Phenolic Profile, Antioxidant and DNA-Protective Capacity, and Microscopic Characters of Ailanthus altissima Aerial Substances
Abstract
1. Introduction
2. Results
2.1. Phenolic Profile (HPLC Analysis) of Aerial Plant Substances of A. altissima
2.2. In Vitro Antioxidant Capacity
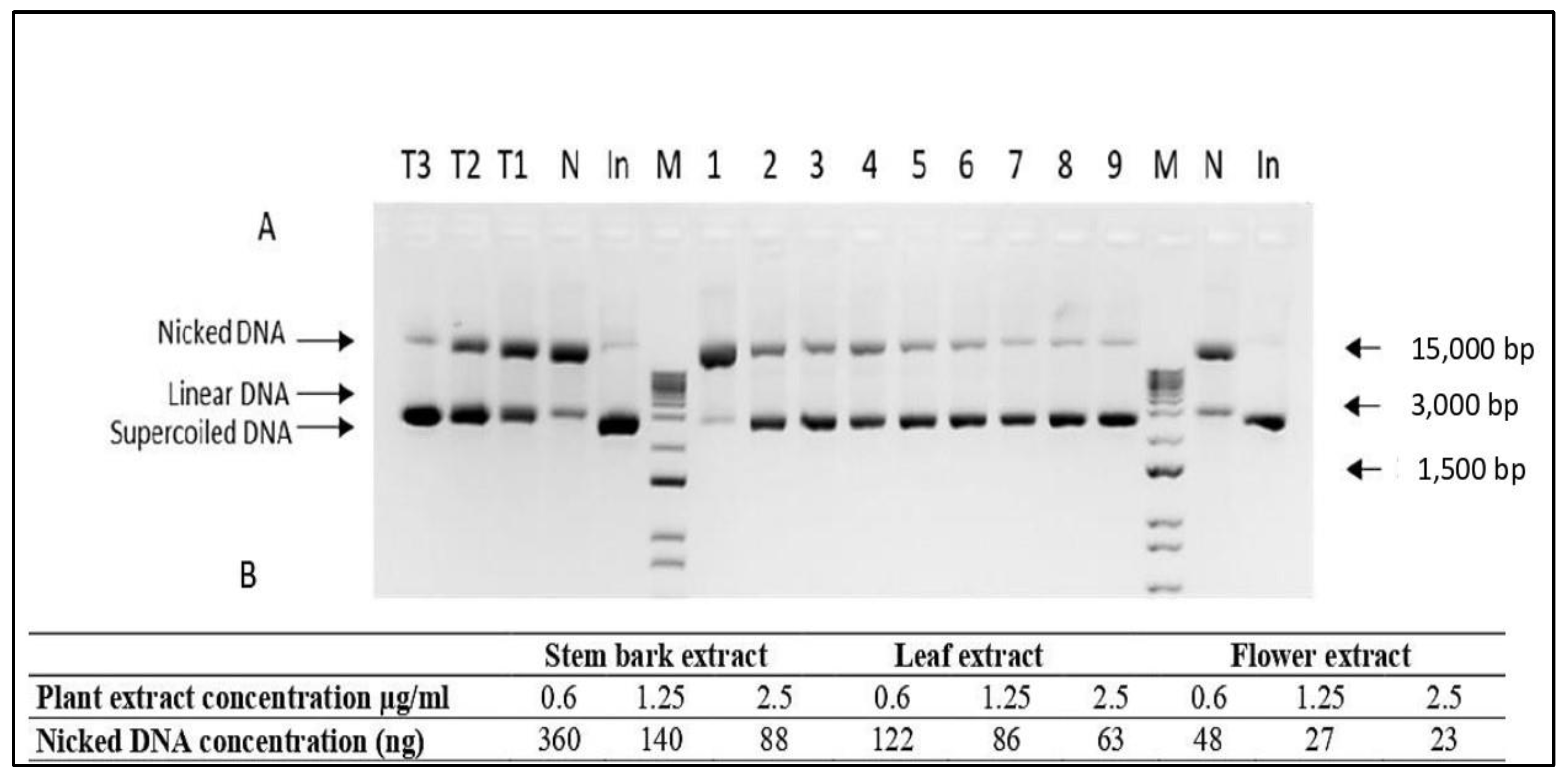
2.3. In Vitro DNA-Protective Capacity
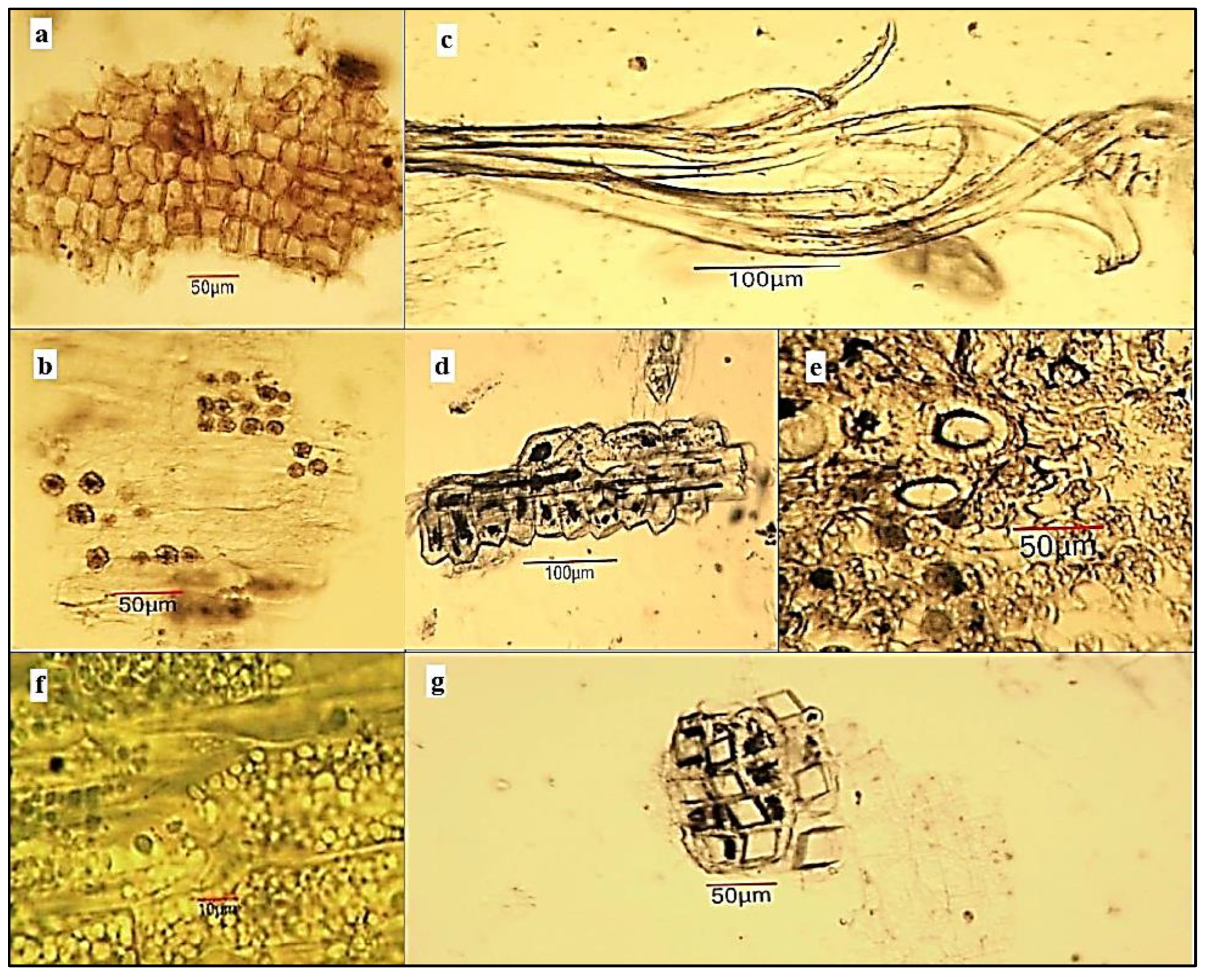
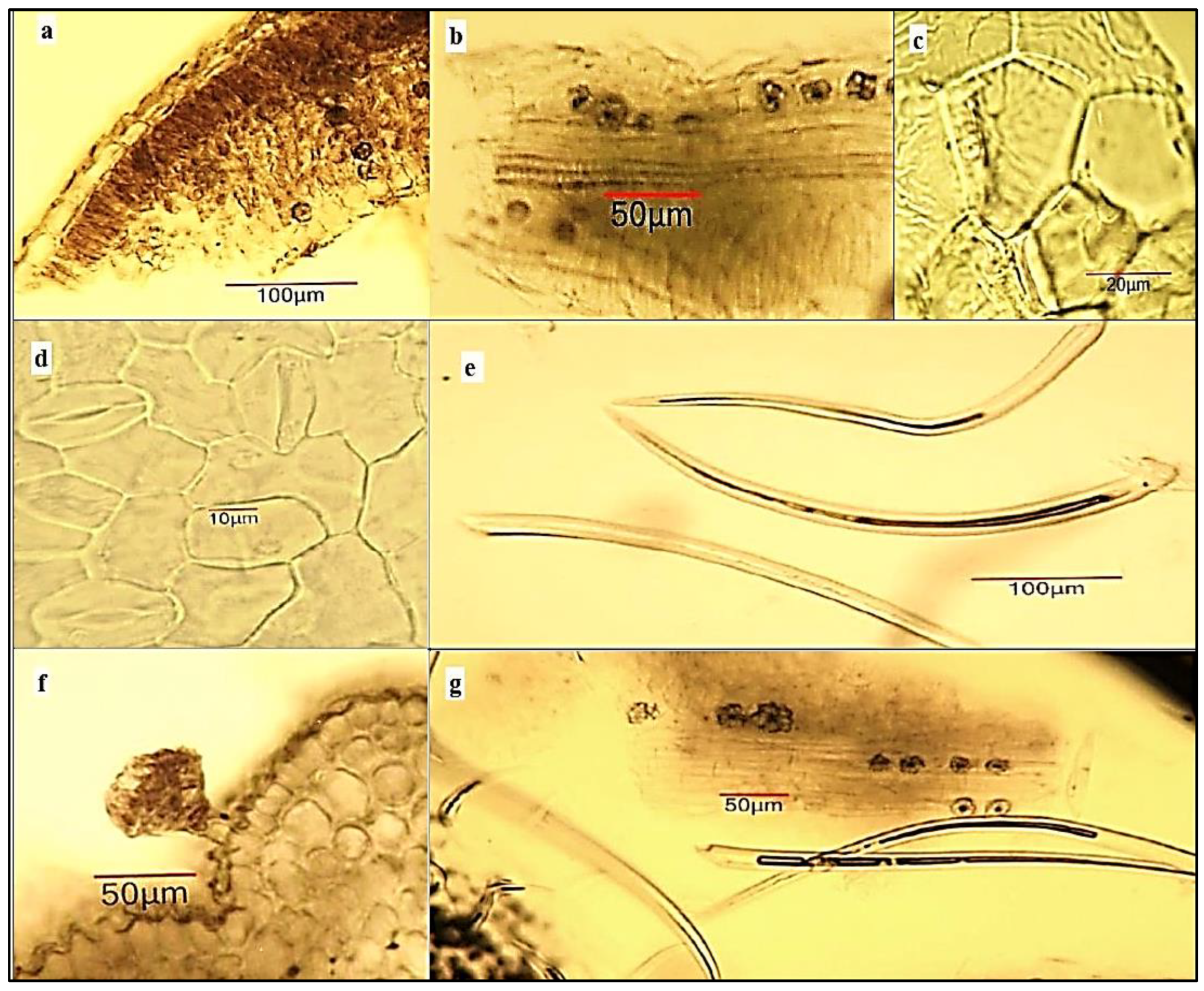
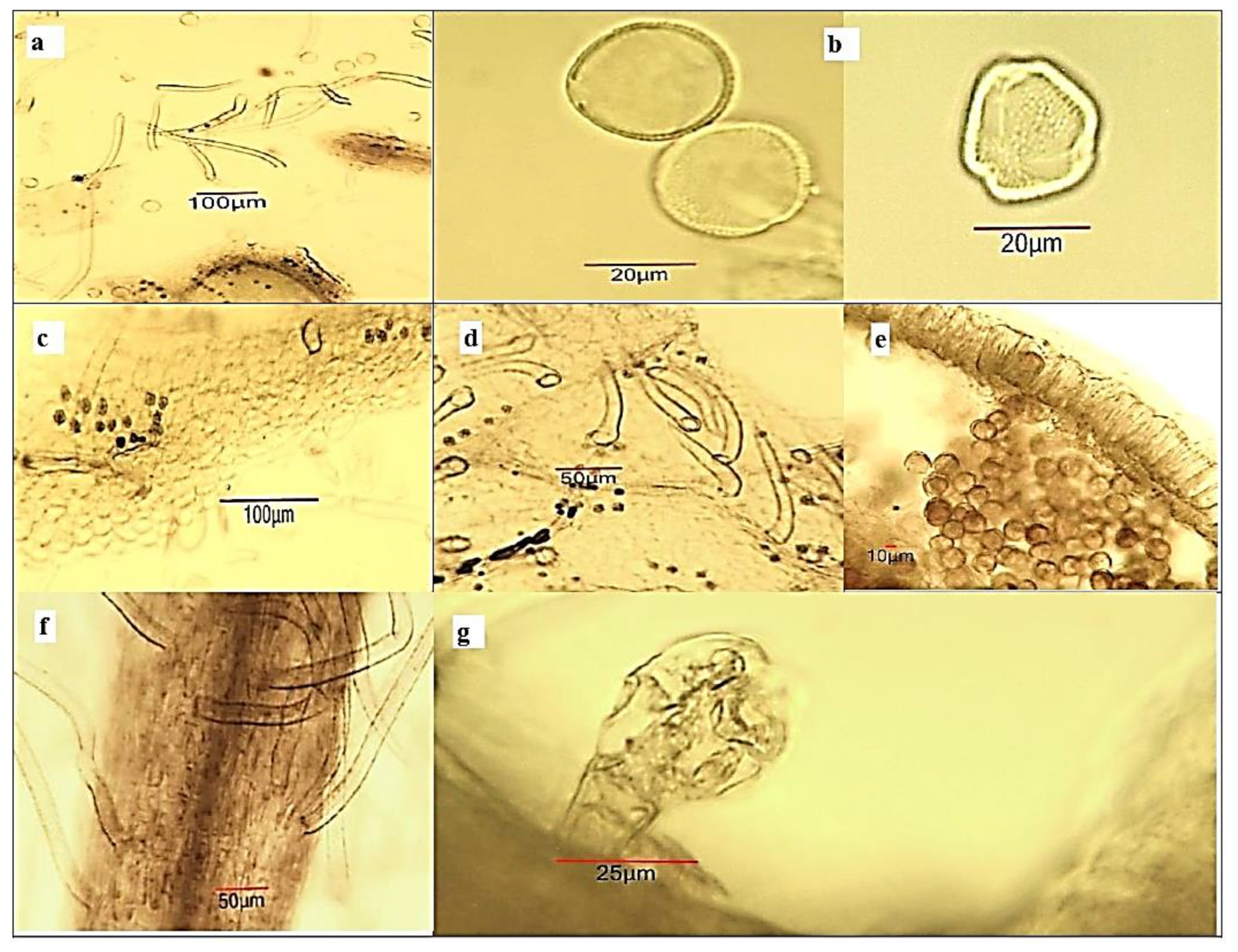
2.4. Light Microscopy Analysis of Ailanthus altissima Aerial Plant Substances
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material Collection and Preparation of the Plant Extracts
4.3. HPLC Analysis for Determination of the Phenolic Profile
4.4. Methods for Determining Antioxidant Activity
4.4.1. ABTS Method
4.4.2. DPPH Method
4.4.3. CUPRAC Method
4.4.4. FRAP Method
4.5. DNA Nicking Protection Assay
4.6. Light-Microscopic Analysis of Powdered Plant Substances
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrova, A.; Vladimirov, V.; Georgiev, V. Invasive Alien Species of Vascular Plants in Bulgaria; Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences: Sofia, Bulgaria, 2013; ISBN 978-954-9746-30-3. [Google Scholar]
- Kožuharova, E.; Lebanova, H.; Getov, I.; Benbassat, N. Ailanthus altissima (Mill.) Swingle—A Terrible Invasive Pest in Bulgaria or Potential Useful Medicinal Plant? Bothalia J. 2014, 44, 18. [Google Scholar]
- Sladonja, B.; Sušek, M.; Guillermic, J. Review on Invasive Tree of Heaven (Ailanthus altissima (Mill.) Swingle) Conflicting Values: Assessment of Its Ecosystem Services and Potential Biological Threat. Environ. Manag. 2015, 56, 1009–1034. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Ma, S.; Zhao, Q.; Wu, J.; Duan, L.; Xie, Y.; Wang, S. Traditional Uses, Phytochemistry, and Pharmacology of Ailanthus altissima (Mill.) Swingle Bark: A Comprehensive Review. J. Ethnopharmacol. 2021, 275, 114121. [Google Scholar] [CrossRef]
- Caramelo, D.; Pedro, S.I.; Marques, H.; Simão, A.Y.; Rosado, T.; Barroca, C.; Gominho, J.; Anjos, O.; Gallardo, E. Insights into the Bioactivities and Chemical Analysis of Ailanthus altissima (Mill.) Swingle. Appl. Sci. 2021, 11, 11331. [Google Scholar] [CrossRef]
- Martino, L.D. PHCOG REV.: Plant Review Chemistry and Biological Activities of Ailanthus altissima Swingle: A Review. Pharmacogn. Rev. 2008, 2, 339. [Google Scholar]
- Kundu, P.; Laskar, S. A Brief Resume on the Genus Ailanthus: Chemical and Pharmacological Aspects. Phytochem. Rev. 2010, 9, 379–412. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.; Yan, Z.; Huang, X. Studies on Chemical Constituents of Ailanthus altissima (Mill.) Swingle and Their Antioxidant Activity. Asian J. Tradit. Med. 2020, 15, 320–327. [Google Scholar]
- Albouchi, F.; Hassen, I.; Casabianca, H.; Hosni, K. Phytochemicals, Antioxidant, Antimicrobial and Phytotoxic Activities of Ailanthus altissima (Mill.) Swingle Leaves. S. Afr. J. Bot. 2013, 87, 164–174. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Sezirahiga, J.; Kwon, J.; Jeong, M.; Lee, D.; Choi, J.-H.; Jang, D. A New Canthinone-Type Alkaloid Isolated from Ailanthus altissima Swingle. Molecules 2016, 21, 642. [Google Scholar] [CrossRef]
- Rahman, A.; Kim, E.L.; Kang, S.C. Antibacterial and Antioxidant Properties of Ailanthis altissima Swingle Leave Extract to Reduce Footborne Pathogens and Spoling Bacteria. J. Food Saf. 2009, 29, 499–510. [Google Scholar] [CrossRef]
- Panasenko, O.I.; Aksonova, I.I.; Denysenko, O.M.; Mozul, V.I.; Holovkin, V.V. Investigation of chemical composition of Ailanthus altissima (Mill.) Swingle. Curr. Issues Pharm. Med. Sci. Pract. 2020, 13, 341–348. [Google Scholar] [CrossRef]
- Lungu, L.; Savoiu, R.; Manolescu, N.; Farcasanu, C.; Popa, V. Phytotoxic and Antioxidant Activities of Leaf Extracts of Ailanthus altissima Swingle. Rev. Chim. 2016, 67, 1928–1931. [Google Scholar]
- Tanasković, S.; Gvozdenac, S.; Kolarov, R.; Bursić, V.; Konstantinović, B.; Prvulović, D. Antifeeding and Insecticidal Activity of Ailanthus altissima and Morus alba Extracts Against Gipsy Moth (Lymantria dispar (L.), Lepidoptera, Lymantridae) Larvae Under Laboratory Conditions. J. Entomol. Res. Soc. 2021, 23, 197–212. [Google Scholar] [CrossRef]
- Todorova, T.; Boyadzhiev, K.; Shkondrov, A.; Parvanova, P.; Dimitrova, M.; Ionkova, I.; Krasteva, I.; Kozuharova, E.; Chankova, S. Screening of Amorpha Fruticosa and Ailanthus altissima Extracts for Genotoxicity/Antigenotoxicity, Mutagenicity/Antimutagenicity and Carcinogenicity/Anticarcinogenicity. BioRisk 2022, 17, 201–212. [Google Scholar] [CrossRef]
- Souiei, S.; Ayeb-Zakhama, A.E.; Belkacem, M.A.; Flamini, G.; Jannet, H.B. Isolation of Essential Oil from Ailanthus altissima (Mill) Stems: Chemical Composition, Separation and Biological Activities. J. Tunis. Chem. Soc. 2017, 19, 303–311. [Google Scholar]
- Mohamed, H.; El-Wakil, E.; Abdel-Hameed, E.-S.; El-Hashash, M.; Shemis, M. Evaluation of Total Phenolics, Flavonoids, and Antioxidant and Cytotoxic Potential of Ailanthus altissima (Mill.) Swingle Leaves. J. Rep. Pharm. Sci. 2021, 10, 130. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, J.; Li, X. Chemical constituents from fruits of Ailanthus altissima. Chin. J. Mater. Med. 2009, 34, 2197–2199. [Google Scholar]
- Ni, J.-C.; Shi, J.-T.; Tan, Q.-W.; Chen, Q.-J. Phenylpropionamides, Piperidine, and Phenolic Derivatives from the Fruit of Ailanthus altissima. Molecules 2017, 22, 2107. [Google Scholar] [CrossRef]
- Al-Hashumi, F.; Al-Khero, A.; Al-daody, A. Investigation of Some Carboxylic Acids and Phenolic Compounds of Ailanthus altissima Leaves and Their Its Effect on Italian Cupressus Seedlings Root Rot Fungi. Rafidain J. Sci. 2018, 27, 8–18. [Google Scholar] [CrossRef]
- Filippi, A.; Petrussa, E.; Boscutti, F.; Vuerich, M.; Vrhovsek, U.; Rabiei, Z.; Braidot, E. Bioactive Polyphenols Modulate Enzymes Involved in Grapevine Pathogenesis and Chitinase Activity at Increasing Complexity Levels. Int. J. Mol. Sci. 2019, 20, 6357. [Google Scholar] [CrossRef]
- Du, Y.-Q.; Yan, Z.-Y.; Chen, J.-J.; Wang, X.-B.; Huang, X.-X.; Song, S.-J. The Identification of Phenylpropanoids Isolated from the Root Bark of Ailanthus altissima (Mill.) Swingle. Nat. Prod. Res. 2021, 35, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Gil, N.; Amaral, M.E.; Domingues, F.; Duarte, A.P.C. Ailanthus altissima (miller) swingle: A source of bioactive compounds with antioxidant activity. BioResources 2012, 7, 2105–2120. [Google Scholar] [CrossRef]
- Lou, K.Q.; Tang, W.Z.; Wang, X.J. Study on chemical constituents from flowers of Ailanthus altissima. Zhong Yao Cai 2012, 35, 1605–1607. [Google Scholar] [PubMed]
- Pekala, K.; Wawrzusiszyn, K.; Bogucka-Kocka, A. Phenolic Substances in Ailanthus Glandulosa Desf. Curr. Issues Pharm. Med. Sci. 2014, 27, 84–87. [Google Scholar] [CrossRef]
- MARINAȘ. The Phenols Content and Phytotoxic Capacity of Various Invasive Plants; University of Bucharest: București, Romania, 2018. [Google Scholar]
- Poljuha, D.; Sladonja, B.; Šola, I.; Dudaš, S.; Bilić, J.; Rusak, G.; Motlhatlego, K.E.; Eloff, J.N. Phenolic Composition of Leaf Extracts of Ailanthus altissima (Simaroubaceae) with Antibacterial and Antifungal Activity Equivalent to Standard Antibiotics. Nat. Prod. Commun. 2017, 12, 1934578X1701201. [Google Scholar] [CrossRef]
- Poljuha, D.; Sladonja, B.; Šola, I.; Šenica, M.; Uzelac, M.; Veberič, R.; Hudina, M.; Famuyide, I.M.; Eloff, J.N.; Mikulic-Petkovsek, M. LC–DAD–MS Phenolic Characterisation of Six Invasive Plant Species in Croatia and Determination of Their Antimicrobial and Cytotoxic Activity. Plants 2022, 11, 596. [Google Scholar] [CrossRef]
- Barakat, H.H. Chemical investigation of the constitutive phenolics of Ailanthus altissima; the structure of a new flavone glycoside gallate. Nat. Prod. Sci. 1998, 4, 153–157. [Google Scholar]
- Aissani, N. Antioxidant Potential and Antimicrobial Activity of Ailanthus altissima (Mill.) Swingle Extracts against Pseudomonas aeruginosa. J. Mol. Biol. Biotechnol. 2018, 3, 3. [Google Scholar]
- Khan, S.; Hussain, A.; Mehmood, A.; Mehmood, R.; Perveen, S.; Imran, M. Ailanthus altissima (Miller) Swingle Fruit—New Acyl β-Sitosteryl Glucoside and In Vitro Pharmacological Evaluation. Nat. Prod. Res. 2016, 30, 2629–2636. [Google Scholar] [CrossRef]
- Jin, M.-I.; Bae, K.-H.; Chang, H.-W.; Son, J.-K. Anti-Inflammatory Compounds from the Leaves of Ailanthus altissima Meihua JIN. Biomol. Ther. 2009, 17, 86–91. [Google Scholar] [CrossRef]
- Zhelev, I.; Georgiev, K.; Dimitrova-Dyulgerova, I. Carotenoid profile of Ailanthus altissima stem bark, in-vitro antioxidant and antineoplastic activities. Word J. Pharm. Res. 2016, 5, 53–60. [Google Scholar]
- Abidi, A.; Aissani, N.; Sebai, H.; Ben Khamsa, S. Anti-Fibrotic and Antioxidant Effects of Ailanthus altissima Wood Aqueous Extract against Bleomycin-Induced Lung Fibrosis in Rat. Eur. Respir. J. 2017, 50, PA4911. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, Y.; Huang, X.; Song, S. Isolation, Structure Elucidation and Antioxidant Activity of Natural Products from the Root Bark of Ailanthus altissima (Mill.) Swingle. Asian J. Tradit. Med. 2020, 15, 217–227. [Google Scholar]
- El Ayeb-Zakhama, A.; Chahdoura, H.; Ziani, B.E.C.; Snoussi, M.; Khemiss, M.; Flamini, G.; Harzallah-Skhiri, F. Ailanthus altissima (Miller) Swingle Seed Oil: Chromatographic Characterization by GC-FID and HS-SPME-GC-MS, Physicochemical Parameters, and Pharmacological Bioactivities. Environ. Sci. Pollut. Res. 2019, 26, 14137–14147. [Google Scholar] [CrossRef]
- Shabbir Awan, S.; Taj Khan, R.; Mehmood, A.; Hafeez, M.; Rizwan Abass, S.; Nazir, M.; Raffi, M. Ailanthus altissima Leaf Extract Mediated Green Production of Zinc Oxide (ZnO) Nanoparticles for Antibacterial and Antioxidant Activity. Saudi J. Biol. Sci. 2023, 30, 103487. [Google Scholar] [CrossRef]
- Mo, Y.; Cheng, F.; Yang, Z.; Shang, X.; Liang, J.; Shang, R.; Hao, B.; Wang, X.; Zhang, H.; Wali, A.; et al. Antioxidant Activity and the Potential Mechanism of the Fruit From Ailanthus altissima Swingle. Front. Vet. Sci. 2021, 8, 784898. [Google Scholar] [CrossRef]
- Bashir, A.; Mohi-ud-din, R.; Farooq, S.; Bhat, Z.A. Pharmacognostic Standardization and Phytochemical Evaluation of Ailanthus altissima (Mill.) Swingle Leaves. J. Drug Deliv. Ther. 2019, 9, 179–183. [Google Scholar]
- Abdel-Baky, A.M.; Darwish, F.M.M.; Ibraheim, Z.Z.; Gouda, Y.G. Macro- and micromorphology of Ailanthus altissima SWINGLE cultivated in Egypt: Leaf, stem and stem-barc. Bull. Pharm. Sci. 1998, 21, 141–159. [Google Scholar] [CrossRef]
- Raja, W.Y.; Bhat, Z.A.; Chashoo, I.A. Pharmacognostic and Phytochemical Characteristics of Ailanthus altissima (Mill.) Swingle Stem and Root Bark: A Comparative Study. Pharmacogn. J. 2017, 9, 668–673. [Google Scholar] [CrossRef]
- Andonova, T.; Muhovski, Y.; Vrancheva, R.; Slavov, I.; Apostolova, E.; Naimov, S.; Pavlov, A.; Dimitrova-Dyulgerova, I. Antioxidant and DNA-Protective Potentials, Main Phenolic Compounds, and Microscopic Features of Koelreuteria Paniculata Aerial Parts. Antioxidants 2022, 11, 1154. [Google Scholar] [CrossRef]
- Krasteva, G. Effect of Basal Medium on Growth and Polyphenols Accumulation by Gardenia Jasminoides Ellis Cell Suspension. BIO Web Conf. 2022, 45, 02006. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food. Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Ivanov, I.G.; Vrancheva, R.Z.; Marchev, A.S.; Petkova, N.T.; Aneva, Y.; Denev, P.P.; Georgiev, V.G.; Pavlov, A.I. Antioxidant Activities and Phenolic Compounds in Bulgarian Fumaria Species. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 296–306. [Google Scholar]
- Kivrak, I.; Duru, M.E.; Öztürk, M.; Mercan, N.; Harmandar, M.; Topçu, G. Antioxidant, Anticholinesterase and Antimicrobial Constituents from the Essential Oil and Ethanol Extract of Salvia Potentillifolia. Food Chem. 2009, 116, 470–479. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Esin Karademir, S.; Erçağ, E. The Cupric Ion Reducing Antioxidant Capacity and Polyphenolic Content of Some Herbal Teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. [2] Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 15–27. ISBN 0076-6879. [Google Scholar]
- Rajiv, C.; Roy, S.S.; Tamreihao, K.; Kshetri, P.; Singh, T.S.; Sanjita Devi, H.; Sharma, S.K.; Ansari, M.A.; Devi, E.D.; Devi, A.K.; et al. Anticarcinogenic and Antioxidant Action of an Edible Aquatic Flora Jussiaea Repens L. Using In Vitro Bioassays and In Vivo Zebrafish Model. Molecules 2021, 26, 2291. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM). Methods in Pharmacognosy. In European Pharmacopoeia (10.0); European Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM): Strasbourg, France, 2019; Volume 1, p. 317. [Google Scholar]
- Vasavada, N. Online Web Statistical Calculators. Available online: http://astatsa.com/ (accessed on 8 May 2022).

| № | Compounds | Plant Substabces | ||
|---|---|---|---|---|
| Leaves | Stem Bark | Flowers | ||
| Flavonoids | ||||
| 1 | Rutin | 1.02 ± 0.026 b | n.d. | 5.68 ± 0.142 a |
| 2 | Hesperidin | 2.67 ± 0.067 a | n.d. | 0.72 ± 0.018 b |
| 3 | Quercetin | 0.19 ± 0.005 b | 0.01 ± 0.001 c | 0.33 ± 0.008 a |
| 4 | Kaempferol | n.d. | 0.11 ± 0.003 | n.d. |
| 5 | (+)-Catechin | 0.95 ± 0.024 c | 2.15 ± 0.054 a | 1.55 ± 0.039 b |
| 6 | (−)-Epicatechin | 0.23 ± 0.006 b | 0.54 ± 0.014 a | 0.13 ± 0.003 c |
| Phenolic acids | ||||
| 7 | Gallic | 0.30 ± 0.008 a | 0.01 ± 0.001 c | 0.16 ± 0.004 b |
| 8 | Protocatechuic | n.d. | n.d. | 0.15 ± 0.004 |
| 9 | Chlorogenic | 0.97 ± 0.024 b | 0.27 ± 0.007 c | 1.40 ± 0.035 a |
| 10 | Vanillic | 0.09 ± 0.002 c | 0.73 ± 0.018 a | 0.16 ± 0.004 b |
| 11 | Caffeic | 0.13 ± 0.003 b | n.d. | 0.23 ± 0.006 a |
| 12 | Syringic | 0.20 ± 0.005 b | 0.08 ± 0.002 c | 0.39 ± 0.010 a |
| 13 | p-Coumaric | 0.24 ± 0.006 b | n.d. | 1.35 ± 0.034 a |
| 14 | Ferulic | 0.11 ± 0.003 b | 0.07 ± 0.002 b | 1.31 ± 0.033 a |
| 15 | Salicilylic | 6.19 ± 0.155 a | 0.07 ± 0.002 c | 3.81 ± 0.095 b |
| 16 | Rosmarinic | 10.32 ± 0.258 a | 0.13 ± 0.003 c | 2.01 ± 0.050 b |
| Plant Extract | ABTS-Assay, mmol TE/g dw 1 | DPPH-Assay, mmol TE/g dw | FRAP-Assay, mmol TE/g dw | CUPRAC-Assay, mmol TE/g dw |
|---|---|---|---|---|
| Leaf | 299.54 ± 4.29 b | 225.62 ± 3.36 b | 906.01 ± 1.53 a | 548.07 ± 1.54 b |
| Flower | 893.14 ± 1.54 a | 729.72 ± 2.04 a | 661.48 ± 1.50 b | 789.54 ± 2.19 a |
| Stem bark | 31.24 ± 1.01 c | 24.96 ± 1.52 c | 16.65 ± 1.02 c | 10.22 ± 0.53 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andonova, T.; Muhovski, Y.; Slavov, I.; Vrancheva, R.; Georgiev, V.; Apostolova, E.; Naimov, S.; Mladenov, R.; Pavlov, A.; Dimitrova-Dyulgerova, I. Phenolic Profile, Antioxidant and DNA-Protective Capacity, and Microscopic Characters of Ailanthus altissima Aerial Substances. Plants 2023, 12, 920. https://doi.org/10.3390/plants12040920
Andonova T, Muhovski Y, Slavov I, Vrancheva R, Georgiev V, Apostolova E, Naimov S, Mladenov R, Pavlov A, Dimitrova-Dyulgerova I. Phenolic Profile, Antioxidant and DNA-Protective Capacity, and Microscopic Characters of Ailanthus altissima Aerial Substances. Plants. 2023; 12(4):920. https://doi.org/10.3390/plants12040920
Chicago/Turabian StyleAndonova, Tsvetelina, Yordan Muhovski, Iliya Slavov, Radka Vrancheva, Vasil Georgiev, Elena Apostolova, Samir Naimov, Rumen Mladenov, Atanas Pavlov, and Ivanka Dimitrova-Dyulgerova. 2023. "Phenolic Profile, Antioxidant and DNA-Protective Capacity, and Microscopic Characters of Ailanthus altissima Aerial Substances" Plants 12, no. 4: 920. https://doi.org/10.3390/plants12040920
APA StyleAndonova, T., Muhovski, Y., Slavov, I., Vrancheva, R., Georgiev, V., Apostolova, E., Naimov, S., Mladenov, R., Pavlov, A., & Dimitrova-Dyulgerova, I. (2023). Phenolic Profile, Antioxidant and DNA-Protective Capacity, and Microscopic Characters of Ailanthus altissima Aerial Substances. Plants, 12(4), 920. https://doi.org/10.3390/plants12040920








