Conserving Citrus Diversity: From Vavilov’s Early Explorations to Genebanks around the World
Abstract
1. Introduction
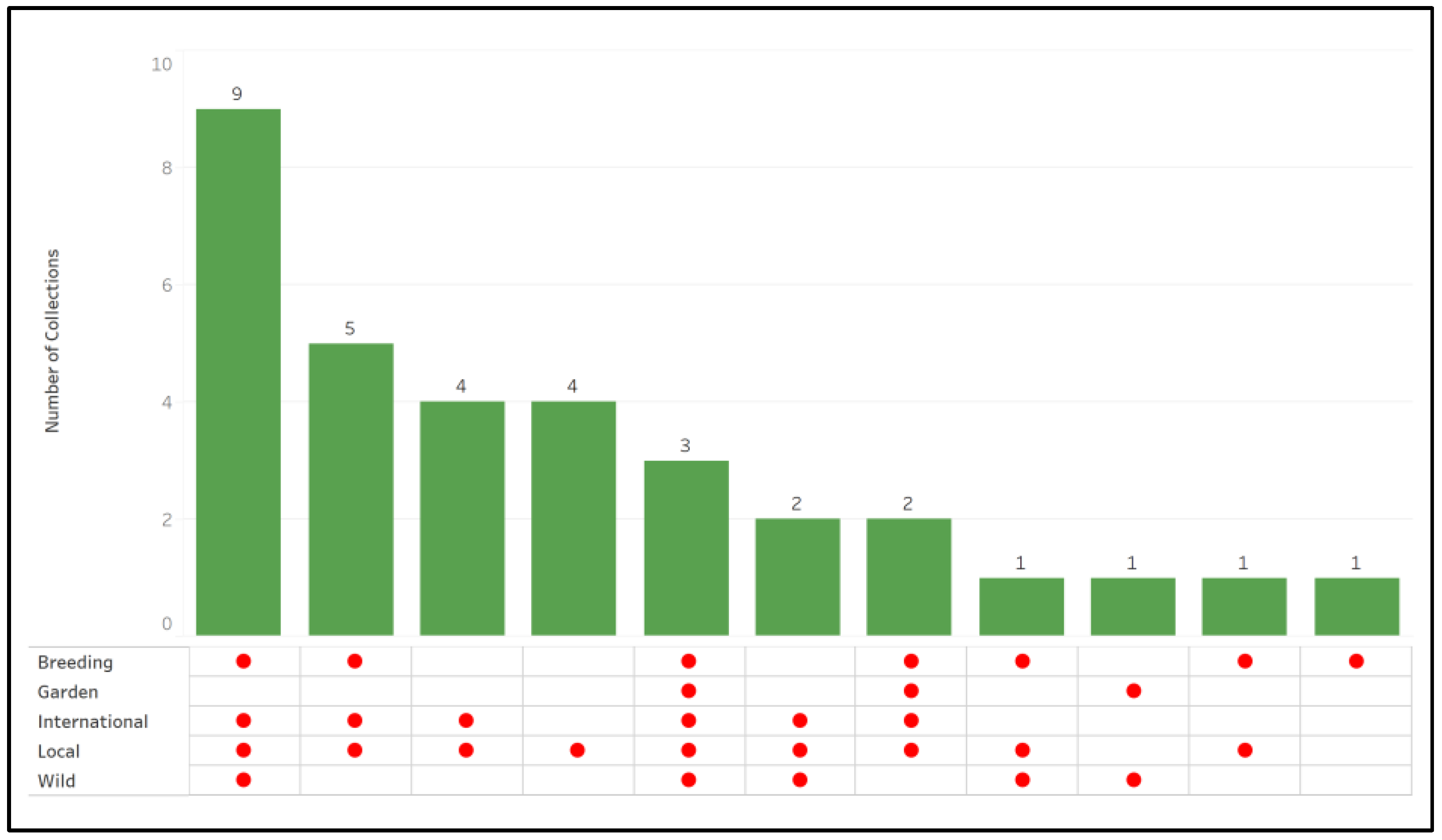
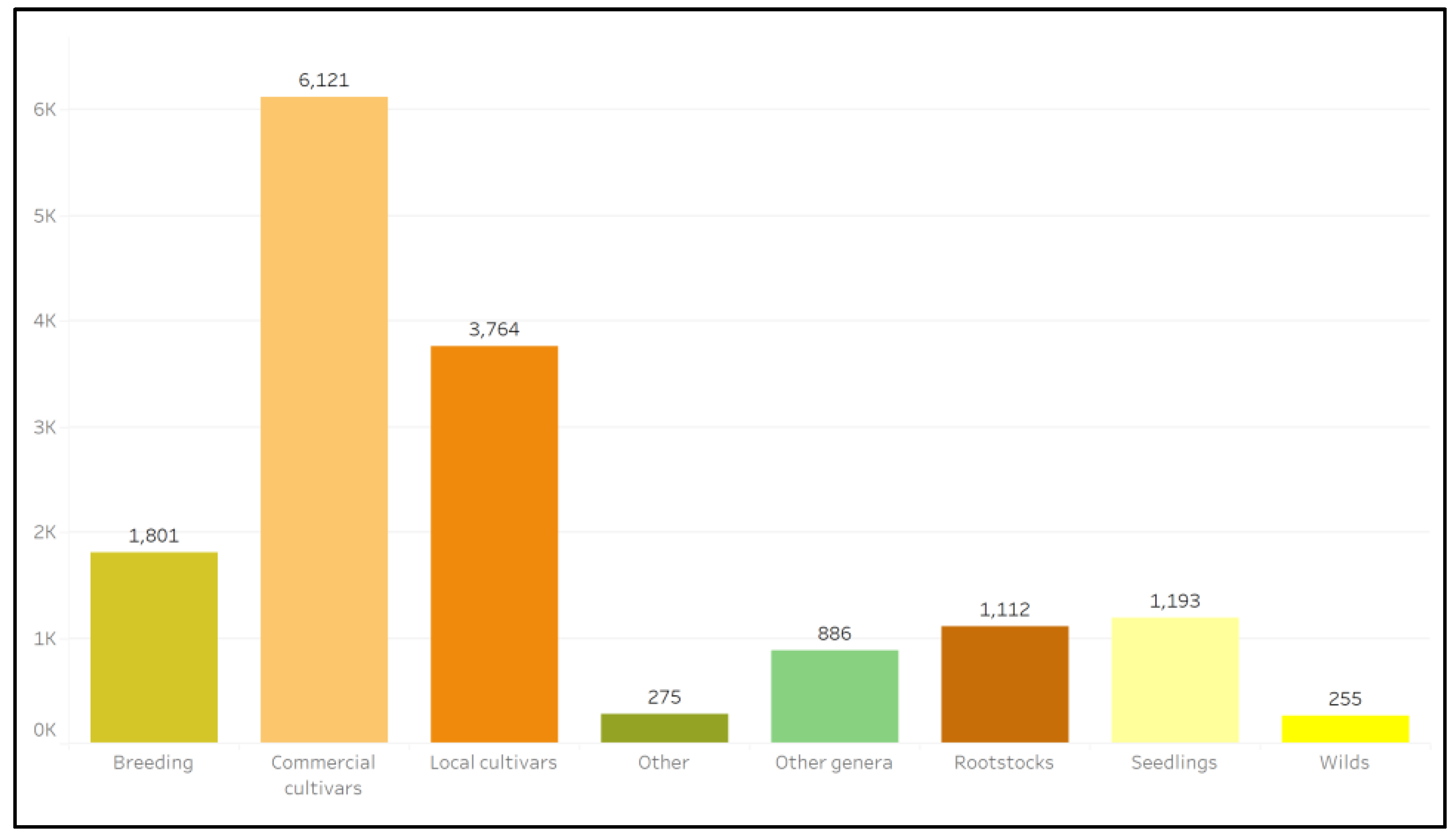
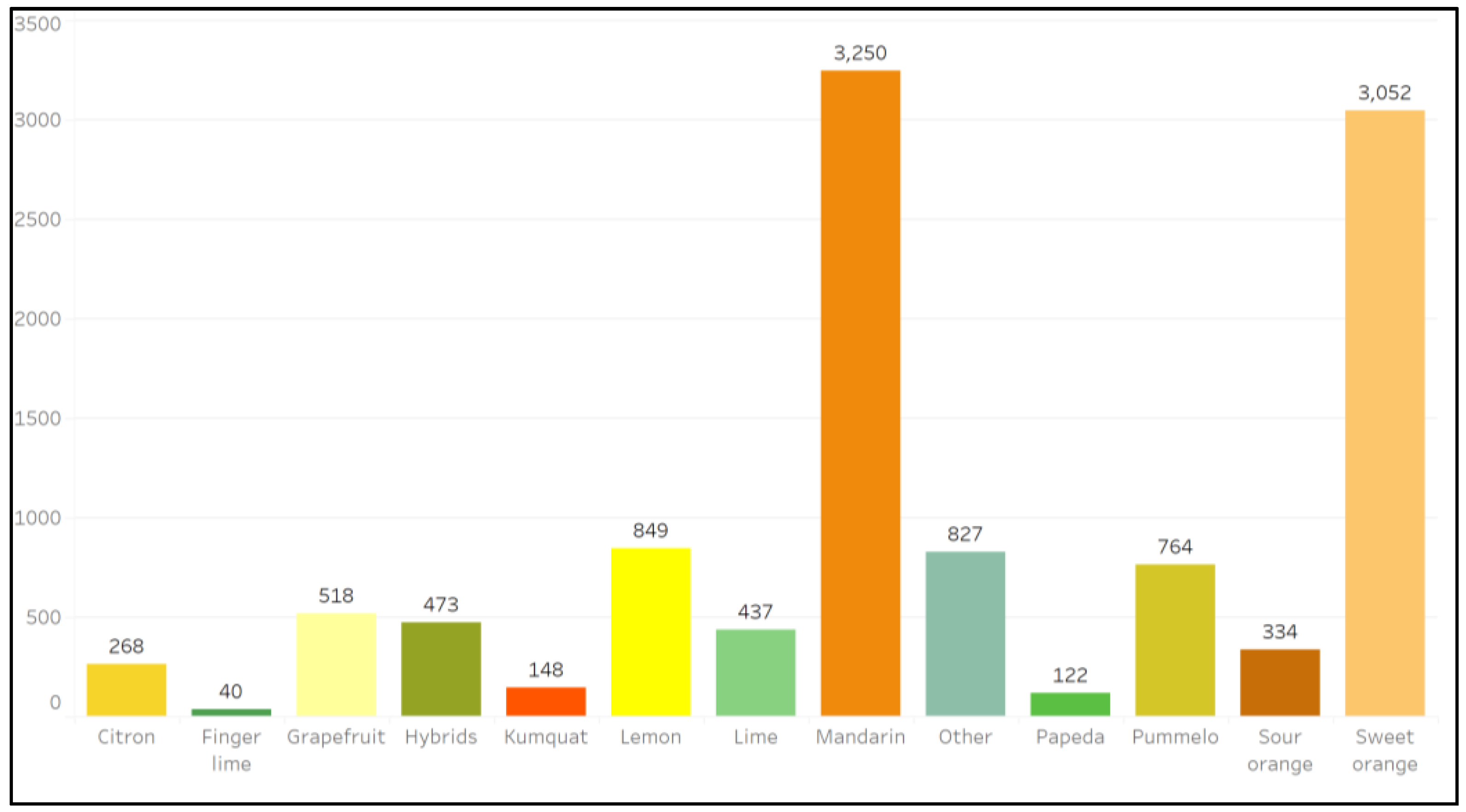
2. Results
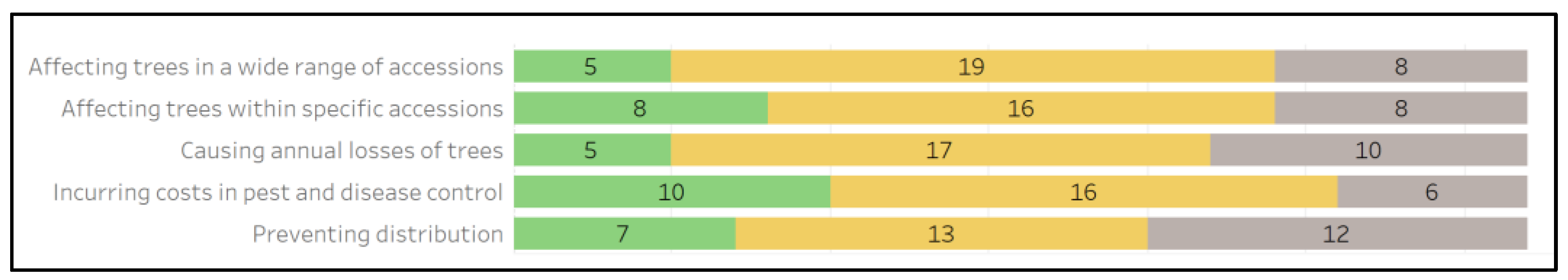
2.1. Collection Health
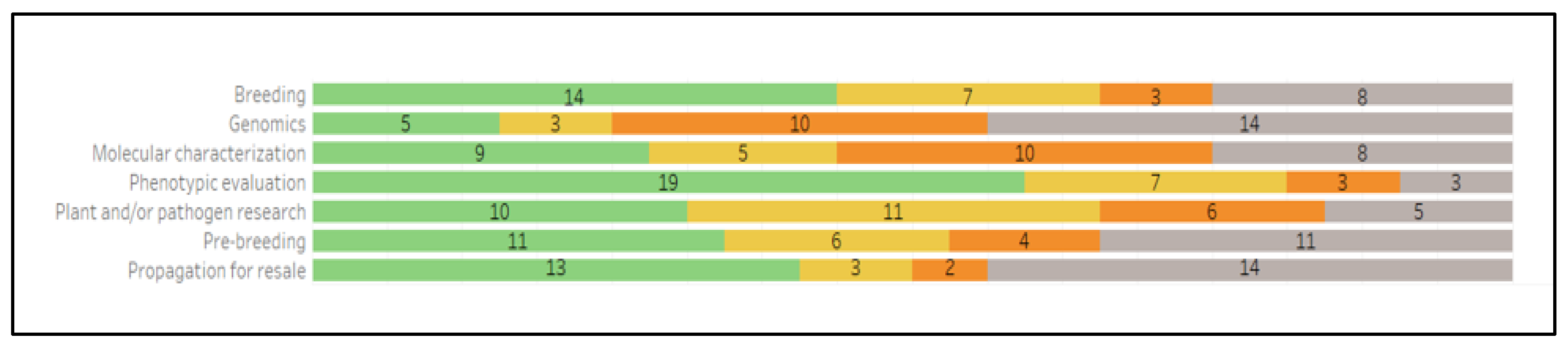
2.2. Collection Evaluation and Characterization
2.3. Data Availability
2.4. Collection Distribution
2.5. Resources
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAOSTAT. License: CC BY-NC-SA 3.0 IGO. 2022. Available online: https://www.fao.org/faostat/en/#home (accessed on 9 October 2022).
- Kruse, J. Citrus. In Mansfelds’ Encyclopedia of Agricultural Crops; Hanelt, P., Ed.; 2001; Volume 2, p. 1019. Available online: https://mansfeld.ipk-gatersleben.de/apex/f?p=257:3:15145524718993::::P2_NAMEID,P2_NAME:7578,Citrus%20L (accessed on 8 February 2023).
- Krueger, R.R.; Navarro, L. Citrus germplasm resources and their use. In Citrus Genetics, Breeding, and Biotechnology; Khan, I., Ed.; CABI: Cambridge, UK, 2007; pp. 45–140. [Google Scholar]
- Wu, G.A.; Terol, J.; Ibanez, V.; López-García, A.; Pérez-Román, E.; Borredá, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Talon, M.; Wu, G.A.; Gmitter, F.G., Jr.; Rokhsar, D.S. The origin of citrus. In The Genus Citrus; Talon., M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 9–31. [Google Scholar] [CrossRef]
- Ramadugu, C.; Keremane, M.L.; Halbert, S.E.; Duan, Y.P.; Roose, M.L.; Stover, E.; Lee, R.F. Long-term field evaluation reveals Huanglongbing resistance in Citrus relatives. Plant Disease 2016, 100, 1858–1869. [Google Scholar] [CrossRef]
- Huang, M.; Roose, M.L.; Yu, Q.; Du, D.; Yu, Y.; Deng, Z.; Stover, E.; Gmitter, F.G., Jr. Construction of high-density genetic maps and detection of QTLs associated with Huanglongbing tolerance in citrus. Front. Plant Sci. 2018, 9, 1694. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Roose, M.L.; Yu, Q.; Stover, E.; Hall, D.G.; Deng, Z.; Gmitter, F.G., Jr. Mapping of QTLs and candidate genes associated with multiple phenotypic traits for Huanglongbing tolerance in citrus. Hortic. Plant J. 2022, in press. [Google Scholar] [CrossRef]
- Stover, E.; Gmitter, F.G., Jr.; Grosser, J.; Baldwin, E.; Wu, G.A.; Bai, J.; Wang, Y.; Chaires, P.; Motamayor, J.C. Rationale for reconsidering current regulations restricting the use of hybrids in orange juice. Hortic. Res. 2020, 7, 38. [Google Scholar] [CrossRef]
- Hummer, K.E.; Hancock, J.F. Vavilovian centers of plant diversity: Implications and impacts. HortScience 2015, 50, 780–783. [Google Scholar] [CrossRef]
- Loskutov, I.G. Vavilov and His institute. A History of the World Col1ection of Plant Genetic Resources in Russia; International Plant Genetic Resources Institute: Rome, Italy, 1999; p. 188. [Google Scholar]
- Vavilov, N.I. Five Continents; Love, D., Translator; International Plant Genetic Resources Institute: Rome, Italy, 1997. [Google Scholar]
- Vavilov, N.I. Theoretical basis for plant breeding. In Origin and Geography of Cultivated Plants, The Phytogeographical Basis for Plant Breeding; Love, D., Translator; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Volk, G.; Samarina, L.; Kulyan, R.; Gorshkov, V.; Malyarovskaya, V.; Ryndin, A.; Polek, M.L.; Krueger, R.; Stover, E. Citrus genebank collections: International collaboration opportunities between the US and Russia. Genet. Resour. Crop Evol. 2018, 65, 433–447. [Google Scholar] [CrossRef]
- Katkoff, V. The Soviet citrus industry. Southern Econ. J. 1952, 18, 374–380. Available online: https://www.jstor.org/stable/1054452 (accessed on 8 February 2023). [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. Available online: https://www.sipav.org/main/jpp/index.php/jpp/article/view/828 (accessed on 8 February 2023).
- Gottwald, T.R. Current epidemiological understanding of citrus huanglongbing. Ann. Rev. Phytopathol. 2010, 48, 119–139. Available online: http://www.stoppinginvasives.org/dotAsset/4243a906-3518-4080-915f-704950d3ff72.pdf (accessed on 8 February 2023). [CrossRef]
- Wang, N. The citrus huanglongbing crisis and potential solutions. Molec. Plant 2019, 12, 607–609. [Google Scholar] [CrossRef]
- Graham, J.; Gottwald, T.; Setamou, M. Status of Huanglongbing (HLB) outbreaks in Florida, California and Texas. Trop. Plant Pathol. 2020, 45, 265–278. [Google Scholar] [CrossRef]
- Moreno, P.; Ambrós, S.; Albiach-Martí, M.R.; Guerri, J.; Pena, L. Citrus tristeza virus: A pathogen that changed the course of the citrus industry. Mol. Plant Pathol. 2008, 9, 251–268. [Google Scholar] [CrossRef]
- Cacciola, S.O.; Lio, G.M.D.S. Management of citrus diseases caused by Phytophthora spp. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Ciancio, A., Mukerji, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 61–84. [Google Scholar]
- Volk, G.M.; Gmitter, F.G., Jr.; Krueger, R. A global strategy for the conservation and use of citrus genetic resources. Glob. Crop Divers. Trust. 2023, accepted. [Google Scholar]
- Vapnek, J. Legislatively Establishing a Health Certification Programme for Citrus; FAO Legal Paper (81). 2009. Available online: https://ssrn.com/abstract=3354974 (accessed on 8 February 2023).
- Obreza, M.; GENESYSR: Genesys PGR Client. R Package Version 1.0.0. 2019. Available online: https://www.genesys-pgr.org/ (accessed on 8 February 2023).
- FAO. WIEWS—World Information and Early Warning System on Plant Genetic Resources for Food and Agriculture. 2023. Available online: https://www.fao.org/wiews/data/ex-situ-sdg-251/overview/en/ (accessed on 8 February 2023).
- Walters, C.; Maschinski, J. The Difference between Orthodox, Intermediate, and Recalcitrant Seed. 2023. Available online: https://saveplants.org/best-practices/difference-between-orthodox-intermediate-and-recalcitrant-seed/#intermediate (accessed on 8 February 2023).
- Graiver, N.; Califano, A.; Zaritsky, N. Partial dehydration and cryopreservation of Citrus seeds. J. Sci. Food Agric. 2021, 92, 544–2550. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.G.; Noor, N.M.; Kim, H.H.; Rao, V.R.; Engelmann, F. Cryopreservation of Citrus aurantifolia seeds and embryonic axes using a desiccation protocol. CryoLetters 2002, 23, 309–316. [Google Scholar]
- FAO. Genebank Standards for Plant Genetic Resources for Food and Agriculture, Revised edition; FAO: Rome, Italy, 2014. [Google Scholar]
- Reed, B.M.; Engelmann, F.; Dulloo, M.E.; Engels, J.M.M. Technical Guidelines for the Management of Field and In Vitro Germplasm Collections; IPGRI Handbooks for Genebanks No. 7; International Plant Genetic Resources Institute: Rome, Italy, 2004. [Google Scholar]
- Frison, E.A.; Taher, M.M. (Eds.) FAO/IBPGR Technical Guidelines for the Safe Movement of Citrus Germplasm; Bioversity International: Rome, Italy, 1991. [Google Scholar]
- Navarro, L.; Juárez, J. Shoot-tip grafting in vitro: Impact in the citrus industry and research applications. In Citrus Genetics, Breeding, and Biotechnology; Khan, I., Ed.; CABI: Cambridge, UK, 2007; pp. 353–364. [Google Scholar]
- Gergerich, R.C.; Welliver, R.A.; Gettys, S.; Osterbauer, N.K.; Kamenidou, S.; Martin, R.R.; Golino, D.A.; Eastwell, K.; Fuchs, M.; Vidalakis, G.; et al. Safeguarding fruit crops in the age of agricultural globalization. Plant Dis. 2015, 99, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Gumpf, D.J. Citrus quarantine, California. In Containment Facilities and Safeguards for Exotic Plant Pathogens and Pests; American Phytopathological Society Press: St. Paul, MN, USA, 1999; pp. 151–156. [Google Scholar]
- IPGRI Descriptors for Citrus. International Plant Genetic Resources Institute, Rome, Italy. 1999. Available online: https://www.bioversityinternational.org/e-library/publications/detail/descriptors-for-citrus-citrus-spp/ (accessed on 8 February 2023).
- USDA. GRIN-Global National Plant Germplasm System. Citrus Descriptors. 2022. Available online: http://npgsweb.ars-grin.gov/gringlobal/cropdetail?type=descriptor&id=112 (accessed on 25 October 2022).
- Caruso, M. Pomological diversity of the Italian blood orange germplasm. Sci. Hort. 2016, 213, 331–339. [Google Scholar] [CrossRef]
- Russo, R.; Caruso, M.; Arlotta, C.; Roberta Lo Piero, A.; Nicolosi, E.; Di Silvestro, S. Identification of field tolerance and resistance to mal secco disease in a Citrus germplasm collection in Sicily. Agronomy 2020, 10, 1806. [Google Scholar] [CrossRef]
- Aparecida da Cruz, M.; Neves, C.S.V.J.; Uilian de Carvalho, D.; Colombo, R.C.; Bai, J.; Yada, I.F.U.; Leite, R.P.; Tazima, Z.H. Five rootstocks for “Emperor” mandarin under subtropical climate in Southern Brazil. Front. Plant Sci. 2021, 20, 777871. [Google Scholar] [CrossRef]
- Aparecida da Cruz, M.; Neves, C.S.V.J.; Uilian de Carvalho, D.; Colombo, R.C.; Leite, R.P.; Tazima, Z.H. ‘Navelina’ sweet orange trees on five rootstocks in Northern Parana state, Brazil. Rev. Bras. Frutic. 2019, 41, 1–9. [Google Scholar] [CrossRef]
- Stenzel, N.M.C.; Veves, C.S.V.J.; Gomes, J.C.; Medina, C.C. Performance of ‘Ponkan’ mandarin on seven rootstocks in Southern Brazil. HortScience 2003, 38, 176–178. [Google Scholar] [CrossRef]
- Tazima, Z.H.; Neves, C.S.V.J.; Yada, I.F.U.; Leite, R.P. Performance of ‘Okitsu’ Satsuma mandarin on nine rootstocks. Sci. Agric. 2013, 70, 422–427. [Google Scholar] [CrossRef]
- Tazima, Z.; Neves, C.S.V.J.; Yada, I.F.U.; Leite, R.P. Performance of ‘Okitsu’ satsuma mandarin trees on different rootstocks in Northwestern Parana State. Semina: Ciencias Agrarias 2014, 35, 2297–2308. [Google Scholar] [CrossRef]
- UPOV International Union for the Protection of New Varieties of Plants. Citrus L. 2003. Available online: http://www.upov.int/en/publications/tg-rom/tg201/tg_201_1.pdf (accessed on 8 February 2023).
- UPOV International Union for the Protection of New Varieties of Plants. Kumquat. 2013. Available online: http://www.upov.int/edocs/tgdocs/en/tg290.pdf (accessed on 8 February 2023).
- Federici, C.T.; Fang, D.Q.; Scora, R.W.; Roose, M.L. Phylogenetic relationships within the genus Citrus (Rutaceae) and related genera as revealed by RFLP and RAPD analysis. Theor. Appl. Genet. 1998, 96, 812–822. [Google Scholar] [CrossRef]
- Sanabam, R.; Singh, N.S.; Sahoo, D.; Devi, H.S. Genetic relationship of rough lemon landraces and under-utilised citrus genotypes from North-East India revealed by SSR and RAPD markers. Trees 2018, 32, 1043–1059. [Google Scholar] [CrossRef]
- Barkley, N.A.; Roose, M.L.; Krueger, R.R.; Federici, C.T. Assessing genetic diversity and population structure in a citrus germplasm collection utilizing simple sequence repeat markers (SSRs). Theor. Appl. Genet. 2006, 112, 1519–1531. [Google Scholar] [CrossRef]
- Luro, F.L.; Costantino, G.; Terol, J.; Argout, X.; Allario, T.; Wincker, P.; Talon, M.; Ollitrault, P.; Morillon, R. Transferability of the EST-SSRs developed on Nules clementine (Citrus clementina Hort ex Tan) to other Citrus species and their effectiveness for genetic mapping. BMC Genom. 2008, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Jannatil, M.; Fotouhil, R.; Abad, A.P.; Salehi, Z. Genetic diversity analysis of Iranian citrus varieties using micro satellite (SSR) based markers. J. Hort. Forest. 2009, 1, 120–125. [Google Scholar] [CrossRef]
- Rohini, M.R.; Sankaran, M.; Rajkumar, S.; Prakash, K.; Gaikwad, A.; Chaudhury, R.; Malik, S.K. Morphological characterization and analysis of genetic diversity and population structure in Citrus × jambhiri Lush. using SSR markers. Genet. Resour. Crop Evol. 2020, 67, 1259–1275. [Google Scholar] [CrossRef]
- Mallick, M.; Bharadwaj, C.; Srivastav, M.; Sharma, N.; Awasthi, O.P. Molecular characterization of Kinnow mandarin clones and mutants using cross genera SSR markers. Indian J. Biotechnol. 2017, 16, 244–249. Available online: http://nopr.niscpr.res.in/handle/123456789/42916 (accessed on 8 February 2023).
- Shahzadi, K.; Naz, S.; Riaz, S. Assessing genetic diversity of Pakistani Citrus varieties using microsatellite markers. J. Animal Plant Sci. 2014, 24, 1752–1757. [Google Scholar]
- Ahmed, D.; Comte, A.; Curk, F.; Costantino, G.; Luro, F.; Dereeper, A.; Mournet, P.; Froelicher, Y.; Ollitrault, P. Genotyping by sequencing can reveal the complex mosaic genomes in gene pools resulting from reticulate evolution: A case study in diploid and polyploid citrus. Ann. Bot. 2019, 123, 1231–1251. [Google Scholar] [CrossRef]
- Hiraoka, Y. Application of High-Density SNP Genotyping Array in Citrus Germplasm Characterization and Genetic Dissection of Traits. PhD Dissertation, University of California Riverside, Riverside, CA, USA, 2020. [Google Scholar]
- Terol, J.; Tadeo, F.; Ventimilla, D.; Talon, M. An RNA-Seq-based reference transcriptome for Citrus. Plant Biotechnol. J. 2016, 14, 938–950. [Google Scholar] [CrossRef]
- Carbonell-Caballero, J.; Alonso, R.; Ibañez, V.; Terol, J.; Talon, M.; Dopazo, J. A phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domestic species within the genus Citrus. Mol. Biol. Evol. 2015, 32, 2015–2035. [Google Scholar] [CrossRef] [PubMed]
- Samarina, L.S.; Kulyan, R.V.; Koninskaya, N.G.; Gorshkov, V.M.; Ryndin, A.V.; Hanke, M.-V.; Flachowsky, H.; Reim, S. Genetic diversity and phylogenetic relationships among citrus germplasm in the Western Caucasus assessed with SSR and organelle DNA markers. Sci. Hortic. 2021, 288, 110355. [Google Scholar] [CrossRef]
- Li, Q.; Qi, J.; Qin, X.; Dou, W.; Lei, T.; Hu, A.; Jia, R.; Jiang, G.; Zou, X.; Long, Q.; et al. CitGVD: A comprehensive database of citrus genomic variations. Hortic. Res. 2020, 7, 12. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Liu, S.; Huang, Y.; Guo, Y.-X.; Xie, W.-Z.; Liu, H.; Qamar, M.T.; Xu, Q.; Chen, L.-L. Citrus Pan-Genome to Breeding Database (CPBD): A comprehensive genome database for citrus breeding. Molec. Plant 2022, 15, 1503–1505. [Google Scholar] [CrossRef]
- Staton, M.; Cannon, E.; Sanderson, L.-A.; Wegrzyn, J.; Anderson, T.; Buehler, S.; Cobo-Simón, I.; Faaberg, K.; Grau, E.; Guignon, V.; et al. Tripal, a community update after 10 years of supporting open source, standards-based genetic, genomic and breeding databases. Brief. Bioinform. 2021, 22, bbab238. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volk, G.M.; Gmitter, F.G., Jr.; Krueger, R.R. Conserving Citrus Diversity: From Vavilov’s Early Explorations to Genebanks around the World. Plants 2023, 12, 814. https://doi.org/10.3390/plants12040814
Volk GM, Gmitter FG Jr., Krueger RR. Conserving Citrus Diversity: From Vavilov’s Early Explorations to Genebanks around the World. Plants. 2023; 12(4):814. https://doi.org/10.3390/plants12040814
Chicago/Turabian StyleVolk, Gayle M., Frederick G. Gmitter, Jr., and Robert R. Krueger. 2023. "Conserving Citrus Diversity: From Vavilov’s Early Explorations to Genebanks around the World" Plants 12, no. 4: 814. https://doi.org/10.3390/plants12040814
APA StyleVolk, G. M., Gmitter, F. G., Jr., & Krueger, R. R. (2023). Conserving Citrus Diversity: From Vavilov’s Early Explorations to Genebanks around the World. Plants, 12(4), 814. https://doi.org/10.3390/plants12040814







