Labellar Structure of the Maxillaria splendens Alliance (Orchidaceae: Maxillariinae) Indicates Floral Polyphenols as a Reward for Stingless Bees
Abstract
1. Introduction
2. Results
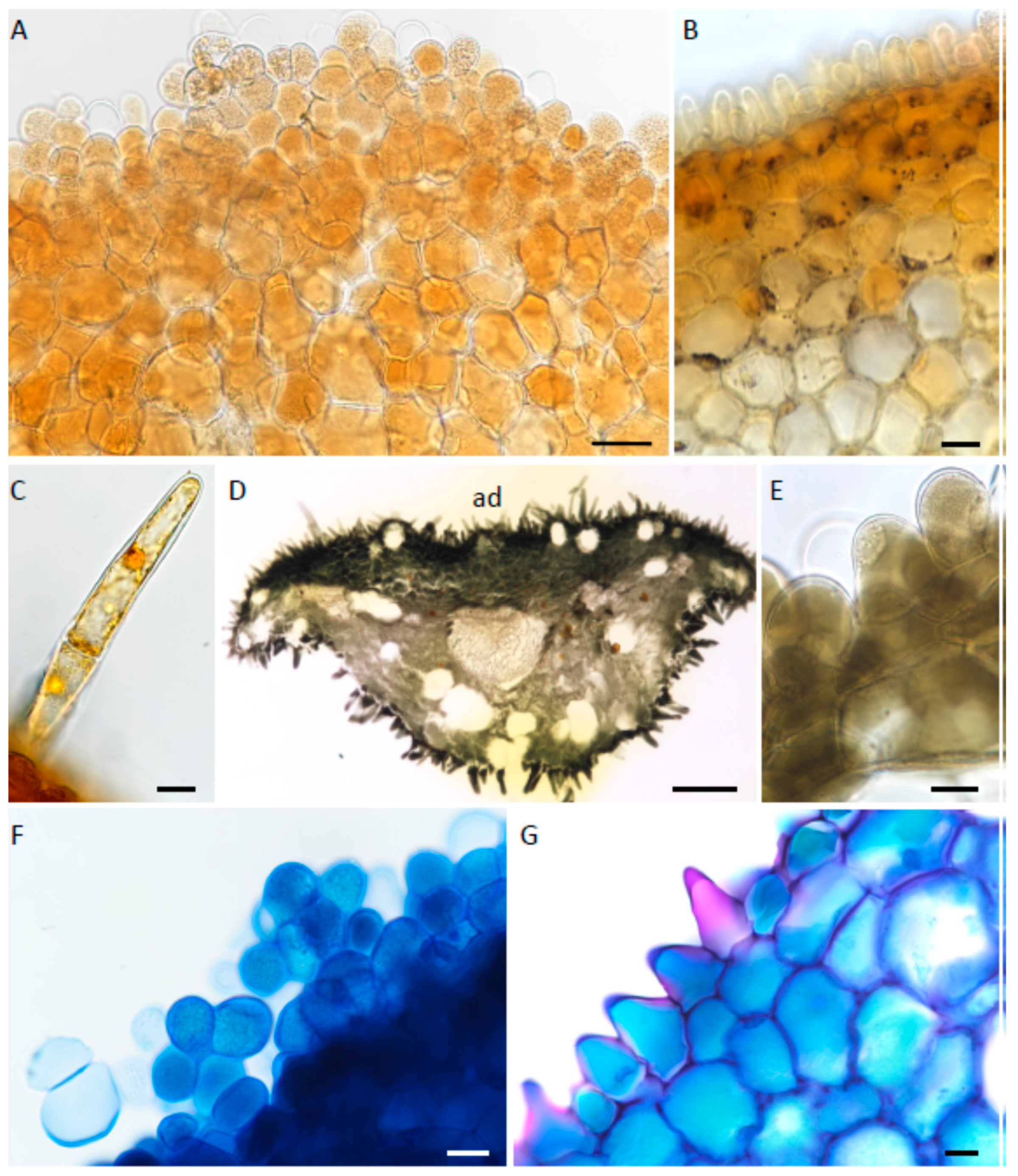
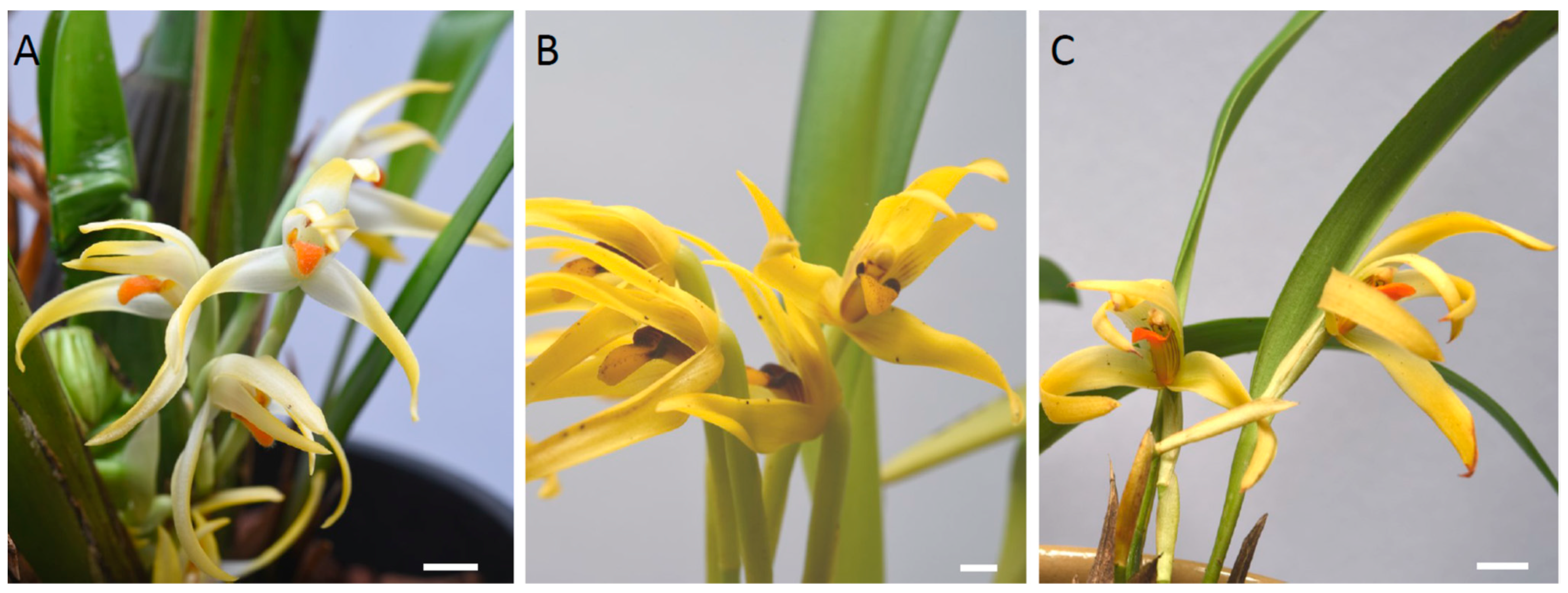
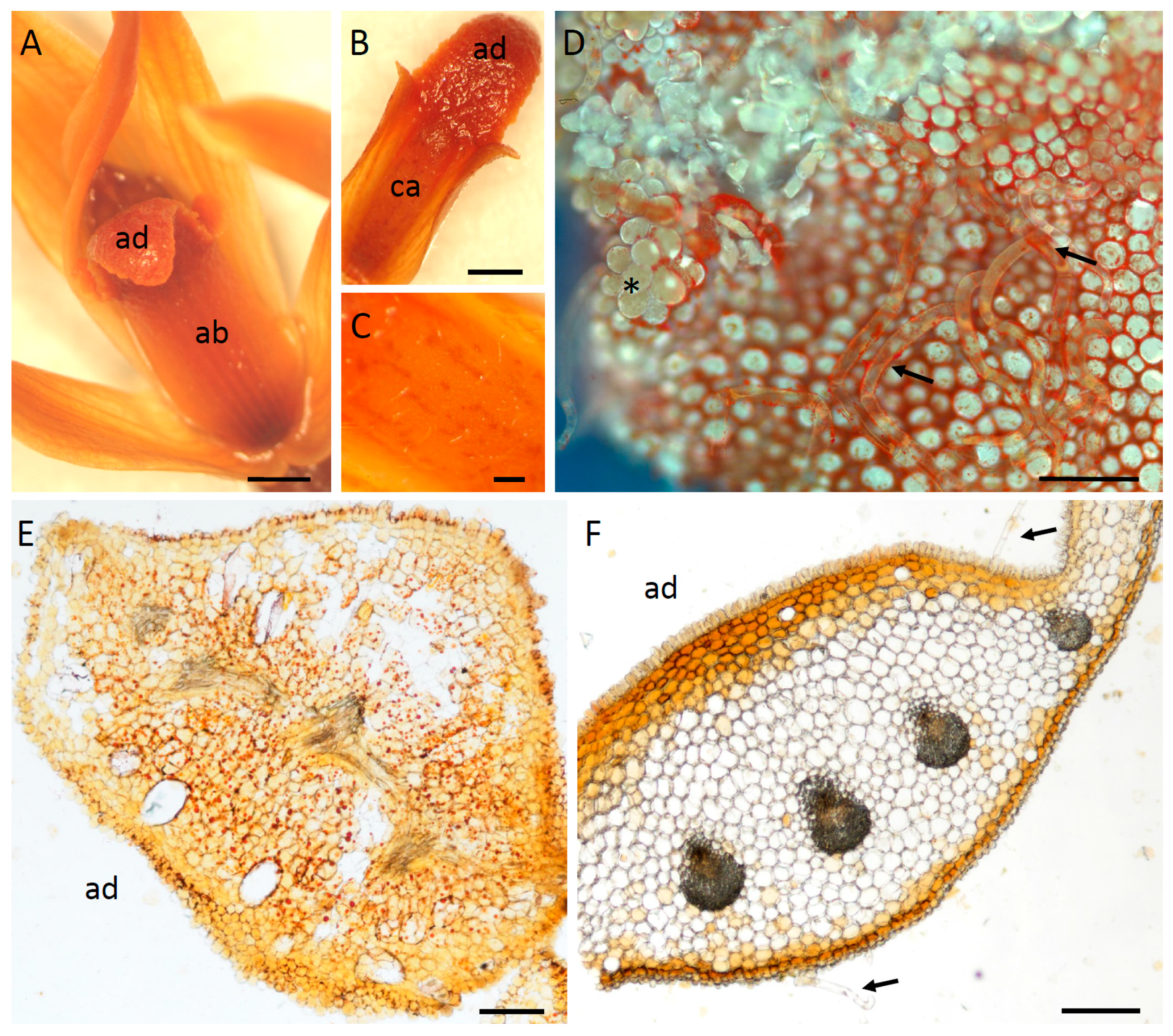
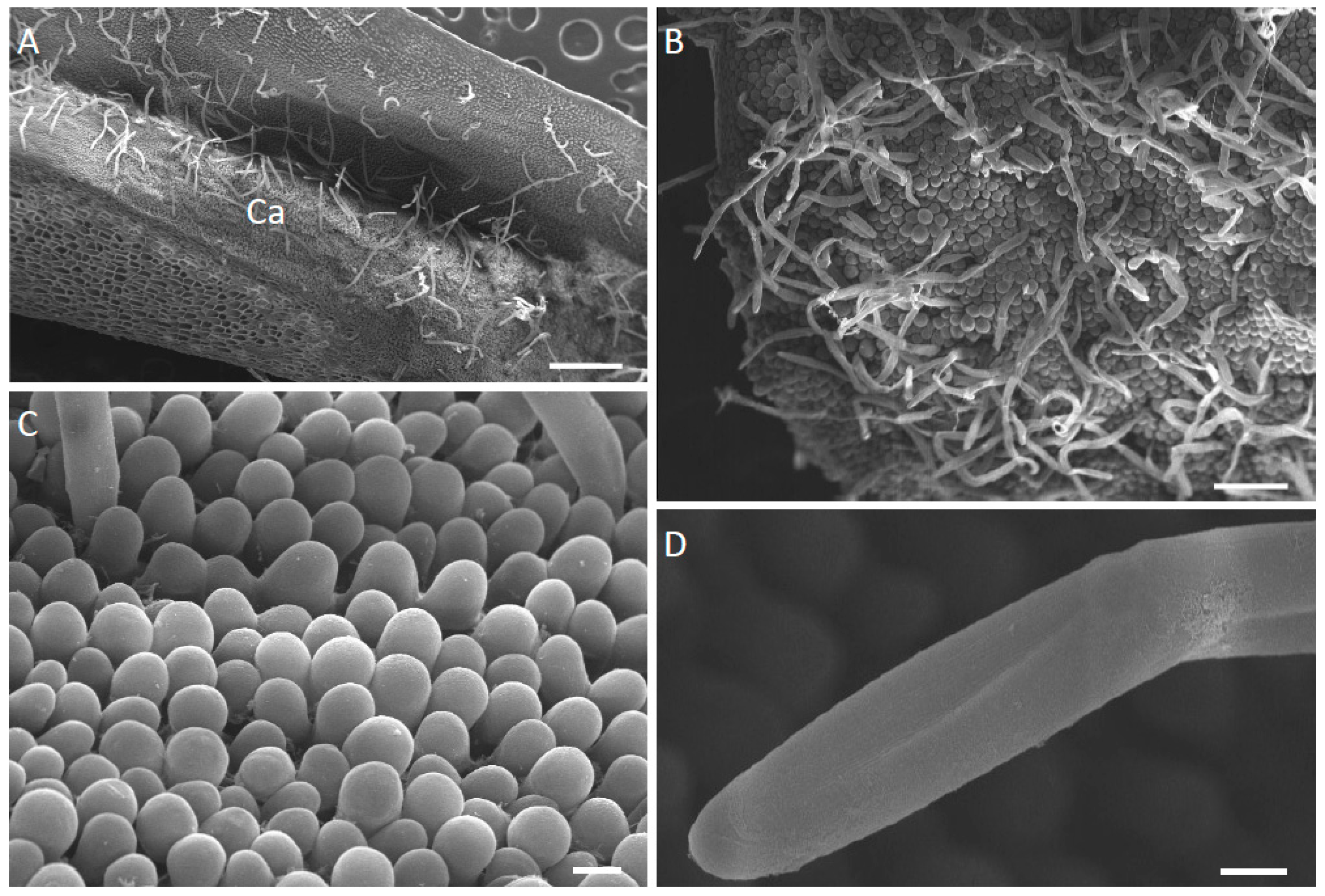
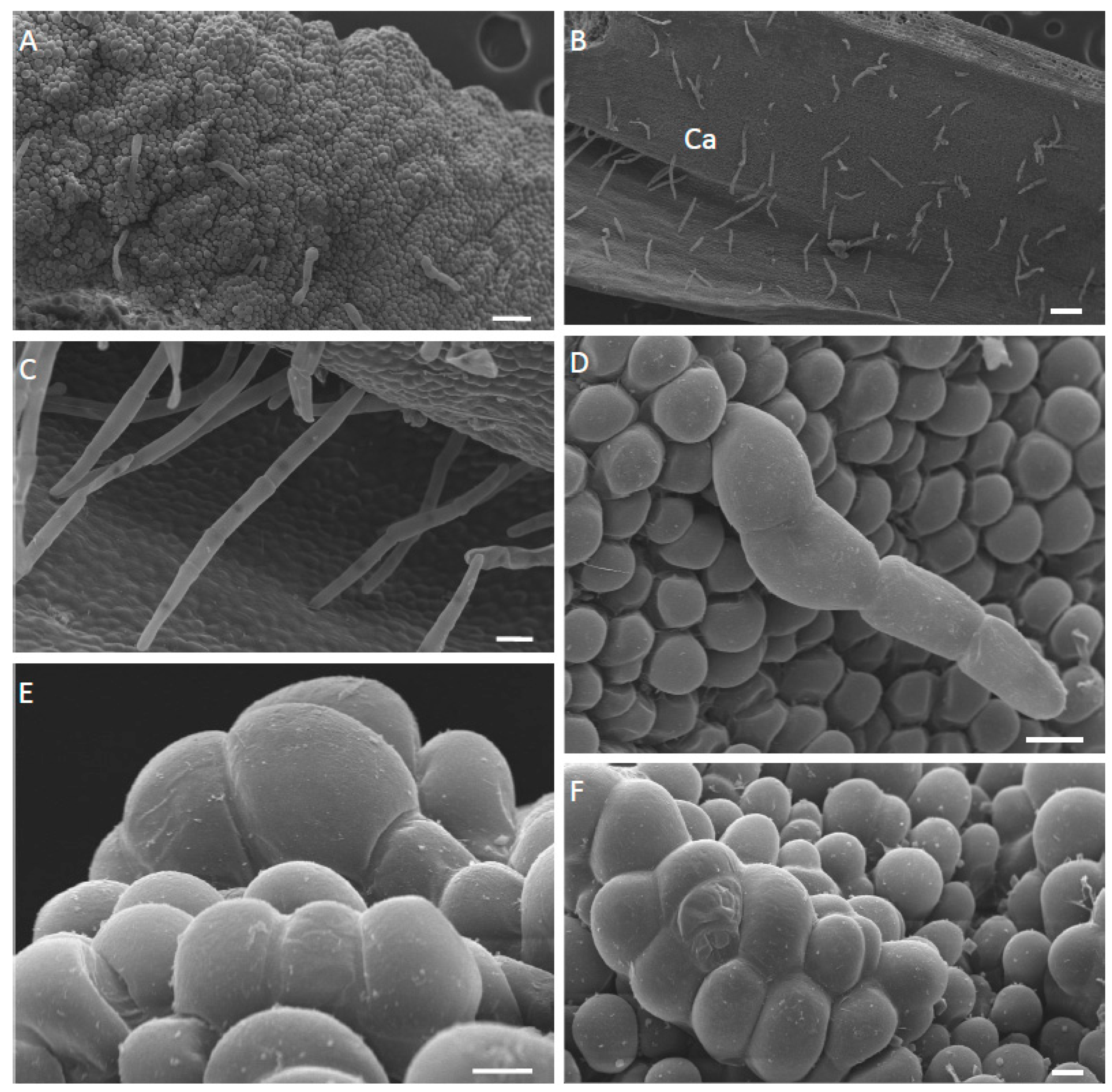
2.1. Micromorphology
2.2. Histochemistry
2.3. Field Observations and Bee Behaviour
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Location of Sites of Fragrance Production
4.3. Histochemistry and Light Microscopy (LM)
4.4. Scanning Electron Microscopy (SEM)
4.5. Field Observations and Bee Behaviour
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stpiczyńska, M.; Davies, K.L.; Gregg, A. Nectary structure and nectar secretion in Maxillaria coccinea (Jacq.) L.O. Williams ex Hodge (Orchidaceae). Ann. Bot. 2003, 93, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.L.; Stpiczyńska, M.; Gregg, A. Nectar-secreting floral stomata in Maxillaria anceps Ames & C. Schweinf. (Orchidaceae). Ann. Bot. 2005, 96, 217–227. [Google Scholar] [PubMed]
- Blanco, M.A.; Carnevali, G.; Whitten, M.W.; Singer, R.B.; Koehler, S.; Williams, N.H.; Ojeda, I.; Neubig, K.M.; Endara, L. Generic realignments in Maxillariinae (Orchidaceae). Lankesteriana 2007, 7, 515–537. [Google Scholar]
- Davies, K.L.; Stpiczyńska, M. Micromorphology of the labellum and floral spur of Cryptocentrum Benth. and Sepalosaccus Schltr. (Maxillariinae: Orchidaceae). Ann. Bot. 2007, 100, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.L.; Stpiczyńska, M. The anatomical basis of floral, food-reward production in Orchidaceae. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues, 1st ed.; Teixeira da Silva, J.A., Ed.; Global Science Books: Isleworth, UK, 2008; Volume 5, pp. 392–407. [Google Scholar]
- Stpiczyńska, M.; Davies, K.L.; Gregg, A. Nectary structure of Ornithidium sophronitis Rchb. f. (Orchidaceae: Maxillariinae). Acta Agrobot. 2009, 62, 3–12. [Google Scholar] [CrossRef]
- Davies, K.L.; Turner, M.P.; Gregg, A. Atypical pseudopollen-forming hairs in Maxillaria Ruiz & Pav. (Orchidaceae). Bot. J. Linn. Soc. 2003, 143, 151–158. [Google Scholar]
- Davies, K.L.; Turner, M.P. Morphology of floral papillae in Maxillaria Ruiz & Pav. (Orchidaceae). Ann. Bot. 2004, 93, 75–86. [Google Scholar]
- Davies, K.L. Food-hair form and diversification in orchids. In Orchid Biology-Reviews and Perspectives; Kull, T., Arditti, J., Wong, S.M., Eds.; Springer Science + Business Media BV: Berlin, Germany, 2009; Volume X, pp. 159–184. [Google Scholar]
- Krahl, A.H.; Holanda, A.S.S.; Krahl, D.R.P.; Webber, A.C. Polinização de Camaridium ochroleucum Lindl. (Orchidaceae: Maxillariinae). Biota Amaz. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Krahl, A.H.; Holanda, A.S.S.; Krahl, D.R.P.; Martucci, M.E.P.; Gobbo-Neto, L.; Webber, A.C.; Pansarin, E.R. Study of the reproductive biology of an Amazonian Heterotaxis (Orchidaceae) demonstrates the collection of resin-like material by stingless bees. Plant Syst. Evol. 2019, 305, 281–291. [Google Scholar] [CrossRef]
- Arévalo, R.; Carlsward, B.S.; Bergquist, G.; Cameron, K.M. Comparative labellar micromorphology of Mormolyca (Maxillariinae: Orchidaceae). In Diversity and Phylogeny of the Monocotyledons: Contributions from Monocots, V., Proceedings of the 5th International Conference on Comparative Biology of Monocotyledons, Memoirs of the New York Botanical Garden, New York, NY, USA, 7–13 July 2013; Campbell, L.M., Davis, J.I., Meerow, A.W., Naczi, R.F.C., Stevenson, D.W., Thomas, W.W., Eds.; New York Botanical Garden Press: New York, NY, USA, 2017; Volume 18. [Google Scholar] [CrossRef]
- Davies, K.L.; Stpiczyńska, M. Comparative floral micromorphology and the ultrastructural basis of fragrance production in pseudocopulatory Mormolyca s.s. and non-pseudocopulatory Maxillaria section Rufescens s.s. (Orchidaceae). Bot. J. Linn. Soc. 2017, 5, 81–112. [Google Scholar] [CrossRef]
- Janse, J.M. Imitirte Pollenkörner bei Maxillaria sp. Dtsch. Bot. Ges. Ber. 1886, 4, 277–283. [Google Scholar]
- Porsch, O. Beiträge zur ‘histologischen’ Blütenbiologie I. Österreichische Bot. Z. 1905, 55, 253–260. [Google Scholar] [CrossRef]
- van der Pijl, L.; Dodson, C.H. Orchid Flowers: Their Pollination and Evolution; University of Miami Press: Coral Gables, FL, USA, 1969. [Google Scholar]
- Davies, K.L.; Winters, C. Ultrastructure of the labellar epidermis in selected Maxillaria species (Orchidaceae). Bot. J. Linn. Soc. 1998, 126, 349–361. [Google Scholar]
- Davies, K.L.; Winters, C.; Turner, M.P. Pseudopollen: Its structure and development in Maxillaria (Orchidaceae). Ann. Bot. 2000, 85, 887–895. [Google Scholar] [CrossRef]
- Matusiewicz, J.; Stpiczyńska, M.; Davies, K.L. Pseudopollen in the flowers of Maxillaria lepidota Lindl. (Orchidaceae). In Proceedings of the 53rd Meeting of the Polish Botanical Society, Toruń, Poland, 6–11 September 2004; p. 14. [Google Scholar]
- Davies, K.L.; Stpiczyńska, M.; Kamínska, M. Dual deceit in pseudopollen-producing Maxillaria s.s. (Orchidaceae: Maxillariinae). Bot. J. Linn. Soc. 2013, 173, 744–763. [Google Scholar] [CrossRef]
- Singer, R.B. Orchid pollination: Recent developments from Brazil. Lankesteriana 2003, 7, 111–114. [Google Scholar]
- Singer, R.B.; Koehler, S. Pollinarium morphology and floral rewards in Brazilian Maxillariinae (Orchidaceae). Ann. Bot. 2004, 93, 39–51. [Google Scholar] [CrossRef]
- Vogel, S. Evolutionary shifts from reward to deception in pollen flowers. In The Pollination of Flowers by Insects; Richards, A.J., Ed.; Academic Press: London, UK, 1979; pp. 89–96. [Google Scholar]
- Davies, K.L.; Turner, M.P.; Gregg, A. Lipoidal labellar secretions in Maxillaria Ruiz & Pav. (Orchidaceae). Ann. Bot. 2003, 91, 439–446. [Google Scholar]
- Stpiczyńska, M.; Davies, K.L. Floral, resin-secreting trichomes in Maxillaria dichroma Rolfe (Orchidaceae: Maxillariinae). Acta Agrobot. 2009, 62, 43–51. [Google Scholar] [CrossRef]
- Davies, K.L.; Stpiczyńska, M. Comparative labellar anatomy of resin-secreting and putative resin-mimic species of Maxillaria s.l. (Orchidaceae: Maxillariinae). Bot. J. Linn. Soc. 2012, 170, 405–435. [Google Scholar] [CrossRef]
- Davies, K.L.; Stpiczyńska, M. Comparative anatomy of putative secretory floral structures in the Camaridium cucullatum complex and Nitidobulbon (Orchidaceae: Maxillariinae). Bot. J. Linn. Soc. 2019, 190, 165–191. [Google Scholar] [CrossRef]
- Mazzoni-Viveiros, S.C.; Salatino, A.; Salatino, M.L.F.; de Barros, F.; Negri, G.; Cardoso-Gustavson, P.; Castro, M.M. Labellar secretion and secretory trichomes of Rhetinantha cerifera (Barb. Rodr.) M.A. Blanco (Orchidaceae, Maxillariinae): Micromorphology and composition. Braz. J. Bot. 2019, 42, 119–134. [Google Scholar] [CrossRef]
- Flach, A.; Dondon, R.C.; Singer, R.B.; Koehler, S.; Amaral, M.C.E.; Marsaioli, A.J. The chemistry of pollination in selected Brazilian Maxillariinae orchids: Floral rewards and fragrance. J. Chem. Ecol. 2004, 30, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- van der Cingel, N.A. An Atlas of Orchid Pollination–America, Africa, Asia and Australia; A.A. Balkema: Rotterdam, The Netherlands, 2001. [Google Scholar]
- Singer, R.B.; Flach, A.; Koehler, S.; Marsaioli, A.J.; Do Carmo EAmaral, M. Sexual mimicry in Mormolyca ringens (Lindl.) Schltr. (Orchidaceae: Maxillariinae). Ann. Bot. 2004, 93, 755–762. [Google Scholar] [CrossRef]
- Flach, A.; Marsaioli, A.J.; Singer, R.B.; Amaral, M.C.E.; Menezes, C.; Kerr, W.E.; Batista-Pereira, L.G.; Corrèa, A.G. Pollination by sexual mimicry in Mormolyca ringens: A floral chemistry that remarkably matches the pheromones of virgin queens of Scaptotrigona sp. J. Chem. Ecol. 2006, 32, 59–70. [Google Scholar] [CrossRef]
- Singer, R.B. The pollination mechanism in Trigonidium obtusum Lindl. (Orchidaceae: Maxillariinae): Sexual mimicry and trap-flowers. Ann. Bot. 2002, 89, 157–163. [Google Scholar] [CrossRef]
- Christenson, E.A. Maxillaria, an Unfinished Monograph; Harding, P.A., McIllmurray, M., Blanco, M., Eds.; PA Harding: Lebanon, OR, USA, 2013; Volume 1–2. [Google Scholar]
- Lipińska, M.M.; Kowalkowska, A.K. Floral morphology and micromorphology of selected Maxillaria species (Maxillariinae, Orchidaceae). Wulfenia 2018, 25, 242–272. [Google Scholar]
- Singer, R.B.; Cocucci, A.A. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana 1999, 14, 47–56. [Google Scholar]
- Pansarin, E.R.; Pedro, S.R.M.; Davies, K.L.; Stpiczyńska, M. Evidence of floral rewards in Brasiliorchis supports the convergent evolution of food-hairs in Maxillariinae. Am. J. Bot. 2022, 109, 806–820. [Google Scholar] [CrossRef]
- Stern, W.L.; Curry, K.J.; Whitten, W.M. Staining fragrance glands in orchid flowers. Bull. Torrey Bot. Club 1986, 113, 288–297. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA; Oxford, UK, 1999. [Google Scholar]
- Jensen, W.A. Botanical Histochemistry: Principle and Practice; W.H. Freeman: San Francisco, CA, USA, 1962. [Google Scholar]
- Fisher, D.B. Protein staining of ribboned epon sections for light microscopy. Histochemie 1968, 16, 92–96. [Google Scholar] [CrossRef]
- Gahan, P.B. Plant Histochemistry and Cytochemistry: An Introduction; Academic Press: London, UK, 1984. [Google Scholar]
- Kirk, P.W. Neutral red as a lipid fluorochrome. Stain Technol. 1970, 45, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Brummitt, R.K.; Powell, C.E. Authors of Plant Names; Royal Botanic Gardens: Kew, UK, 1992. [Google Scholar]
- Kjellsson, G.; Rasmussen, F.N. Does the pollination of Dendrobium unicum Seidenf. involve pseudopollen? Die Orchid. 1987, 38, 183–187. [Google Scholar]
- Davies, K.L.; Turner, M.P. Pseudopollen in Dendrobium unicum Seidenf. (Orchidaceae): Reward or Deception? Ann. Bot. 2004, 94, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, A.; de Sousa, M.H.O.; dos Freitas, M.S.D.; Cesca, K.; de Moura, N.F. Chemical composition and biological activity of propolis from Melipona quadrifasciata. Res. Soc. Dev. 2022, 11, e193111234175. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 6, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Tuksitha, L.; Chen, Y.L.S.; Chen, Y.L.; Wong, K.Y.; Peng, C.C. Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak). J. Asia-Pac. Entomol. 2018, 21, 563–570. [Google Scholar] [CrossRef]
- Biluca, F.C.; Silva B da Caon, T.; Mohr, E.T.B.; Vieira, G.N.; Gonzaga, L.V.; Vitali, L.; Micke, G.; Fett, R.; Dalmarco, E.M.; Costa, A.C.O. Investigation of phenolic compounds, antioxidant and anti-inflammatory activities in stingless bee honey (Meliponinae). Food Res. Int. 2020, 129, 108756. [Google Scholar] [CrossRef]
- Shanahan, M.; Spivak, M. Resin use by stingless bees: A review. Insects 2021, 12, 719. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davies, K.L.; Pansarin, E.R.; Stpiczyńska, M. Labellar Structure of the Maxillaria splendens Alliance (Orchidaceae: Maxillariinae) Indicates Floral Polyphenols as a Reward for Stingless Bees. Plants 2023, 12, 921. https://doi.org/10.3390/plants12040921
Davies KL, Pansarin ER, Stpiczyńska M. Labellar Structure of the Maxillaria splendens Alliance (Orchidaceae: Maxillariinae) Indicates Floral Polyphenols as a Reward for Stingless Bees. Plants. 2023; 12(4):921. https://doi.org/10.3390/plants12040921
Chicago/Turabian StyleDavies, Kevin L., Emerson R. Pansarin, and Małgorzata Stpiczyńska. 2023. "Labellar Structure of the Maxillaria splendens Alliance (Orchidaceae: Maxillariinae) Indicates Floral Polyphenols as a Reward for Stingless Bees" Plants 12, no. 4: 921. https://doi.org/10.3390/plants12040921
APA StyleDavies, K. L., Pansarin, E. R., & Stpiczyńska, M. (2023). Labellar Structure of the Maxillaria splendens Alliance (Orchidaceae: Maxillariinae) Indicates Floral Polyphenols as a Reward for Stingless Bees. Plants, 12(4), 921. https://doi.org/10.3390/plants12040921





