA StyleKhan, S., Agarwal, S., Singh, K., Chuturgoon, A., & Pareek, A. (2023). Molecular Fingerprinting and Phytochemical Investigation of Syzygium cumini L. from Different Agro-Ecological Zones of India. Plants, 12(4), 931. https://doi.org/10.3390/plants12040931








